Abstract
Lower gastrointestinal hemorrhage remains a common cause of hospitalization, with significant health care costs. Initial management should include aggressive resuscitation followed by localization of bleeding with nuclear scintigraphy, colonoscopy, or computed tomography (CT) angiography. If bleeding does not resolve spontaneously, expeditious intervention with minimally invasive endoscopic or angiographic methods is necessary with surgical intervention as a last resort.
Keywords: lower gastrointestinal bleed, angiography, colonoscopy, CT angiography
Lower gastrointestinal bleeding (LGIB) is a significant cause of morbidity and mortality, specially in the aging population. It accounts for approximately 20.5 to 27 per 100,000 persons per year 1 and is estimated to lead to 36/100,000 admissions to the hospital, 2 with numbers increasing in the elderly population. The mortality rate from colonic bleeding ranges from 2.4 to 3.9% but can increase to as high as 40% in the elderly population with multiple comorbidities. 3 4 The mean cost of admission, work-up, and treatment ranges from US$9,700 to US$11,800. 5 6
LGIB is defined as bleeding originating distal to the ligament of Treitz. It is often self-limited and resolves spontaneously, allowing for a nonurgent evaluation. However, LGIB can lead to life-threatening hemorrhage or recurrent bleeding and, in many cases, may require expeditious work-up, decision-making, and treatment. Severe hematochezia is defined by continued bleeding in the first 24 hours of hospitalization, decrease in hemoglobin by at least 2 g/dL, and transfusion requirement of at least 2 units of packed red blood cells.
The disease process is specific to the age of the patient, and familiarity with it may help in guiding the diagnostic work-up. Juvenile polyps, Meckel's diverticulum, and inflammatory bowel disease account for most cases of LGIB in children and adolescents. 5 Adults are most likely to present with bleeding secondary to diverticular disease or vascular malformations. Neoplasms become more prevalent with increasing age. Ischemic colitis, postpolypectomy bleeding, small and large bowel ulcers, radiation changes, inflammatory bowel disease, infection, and diversion proctitis are less common causes of LGIB. 3 One entity that requires a special mention is postcolorectal surgery anastomotic bleeding. Although most bleeding episodes resolve with conservative measures, evaluation and management vary depending on the etiology of the LGIB.
The principles of nonoperative management of a lower gastrointestinal (GI) hemorrhage remain largely unchanged, though the medication and technology used have evolved with contemporary medical care. The management of LGIB can be divided into the following three components: recognition/initial assessment/resuscitation, localization, and therapeutic intervention ( Fig. 1 ). Through careful history and physical examination, a physician's goal is to determine both the potential etiology of a GI bleed and the extent of a patient's volume loss. After reversal of potentially modifiable risk factors for bleeding, such as pharmaceutical anticoagulation, the optimal choice of procedure is based on, first, diagnosing the source of bleeding and then a therapeutic intervention to control it. Finally, vigilant monitoring is required in the postprocedure period for early recognition of signs of repeat bleeding.
Fig. 1.
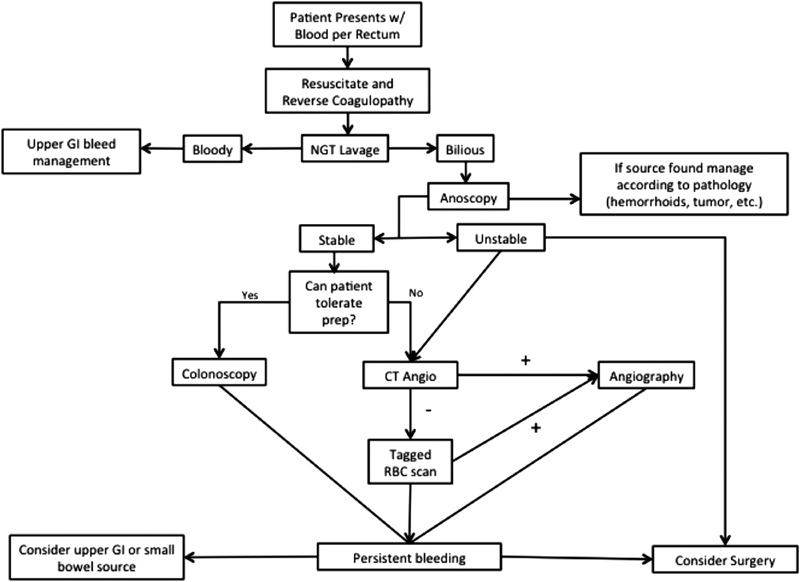
Algorithm for the initial assessment and management of lower gastrointestinal breathing.
Clinical Evaluation
The clinical presentation of a patient with acute GI hemorrhage classically involved the passage of maroon stools or hematochezia, depending on the source of hemorrhage within the lower GI tract. Nasogastric lavage should be considered even with patients with hematochezia, as this may be the result of a brisk upper GI bleed with rapid transit through the small bowel. 5
Patients may present with one or more aberrant vital signs such as orthostatic hypotension or tachycardia, and this should prompt the physician to use aggressive volume resuscitation and rapid diagnostic modalities. Early resuscitation in patients who are hemodynamically unstable or have evidence of reduced end-organ perfusion should be centered on fluid replacement and blood product administration to maintain intravascular volume. In hemodynamically stable patients, the presence of chronic anemia may suggest a slow or indolent source of bleeding.
Role of Radiology in Lower Gastrointestinal Hemorrhage
The two common radiological modalities available to diagnose lower GI hemorrhage are a nuclear scintigraphy and computed tomography (CT) angiography.
Nuclear Scintigraphy
Nuclear scintigraphy, also known as a nuclear medicine bleeding scan (NMBS), allows for identification of those patients who are actively bleeding. The two common techniques used to detect active bleeding include 99m Tc-labeled autologous erythrocytes and 99m Tc sulfur colloid, both tests being considered equivalent. 6
Compared to CT angiography, NMBS is more sensitive in detecting an acute bleed with the ability to detect hemorrhages that are occurring at a rate of 0.3 mL/minute. There is evidence to suggest that if more than 3 mL is pooled at a single location, then a rate as low as 0.1 mL/minute can be identified. 6 The advantages of nuclear scintigraphy relative to CT angiography include the lower threshold rate for acute bleeding, as well as the blood pool phase, which allows for either continuous or intermittent imaging over an extended period of time (determined by the half-life of the radiotracer) that can be used to detect intermittent bleeding. In addition, NMBS may be an ideal modality to detect bleeding in patients with acute kidney injury who cannot tolerate the renal toxicity of intravenous contrast used in CT angiography. 7 However, while sensitive to active bleeding, there has been variability from 40 to 100% for NMBS to actually localize the source of bleeding. 6 Furthermore, the number of positive scans in many published series ranges from 25 to 82% depending on patient factors such as timing of study, ongoing blood transfusion requirement, and etiology of hemorrhage rather than the study itself. 8
The ideal use of nuclear scintigraphy remains unclear. Many guidelines for GI hemorrhage use bleeding scan as an early diagnostic modality to determine whether active hemorrhage is present or to identify patients who will be positive on angiography. For indolent GI hemorrhages where continuous imaging may be advantageous or in patients with acute kidney injury, then the use of NMBS relative to CT angiography is clearly beneficial. However, NMBS has had mixed performance in determining which patients are likely to have subsequent positive angiography largely dependent on patient characteristics, timing of imaging, and skill of the interventional radiologist. One study of 86 patients suggested a 75% positive predictive value and a 93% negative predictive value for detectable bleeding on angiography if an NMBS is positive within the first 2 minutes. 9 Given the 24-hour accessibility to CT at most institutions, the role of NMBS may be reserved for patients who are initially CT angiography negative but have ongoing evidence of GI hemorrhage or those with insufficient renal function to tolerate intravenous contrast.
Computed Tomography Angiography
Multidetector CT (MDCT) has become increasingly accepted as an early diagnostic modality to localize the source of active lower GI hemorrhage. Relative to endoscopy, it requires no special patient preparation, provides wide spatial and temporal resolution, provides assessment of the entire GI tract, has a high diagnostic accuracy, and is readily available in the majority of emergency departments. The hallmark finding used to identify the source of hemorrhage is the presence of a “blush” or extravasation of contrast between an arterial phase and a venous phase. Secondary findings include focal hyperattenuation within the bowel on a nonenhanced CT, which may represent a “sentinel clot” or sign of recent bleeding. 10 MDCT has a reported ability to detect active bleeding at a rate of 0.3 to 0.4 ml/minute. In multiple systematic reviews, MDCT has demonstrated a sensitivity of 85 to 87% and a specificity of 92 to 95% for detecting acute GI hemorrhage. 11 As with NMBS, the severity of GI hemorrhage is the most important factor in predicting the success of the study, where brisk or large volume bleeds are more likely to be positive. Active bleeding on MDCT can be used to localize and guide further intervention, typically through invasive angiography or endoscopic/surgical exploration. The primary limitations of this modality include radiation exposure, contrast-related allergies, and inability to administer intravenous contrast to patients with acute kidney injury.
Invasive Angiography
Invasive angiography was first described in 1965 as a modality to evaluate the mesenteric vessels supplying the GI tract. 12 It is able to identify bleeding that occurs at a rate of 0.5 to 1 mL/minute. It has a sensitivity of 60% and a specificity of 100%. 13 Access is commonly gained through the common femoral artery, with evaluation of the superior mesenteric arteries (SMA) and inferior mesenteric arteries (IMA). If negative for acute bleeding, the celiac artery may be evaluated to exclude upper GI sources of bleeding. Finally, the internal iliac, middle, and inferior rectal arteries can also be evaluated as a tributary to a source of hemorrhage. Positive findings include extravasation of contrast, mucosal blushes with abnormal vessels may suggest a tumor, prolonged contrast may be suggestive of inflammation, and arteriovenous malformations are demonstrated by the presence of arteries and veins during the same phase of the study.
Hemostasis through angiography is achieved by a reduction in blood flow through the vessel supplying the bleeding GI tract; this reduces the perfusion pressure and facilitates clot formation. To reduce the risk of bowel infarction, superselective embolization is used, allowing adequate collateral circulation while still reducing blood flow to the bleeding segment. Bleeding from the ascending colon will usually involve cannulation of the SMA and then advancement of the catheter to the vasa rectum. For the descending colon, the IMA is cannulated, and the catheter is advanced to the marginal or terminal artery, with the catheter situated at the mesenteric border of the colon. One method of blood flow reduction is with transcatheter embolization (TAE) using coils, Gelfoam (absorbable compressed sponge), polyvinyl alcohol particles, vascular plugs, or glue. Routinely, multiple embolic agents will be used together to reduce the risk of recurrent bleeding post procedure. Chemical arterial constriction using vasopressin is another modality to reduce blood flow and is more frequently used for lower GI bleeds compared with upper GI bleeds due to the smaller caliber vessels. The primary complication of note from TAE is bowel ischemia; this has been significantly mitigated with the advancement of superselective vessel catheterization. An important note is that while successful angiography and TAE may stop the source of bleeding, it ultimately does not provide a definitive diagnosis of the exact etiology, whether tumor or diverticular/vascular ectasia.
Provocative Angiography
Lower GI hemorrhage poses a challenging clinical dilemma as more than 80% of patients will spontaneously stop but then will have recurrent hemorrhage without any obvious predisposing factors. 3 This can result in multiple hospitalizations and diagnostic studies without ever localizing the source of bleeding. Provocative angiography uses vasodilating, thrombolytic, or anticoagulation agents to elicit active bleeding. The technique was first reported in 1982 and has not gained widespread popularity, likely due to the presumed risk of hemorrhagic complications. 13 At present, it is not routinely used in the evaluation of an acute GI hemorrhage and is more commonly reserved for patients with a chronic GI bleed who have undergone multiple negative radiographic and endoscopic evaluations. Success rates of identifying an actively bleeding vessel are highly proceduralist and patient dependent, varying from 29 to 80%. 13
Radiographic Conclusion
The success of identifying and stopping a lower GI hemorrhage is highly dependent on the rate of bleeding from the vessel. In patients with GI bleed where immediate endoscopic evaluation is not possible, diagnosis with MDCT imaging affords a fast and potentially high-yield diagnostic modality. If positive, therapeutic intervention can be pursued through invasive angiography in the interventional radiology suite. For patients with slow bleeds that are not as readily apparent on MDCT, the use of nuclear scintigraphy provides the ability to detect a slower rate of bleeding over a longer period of time at the expense of exact localization. In patients with lower GI bleeding due to vascular malformation or diverticulosis, invasive angiography can be therapeutic without the need for further diagnostic evaluation. Lower GI bleeds secondary to a neoplasm or polyp will still require endoscopic and/or surgical intervention.
Colonoscopy
Colonoscopy plays a key diagnostic role in localizing the source of LGIB and can be used to offer a less invasive therapeutic alternative to surgery to achieve hemostasis in the setting of significant hemorrhage. 14 The diagnostic value of colonoscopy is significant and has been documented to determine the source of bleeding in 48 to 90% of cases. 4 15
Timing of Colonoscopy
Colonoscopy should be performed within 12 to 24 hours of presentation. Adequate bowel preparation and resuscitation should be performed prior to colonoscopy to maximize diagnostic and therapeutic value. 16 17 18 Colonoscopy performed early within 24 hours of admission has been shown to reduce length of hospital stay. This is due, in part, to the early identification of low-risk patients, leading to early discharge after negative examination. It is unclear, however, if early colonoscopy changes outcomes including rebleeding, mortality, and need for invasive surgery. More studies are needed to further delineate the benefits of early endoscopy versus initial observation. 16 19 20 21
General principles of an adequate technique include careful inspection of the colonic mucosa during insertion and withdrawal of the scope with the assistance of saline lavage to ensure good visualization. The terminal ileum should be intubated to rule out bleeding pathology proximal to the colon. A colonoscope with a large working channel should be used to accommodate pulse saline lavage; suction of stool, blood, and debris; and instruments of hemostasis. 22
Bowel Preparation
Bowel preparation when the patient is hemodynamically stable should be performed so that there is adequate visualization of the colon wall. 17 18 23 Some studies suggest using a prokinetic agent just prior to administration of prep, such as Reglan 10 mg, as an adjunct to prevent nausea or vomiting. 18 If the patient is unable to drink, a nasogastric tube can be placed to administer the preparation, which is typically 4 to 6 L of polyethylene glycol. High-risk patients must be monitored closely for the development of rare complications including aspiration secondary to emesis and electrolyte–fluid balance abnormalities. 23 Studies have demonstrated lower cecal intubation rates in patients with unprepped bowel, indicating that the diagnostic and therapeutic efficacy is inferior in unprepped bowel. 24 25 26 In patients unable to undergo bowel preparation, an alternative method using a water jet pump for aggressive irrigation after three 1 L tap water enemas have been proposed. 27 Further investigation is necessary to determine the efficacy of this technique.
Therapeutic Interventions
Currently, there are several endoscopic hemostatic techniques that are used, including injection of epinephrine, bipolar/monopolar electrocoagulation, argon plasma coagulation, application of clips, and band ligation. Alternative treatment options under development include the application of various hemostatic agents and advanced over-the-scope clip (OTSC) application devices. 28 29
Complication rates associated with endoscopic interventions for hemostasis are favorable and range from 0.3 to 1.3%. 22 30 Reported complications associated with colonoscopy in the setting of LGIB include perforation, congestive heart failure, aspiration, and electrolyte imbalance. Unfortunately, there is a lack of investigation comparing different endoscopic hemostatic techniques individually or in combination in the setting of lower GI hemorrhage. Choice of endoscopic intervention is left to the clinician's expertise, resources, and the pathological cause of bleeding. The majority of research literature investigating the role of colonoscopy in lower GI hemorrhage is focused on endoscopic management of diverticular bleeding, angioectasias, and postpolypectomy bleeding.
Diverticular bleeding is the most common cause of LGIB in adults, accounting for 30 to 50% of cases. 31 Four-quadrant injection of epinephrine 1:20,000 concentration in 1- to 2-mL aliquots into the submucosal layer causes vasoconstriction and tamponade of bleeding. 18 Monotherapy with epinephrine alone is associated with a significant rebleeding rate. Therefore, it is recommended that epinephrine injection should be supplemented with a second form of hemostasis, including clip or thermal energy application. 16 18 Alternative therapy includes the application of bipolar thermal coagulation at 10 to 15 W in 1-second bursts. Careful consideration should be taken when using thermal treatment for hemostasis, as transmural damage to the colon wall can rarely occur. If the bleeding diverticulum is identified, placement of a clip or band can achieve hemostasis and avoid the risk of thermal injury. The clip is placed directly on the bleeding vessel but can also be used to close the orifice of the diverticulum, resulting in tamponade of bleeding. 32 Endoscopic banding entails involution of the diverticulum with the placement of a band. The band device typically fits on the end of the colonoscope. Once the bleeding source has been identified, the target area is marked with ink or placement of a clip. The scope is withdrawn and the band device is placed on the tip of the scope followed by reinsertion to the target and deployment of the band. 33 34 A Doppler ultrasound probe can be used to monitor arterial flow before and after treatment. In patients who underwent hemostatic treatment, arterial flow was noted to be absent posttreatment. 35 Further studies are necessary to delineate the role of Doppler monitoring in endoscopy for diverticular hemorrhage control.
Argon plasma coagulation is a noncontact modality of thermal hemostasis that offers control over depth of energy penetration, making it an effective treatment option for angioectasias and radiation proctitis. The device emits a spray of argon gas that is ionized with a spark from the applicator delivering thermal energy. Typical power settings used range from 20 to 60 W, with argon gas flow rate ranging from 1 to 2.5 L/minute. 36 37
Postpolypectomy bleeding is the most common complication after polypectomy, occurring in 0.5 to 6% of cases. Surgery is rarely necessary, and endoscopic clip placement, epinephrine injection, or banding is effective. 38 39 Newer treatment options are being developed, including application of hemostatic agents including hemostatic agent TC-325 (Hemospray), polysaccharide hemostatic system (EndoClot), and Ankaferd BloodStopper. Small case series have suggested that these agents can be used as first- or second-line treatment for lesions that are difficult to access. 39 OTSC application is a new modality for the management of bleeding, fistulas, and leaks. The clip consists of an elastic material, Nitinol, and has the shape of a bear trap that is mounted on the end of the scope and is applied similar to a band. Treatment success has been reported in up to 89% of cases. Currently, there are no randomized control trials to compare treatment with the OTSC device to other modalities. 40
Postoperative bleeding is a rare complication after colectomy. 41 The best treatment of postoperative anastomotic bleeding is prevention. Procedure performed, comorbidities of the patient, clotting profile, and resumption of anticoagulation all play a role in increasing the risk of postsurgery bleeding. Hemodynamic instability, decrease in blood pressure, and ensuing arrhythmias can compromise the newly created anastomosis, leading to a more dreaded complication of anastomotic leak. Postcolectomy bleeding can be separated into minor and major bleedings. Minor bleeding is defined as passage of blood-streaked or dark stools in the first 24 hours without hemodynamic instability or change in hemoglobin. It does not require any intervention other than close observation. Major bleeding is bleeding accompanied by hemodynamic instability, change in hemoglobin, and requirement for transfusion and/or fluid resuscitation. Although most of the major bleedings will cease spontaneously, certain patients will continue to bleed, requiring intervention. It is estimated that 0.5 to 4.2% of patients will develop major bleeding after a colectomy with anastomosis. 41 Most of the data are limited to ileocolic anastomosis in patients with inflammatory bowel disease. 42 There seems to be no difference in the way the anastomosis is constructed (stapled vs. hand-sewn), and specific risk factors for bleeding have not been identified secondary to limited available data. Postoperatively, the patient's vital signs should be assessed at regular intervals, hemoglobin should be measured, and bloody bowel movements should be documented. Initial management should include supportive care and correction of coagulopathies, if present. Surgical intervention is reserved for unstable patients or those who fail nonoperative treatment, but it should be considered early in this patient population due to the potential for anastomotic leak.
Patients with a low anastomosis should undergo proctoscopy with evacuation of clots and possible transanal ligation of bleeding points. Patients with higher colorectal or ileocolic anastomosis should be evaluated through colonoscopy. A retrospective review of 1,389 patients who underwent a colorectal resection identified 7 (0.5%) patients with postoperative LGIB. Six patients were treated endoscopically to control bleeding, and one required operative management. 43 Endoscopic treatment of postoperative bleeding includes isotonic saline washout, electrocoagulation, epinephrine injection, and application of hemostatic clips. Angiography and intra-arterial vasopressin injection have been effective in the management of anastomotic bleeding that fails other measures. 44 However, these modalities carry a higher risk of ischemia and anastomotic disruption. 43
Reoperation to obtain control of bleeding is the last resort when bleeding cannot be controlled with other methods. Patients who remain hemodynamically unstable, require repeated blood transfusions, or show signs of sepsis should be taken promptly to the operating room where resection of the bleeding anastomosis should be performed, either revised or diverted based on patient's overall status. It should be noted that there is no prospective data addressing LGIB after colorectal anastomosis. Most recommendations are extrapolated from case reports and retrospective reviews.
Restarting Anticoagulation
As more patients are on anticoagulation for CVA (cerebrovascular accident) prevention, arrhythmias, and heart conditions, there is an urgency to resume or continue antiplatelet and anticoagulant therapy. The American Heart Association recommends stopping and resuming therapy within 48 hours.
Conclusion
Prompt recognition and treatment of LGIB will lead to improved patient outcomes and decreased health care costs.
Footnotes
Conflict of Interest None.
References
- 1.Manning-Dimmitt L L, Dimmitt S G, Wilson G R. Diagnosis of gastrointestinal bleeding in adults. Am Fam Physician. 2005;71(07):1339–1346. [PubMed] [Google Scholar]
- 2.Peery A F, Dellon E S, Lund J et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(05):1179–1.187E6. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGuire H H., Jr Bleeding colonic diverticula. A reappraisal of natural history and management. Ann Surg. 1994;220(05):653–656. doi: 10.1097/00000658-199411000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davila R E, Rajan E, Adler D G et al. ASGE Guideline: the role of endoscopy in the patient with lower-GI bleeding. Gastrointest Endosc. 2005;62(05):656–660. doi: 10.1016/j.gie.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Zuccaro G, Jr; American College of Gastroenterology. Practice Parameters Committee.Management of the adult patient with acute lower gastrointestinal bleeding Am J Gastroenterol 199893081202–1208. [DOI] [PubMed] [Google Scholar]
- 6.Howarth D M. The role of nuclear medicine in the detection of acute gastrointestinal bleeding. Semin Nucl Med. 2006;36(02):133–146. doi: 10.1053/j.semnuclmed.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Zuckerman G R, Prakash C, Askin M P, Lewis B S. AGA technical review on the evaluation and management of occult and obscure gastrointestinal bleeding. Gastroenterology. 2000;118(01):201–221. doi: 10.1016/s0016-5085(00)70430-6. [DOI] [PubMed] [Google Scholar]
- 8.Currie G M, Kiat H, Wheat J M. Scintigraphic evaluation of acute lower gastrointestinal hemorrhage: current status and future directions. J Clin Gastroenterol. 2011;45(02):92–99. doi: 10.1097/MCG.0b013e3181f39d46. [DOI] [PubMed] [Google Scholar]
- 9.Ng D A, Opelka F G, Beck D E et al. Predictive value of technetium Tc 99m-labeled red blood cell scintigraphy for positive angiogram in massive lower gastrointestinal hemorrhage. Dis Colon Rectum. 1997;40(04):471–477. doi: 10.1007/BF02258395. [DOI] [PubMed] [Google Scholar]
- 10.Artigas J M, Martí M, Soto J A, Esteban H, Pinilla I, Guillén E. Multidetector CT angiography for acute gastrointestinal bleeding: technique and findings. Radiographics. 2013;33(05):1453–1470. doi: 10.1148/rg.335125072. [DOI] [PubMed] [Google Scholar]
- 11.Geffroy Y, Rodallec M H, Boulay-Coletta I, Jullès M-C, Ridereau-Zins C, Zins M. Multidetector CT angiography in acute gastrointestinal bleeding: why, when, and how. Radiographics. 2011;31(03):E35–E46. doi: 10.1148/rg.313105206. [DOI] [PubMed] [Google Scholar]
- 12.Boley S J, Brandt L J, Sammartano R J. History of mesenteric ischemia. The evolution of a diagnosis and management. Surg Clin North Am. 1997;77(02):275–288. doi: 10.1016/s0039-6109(05)70548-x. [DOI] [PubMed] [Google Scholar]
- 13.Johnston C, Tuite D, Pritchard R, Reynolds J, McEniff N, Ryan J M. Use of provocative angiography to localize site in recurrent gastrointestinal bleeding. Cardiovasc Intervent Radiol. 2007;30(05):1042–1046. doi: 10.1007/s00270-007-9107-5. [DOI] [PubMed] [Google Scholar]
- 14.Green B T, Rockey D C. Lower gastrointestinal bleeding--management. Gastroenterol Clin North Am. 2005;34(04):665–678. doi: 10.1016/j.gtc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Strate L L. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005;34(04):643–664. doi: 10.1016/j.gtc.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Strate L L, Syngal S. Timing of colonoscopy: impact on length of hospital stay in patients with acute lower intestinal bleeding. Am J Gastroenterol. 2003;98(02):317–322. doi: 10.1111/j.1572-0241.2003.07232.x. [DOI] [PubMed] [Google Scholar]
- 17.Green B T, Rockey D C, Portwood G et al. Urgent colonoscopy for evaluation and management of acute lower gastrointestinal hemorrhage: a randomized controlled trial. Am J Gastroenterol. 2005;100(11):2395–2402. doi: 10.1111/j.1572-0241.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- 18.Jensen D M, Machicado G A, Jutabha R, Kovacs T O. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. 2000;342(02):78–82. doi: 10.1056/NEJM200001133420202. [DOI] [PubMed] [Google Scholar]
- 19.Nagata N, Niikura R, Sakurai T et al. Safety and effectiveness of early colonoscopy in management of acute lower gastrointestinal bleeding on the basis of propensity score matching analysis. Clin Gastroenterol Hepatol. 2016;14(04):558–564. doi: 10.1016/j.cgh.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Schmulewitz N, Fisher D A, Rockey D C. Early colonoscopy for acute lower GI bleeding predicts shorter hospital stay: a retrospective study of experience in a single center. Gastrointest Endosc. 2003;58(06):841–846. doi: 10.1016/s0016-5107(03)02304-6. [DOI] [PubMed] [Google Scholar]
- 21.Navaneethan U, Njei B, Venkatesh P G, Sanaka M R. Timing of colonoscopy and outcomes in patients with lower GI bleeding: a nationwide population-based study. Gastrointest Endosc. 2014;79(02):297–3.06E14. doi: 10.1016/j.gie.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Strate L L, Naumann C R.The role of colonoscopy and radiological procedures in the management of acute lower intestinal bleeding Clin Gastroenterol Hepatol 2010804333–343., quiz e44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen D M, Machicado G A. Diagnosis and treatment of severe hematochezia. The role of urgent colonoscopy after purge. Gastroenterology. 1988;95(06):1569–1574. doi: 10.1016/s0016-5085(88)80079-9. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhry V, Hyser M J, Gracias V H, Gau F C. Colonoscopy: the initial test for acute lower gastrointestinal bleeding. Am Surg. 1998;64(08):723–728. [PubMed] [Google Scholar]
- 25.Ohyama T, Sakurai Y, Ito M, Daito K, Sezai S, Sato Y. Analysis of urgent colonoscopy for lower gastrointestinal tract bleeding. Digestion. 2000;61(03):189–192. doi: 10.1159/000007756. [DOI] [PubMed] [Google Scholar]
- 26.Tada M, Shimizu S, Kawai K. Emergency colonoscopy for the diagnosis of lower intestinal bleeding. Gastroenterol Jpn. 1991;26(03) 03:121–124. doi: 10.1007/BF02779279. [DOI] [PubMed] [Google Scholar]
- 27.Repaka A, Atkinson M R, Faulx A L et al. Immediate unprepared hydroflush colonoscopy for severe lower GI bleeding: a feasibility study. Gastrointest Endosc. 2012;76(02):367–373. doi: 10.1016/j.gie.2012.03.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barkun A N, Moosavi S, Martel M. Topical hemostatic agents: a systematic review with particular emphasis on endoscopic application in GI bleeding. Gastrointest Endosc. 2013;77(05):692–700. doi: 10.1016/j.gie.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Leung Ki E-L, Lau J Y. New endoscopic hemostasis methods. Clin Endosc. 2012;45(03):224–229. doi: 10.5946/ce.2012.45.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuckerman G R, Prakash C. Acute lower intestinal bleeding: part I: clinical presentation and diagnosis. Gastrointest Endosc. 1998;48(06):606–617. doi: 10.1016/s0016-5107(98)70043-4. [DOI] [PubMed] [Google Scholar]
- 31.Gayer C, Chino A, Lucas Cet al. Acute lower gastrointestinal bleeding in 1,112 patients admitted to an urban emergency medical center Surgery 200914604600–606., discussion 606–607 [DOI] [PubMed] [Google Scholar]
- 32.Kumar A, Artifon E, Chu A, Halwan B. Effectiveness of endoclips for the treatment of stigmata of recent hemorrhage in the colon of patients with acute lower gastrointestinal tract bleeding. Dig Dis Sci. 2011;56(10):2978–2986. doi: 10.1007/s10620-011-1683-1. [DOI] [PubMed] [Google Scholar]
- 33.Ishii N, Setoyama T, Deshpande G A et al. Endoscopic band ligation for colonic diverticular hemorrhage. Gastrointest Endosc. 2012;75(02):382–387. doi: 10.1016/j.gie.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 34.Setoyama T, Ishii N, Fujita Y. Enodoscopic band ligation (EBL) is superior to endoscopic clipping for the treatment of colonic diverticular hemorrhage. Surg Endosc. 2011;25(11):3574–3578. doi: 10.1007/s00464-011-1760-8. [DOI] [PubMed] [Google Scholar]
- 35.Jensen D M, Ohning G V, Kovacs T O et al. Natural history of definitive diverticular hemorrhage based on stigmata of recent hemorrhage and colonoscopic Doppler blood flow monitoring for risk stratification and definitive hemostasis. Gastrointest Endosc. 2016;83(02):416–423. doi: 10.1016/j.gie.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwan V, Bourke M J, Williams S J et al. Argon plasma coagulation in the management of symptomatic gastrointestinal vascular lesions: experience in 100 consecutive patients with long-term follow-up. Am J Gastroenterol. 2006;101(01):58–63. doi: 10.1111/j.1572-0241.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 37.Olmos J A, Marcolongo M, Pogorelsky V, Herrera L, Tobal F, Dávolos J R. Long-term outcome of argon plasma ablation therapy for bleeding in 100 consecutive patients with colonic angiodysplasia. Dis Colon Rectum. 2006;49(10):1507–1516. doi: 10.1007/s10350-006-0684-1. [DOI] [PubMed] [Google Scholar]
- 38.Gibbs D H, Opelka F G, Beck D E, Hicks T C, Timmcke A E, Gathright J B., Jr Postpolypectomy colonic hemorrhage. Dis Colon Rectum. 1996;39(07):806–810. doi: 10.1007/BF02054448. [DOI] [PubMed] [Google Scholar]
- 39.Parra-Blanco A, Kaminaga N, Kojima T, Endo Y, Tajiri A, Fujita R. Colonoscopic polypectomy with cutting current: is it safe? Gastrointest Endosc. 2000;51(06):676–681. doi: 10.1067/mge.2000.105203. [DOI] [PubMed] [Google Scholar]
- 40.Bustamante-Balén M, Plumé G. Role of hemostatic powders in the endoscopic management of gastrointestinal bleeding. World J Gastrointest Pathophysiol. 2014;5(03):284–292. doi: 10.4291/wjgp.v5.i3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirchhoff P, Clavien P-A, Hahnloser D. Complications in colorectal surgery: risk factors and preventive strategies. Patient Saf Surg. 2010;4(01):5. doi: 10.1186/1754-9493-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choy P YG, Bissett I P, Docherty J G, Parry B R, Merrie A E. Stapled versus handsewn methods for ileocolic anastomoses. Cochrane Database Syst Rev. 2007;(03):CD004320. doi: 10.1002/14651858.CD004320.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Martínez-Serrano M-A, Parés D, Pera M et al. Management of lower gastrointestinal bleeding after colorectal resection and stapled anastomosis. Tech Coloproctol. 2009;13(01):49–53. doi: 10.1007/s10151-009-0458-6. [DOI] [PubMed] [Google Scholar]
- 44.Atabek U, Pello M J, Spence R K, Alexander J B, Camishion R C. Arterial vasopressin for control of bleeding from a stapled intestinal anastomosis. Report of two cases. Dis Colon Rectum. 1992;35(12):1180–1182. doi: 10.1007/BF02251974. [DOI] [PubMed] [Google Scholar]


