Abstract
Background:
This systematic review aims to evaluate the efficacy of Gegen Qinlian Decoction (GQD) for ulcerative colitis (UC).
Methods:
PubMed, EMBASE, Springer LINK, Cochrane Library, the China National Knowledge Infrastructure, Chongqing Weipu Database for Chinese Technical Periodicals, Wan-fang Database, and Chinese Biomedicine Database were searched from their inception to December 2018 for randomized controlled trials comparing the use of GQD alone or in combination with western medicine (WM) with that of WM therapies for UC. Outcomes on the therapy's effectiveness rate, ulcerative colitis endoscopic index of severity (UCEIS), recurrence rate, and adverse events were extracted and analyzed by Review Manager 5.3 software. Meta-analysis was combined with fixed or random-effects model, and risk ratios (RR) and 95% confidence intervals (CI) were calculated for all outcomes. Two researchers independently reviewed each trial to determine its inclusion. The Cochrane risk of bias assessment tool was used for quality assessment.
Results:
We included 22 trials involving 2028 patients with UC. When compared with WM therapy, GQD significantly improved the clinical effectiveness (n = 591, RR = 1.21, 95% CI: 1.12–1.31, P < .00001) and recurrence rate (n = 94, RR = 0.23, 95% CI: 0.10–0.54, P = .0006). GQD plus WM was more effective in improving the clinical effectiveness (n = 1337, RR = 1.21, 95% CI: 1.16–1.27, P < .00001), and decreasing UCEIS scores (n = 384, mean difference = −0.63, 95% CI: −1.26–−0.01, P = .05), recurrence rate (n = 179, RR = 0.18, 95% CI: 0.06–0.61, P = .006). In addition, the adverse events for GQD (n = 238, RR = 0.20, 95% CI: 0.02–1.68, P = .14) and GQD plus WM (n = 427, RR = 0.37, 95% CI: 0.15–0.90, P = .03) was significantly lower than that for WM alone. Noted adverse events primarily included gastrointestinal symptoms, headache, dizziness, and leukocytopenia.
Conclusions:
This meta-analysis shows that GQD used alone or in combination with WM might have potential benefits in curing UC. However, there is no sufficient evidence to draw definite conclusion supporting the effect of GQD for UC due to poor methodological quality of the included trials. More rigorously designed investigations and studies with large sample sizes should be conducted to establish clinical evidence further.
Keywords: Gegen Qinlian Decoction, inflammatory bowel disease, meta-analysis, ulcerative colitis
1. Introduction
Ulcerative colitis (UC) is a chronic idiopathic intestinal disorder with an unclear etiology, characterized by recurring episodes of mucosal inflammation restricted to the rectum and colon.[1] The typical manifestations of UC include bloody diarrhea, abdominal pain, and rectal urgency.[2] At present, the pathogenesis of UC is regarded as multifactorial, with genetic, environmental, and gut microbiome influences considered to play a role.[3] The incidence and prevalence of UC also differ by region, with a maximal annual incidence of 19.2 to 24.3 per 100,000 in Europe and North America and only 6.3 per 100,000 in Asia and other developing countries.[4] UC has become a global disease, with an accelerating incidence in western countries and with rates in newly industrialized countries evidencing an even greater increase in incidence.[5]
Current conventional treatment for UC primarily includes amino salicylic acid, steroid hormones, and immune-regulatory medications, and it may help in maintaining partial remission for patients.[6,7,8] However, these therapies have poor long-term therapeutic efficacy, and even when medicated, patients with UC often remain chronically unwell and suffer from a high short-term recurrence rate.[9,10] In recent years, new biological agents have been shown to be effective in inducing remission in patients with moderate to severe active UC, even preventing the need for a colectomy in some cases.[11,12] However, considering the need for the long-term maintenance of UC treatment, the high financial cost of these biological therapies is a barrier for many patients.[13] Thus, conventional therapies alone may not fully meet the needs of UC patients. A long-term strategy for managing or even slowing the progression of UC with minimal complications remains to be developed.
Contemporary research has suggested that Chinese herbal medicines can be adopted as an auxiliary treatment for UC, with potential benefits including high efficacy and acceptability by patients, safety, and a relatively low associated financial cost.[14] According to the traditional Chinese medicine (TCM) theory, UC, which is a Yangming meridians’ disease, is categorized as Chang Pi (spouting bleeding from the anus).[15] Gegen Qinlian decoction (GQD), which stems from Zhang Zhongjing's Treatise on febrile diseases, is a well-known Chinese medicinal formula that dates to the Eastern Han dynasty, during which it was used for approximately 2000 years. During this time, GQD was used for the management of the symptoms of infectious diseases such as pyrexia and diarrhea.[16] The Chinese medicine preparation of GQD consists of 4 Chinese medicinal herbs: Radix Puerariae (Gegen in Chinese), Radix Scutellariae (Huangqin in Chinese), Rhizoma Coptidis (Huanglian in Chinese), and Radix Glycyrrhizae (Gancao in Chinese).[17] Many clinical studies have shown that GQD alone or in combination with other therapeutics is widely used to treat UC across the Chinese mainland.[18,19] Experimental trials have further reported that GQD may successfully be used for the management of UC and gastrointestinal function and has anti-inflammatory and antibacterial properties.[20,21] However, only a small number of cases have been included in most clinical trials on this subject. Furthermore, there are no systematic evaluations of the efficacy and safety of GQD for the treatment of UC. Given this background, we conducted the present systematic review, which includes relevant randomized controlled trials (RCTs) assessing the efficacy and safety of GQD alone or in combination with western medicine (WM) for the treatment of UC.
2. Methods
2.1. Databases and search strategy
We searched all relevant articles published through December 2018 and available in the following electronic databases: PubMed, EMBASE, Springer LINK, Cochrane Library, the China National Knowledge Infrastructure, Chongqing Weipu Database for Chinese Technical Periodicals, Wan-fang database, and the Chinese Biomedicine Database. The search terms and text words were as follows: (“gegen qinlian” OR “gegen qinlian decoction” OR “gegen qinlian powder”) AND (“ulcerative colitis” OR “colitis” OR “colitis gravis” OR “ulcer colonitis” OR “inflammatory bowel disease”) AND (“randomized controlled trial” OR “random∗”).
2.2. Inclusion criteria
-
(1)
Type of study: Only RCTs were considered eligible.
-
(2)
Patients: Studies including individuals with UC or chronic UC diagnosed as per appropriate diagnostic criteria[22] and verified via colonoscopy and barium enema examination.
-
(3)
Intervention: The experimental group in included studies comprised recipients of GQD alone or in combination with WM therapeutics. There was no distinction between an oral or enema-based route of treatment. If modified by the addition of Chinese herbal additives, GQD was determined to be the primary herbal medicine on the basis of TCM syndrome differentiation.
-
(4)
Controls: patients received WM alone.
-
(5)
Outcomes: The primary outcome was the total effectiveness rate of the given treatment, while secondary outcomes included ulcerative colitis endoscopic index of severity (UCEIS),[23] the recurrence rate, and adverse events.
2.3. Exclusion criteria
-
(1)
Irrelevant studies,
-
(2)
Literature reviews,
-
(3)
Case and expert reports,
-
(4)
Animal studies,
-
(5)
Studies related to Crohn disease and other colitis diseases,
-
(6)
GQD combined with other decoction(s), and
-
(7)
Duplicate publications were excluded.
2.4. Literature selection and data extraction
On the basis of inclusion and exclusion criteria, 2 researchers independently reviewed all search results. Differences were resolved by a third party. Both researchers independently extracted data from the included studies. Data included author names, year of publication, study samples, interventional measures from experimental and control groups, efficacy evaluation indicators, treatment course, follow-up duration, and other methods.
2.5. Methodological quality assessment
The methodological quality of included studies was evaluated using the “risk of bias assessment tool” (Cochrane Handbook Version 5.3).[24] Evaluation metrics included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias.
2.6. Statistical analyses
Review Manager Software (Version 5.3) was used for data-analyses. Z-tests and I 2 tests were applied to assess the overall heterogeneity of included studies. A fixed-effect model or random-effect model was used across all studies. When no statistical heterogeneity was detected among trials (P > .10, I 2 < 50%), a fixed-effect model was employed. If clinical/methodological heterogeneity was detected, a random effect model was employed. A pooled relative risk (RR) was assessed with a 95% confidence interval (CI) for dichotomous data. If continuous data were available, a weighted mean difference (MD) or standardized mean difference was calculated. Subgroup analyses were conducted to evaluate the robustness of results when heterogeneity was present. Bias was assessed via a funnel plot.
3. Results
3.1. Search results
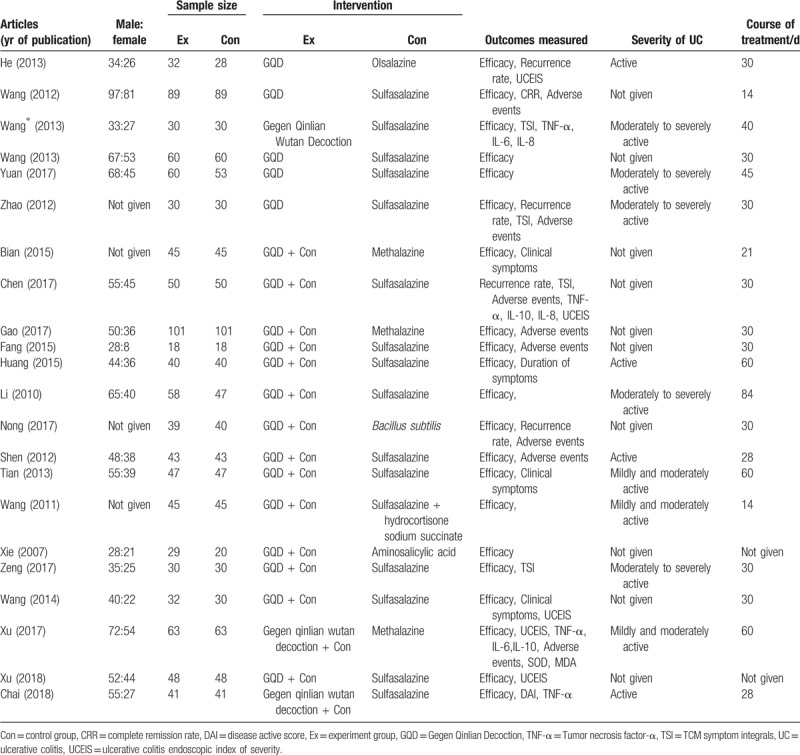
The workflow followed for study selection is illustrated in Figure 1. A total of 142 articles were retrieved according to the search strategy and data collection methods detailed above. Per our inclusion and exclusion criteria, 22 primary studies with a total of 2028 participants were included in the final systematic review. All included studies were published in China. The basic characteristics of these included studies are shown in Table 1.
Figure 1.
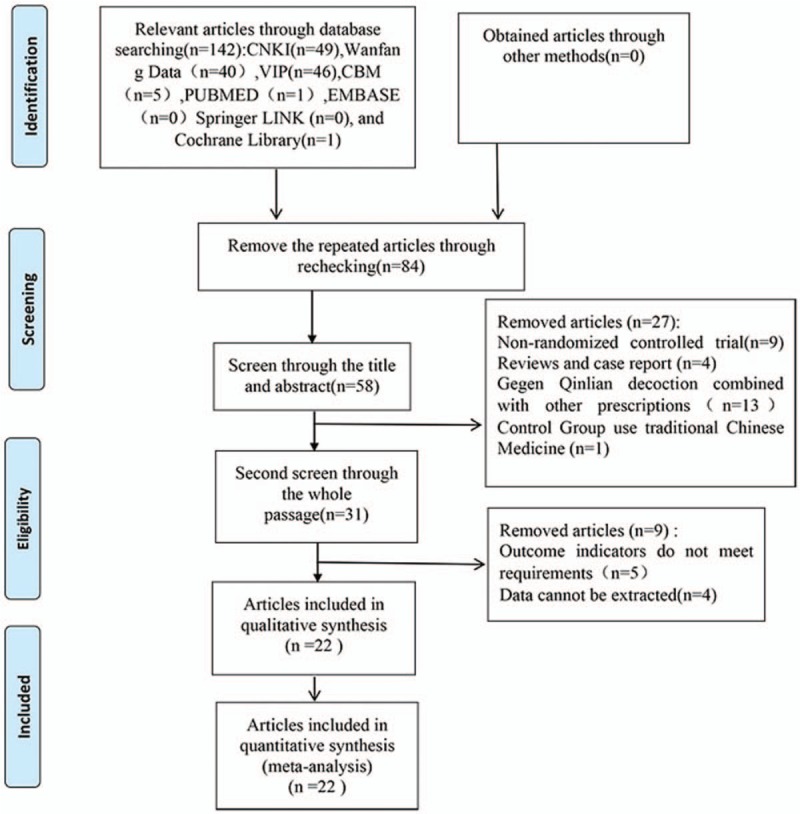
Literature retrieval process.
Table 1.
Characteristics of the included studies in a meta-analysis for determining the efficacy of Gegen Qinlian Decoction for ulcerative colitis treatment.
3.2. Quality evaluation of included articles
All of the 22 included RCTs[18,19,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44] reported no significant differences between experimental and control groups at baseline. However, only 5 studies[18,26,34,38,42] used a randomization technique (eg, random number table), while 1[33] used randomization based on registration order and 1[27] randomized according to the parity number. The remaining studies simply mentioned the term “random” within the text. Moreover, none of the 22 trials described double-blinding and/or allocation concealment, nor did they provide any information on drop-out or loss to follow-up, incomplete outcome data, selective reporting, or other biases. The risk bias assessment of methodological quality is shown in Figures 2 and 3.
Figure 2.
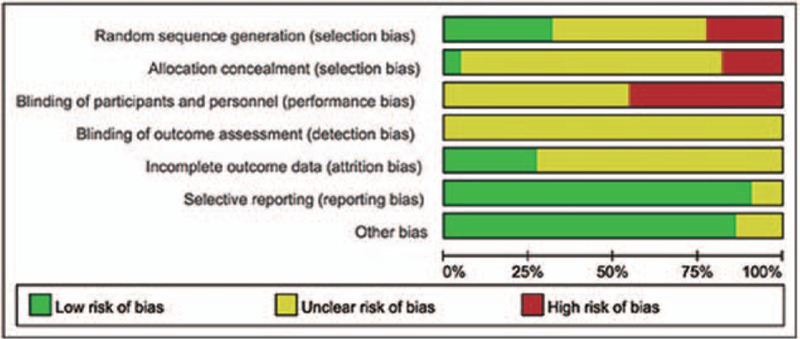
Graph showing the risk of bias in studies assessing the efficacy of Gegen Qinlian Decoction for ulcerative colitis treatment.
Figure 3.
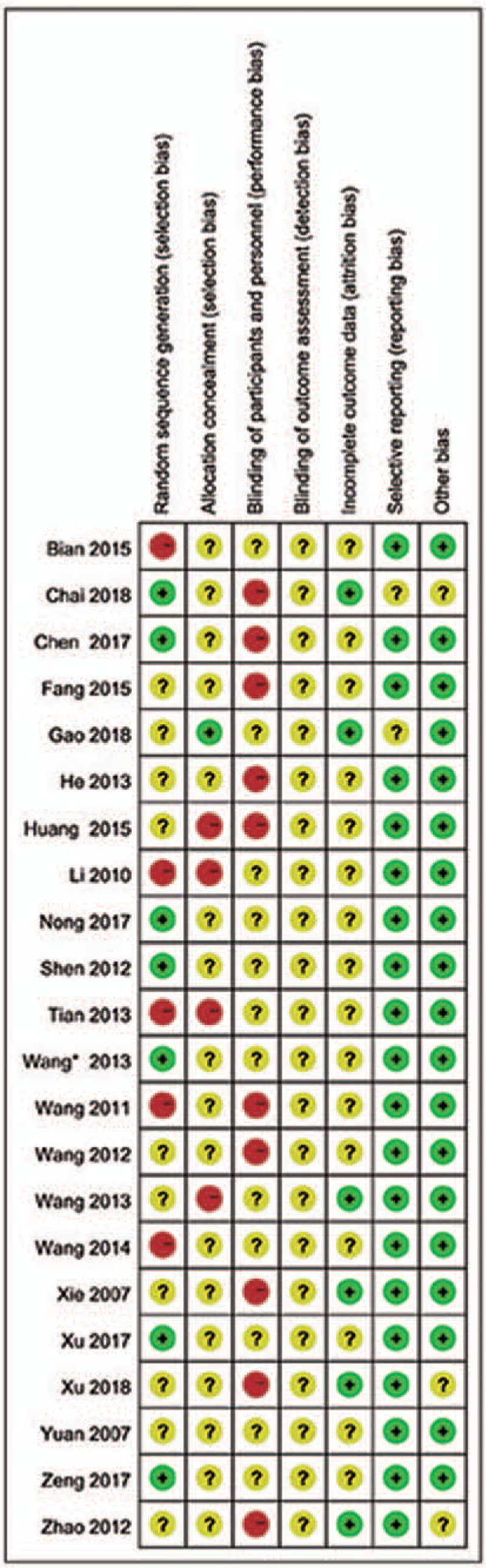
Risk of bias summary for studies assessing the efficacy of Gegen Qinlian Decoction for ulcerative colitis treatment.
3.3. Results of meta-analysis
3.3.1. Clinical effectiveness rate
3.3.1.1. GQD versus WM
Six trials[30,37,38,39,43,44] including 591 patients were evaluated for the overall clinical efficacy of GQD for UC treatment. No statistically significant heterogeneity was detected among the 6 trials comparing GQD and WM alone (P = .75, I 2 = 0%; Fig. 4A). The results of fixed effects modeling combined with effect sizes showed that the clinical effectiveness rate was significantly higher for the experimental group than for the control group (RR = 1.21, 95% CI: 1.12–1.31, P < .00001).
Figure 4.
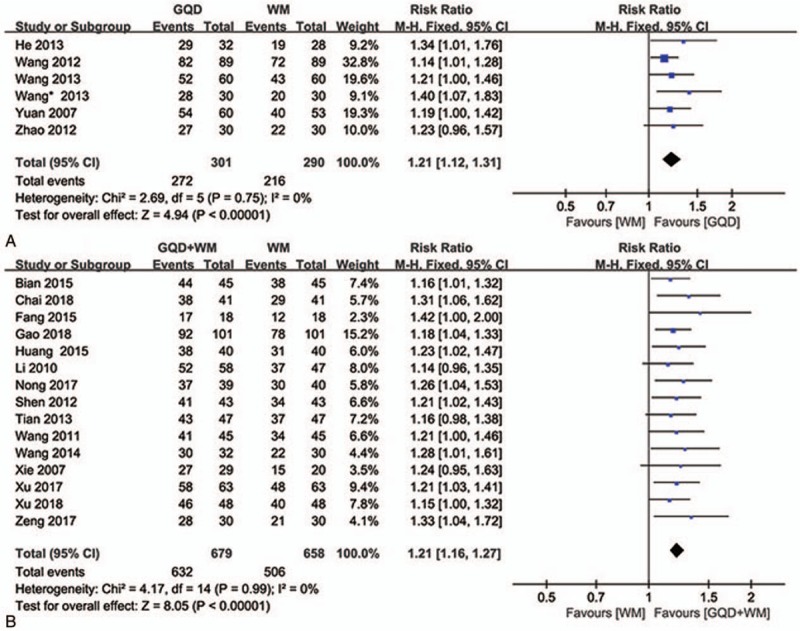
Forest plot showing the clinical efficacy for ulcerative colitis. (A: GQD vs WM, B: GQD + WM vs WM). GQD = Gegen Qinlian Decoction, WM, western medicine.
3.3.1.2. GQD + WM versus WM
In 15 studies[18,19,25,26,28,29,31,32,33,34,35,36,40,41,42] comparing GQD plus WM and WM alone, no statistically significant heterogeneity was detected (P = .99, I 2 = 0%; Fig. 4B). Furthermore, the results of fixed effects modeling combined with effect sizes demonstrated that the clinical effectiveness rate was significantly higher for the experimental group than for the control group (RR = 1.21, 95% CI: 1.16–1.27, P < .00001).
3.3.2. UCEIS
3.3.2.1. GQD versus WM
With regard to the comparison between GQD and WM alone, only 1 trial[30] reported that GQD reduced UCEIS to a greater extent than did WM alone (Fig. 5A).
Figure 5.
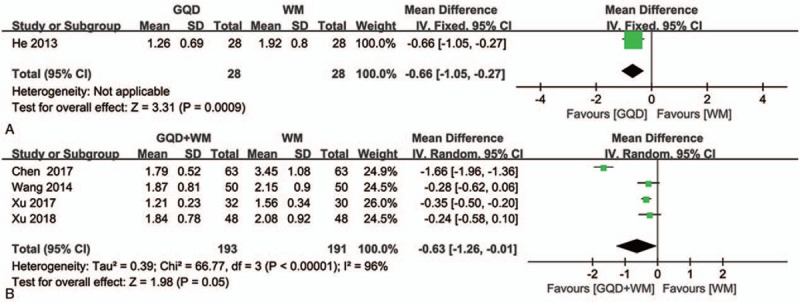
Forest plot showing the effects of Gegen Qinlian Decoction and Gegen Qinlian Decoction plus Western medicine therapy on UCEIS in patients with ulcerative colitis. (A: GQD vs WM, B: GQD + WM vs WM). GQD = Gegen Qinlian Decoction, UCEIS = ulcerative colitis endoscopic index of severity, WM, western medicine.
3.3.2.2. GQD + WM versus WM
Only 4 studies[19,27,41,42] reported data on changes in endoscopic index of severity in UC after GQD plus WM or WM alone. These data were found to be significantly heterogeneous (P < .00001, I 2 = 96%; Fig. 5B). Given this, a random-effects model was applied, from which we concluded that changes in UCEIS significantly differed between the experimental and control groups (MD = −0.63, 95%CI: −1.26 to −0.01, P = .05).
3.3.3. Recurrence rate
3.3.3.1. GQD versus WM
Two trials compared the recurrence rates between GQD and WM alone,[30,44] with no significant heterogeneity in data (P = .29, I 2 = 9%; Fig. 6A). The results of fixed effects modeling combined with effect sizes confirmed a significant difference between the experimental and control groups (RR = 0.23, 95% CI: 0.10–0.54, P = .0006), with the experimental group outperforming the control group.
Figure 6.
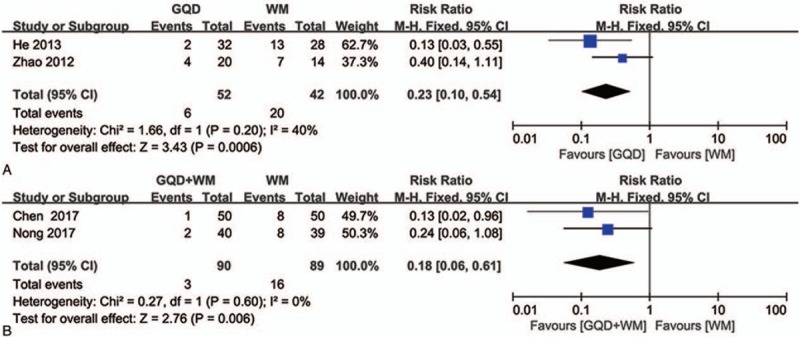
Forest plot showing the effect of Gegen Qinlian Decoction and Gegen Qinlian Decoction plus Western medicine therapy on the recurrence rate for ulcerative colitis. (A: GQD vs WM, B: GQD + WM vs WM). GQD = Gegen Qinlian Decoction, WM, western medicine.
3.3.3.2. GQD + WM versus WM
With regard to studies comparing recurrence rates between GQD plus WM and WM alone, no statistically significant heterogeneity was detected between the 2 included trials[27,33] (P = .63, I 2 = 0%; Fig. 6B). The results of fixed effects modeling combined with effect sizes indicated a significant difference between the experimental and control groups (RR = 0.18, 95% CI: 0.06–0.61, P = .006), with the experimental group outperforming the control group.
3.3.4. Adverse events
3.3.4.1. GQD versus WM
Among studies[37,44] comparing adverse events between GQD and WM alone, 2 showed no statistically significant heterogeneity in data (P = 1.00, I 2 = 0%; Fig. 7A). The results of fixed effects modeling showed a significant difference between the experimental and control groups (RR = 0.20, 95% CI: 0.02–1.68, P = .14), with a significantly lower adverse event rate in the experimental group than in the control group.
Figure 7.
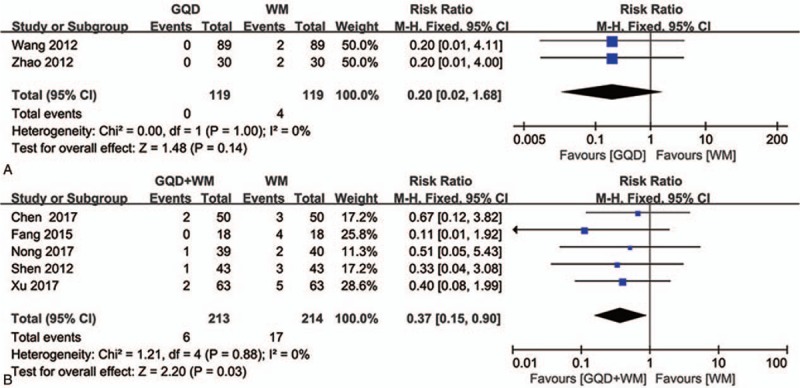
Forest plot showing the adverse events after Gegen Qinlian Decoction and Gegen Qinlian Decoction plus Western medicine therapy for ulcerative colitis. (A: GQD vs WM, B: GQD + WM vs WM). GQD = Gegen Qinlian Decoction, WM, western medicine.
3.3.4.2. GQD + WM versus WM
Among studies comparing adverse events between GQD plus WM and WM alone, 5[27,28,33,34,42] showed no statistically significant heterogeneity in data (P = .88, I 2 = 0%; Fig. 7B). When these 5 trials were analyzed, a significant difference was detected between the experimental and control groups (RR = 0.37, 95% CI: 0.15–0.90, P = .03), with the former showing a significantly lower rate than the latter.
In total, adverse events in gastrointestinal symptoms were mainly reported in 5 trials.[28,33,34,42,44] primarily including abdominal distention, nausea, emesis. Two trials reported leukocytopenia.[27,44] Headache and dizziness symptoms were also mentioned in 7 trials.[28,33,34,37,42] No serious side effects or abnormal laboratory parameters, including markers of liver and renal function, were reported. Treatment with WM alone was more likely to be associated with adverse side effects such as gastrointestinal symptoms, headache, and leukocytopenia.
3.3.5. Funnel plot analysis
A funnel plot analysis of the clinical efficacy of the assessed treatments including GQD versus WM and GQD + WM versus WM suggested that there was possibility certain publication bias in the literature included here and trials with negative results may not be published (Fig. 8). This result indicates the present analysis of clinical efficacy needs more high-quality literatures to identify.
Figure 8.
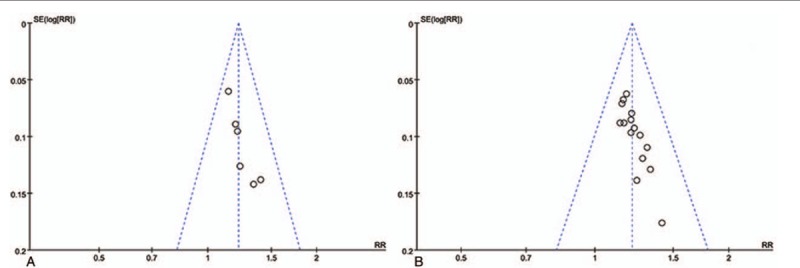
Funnel plot showing the overall clinical efficacy of Gegen Qinlian Decoction and Gegen Qinlian Decoction plus Western medicine therapy for ulcerative colitis.
4. Discussion
The present systematic review provided a quantitative synthesis of the clinical efficacy and safety of GQD for the treatment of UC by integrating outcomes from 22 clinical trials involving 2028 individuals with UC. The results of analyses demonstrated that the therapeutic efficacy of GQD alone or in combination with traditional WM may significantly better than that of conventional WM alone for the treatment of UC. These beneficial effects are evidenced by the facts that GQD had greater beneficial effects than did WM therapy, that GQD plus WM therapy significantly reduced UCEIS scores compared with WM therapy alone, and that GQD alone or in combination with WM therapy was effective in treating UC and maintaining low recurrence and adverse events. Notably, although GQD therapy appears to be more effective than WM therapy alone, only 1 trial[30] used a UCEIS score to define the patient response to treatment. Because there were only 60 patients in this trial, it does not allow us to draw a definitive conclusion. Given the limited number of available studies, the present systematic review attempted to offer an evidence-based approach to confirm that GQD may be a promising adjunct option for patients with UC.
UC, a chronic inflammatory bowel disease, degrades the patient's quality of life because of its long duration, high recurrence rate, and wide range of associated pathological symptoms and clinical features.[45] However, because of its complexity, the underlying pathogenesis of UC remains unclear. One possibility, indicated by previous studies, is that susceptibility gene variants and environmental changes play a significant role in the pathogenesis of UC.[46,47] Furthermore, a hyperactive mucosal immune response to intestinal microorganisms in genetically susceptible individuals with microbial dysbiosis and reduced microbial diversity has been shown to impact the development of inflammatory bowel disease.[48] Sustained dysregulation of mucosal immunity, particularly in terms of pro-inflammatory cytokine overproduction and active inflammation, has further been implicated in the damage to intestinal mucosa seen in UC.[49] All the time clinical symptoms and colonoscopy have been the vital diagnostic criteria used by physicians to evaluate the severity and extent of UC.[50] Presently, the goal of UC clinical treatment also tends to mucosal healing.[51] In this context, UCEIS scores were employed as an outcome, which is extremely useful for evaluating the efficacy of GQD for patients with UC.[23] Present study showed that GQD plus WM therapy significantly reduced UCEIS scores may be associated with the pharmacological action of GQD.
Indeed, several studies have sought to shed light on the pharmacological aspects GQD's role in UC treatment. Li et al reported that GQD relieved UC symptoms and repaired of intestinal epithelial barrier via the inhibition of Toll-like receptor 4/ Nuclear factor-κB signaling, which serves to suppress the production of pro-inflammatory cytokines ( [Interleukin] IL-6, Tumor necrosis factor-α, IL-1β).[52] And active components of GQD,[53] such as puerarin, baicalin, glycyrrhizic acid, and berberine, exert broad antipyretic, antiviral, and antidiarrheal effects, which may further ameliorate the clinical symptoms of UC. In addition, the combination of pueraria, rhizoma coptis, and glycyrrhiza in GQD may drive the reconstruction and repair of colonic mucosa, according to endoscopic assessments.[54]
Certainly, WM is still the mainstream drug for the treatment of UC, such as mesalazine, sulfasalazine, and adalimumab, which also play a vital role in anti-inflammation and regulation of immune apparatus.[55,56] In this study, the combination of WM and GQD provided substantial benefit beyond that of WM alone, including low recurrence rate and adverse event. But unfortunately, a definite mechanism by which GQD and WM synergistically impact the progression of UC has not yet been described. Thus, we can only guess that, owing to the powerful pharmacological effects of GQD, it may alleviate these adverse events produced by long-term WM treatment[57] and help patient tolerate the prolonged therapy to reduce recurrence rate.
Meanwhile, GQD, in TCM theory, is designed to regulate dampness-heat and its associated syndromes by restoring the conductive function of the large intestine. TCM has a long history of clinical application in Asian countries.[58] In terms of TCM theory, “dampness-heat” serves as a main pathogenic factor underlying UC. Accumulation of dampness-heat in the gut may thus provoke qi stagnation and blood stasis, which in turn generates damage to intestinal mucosa, diarrhea, and purulent bloody stool.[15] Theoretically, eliminating damp-heat and activating blood circulation using TCM techniques may thus heal diseased intestinal mucosa.[59] And, GQD has served as a useful medicine for the treatment of UC for a long time.[60] GQD is often modified with Chinese herbal additions based on the TCM syndromes a patient presents with. Given this, the exact mechanism by which GQD treats UC may involve multiple herbal formulations with various, integrative and synergistic effects.
Collectively, evidence from the present systematic review supports the use of GQD alone or in conjunction with WM therapies for the treatment of UC. However, the RCTs included in this systematic review do have several significant limitations. First, all were conducted in China, and no negative results were reported. Second, the included studies were of low methodological quality. The lack of practitioner and assessor blinding to the participant status likely affected the outcomes, which may have resulted in selection bias. Third, we were unable to assess variations in the composition or dosage of TCM or its route of administration among the included RCTs. These factors contribute to increased heterogeneity and further decrease the reliability.
5. Conclusion
The present systematic review suggests that the Chinese herbal medicine GQD and its use in conjunction with standard WM therapies may benefits in correcting clinical symptoms and promote endoscopic healing in patients with UC. In addition, GQD is associated with a low incidence of adverse reactions and UC recurrence rates. Despite these benefits, limitations exist. Before it was admitted as an evidence-based treatment option in clinical practice, there need more data to determine the pros and cons.
6. Implication on clinical practice
The result in this analysis suggest that the herbal compounds of GQD have a positive, therapeutic effect on UC. And GQD, as complementary and alternative medicine treatment option, may serve as viable alternatives for the prevention and treatment of UC or as add-on approaches to existing conventional WM. Thus, in clinical practice, the development of a more integrative, individualized treatment approach should be widely promoted to improve the effectiveness of clinical treatment.
7. Implication on future research
The included studies in this system review were of low methodological quality, which reduced the recommendation level and the strength of evidence for systematic evaluation. Therefore, the future clinical research should note as follows.
-
(1)
Randomization should be described in detail specific schemes (including methods for generating random sequences, etc).
-
(2)
Concealment of allocation and blinding should be described in detail.
-
(3)
Further methodological standardization of RCTs assessing TCM therapies, particularly with regard to the composition and dosage, is required.
-
(4)
Future well-designed, large-scale, high-quality, multicenter RCTs are necessary for more reliable assessment of the research.
Author contributions
Conceptualization: Zhigang Mei.
Data curation: Yuling Fan, Wen Yi, Han Huang.
Formal analysis: Zhitao Feng.
Investigation: Yuling Fan.
Methodology: Zhigang Mei, Zhitao Feng.
Writing – original draft: Yuling Fan, Wen Yi.
Writing – review and editing: Zhigang Mei, Zhitao Feng.
Zhigang Mei orcid: 0000-0002-9099-7099.
Footnotes
Abbreviations: CI = confidence interval, GQD = Gegen Qinlian Decoction, MD = mean differences, RCTs = randomized controlled clinical trials, TCM = traditional Chinese medicine, UC = ulcerative colitis, UCEIS = ulcerative colitis endoscopic index of severity, WM = western medicine.
How to cite this article: Fan Y, Yi W, Huang H, Mei Z, Feng Z. Efficacy of herbal medicine (Gegen Qinlian Decoction) on ulcerative colitis: A systematic review of randomized controlled trials. Medicine. 2019;98:52(e18512).
YF and WY contributed equally to this paper.
The present study was supported by the Natural Science Foundation of Hubei province, China, No. 2012FFC123.
The authors have no conflicts of interest to disclose.
References
- [1]. Feuerstein JD, Cheifetz AS. Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin Proc 2014;89:1553–63. [DOI] [PubMed] [Google Scholar]
- [2]. Assadsangabi A, Lobo AJ. Diagnosing and managing inflammatory bowel disease. Practitioner 2013;257:13. [PubMed] [Google Scholar]
- [3]. Ordas I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet 2012;380:1606–19. [DOI] [PubMed] [Google Scholar]
- [4]. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2011;142:46–54. [DOI] [PubMed] [Google Scholar]
- [5]. Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017;152:313–21. [DOI] [PubMed] [Google Scholar]
- [6]. Sandborn WJ, Bosworth B, Zakko S, et al. Budesonide foam induces remission in patients with mild to moderate ulcerative proctitis and ulcerative proctosigmoiditis. Gastroenterology 2015;148:740–50. [DOI] [PubMed] [Google Scholar]
- [7]. Ford AC, Khan KJ, Achkar JP, et al. Efficacy of oral vs. topical, or combined oral and topical 5-aminosalicylates, in ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol 2012;107:167–76. [DOI] [PubMed] [Google Scholar]
- [8]. Timmer A, Mcdonald JW, Macdonald JK, et al. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 2007;1:CD000478. [DOI] [PubMed] [Google Scholar]
- [9]. Manson SC, Brown RE, Cerulli A, et al. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med 2009;103:975–94. [DOI] [PubMed] [Google Scholar]
- [10]. Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 2007;369:1641 . [DOI] [PubMed] [Google Scholar]
- [11]. Warner B, Harris AW. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142:257–65.e3. [DOI] [PubMed] [Google Scholar]
- [12]. Teixeira FV, Hossne RS, Kotze PG, et al. Biological therapy in the treatment of moderate-to severe ulcerative colitis patients: can colectomy be prevented? J Coloproctol 2011;31:325–9. [Google Scholar]
- [13]. Beilman CL, Xuan TN, Victoria U, et al. Real-life treatment paradigms show adalimumab is cost-effective for the management of ulcerative colitis. Can J Gastroenterol Hepatol 2016;2016:5315798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Ke F, Yadav PK, Ju LZ. Herbal medicine in the treatment of ulcerative colitis. Saudi J Gastroenterol 2012;18:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Wu PT. What should be kept in mind in the TCM differential treatment for ulcerative colitis? J Tradit Chin Med 2008;28:308–9. [DOI] [PubMed] [Google Scholar]
- [16]. Xiaojie D, Zhengping D. Research progress on Gegen Qinlian Decoction in the treatment of ulcerative colitis. Chin Med Mod Distance Edu China 2016;14:144–6. [Google Scholar]
- [17]. Wang N, Feng Y, Cheung F, et al. A Chinese medicine formula Gegen Qinlian decoction suppresses expansion of human renal carcinoma with inhibition of matrix metalloproteinase-2. Integr Cancer Ther 2015;14:75–85. [DOI] [PubMed] [Google Scholar]
- [18]. Zeng Y. Analysis of curative effect of Gegen Qinlian decoction combined with bletilla enema on acute attack of chronic ulcerative colitis. China Foreign Med Treat 2017;36:180–4. [Google Scholar]
- [19]. Wang QQ, Yu L. Clinical observation of 32 cases of chronic nonspecific ulcerative colitis treated by Gegen Qinlian decoction enema combined with sulfasalazine. Hebei J Tradit Chin Med 2014;36:1023–4. [Google Scholar]
- [20]. Mao Y, Zhang G, Peng H, et al. Pharmacodynamics study on antipyreticand anti-inflammatory effects of active compositions alignment in Gengen Qinlian decoction. J Liaoning Univ Tradit Chin Med 2014;16:30–2. [Google Scholar]
- [21]. Mao Y, Zhang G, Peng H, et al. Pharmacodynamics study on anti-diarrhea of active compositions alignment in Gengen Qinlian decoction. Liaoning J Tradit Chin Med 2013;40:1433–5. [Google Scholar]
- [22]. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: Current Management. J Crohns Colitis 2017;11:769–84. [DOI] [PubMed] [Google Scholar]
- [23]. Travis SP, Schnell D, Krzeski P, et al. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology 2013;145:987–95. [DOI] [PubMed] [Google Scholar]
- [24]. Savović J, Weeks L, Sterne JA, et al. Evaluation of the Cochrane Collaboration's tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Syst Rev 2014;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Bian YY. Analysis of effect of Gegen Qinlian decoction combined with mesalazine for ulcerative colitis. Contemp Med 2015;13:182–3. [Google Scholar]
- [26]. Chai S, Du X, Zhou X. Gegen Qinlian Wutan decoction combined with sulfasalazine in treatment of active ulcerative colitis randomized controlled study. Zhejiang J Integrated Tradit Chin Western Med 2018;3:191–2. [Google Scholar]
- [27]. Chen S. Clinical research of Gegen Qinlian Decoction combined with sulfasalazine for treatment of ulcerative colitis. Shaanxi J Tradit Chin Med 2017;38:1110–1. [Google Scholar]
- [28]. Fang CM. Chinese and western medicine in treatment of ulcerative colitis for 36 cases. J World Newest Med Inf Dig 2015;15:17–8. [Google Scholar]
- [29]. Gao D, Shao X. Efficacy of Puerariae and Scutellariae and Coptidis Decoction in treatment of patients with ulcerative colitis and its effects on inflammatory reaction and immune function. Med Pharm J Chin People's Liberation Army 2018;30:89–92. [Google Scholar]
- [30]. He J. Observation on clinical effects of modified Gegen Qinlian Decoction for damp-heat ulcerative colitis. J Sichuan Tradit Chin Med 2013;31: [Google Scholar]
- [31]. Huang D. The clinical study of Gegen Qinlian Wutan Decoction combined with sulfasalazine in treatment of active ulcerative colitis. J Bethune Med Sci 2015;13:5. [Google Scholar]
- [32]. Li SB. Modified Gegen Qinlian decoction in the treatment of ulcerative colitis for 58 cases. Ch Med Mod Distance Edu China 2010;8:76. [Google Scholar]
- [33]. Nong Y. Clinical observation of 40 cases of ulcerative colitis treated by combination of probiotics and Gegen Qinlian decoction combined with probiotics. Chin J Ethnomed Ethnopharm 2017;26:106–7. [Google Scholar]
- [34]. Shen Y. Integrated traditional Chinese and Western medicine in the treatment of ulcerative colitis for 43 cases. Tradit Chin Med Res 2012;25:41–2. [Google Scholar]
- [35]. Tian Z. Analysis of the effect of Gegen qinlian decoction on ulcerative colitis. J World Newest Med Inf Dig 2013;15:271–2. [Google Scholar]
- [36]. Wang J. Observation of the curative effect of Gegen Qinlian Decoction in treating ulcerative colitis. Mod Med Health 2011;22:3462. [Google Scholar]
- [37]. Wang J. Modified Gegen Qinlian Decoction for damp-heat ulcerative colitis: clinical observation of 89 cases. J Sichuan Tradit Chin Med 2012;30:88–9. [Google Scholar]
- [38]. Wang Y. Clinical observation of 30 cases for active ulcerative colitis by Gegen Qinlian Wu Decoction oral and enema. Tradit Chin Med Res 2013;26:29–32. [Google Scholar]
- [39]. Wang ZM, Zhang F. Modified Gegen Qinlian decoction retention enema in the treatment of ulcerative colitis for 60 cases. Jiangsu J Tradit Chin Med 2014;46:44–5. [Google Scholar]
- [40]. Xie JJ, Xie JH. Chinese and western medicine in the treatment of Ulcerative Colitis for 29 cases. Henan Tradit Chin Med 2007;4:50–1. [Google Scholar]
- [41]. Xu AJ. Efficacy observation of Gegenqinlian Wutan Decoction enema combined with sulfasalazine in treating chronic ulcerative colitis. J North Pharm 2018;15:60–1. [Google Scholar]
- [42]. Xu C. The effects of Gegen Qinlian Wutan decoction on inflammatory factors and oxidative stress in patients with ulcerative colitis. Acta Chin Med 2017;32:1067–71. [Google Scholar]
- [43]. Yuan Y. Clinical observation on Puerariae and Scutellariae and Coptidis Decoction in treatment of 60 patients with ulcerative colitis. Progress Mod Biomed 2007;9:1336–7. [Google Scholar]
- [44]. Zhao CL, Zhang LW, Yang YC. Gegenqinlian decoction combined with bletilla enema on acute exacerbation of chronic ulcerative colitis for 30 cases. Guiding J Tradit Chin Med Pharm 2012;18:31–3. [Google Scholar]
- [45]. Zheng K, Zhang S, Wang C, et al. Health-related quality of life in Chinese patients with mild and moderately active ulcerative colitis. Plos One 2015;10:e0124211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Souza HSPD, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 2016;13:13–27. [DOI] [PubMed] [Google Scholar]
- [47]. Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 2006;3:390–407. [DOI] [PubMed] [Google Scholar]
- [48]. Xu XR, Liu C, Feng B, et al. Dysregulation of mucosal immune response in pathogenesis of inflammatory bowel disease. World J Gastroenterol 2014;20:3255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Parray FQ, Wani ML, Malik AA, et al. Ulcerative colitis: a challenge to surgeons. Int J Prev Med 2012;3:749–63. [PMC free article] [PubMed] [Google Scholar]
- [50]. Naganuma M, Ichikawa H, Inoue N, et al. Novel endoscopic activity index is useful for choosing treatment in severe active ulcerative colitis patients. J Gastroenterol 2010;45:936–43. [DOI] [PubMed] [Google Scholar]
- [51]. Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, et al. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol 2010;7:15–29. [DOI] [PubMed] [Google Scholar]
- [52]. Li R, Chen Y, Shi M, et al. Gegen Qinlian decoction alleviates experimental colitis via suppressing TLR4/NF-κB signaling and enhancing antioxidant effect. Phytomedicine 2016;23:1012–20. [DOI] [PubMed] [Google Scholar]
- [53]. Xu B, Zhang G, Ji Y. Active components alignment of Gegen Qinlian decoction protects ulcerative colitis by attenuating inflammatory and oxidative stress. J Ethnopharmacol 2015;162:253–60. [DOI] [PubMed] [Google Scholar]
- [54]. Wen Y. Effects of GegenQinlian decoction and its disassembled prescriptions on P selectin and colonic mucosal ultrastructure in rats with ulcerative colitis. Lishizhen Med Mater Med Res 2012;23:635–6. [Google Scholar]
- [55]. Hauso Ø, Martinsen TC, Waldum H. 5-Aminosalicylic acid, a specific drug for ulcerative colitis. Scand J Gastroenterol 2015;50:933–41. [DOI] [PubMed] [Google Scholar]
- [56]. Su C, Salzberg B, Lewis JD, et al. Efficacy of anti-tumor necrosis factor therapy in patients with ulcerative colitis. Am J Gastroenterol 2002;97:2577–84. [DOI] [PubMed] [Google Scholar]
- [57]. Timmer A, Patton PH, Chande N, et al. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev 2016;18:CD000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Peltzer K, Pengpid S. Utilization and practice of traditional/complementary/alternative medicine (T/CAM) in Southeast Asian Nations (ASEAN) member states. Stud Ethno-Med 2015;9:209–18. [Google Scholar]
- [59]. Ye B, Shen H, Le L. Clinical observation son 100 cases of ulcerative colitis treated with the method of clearing away heat, expelling dampness, promoting blood circulation and healing ulcer. J Tradit Chin Med 2010;30:98–102. [DOI] [PubMed] [Google Scholar]
- [60]. Chen LH, Tang YP, Wang Q. Advances in studies on Gegen Qinlian decoction. Chin Trad Herb Drugs 2010;41:676–80. [Google Scholar]


