Abstract
Background:
The risk of recurrent herniation after lumbar discectomy is highest during the first postoperative year. The purpose of this study was to determine whether implantation of a bone-anchored annular closure device (ACD) following limited lumbar discectomy reduced the risk of recurrent herniation and complications during the first year of follow-up compared to limited lumbar discectomy alone (Controls) and whether this risk was influenced by patient characteristics.
Methods:
In this randomized multicenter trial, patients with symptomatic lumbar disc herniation and with a large annular defect following limited lumbar discectomy were randomized to bone-anchored ACD or Control groups. The risks of symptomatic reherniation, reoperation, and device- or procedure-related serious adverse events were reported over 1 year of follow-up.
Results:
Among 554 patients (ACD 276; Control 278), 94% returned for 1-year follow-up. Bone-anchored ACD resulted in lower risks of symptomatic reherniation (8.4% vs. 17.3%, P = .002) and reoperation (6.7% vs. 12.9%, P = .015) versus Controls. Device- or procedure-related serious adverse events through 1 year were reported in 7.1% of ACD patients and 13.9% of Controls (P = .009). No baseline patient characteristic significantly influenced these risks.
Conclusions:
Among patients with large annular defects following limited lumbar discectomy, additional implantation with a bone-anchored ACD lowered the risk of symptomatic reherniation and reoperation over 1 year follow-up. Device- or procedure-related serious adverse events occurred less frequently in the ACD group. These conclusions were not influenced by patient characteristics. ClinicalTrials.gov (NCT01283438).
Keywords: annulus fibrosus, lumbar discectomy, randomized controlled trial, sciatica
1. Introduction
Recurrent lumbar disc herniation occurs in 7% to 18% of patients after lumbar discectomy.[1–3] Most recurrent cases require additional surgery to adequately resolve symptoms[4] yet the magnitude of clinical improvement after repeat surgery is less compared to primary surgery.[5,6] Patients with large defects (≥6 mm width) in the annulus fibrosus after lumbar discectomy are particularly prone to reherniation and represent an under-served, high-risk subgroup.[4] The first postoperative year appears to be the most critical to long-term surgical success since pain recurs at a proportionally higher rate within 1 year of primary discectomy compared to during subsequent years.[7] Techniques intended to prevent reherniation and associated reoperations during this critical period have potential to significantly improve patient outcomes and may reduce medical costs since revision surgeries tend to be more expensive and technically demanding compared to primary procedures.[2,8] Clinical outcomes during the first year after surgery are also important metrics utilized by healthcare payers for making coverage decisions on new medical technologies. A bone-anchored annular closure device (ACD) received marketing approval by the Food and Drug Administration in February 2019 for prevention of lumbar disc reherniation. An analysis of 1-year results with the ACD and determination of whether patient characteristics might influence clinical outcomes is warranted. The primary objective of this study was to determine if additional use of a bone-anchored ACD that is intended to occlude large postsurgical annular defects could reduce recurrence and complication rates in a high-risk patient population during the critical first year after limited lumbar discectomy. A secondary objective was to determine whether these risks were influenced by patient characteristics.
2. Methods
2.1. Trial design
This was a multicenter randomized trial with the primary objective to determine whether implantation of a bone-anchored ACD following lumbar discectomy (ACD group) reduced the risk of recurrent herniation compared to lumbar discectomy alone (Control group). Local ethics review boards approved the study and participants provided written informed consent. The study was prospectively registered at ClinicalTrials.gov (NCT01283438) and the study protocol was previously published.[9]
2.2. Participants
Preoperative imaging tests included magnetic resonance imaging (MRI) with T1- and T2-weighted axial and sagittal images of the lumbar spine, low-dose multiplanar computed tomography (CT) of the target level only, and flexion/extension x-rays. Eligible patients presented with imaging evidence of lumbar disc herniation at a single level between L1 and S1. Radicular symptoms were confirmed by a positive straight leg raise or femoral stretch test. Patients reported leg pain severity at least 40/100 on a visual analogue scale and back-related dysfunction at least 40/100 on the Oswestry Disability Index (ODI) despite at least 6 weeks of attempted nonsurgical management. Important exclusion criteria were previous spinal surgery at the level of the herniation, spondylolisthesis with ≥25% slip, and lumbar osteoporosis. Patients who met all preoperative eligibility criteria were scheduled for limited lumbar discectomy during which the final eligibility criterion regarding annular defect size would be assessed. A complete list of eligibility criteria is provided in Table 1.
Table 1.
Inclusion and exclusion criteria.

2.3. Lumbar discectomy procedure
All patients were treated on an inpatient basis by experienced spine surgeons with limited lumbar microdiscectomy using an interlaminar transflaval approach as described by Spengler.[10] The limited disc excision procedure involved exposure of the ligamentum flavum and, if necessary, a small unilateral foraminotomy was performed to expose the affected disc space. Via an incision in the annulus fibrosus, disc material fragments in the extradiscal space on the affected side were removed with no or minimal removal of tissue fragments from the intervertebral disc space.
2.4. Intraoperative randomization
At completion of the limited discectomy procedure, patients were then evaluated for ACD eligibility by measuring the size of the annular defect. Patients with a large annular defect (4–6 mm tall and 6–10 mm wide) were randomly allocated (1:1) to ACD or Control groups using a central web-based system. Patients whose annular defects did not meet these size thresholds were excluded from further study participation. Patients assigned to the Control group received no additional treatment and the limited discectomy procedure was concluded in standard fashion.
2.5. Annular closure device and implantation procedure
Patients assigned to the ACD group received a permanent implant (Barricaid, Intrinsic Therapeutics) consisting of two components—an occlusion component to block the annular defect and a titanium anchor to secure the occlusion component to an adjacent vertebral body (Fig. 1). The occlusion component is comprised of a flexible polymer that is designed to prevent reherniation by physically blocking the annulus at the post-surgery defect and maintaining hydrostatic pressure inside the nucleus pulposus,[11] and contains a platinum-iridium radiopaque marker to enable radiographic visualization. The anchor component is comprised of a saw-toothed titanium alloy that is carefully hammered into either the caudal-adjacent or cranial-adjacent vertebral body to resist migration.
Figure 1.
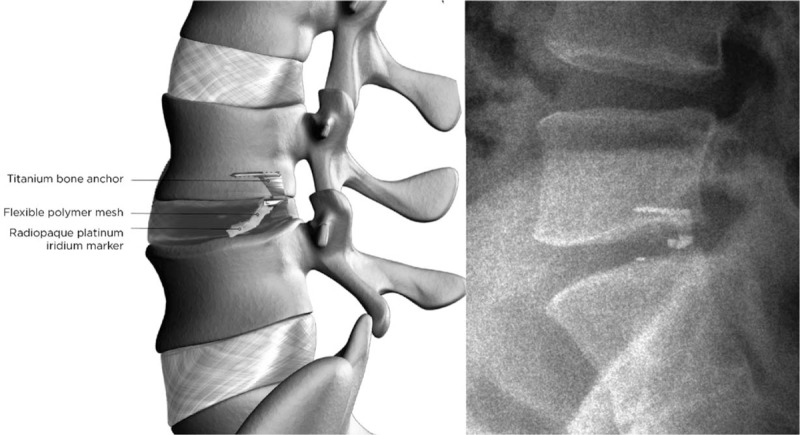
Lateral radiograph showing the bone-anchored annular closure device implanted at L4-L5. Graphic representation of the boneanchored annular closure device, with a titanium bone anchor holding the polyester mesh in place (left panel). Lateral radiograph showing the boneanchored annular closure device implanted at L4L5 (right panel).
After confirmation of a large annular defect, a sizing trial was performed under fluoroscopic control to establish the correct position and angle of the ACD. Next, the device was implanted under fluoroscopic guidance by impacting the anchor into the vertebral body while the occlusion component was placed in the annular defect to prevent expulsion of disc material into the extradiscal space. After fluoroscopic confirmation of correct device placement, the surgical site was inspected and standard wound closure was performed.
2.6. Outcomes
Clinical follow-up occurred at 6 weeks, 3 months, 6 months, and 1-year follow-up, with imaging tests (CT, MRI, and flexion-extension x-rays) performed at 1 year. Symptomatic reherniation was either confirmed during reoperation, or diagnosed based on an independent imaging core laboratory confirmation of reherniation combined with associated clinical symptoms. Reoperations included any surgical procedure that was performed at the level of the original herniation, regardless of side, during follow-up. The decision to reoperate was based on patient-physician shared decision-making. The occurrence of adverse events was evaluated at each visit and adjudicated for seriousness and relation to the procedure or device by an independent Data Safety Monitoring Board.
2.7. Statistical analysis
The sample size of this trial was determined using Bayesian techniques. Baseline patient characteristics were presented as means and standard deviations for continuous variables and counts and percentages for categorical variables. Baseline group comparisons were made with independent samples t test for continuous data or Fisher exact test for categorical data. We additionally reported the absolute standardized difference (ASD) statistic, which is calculated as the difference in means or proportions between groups divided by the pooled standard deviation. A small difference between treatment groups is defined as an ASD of less than 0.2. Time-to-event data were analyzed using Kaplan–Meier methods with log-rank tests for group comparisons. Cox regression models were developed for interaction testing to evaluate the consistency of treatment effects between patient characteristic subgroups. Statistical significance was set at P < .05 and hypothesis testing was two-sided. Statistical analyses were performed using SAS v9.4 (SAS Institute).
3. Results
3.1. Patient characteristics and follow-up
Between December 2010 and October 2014, 554 patients were randomly allocated to ACD (n = 276) or Control (n = 278) groups. ACD implantation was not attempted in 4 patients due to proximity of the nerve root; these patients were excluded from the analysis. Randomization effectively balanced baseline patient characteristics between treatment groups, which were typical of the general population undergoing lumbar discectomy. Mean patient age was 43 years, 59% were male, and patients generally presented with severe leg pain and disability. The proportion of patients with herniation at L5-S1 was statistically higher in the Control group compared to the ACD group (P = .04), but the magnitude of this difference was small (ASD = .23) (Table 2). Patient compliance with follow-up visits was high in each group, with 94% returning for the 1-year visit (Fig. 2).
Table 2.
Baseline patient characteristics∗.
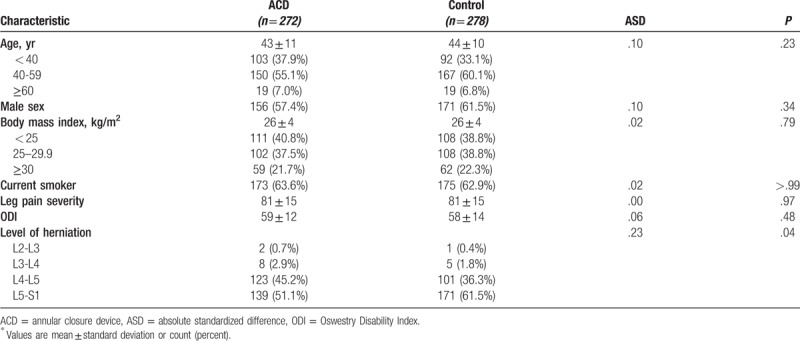
Figure 2.
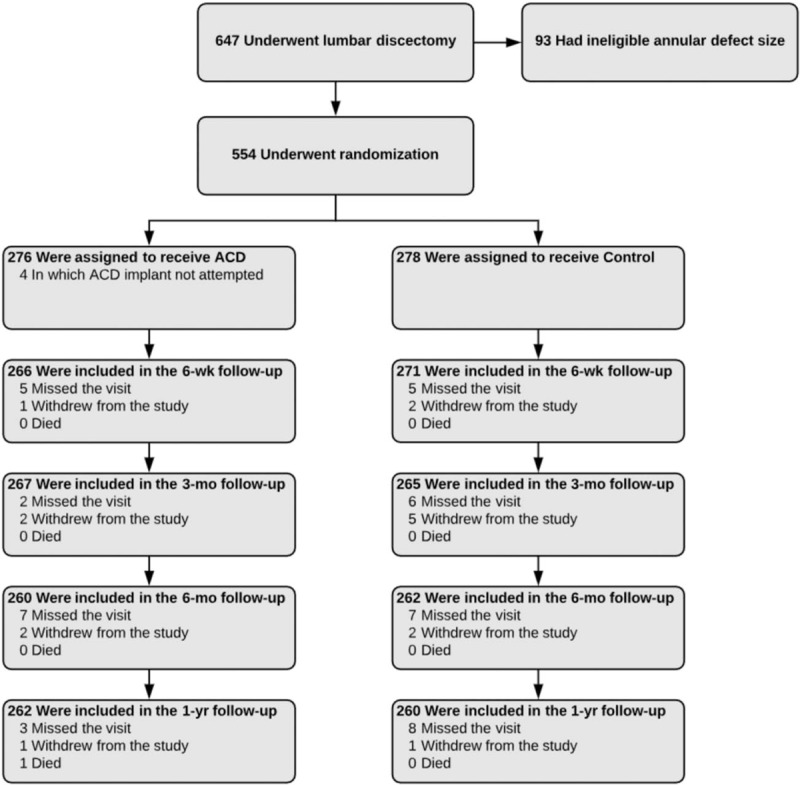
CONSORT flow diagram of patient enrollment and randomization into treatment groups. Compliance with clinical follow-up at 1 year was 95% with annular closure device and 94% with controls.
3.2. Symptomatic reherniation, reoperation, and complications
The benefit of the ACD in preventing symptomatic reherniation was realized soon after surgery and maintained throughout the 1-year follow-up period. Over 1 year, the cumulative risk of experiencing a symptomatic reherniation was 8.4% in the ACD group and 17.3% in the Control group (P = .002) (Fig. 3). The corresponding hazard ratio was 0.45 (95% CI: 0.27, 0.75), indicating a 55% risk reduction with ACD. There was no modification of this risk estimate according to age (P = .11), sex (P = .82), body mass index (BMI) (P = .66), smoking status (P = .51), level of herniation (P = .12), leg pain severity at baseline (P = .64), or ODI at baseline (P = .82) (Fig. 4).
Figure 3.
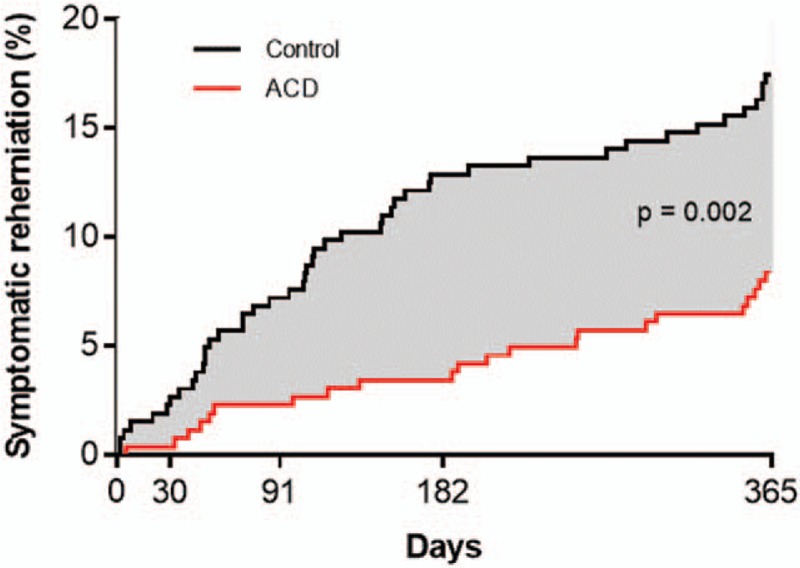
Cumulative risk of symptomatic reherniation over 1-year post-treatment. Risk of symptomatic herniation was 8.4% (standard error 1.7%) with annular closure device and 17.3% (standard error 2.3%) with Controls. Log-rank P = .002.
Figure 4.
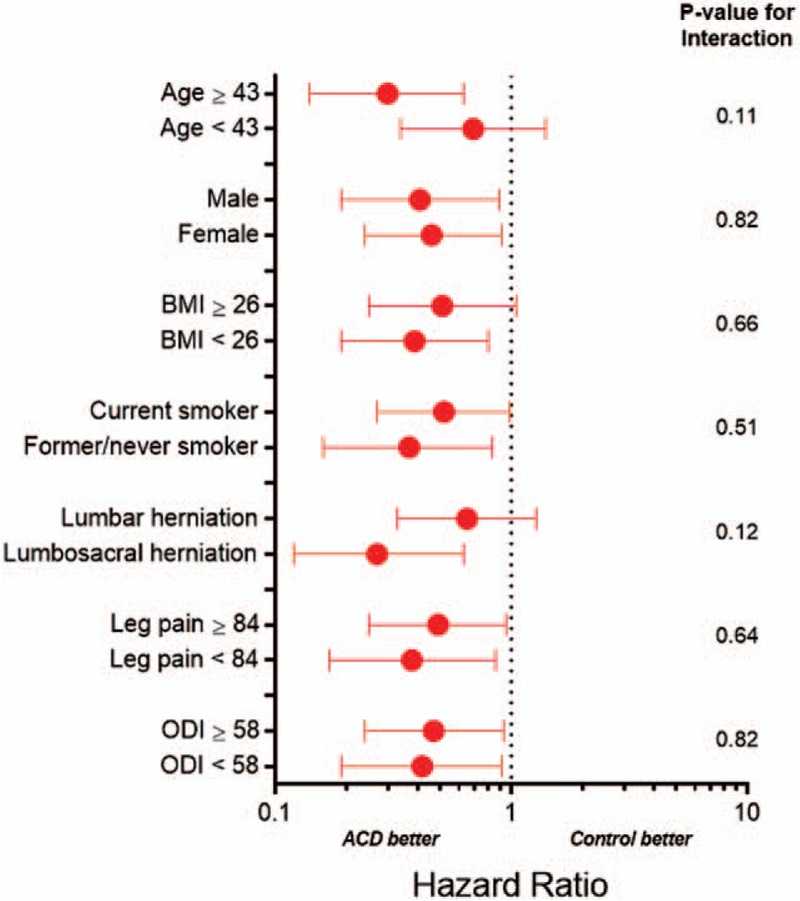
Hazard ratio for symptomatic reherniation with annular closure device vs controls by baseline patient characteristics. ACD = annular closure device; BMI = body mass index; ODI = Oswestry Disability Index. Plotted values are hazard ratio and 95% confidence interval. Hazard ratio < 1 indicates lower risk with ACD; > 1 indicates higher risk with ACD. Continuous variables are categorized by the median value.
The ACD was similarly efficacious in reducing the risk of reoperation with similar observation of early benefit that was maintained over 1 year. There were 21 reoperations in 18 ACD patients and 41 reoperations in 35 Control patients during this period. The cumulative risk of reoperation was 6.7% in the ACD group and 12.9% in the Control group (P = .015) (Fig. 5). The corresponding hazard ratio was 0.50 (95% CI: 0.28, 0.88), indicating a 50% risk reduction with ACD. There was no modification of this risk estimate according to age (P = .21), sex (P = .64), BMI (P = .38), smoking status (P = .43), level of herniation (P = .26), leg pain severity at baseline (P = .39), or ODI at baseline (P = .54) (Fig. 6).
Figure 5.
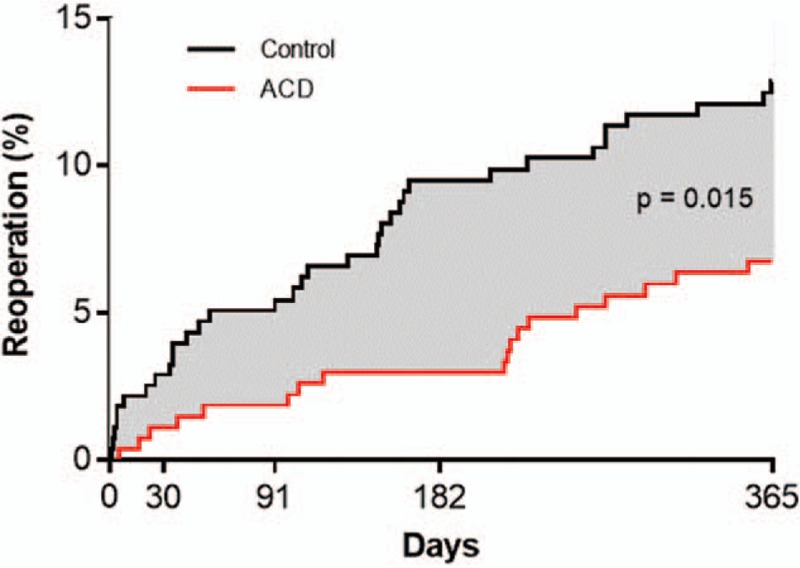
Cumulative risk of reoperation over 1-year post-treatment. Risk of symptomatic herniation was 6.7% (standard error 1.5%) with annular closure device and 12.9% (standard error 2.0%) with controls. Log-rank P = .015.
Figure 6.
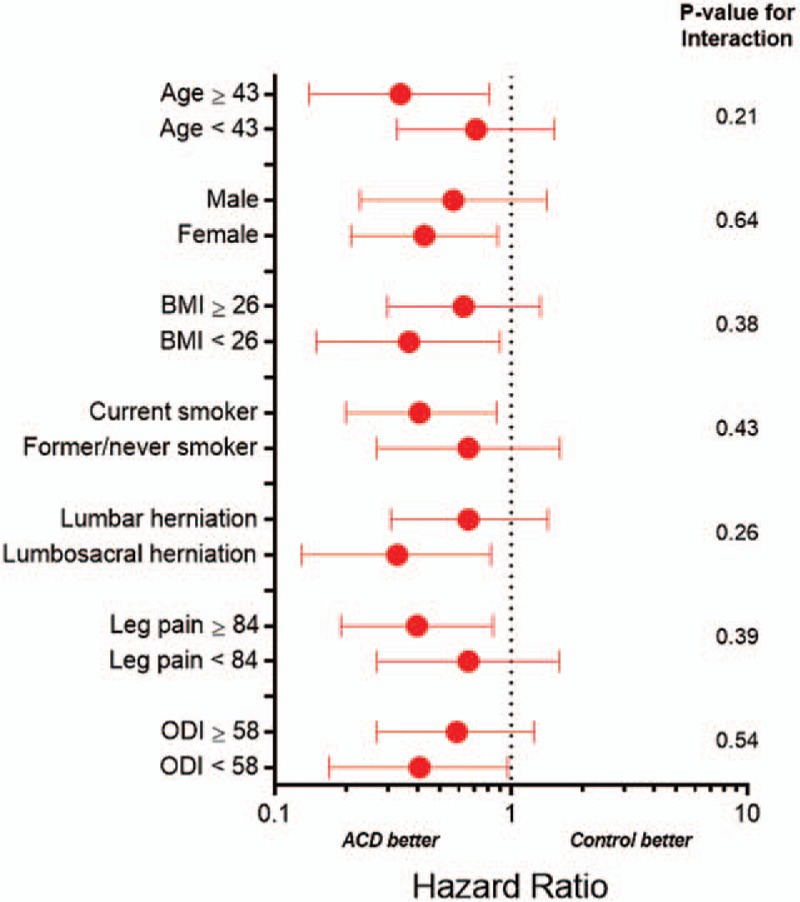
Hazard ratio for reoperation with annular closure device vs. controls by baseline patient characteristics. ACD = annular closure device; BMI = body mass index; ODI = Oswestry Disability Index. Plotted values are hazard ratio and 95% confidence interval. Hazard ratio < 1 indicates lower risk with ACD; > 1 indicates higher risk with ACD. Continuous variables are categorized by the median value.
Implantation of the ACD was unsuccessful in 5 patients, including 4 patients in whom the flexible polymer component did not fully enter the disc and in 1 patient with nerve root injury during attempted implantation. The cumulative risk of a procedure- or device-related serious adverse event over 1 year was 7.1% for ACD and 13.9% for Controls (P = .009) (Fig. 7). The corresponding hazard ratio was 0.49 (95% CI: 0.28, 0.85), indicating a 51% risk reduction with ACD. There was no modification of this risk estimate according to age (P = .10), sex (P > .99), BMI (P = .36), smoking status (P = .77), level of herniation (P = .17), leg pain severity at baseline (P = .85), or ODI at baseline (P = .52) (Fig. 8). This difference was primarily due to the lower incidence of symptomatic reherniation in the ACD group. Comparing the ACD to Control group across subcategories of procedure- or device-related serious adverse events, the corresponding frequencies were 0% vs 0.7% for cardiac/vascular, 2.2% vs 0% for device deficiency, 3.7% for 12.9% for disc herniation, 0.4% vs 0% for neurological, 1.5% vs 0.7% for pain, and 1.1% vs 1.8% for wound complications.
Figure 7.
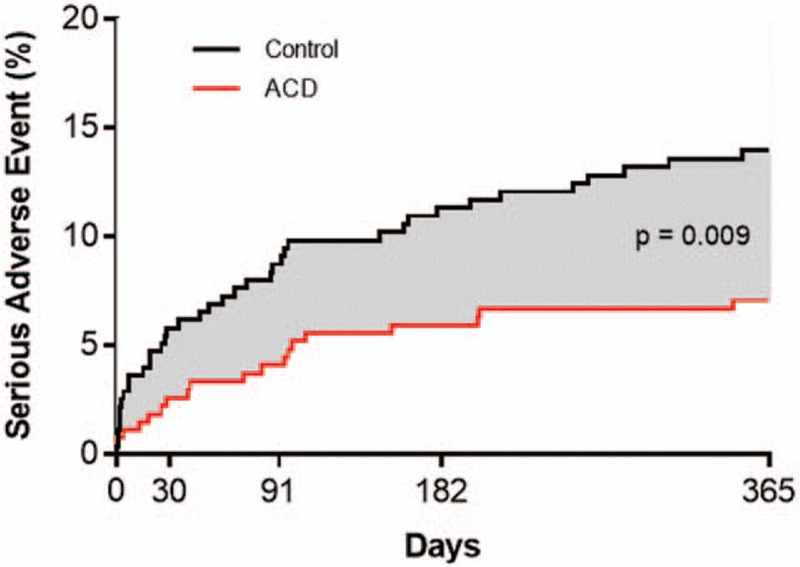
Cumulative risk of device- or procedure-related serious adverse event over 1-year post-treatment. Risk of device- or procedure-related serious adverse event was 7.1% (standard error 1.6%) with annular closure device and 13.9% (standard error 2.1%) with controls. Log-rank P = .009.
Figure 8.
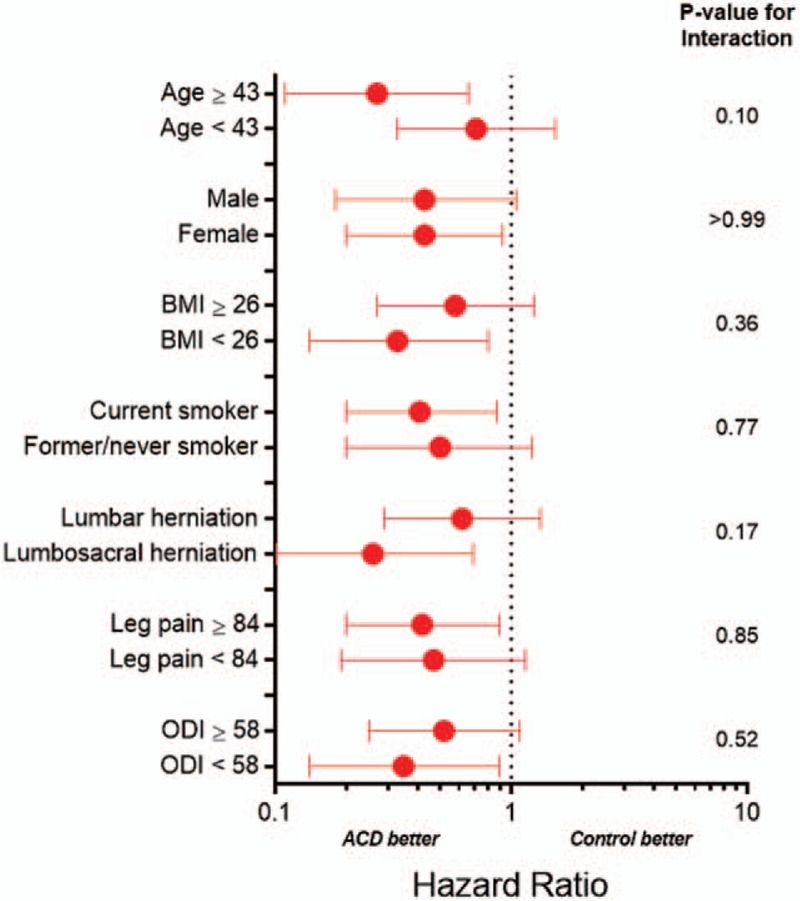
Hazard ratio for device- or procedure-related serious adverse event with annular closure device vs. controls by baseline patient characteristics. ACD = annular closure device; BMI = body mass index; ODI = Oswestry Disability Index. Plotted values are hazard ratio and 95% confidence interval. Hazard ratio < 1 indicates lower risk with ACD; > 1 indicates higher risk with ACD. Continuous variables are categorized by the median value.
The percentage of patients experiencing symptomatic reherniation, reoperation, or a procedure- or device-related serious adverse event over 1 year was 11.2% for ACD and 20.9% for Controls (P = .002). The corresponding hazard ratio was 0.50 (95% CI: 0.32, 0.78), indicating a 50% risk reduction with ACD.
4. Discussion
Lumbar herniation recurrence remains a challenging therapeutic problem, particularly in the high-risk subset of patients with a large defect in the annulus fibrosus following lumbar discectomy. This randomized trial was designed specifically to determine whether recurrence risk could be lowered by additional implantation of a device that physically occludes the defect in the annulus following surgery. During the first postoperative year when recurrence risk is the greatest, patients who received lumbar discectomy with bone-anchored ACD implantation had a lower risk of symptomatic reherniation, reoperation, and serious adverse events compared to patients treated with lumbar discectomy only. Further, the treatment benefit of the ACD was realized early, was durably maintained, and was not influenced by baseline patient characteristics. These results have important clinical and economic implications for patients, physicians, and healthcare payers.
The results reported here are in agreement with a recent meta-analysis that reported annular closure resulted in a 66% reduction in the risk of symptomatic reherniation.[12] An interesting aspect of these results was the almost immediate benefit derived in the ACD group relative to Controls. For example, in the first 30 days after surgery, only 1 patient suffered symptomatic reherniation in the ACD group compared to 7 patients in the Control group. Notably, the benefits of ACD were derived without an increase in associated serious complications and, thus, the benefit-to-risk profile of this device appears favorable in well-selected patients.
There is mounting evidence that additional implantation of a bone-anchored ACD implantation is cost effective in high-risk patients relative to lumbar discectomy alone. Parker and colleagues reported that direct and indirect medical costs associated with the management of recurrent disc herniation totaled approximately $38,000 per case and that bone-anchored ACD use resulted in a $2,200 savings per person compared to discectomy alone.[13] More recently, Ament and colleagues[14] derived similar conclusions in which lumbar discectomy with ACD was $2,100 less expensive than with discectomy alone. Further, patients treated with ACD reported better health-related quality of life. Thus, in the context of a health economics evaluation, the ACD was deemed dominant in which greater quality of life was attained at less cost. Although evaluation of medical costs incurred over 1 year of follow-up are beyond the scope of this paper, the risk reductions in symptom recurrence, reoperations, and serious complications suggest potential for cost savings over 1 year when an ACD is added to the surgical management strategy of patients undergoing lumbar discectomy with large postsurgical annular defects.
It is important to note that careful patient selection is mandatory when considering treatment with an ACD. Patients with small postsurgical annular defects are poor candidates for treatment with the ACD since reherniation risk in such patients is comparatively low.[4] Posterior disc height must be at least 5 mm to accommodate device placement. Patients with lumbar osteoporosis, spinal malignancy, or significant metabolic bone disease may not have adequate bone stock to facilitate sufficient device purchase into the adjacent vertebral body. Meticulous surgical technique is also a prerequisite for safe device placement. The ACD should not be implanted if the location of the nerve root prevents delivery of the anchor. Also, surgical instrument alignment under fluoroscopic guidance prior to ACD implantation allows the surgeon to select the correct implantation trajectory, which may lower the risk for complications due to device malpositioning. Attention to these patient- and procedure-related recommendations is mandatory in order to ensure patient safety and optimize clinical efficacy.
Strengths of the trial design included data monitoring, safety oversight by an independent committee, adverse event adjudication, and independent imaging review. There were also several aspects of this study that may be considered limitations. First, this report was focused on results during the first postoperative year since the risk of herniation recurrence is greatest during this period. Thus, the influence of patient characteristics on long-term results remains unclear. Second, all patients were treated with limited lumbar discectomy and, therefore, it is plausible that aggressive disc resection may also lower reherniation rates albeit at the risk of developing intervertebral instability and acceleration of spondylosis.[15] Third, approximately 1 in 3 patients have a large annular defect after lumbar discectomy[4] and, therefore, ACD implantation is only appropriate in this high-risk subset of the population undergoing lumbar discectomy. Patients with annular defect widths greater than 10 mm were not studied due to device size limitations. Finally, it is plausible that baseline patient characteristics had a small influence on the risk of symptomatic reherniation and reoperation risk with ACD since the study was not prospectively designed for these subgroup comparisons.
5. Discussion
Among patients with large annular defects following limited lumbar discectomy, additional implantation with a bone-anchored ACD lowered the risk of symptomatic reherniation and reoperation over 1-year follow-up. Device- or procedure-related serious adverse events occurred less frequently in the ACD group. These conclusions were not influenced by patient characteristics.
Author contributions
Conceptualization: Wimar van den Brink, Charlotte Flüh, Larry E Miller, Peter D. Klassen, Richard Bostelmann.
Formal analysis: Larry E Miller.
Investigation: Wimar van den Brink, Charlotte Flüh, Peter D. Klassen, Richard Bostelmann.
Methodology: Larry E Miller.
Project administration: Wimar van den Brink, Charlotte Flüh, Peter D. Klassen, Richard Bostelmann.
Supervision: Wimar van den Brink, Charlotte Flüh, Peter D. Klassen, Richard Bostelmann.
Writing - Original Draft: Larry E Miller.
Writing - Review & Editing: Wimar van den Brink, Charlotte Flüh, Larry E Miller, Peter D. Klassen, Richard Bostelmann.
Footnotes
Abbreviations: ACD = annular closure device, ASD = absolute standardized difference, BMI = body mass index, CT = computed tomography, MRI = magnetic resonance imaging, ODI = Oswestry Disability Index.
How to cite this article: van den Brink W, Flüh C, Miller LE, Klassen PD, Bostelmann R. Lumbar disc reherniation prevention with a bone-anchored annular closure device: 1-year results of a randomized trial. Medicine. 2019;98:44(e17760).
This research was supported by Intrinsic Therapeutics (Woburn, MA, United States).
LM and PK received personal fees from Intrinsic Therapeutics. WB, CF, and RB report no conflicts of interest.
References
- [1].Carragee EJ, Spinnickie AO, Alamin TF, et al. A prospective controlled study of limited versus subtotal posterior discectomy: short-term outcomes in patients with herniated lumbar intervertebral discs and large posterior anular defect. Spine (Phila Pa 1976) 2006;31:653–7. [DOI] [PubMed] [Google Scholar]
- [2].Ambrossi GL, McGirt MJ, Sciubba DM, et al. Recurrent lumbar disc herniation after single-level lumbar discectomy: incidence and health care cost analysis. Neurosurgery 2009;65:574–8. discussion 578. [DOI] [PubMed] [Google Scholar]
- [3].McGirt MJ, Eustacchio S, Varga P, et al. A prospective cohort study of close interval computed tomography and magnetic resonance imaging after primary lumbar discectomy: factors associated with recurrent disc herniation and disc height loss. Spine (Phila Pa 1976) 2009;34:2044–51. [DOI] [PubMed] [Google Scholar]
- [4].Miller LE, McGirt MJ, Garfin SR, et al. Association of annular defect width after lumbar discectomy with risk of symptom recurrence and reoperation: systematic review and meta-analysis of comparative studies. Spine (Phila Pa 1976) 2018;43:E308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Abdu RW, Abdu WA, Pearson AM, et al. Reoperation for recurrent intervertebral disc herniation in the spine patient outcomes research trial: analysis of rate risk factors, and outcomes. Spine (Phila Pa 1976) 2017;42:1106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fritzell P, Knutsson B, Sanden B, et al. Recurrent versus primary lumbar disc herniation surgery: patient-reported outcomes in the Swedish spine register Swespine. Clin Orthop Relat Res 2015;473:1978–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Suri P, Pearson AM, Zhao W, et al. Pain recurrence after discectomy for symptomatic lumbar disc herniation. Spine (Phila Pa 1976) 2017;42:755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Parker SL, Mendenhall SK, Godil SS, et al. Incidence of low back pain after lumbar discectomy for herniated disc and its effect on patient-reported outcomes. Clin Orthop Relat Res 2015;473:1988–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Klassen PD, Hes R, Bouma GJ, et al. A multicenter, prospective, randomized study protocol to demonstrate the superiority of a bone-anchored prosthesis for anular closure used in conjunction with limited discectomy to limited discectomy alone for primary lumbar disc herniation. Int J Clin Trials 2016;3:120–31. [Google Scholar]
- [10].Spengler DM. Lumbar discectomy. Results with limited disc excision and selective foraminotomy. Spine (Phila Pa 1976) 1982;7:604–7. [PubMed] [Google Scholar]
- [11].Bostelmann R, Steiger HJ, Cornelius JF. Effect of annular defects on intradiscal pressures in the lumbar spine: an in vitro biomechanical study of diskectomy and annular repair. J Neurol Surg A Cent Eur Neurosurg 2017;78:46–52. [DOI] [PubMed] [Google Scholar]
- [12].Choy WJ, Phan K, Diwan AD, et al. Annular closure device for disc herniation: meta-analysis of clinical outcome and complications. BMC Musculoskelet Disord 2018;19:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Parker SL, Grahovac G, Vukas D, et al. Cost savings associated with prevention of recurrent lumbar disc herniation with a novel annular closure device: a multicenter prospective cohort study. J Neurol Surg A Cent Eur Neurosurg 2013;74:285–9. [DOI] [PubMed] [Google Scholar]
- [14].Ament J, Thaci B, Yang Z, et al. Cost-effectiveness of a bone-anchored annular closure device versus conventional lumbar discectomy in treating lumbar disc herniations. Spine (Phila Pa 1976) 2019;44:5–16. [DOI] [PubMed] [Google Scholar]
- [15].Mochida J, Nishimura K, Nomura T, et al. The importance of preserving disc structure in surgical approaches to lumbar disc herniation. Spine (Phila Pa 1976) 1996;21:1556–63. discussion 1563–1554. [DOI] [PubMed] [Google Scholar]


