Abstract
We present a bio-inspired late stage C–H oxidation of the ent-trachylobane natural product mitrephorone B to mitrephorone A. The realization of this unprecedented transformation was accomplished by either an iron-catalyzed or electrochemical oxidation and enabled access to the densely substituted oxetane in one step. Formation of mitrephorone C, which is lacking the central oxetane unit but features a keto-function at C2, was not formed under these conditions.
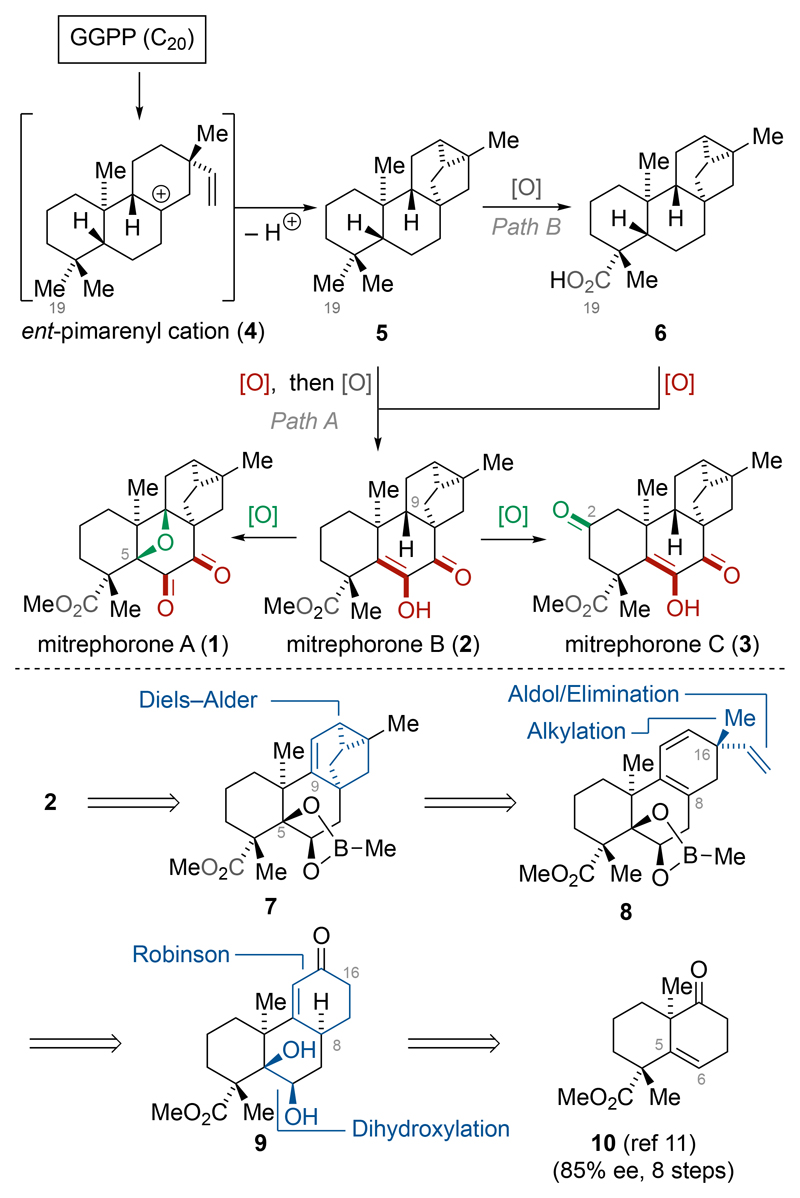
The ent-trachylobane natural products mitrephorone A (1), B (2) and C (3) are structurally related to the diterpenoids ent-atiserene, ent-beyerene, and ent-kaurene, but they display a rare and synthetically challenging oxidation pattern (Scheme 1).1,2 An initial bioactivity screen revealed moderate anti-microbial and anti-cancer activities for 1–3.2 The proposed biosynthesis begins with the cyclization of geranylgeranyl pyrophosphate (GGPP, C20) to the ent-pimarenyl carbocation 4. The mechanism for the conversion of 4 into the ent-trachylobane skeleton 5 was clarified by Tantillo and excludes the intermediacy of a previously postulated secondary carbocation.3 After the initial cyclization phase, 5 is enzymatically postmodified. As shown for the formation of mitrephorone B (2), it is reasonable that either the trans-decalin framework is oxidized first (Path A) or that functionalization of C19 to give ent-trachyloban-19-oic acid (6) precedes this event (Path B). At the outset of this project, we hypothesized that 2 represents the pivotal intermediate from which 1 and 3 are generated via selective oxidation. Based on this consideration and our continued interest to constructing synthetically challenging oxetanes,4 we envisioned investigation of this oxidation process in the chemical laboratory. Notably, mimicking the putative oxidation of mitrephorone B (2) to mitrephorone A (1) would allow us to circumvent the limited substrate scope generally associated with conventional oxetane formation methods such as ring-expansion, [2+2]-cycloaddition, and nucleophilic displacement reactions.5,6 Carreira recently disclosed the synthesis of 1 employing an elegant Umpolung strategy.5 Here we present the first total synthesis of mitrephorone B (2) and show its conversion to mitrephorone A (1) via a bio-inspired late-stage C–H oxidation.7,8
Schema 1. Schematic Biosynthesis of Mitrephorone A (1), B (2), and C (3) and Retrosynthetic Analysis.
Retrosynthetic bond disconnections of our initial target, mitrephorone B (2), revealed tricyclo-[3.2.1.02,7]oct-3-ene 7 as the precursor. For the installation of this motif, 7 was traced back to 5-vinyl-1,3-cyclohexadiene 8 by employing an intramolecular retro-Diels–Alder (IMDA) reaction.
This powerful type of IMDA was previously emulated in related systems by Trauner9 and has proven its efficiency in the synthesis of 1.5 Removal of the C16 quaternary stereocenter revealed enone 9, which contains the retron for a simplifying Robinson annulation.10 Further disconnection of 9 generated known ketone 1011 as the ideal starting point for our investigations.
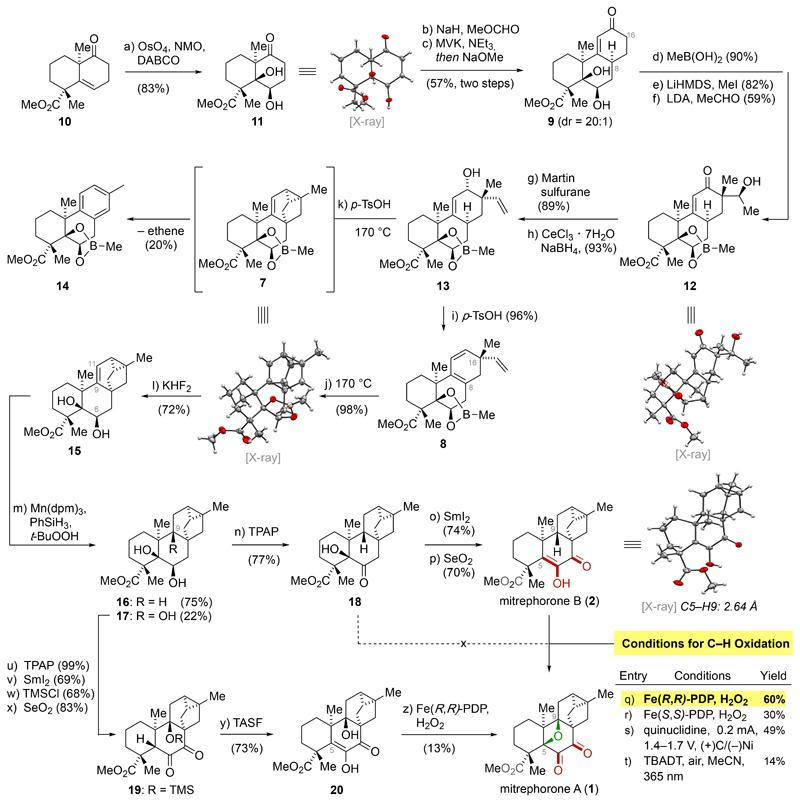
As shown in Scheme 2, asymmetric synthesis of 10 was carried out on multigram scale (4.4 g) in good overall yield. The Upjohn dihydroxylation of the neopentylic alkene was exceptionally challenging and no conversion of the starting material took place at ambient temperature. Fortunately, it was found that 1,2-diol 11 was obtained in good yield and as a single diastereomer by conducting the dihydroxylation at elevated temperature (90 °C) using 1,4-diazabicyclo[2.2.2]octane (DABCO) as a crucial additive in this transformation.12 The early dihydroxylation of the C5–C6 alkene enabled investigation of the limitation of oxetane formation via nucleophilic substitution and revealed valuable information about the structural requirements for the realization of the C–H oxidation (vide infra). Conversion of 11 to tricyclic enone 9 was accomplished by employing a two-step Robinson annulation protocol.10 First, 11 was activated by installation of a β-keto aldehyde (NaH, methyl formate). Subsequent exposure of this intermediate to methyl vinyl ketone (MVK), followed by addition of sodium methoxide gave 9 in reproducibly good yield (57% over two steps) and excellent diastereoselectivity (20:1).13
Schema 2. Total Synthesis of Mitrephorone B (2) and Mitrephorone A (1)a.
aReagents and conditions: (a) OsO4, NMO, DABCO, acetone–H2O, 90 °C, 72 h, 83%; (b) NaH, then MeOCHO, THF–PhMe, 0 °C to 23 °C, 4 h; (c) MVK, NEt3, CH2Cl2, 23 °C, 96 h, then NaOMe, MeOH, 44 °C, 4 h, 57% over two steps; (d) MeB(OH)2, C6H6, 30 °C, 2 h, 90%; (e) LiHMDS, then MeI, THF, –50 °C to 23 °C, 13 h, 82%; (f) LDA, TMEDA, then MeCHO, THF, –20 °C to –78 °C, 3 h, 59%; (g) Martin sulfurane, C6H6, 23 °C, 2 h, 89%; (h) NaBH4, CeCl3•7H2O, MeOH, 0 °C, 3 h, 93%; (i) p-TsOH, 4 Å MS, PhMe, 23 °C, 40 h, 96%; (j) PhMe, 170 °C, 3 h, 98%; (k) p-TsOH, 4 Å MS, PhMe, 23 °C to 170 °C, 72 h, 20%; (l) KHF2, MeOH–H2O, 40 °C, 24 h, 72% over three cycles; (m) Mn(dpm)3, PhSiH3, t-BuOOH, i-PrOH, 23 °C, 17 h, 97%; (n) TPAP, NMO, 4 Å MS, CH2Cl2, 23 °C, 3 h, 77%; (o) SmI2, THF–MeOH, 23 °C, 20 min, 74%; (p) SeO2, 1,4-dioxane, 100 °C, 7 h, 70%; (q) Fe(R,R)-PDP, H2O2 (aq.), AcOH, MeCN, 23 °C, 1 h, 60%; (r) Fe(S,S)-PDP, H2O2 (aq.), AcOH, MeCN, 23 °C, 1 h, 30%; (s) (+)RVC foam/(–)Ni, Me4NBF4, quinuclidine, air, HFIP, MeCN, 0.2 mA, 23 °C, 4 h, 49%; (t) TBADT, HCl (aq.), 365 nm, 23 °C, 9 h, 14%; (u) TPAP, NMO, CH2Cl2, 23 °C, 3 h, 99%; (v) SmI2, THF–MeOH, 23 °C, 12 min, 69%; (w) TMSCl, DMAP, imidazole, DMF, 90 °C, 6 d, 68%; (x) SeO2, 1,4-dioxane, 100 °C, 2 h, 83%; (y) TASF, H2O, DMF, 0 °C; 30 min, 73%; (z) Fe(R,R)-PDP, H2O2 (aq.), AcOH, MeCN, 23 °C, 1 h, 13%.
Further functionalization and installation of the quaternary carbon center at C16 required protection of the 1,2-diol motif. From a survey of protecting groups (e.g. dialkylsilylenes, acetonides, benzylidene acetal, cyclic carbonates), installation of a rarely used cyclic boronic ester14 emerged as the sole solution (MeB(OH)2, benzene, 30 °C). The boronic ester was stable enough to undergo the following alkylation/aldol sequence and was amenable to selective cleavage at a later stage. While the α-methylation (LiHMDS, MeI, 82%) proceeded efficiently, all attempts to realize a subsequent direct α-vinylation15 resulted in complex reaction mixtures and low isolated yields. Therefore, a two-step protocol was employed. First, aldol reaction with acetaldehyde as an inexpensive C2 synthon (LDA, TMEDA, acetaldehyde, –78 °C)16 gave 12, whose single crystal structure validated the depicted stereochemistry. Sequential exposure of 12 to Martin sulfurane17 and Luche conditions18 provided the corresponding allylic alcohol 13. Treatment of 11 with p-toluenesulfonic acid (40 mol%) at 23 °C effected clean elimination to furnish the desired 5-vinyl-1,3-cyclohexadiene 8 (96%). Subsequent heating at 170 °C induced smooth Diels–Alder reaction to afford 7 in excellent yield (98%). Crystallization from n-pentane–ethyl acetate enabled single-crystal structure analysis to validate the depicted structure. For the formation of 7 it was crucial to separate the elimination from the cycloaddition step, as cyclopropane ring-opening followed by extrusion of ethylene and aromatization to 14 was observed at elevated temperatures in the presence of acid.3,19 In this context it is interesting to note that the formed carbocyclic framework was recently also found in natural products isolated from Burmese amber, raising questions about their (biosynthetic) origin.20 For the cleavage of the cyclic boronic ester, 7 was treated with potassium bifluoride (20.0 equiv) to give diol 15 (47%). Unreacted starting material was recovered (39%) and resubmitted to the reaction conditions to provide 15 in 72% overall yield after three cycles. Having gained access to 15 also enabled investigation of the limitation of oxetane formation based on nucleophilic substitution.21 Attempts to initiate ring-closure of diol 15 or its C6-keto derivative by protonation, halogenation or epoxidation of the C9–C11 alkene were met with failure. In most cases, unreacted starting material was recovered and decomposition prevailed under more forcing conditions.
For the selective alkene reduction of 15, Shenvi’s direct hydrogen atom transfer (HAT) protocol22 was identified to be method of choice. Under these conditions (Mn(dpm)3, PhSiH3, t-BuOOH, 23 °C) hydrogenolysis of the cyclopropane was not observed and 16 was isolated in 75% yield as a single diastereomer at C9 together with only minor amounts of diastereomerically pure triol 17 (22%, vide infra).
Completion of the synthesis of mitrephorone B (2) involved oxidation (tetrapropylammonium perruthenate (TPAP), NMO, 4 Å molecular sieves) of 16 to 18 (77%), removal of the tertiary alcohol using samarium(II) iodide (74%)4,23 and installation of the 1,2-diketone (SeO2, 100 °C, 1,4-dioxane).24 The analytical data obtained for synthetic mitrephorone B (2) were in full agreement with those reported for the natural material.
Having secured ample amounts of 2, we set out to test the feasibility of the bio-inspired C–H oxidation to 1. Gratifyingly, exposure of mitrephorone B (2) to the White–Chen catalyst system (25 mol% Fe(R,R)-PDP, H2O2, AcOH, MeCN, 23 °C, 1 h) afforded mitrephorone A (1) in 60% yield.25 To the best of our knowledge, this represents the first example of oxetane formation via C–H oxidation. The use of the enantiomeric Fe(S,S)-PDP catalyst appears to represent the mismatched case for this C–H oxidation and only 30% of mitrephorone A (1) were obtained. In the absence of the iron catalyst, no oxidation was observed. In addition, we were also able to access 1 by electrochemical oxidation of 2 (49%) using the protocol described by Baran (quinuclidine, Me4NBF4, RVC foam anode, Ni cathode, 0.2 mA, 1.4–1.7 V, 2 F/mol, HFIP, MeCN, 23 °C, 4 h).26 Alternatively, applying photocatalysis (TBADT, aq. HCl; 365 nm)27 we observed significantly lower yields of 1 (14%).
We found that the presence of the enol is crucial to furnish the oxetane. All attempts to effect oxetane formation employing the α-hydroxyketone 18 failed, and only decomposition was observed or starting material was recovered. Further insight was obtained by investigating the iron-mediated C–H oxidation of known diosphenol 20.5 For this purpose, triol 17 was first converted to 19 in four steps. Exposure of 19 to tris(dimethylamino)sulfonium difluorotrimethylsilicate (TASF) liberated the tertiary alcohol and induced tautomerization to give 20. This intermediate was subjected to the White–Chen system as before to promote cyclization to mitrephorone A (1) in 13%.28
The selectivity towards oxetane formation of 2 might be a result of the short distance between C5 of the enol and H9 (2.64 Å). In agreement with the literature, we believe that the sequence is initiated by oxidation of the enone to a tertiary radical.26,29 The subsequent incorporation of oxygen might occur directly at C5 or, after radical translocation,30 at C9. Final ring-closure to the oxetane can occur for both pathways (see Supporting Information for details).
In summary, we were able to mimic a bio-inspired C–H oxidation of mitrephorone B (2) to mitrephorone A (1) using either iron-catalysis or electrochemical oxidation. Despite a careful screen of oxidants as well as literature precedence for structurally related substrates,25,26 oxidation of the C2-position to give mitrephorone C (3) was never observed.31 Our inability to realize this transformation might reveal current limitations of modern C–H oxidation methods or indicate an alternative biosynthetic pathway. For the latter scenario, oxidation of C2 would precede formation of the delicate diosphenol motif thus ruling out mitrephorone B (2) as the parent compound.32
Supplementary Material
The Supporting Information is available free of charge on the ACS Publications website.
Acknowledgment
This work was supported by the Austrian Science Fund FWF (P31023-NBL to T.M.) and the Center for Molecular Biosciences CMBI. Furthermore, we thank Dr. Kevin Mellem (Maze Therapeutics) and Dr. Cedric Hugelshofer (Merck) for assistance during the preparation of this paper, Juri Skotnitzki (LMU Munich) for chiral GC analysis and Dr. Matthias Schmid (University of Innsbruck) for helpful discussions.
Footnotes
ORCID
Thomas Magauer: 0000-0003-1290-9556
Notes
The authors declare no competing financial interest.
References
- (1).(a) Hanson JR. Diterpenoids. Nat Prod Rep. 2006;23:875–885. doi: 10.1039/b516326a. [DOI] [PubMed] [Google Scholar]; (b) Hanson JR. Diterpenoids. Nat Prod Rep. 2007;24:1332–1341. doi: 10.1039/b705951p. [DOI] [PubMed] [Google Scholar]; (c) Roy A, Roberts FG, Wilderman PR, Zhou K, Peters RJ, Coates RM. 16-Aza-ent-beyerane and 16-Aza-ent-trachylobane: potent mechanism-based inhibitors of recombinant ent-kaurene synthase from Arabidopsis thaliana. J Am Chem Soc. 2007;129:12453–12460. doi: 10.1021/ja072447e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Li C, Lee D, Graf TN, Phifer SS, Nakanishi Y, Burgess JP, Riswan S, Setyowati FM, Saribi AM, Soejarto DD, Farnsworth NR, et al. A Hexacyclic ent-Trachylobane Diterpenoid Possessing an Oxetane Ring from Mitrephora glabra. Org Lett. 2005;7:5709–5712. doi: 10.1021/ol052498l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Hong YJ, Tantillo DJ. Formation of beyerene, kaurene, trachylobane, and atiserene diterpenes by rearrangements that avoid secondary carbocations. J Am Chem Soc. 2010;132:5375–5386. doi: 10.1021/ja9084786. [DOI] [PubMed] [Google Scholar]
- (4).(a) Hugelshofer CL, Magauer T. A Bioinspired Cyclization Sequence Enables the Asymmetric Total Synthesis of Dictyoxetane. J Am Chem Soc. 2016;138:6420–6423. doi: 10.1021/jacs.6b03720. [DOI] [PubMed] [Google Scholar]; (b) Hugelshofer CL, Magauer T. A Divergent Approach to the Marine Diterpenoids (+)-Dictyoxetane and (+)-Dolabellane V. Chem Eur J. 2016;22:15125–15136. doi: 10.1002/chem.201603061. [DOI] [PubMed] [Google Scholar]
- (5).Richter MJR, Schneider M, Brandstätter M, Krautwald S, Carreira EM. Total Synthesis of (–)-Mitrephorone A. J Am Chem Soc. 2018;140:16704–16710. doi: 10.1021/jacs.8b09685. [DOI] [PubMed] [Google Scholar]
- (6).(a) Burkhard JA, Wuitschik G, Rogers-Evans M, Müller K, Carreira EM. Oxetanes as versatile elements in drug discovery and synthesis. Angew Chem Int Ed. 2010;49:9052–9067. doi: 10.1002/anie.200907155. [DOI] [PubMed] [Google Scholar]; (b) Bull JA, Croft RA, Davis OA, Doran R, Morgan KF. Oxetanes: Recent Advances in Synthesis, Reactivity, and Medicinal Chemistry. Chem Rev. 2016;116:12150–12233. doi: 10.1021/acs.chemrev.6b00274. [DOI] [PubMed] [Google Scholar]
- (7).Gutekunst WR, Baran PS. C–H functionalization logic in total synthesis. Chem Soc Rev. 2011;40:1976–1991. doi: 10.1039/c0cs00182a. [DOI] [PubMed] [Google Scholar]
- (8).Abrams DJ, Provencher PA, Sorensen EJ. Recent applications of C–H functionalization in complex natural product synthesis. Chem Soc Rev. 2018;47:8925–8967. doi: 10.1039/c8cs00716k. [DOI] [PubMed] [Google Scholar]
- (9).(a) Ng SM, Beaudry CM, Trauner D. Intramolecular Diels-Alder reactions of 5-vinyl-1,3-cyclohexadienes. Org Lett. 2003;5:1701–1704. doi: 10.1021/ol0343414. [DOI] [PubMed] [Google Scholar]; (b) Kelli SK, Beaudry CM, Trauner D, Houk KN. Dienophile Twisting and Substituent Effects Influence Reaction Rates of Intramolecular Diels–Alder Cycloadditions: A DFT Study. J Am Chem Soc. 2005;127:3688–3689. doi: 10.1021/ja050135a. [DOI] [PubMed] [Google Scholar]
- (10).Gallier F, Martel A, Dujardin G. Enantioselective Access to Robinson Annulation Products and Michael Adducts as Precursors. Angew Chem Int Ed. 2017;56:12424–12458. doi: 10.1002/anie.201701401. [DOI] [PubMed] [Google Scholar]
- (11).Deschamp J, Hermant T, Riant O. An easy route toward enantioenriched polycyclic derivatives via an asymmetric domino conjugate reduction–aldol cyclization catalyzed by a chiral Cu(I) complex. Tetrahedron. 2012;68:3457–3467. [Google Scholar]
- (12).Zhou B, Miao Z, Deng G, Ding J, Yang Y, Feng H, Li Y. Synthesis and biological evaluation of novel triptolide analogues for anti-cancer activity. Bioorg Med Chem Lett. 2010;20:6217–6221. doi: 10.1016/j.bmcl.2010.08.106. [DOI] [PubMed] [Google Scholar]
- (13).Yu J, Yu B. Synthesis of the ABC skeleton of the aglycon of Echinoside A. Chin Chem Lett. 2015;26:1331–1335. [Google Scholar]; (b) Utilizing alkene 10 under identical reaction conditions did not provide the Robinson product, but lead to a complex product mixture.
- (14).Xu C, Han A, Virgil SC, Reisman SE. Chemical Synthesis of (+)-Ryanodine and (+)-20-Deoxyspiganthine. ACS Cent Sci. 2017;3:278–282. doi: 10.1021/acscentsci.6b00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Chieffi A, Kamikawa K, Ahman J, Fox JM, Buchwald SL. Catalytic asymmetric vinylation of ketone enolates. Org Lett. 2001;3:1897–1900. doi: 10.1021/ol0159470. [DOI] [PubMed] [Google Scholar]
- (16).Corey EJ, Cheng X-M. Logic of chemical synthesis. Wiley; New York: 1995. [Google Scholar]
- (17).Kuramochi A, Usuda H, Yamatsugu K, Kanai M, Shibasaki M. Total synthesis of (±)-garsubellin. J Am Chem Soc. 2005;127:14200–14201. doi: 10.1021/ja055301t. [DOI] [PubMed] [Google Scholar]
- (18).Ramesh R, Bell V, Twidle AM, Gonnade R, Reddy DS. Enantiospecific Synthesis of Both Enantiomers of the Longtailed Mealybug Pheromone and Their Evaluation in a New Zealand Vineyard. J Org Chem. 2015;80:7785–7789. doi: 10.1021/acs.joc.5b01131. [DOI] [PubMed] [Google Scholar]
- (19).Wu Z-Y, Zhang Y-B, Zhu K-K, Luo C, Zhang J-X, Cheng C-R, Feng R-H, Yang W-Z, Zeng F, Wang Y, Xu P, et al. Anti-inflammatory diterpenoids from the root bark of Acanthopanax gracilistylus. J Nat Prod. 2014;77:2342–2351. doi: 10.1021/np500125x. [DOI] [PubMed] [Google Scholar]
- (20).Shimizu E, Koshino H, Noro A, Maruyama M, Shimoda N, Uesugi S, Ohnishi M, Kimura K. Isolation of a spirolactone norditerpenoid as a yeast Ca2+ signaltransduction inhibitor from Kuji amber and evaluation of its effects on PPM1A activity. Filoterapia. 2019;134:290–296. doi: 10.1016/j.fitote.2019.02.027. [DOI] [PubMed] [Google Scholar]
- (21).(a) Sigrist R, Rey M, Dreiding AS. Kurze Totalsynthesen von (±)-Sativen und (±)-cis-Sativendiol. Helv Chim Acta. 1988;71:788–807. [Google Scholar]; (b) Galatsis P, Parks DJ. Stereoselective synthesis of substituted oxetanes. Tet Lett. 1994;35:6611–6614. [Google Scholar]
- (22).Iwasaki K, Wan KK, Oppedisano A, Crossley SWM, Shenvi RA. Simple, chemoselective hydrogenation with thermodynamic stereocontrol. J Am Chem Soc. 2014;136:1300–1303. doi: 10.1021/ja412342g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Efforts to deoxygenate 16 via a pinacol-like rearrangement remained unsuccessful. For recent examples, see: Defaut B, Parsons TB, Spencer N, Male L, Kariuki BM, Grainger RS. Synthesis of the trans-hydrindane core of dictyoxetane. Org Biomol Chem. 2012;10:4926–4932. doi: 10.1039/c2ob25384d. Liu S-A, Trauner D. Asymmetric Synthesis of the Antiviral Diterpene Wickerol A. J Am Chem Soc. 2017;139:9491–9494. doi: 10.1021/jacs.7b05046.
- (24).Riley HL. Oxidation Activity of Selenium Dioxide. Nature. 1947;159:571–572. [Google Scholar]
- (25).Chen MS, White MC. Combined effects on selectivity in Fe-catalyzed methylene oxidation. Science. 2010;327:566–571. doi: 10.1126/science.1183602. [DOI] [PubMed] [Google Scholar]
- (26).Kawamata Y, Yan M, Liu Z, Bao D-H, Chen J, Starr JT, Baran PS. Scalable, Electrochemical Oxidation of Unactivated C–H Bonds. J Am Chem Soc. 2017;139:7448–7451. doi: 10.1021/jacs.7b03539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Laudadio G, Govaerts S, Wang Y, Ravelli D, Koolman HF, Fagnoni M, Djuric SW, Noël T. Selective C(sp3)-H Aerobic Oxidation Enabled by Decatungstate Photocatalysis in Flow. Angew Chem Int Ed. 2018;57:4078–4082. doi: 10.1002/anie.201800818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Electrochemical oxidation of diosphenol 20 provided 1 in 28% yield.
- (29).(a) Dantignana V, Serrano-Plana J, Draksharapu A, Magallón C, Banerjee S, Fan R, Gamba I, Guo Y, Que L, Jr, Costas M, Company A. Spectroscopic and Reactivity Comparisons between Nonheme Oxoiron(IV) and Oxoiron(V) Species Bearing the Same Ancillary Ligand. J Am Chem Soc. 2019;141:15078–15091. doi: 10.1021/jacs.9b05758. [DOI] [PubMed] [Google Scholar]; (b) White MC, Zhao J. Aliphatic C–H Oxidations for Late-Stage Functionalization. J Am Chem Soc. 2018;140:13988–14009. doi: 10.1021/jacs.8b05195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).(a) Robertson J, Pillai J, Lush RK. Radical translocation reactions in synthesis. Chem Soc Rev. 2001;30:94–103. [Google Scholar]; (b) Hioe J, Zipse H. Radical stability and its role in synthesis and catalysis. Org Biomol Chem. 2010;8:3609–3617. doi: 10.1039/c004166a. [DOI] [PubMed] [Google Scholar]
- (31).We also attempted C–H oxidation of alkene 7, but only observed formation of a complex product mixture.
- (32).The recent isolation of a series of closely related natural products corroborates this hypothesis: Dal Piaz F, Bader A, Malafronte N, D’Ambola M, Petrone AM, Porta A, Ben Hadda T, de Tommasi N, Bisio A, Severino L. Phytochemistry of compounds isolated from the leaf-surface extract of Psiadia punctulata (DC.) Vatke growing in Saudi Arabia. Phytochemistry. 2018;155:191–202. doi: 10.1016/j.phytochem.2018.08.003.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.