Abstract
Objectives
Acupuncture or electroacupuncture (EA) has been applied for treating chemotherapy-induced emesis with limited success. The aims of this study were to investigate the anti-emetic effect of EA and to explore underlying anti-emetic mechanisms.
Materials and Methods
Rats were chronically implanted with a pair of stainless steel leads at acupoint PC6. Effects of EA with different parameters on cisplatin-induced nausea were assessed by pica (intake of kaolin, a surrogate of nausea in species without vomiting reflex). C-fos expressions in the area postrema (AP) and nucleus tractus solitarii (NTS) were analyzed. Subdiaphragmatic vagotomy was used to study involvement of the vagal pathway.
Results
1) EA at 20Hz/0.6ms reduced kaolin intake in the first and second days after cisplatin injection compared with the sham-EA group (first day: 1.0±0.2g vs. 1.9±0.3g, p=0.017; second day: 0.4±0.2g vs.1.1±0.3g, p=0.010). However, EA at 10Hz/1.2ms was ineffective on both days. 2) Subdiaphragmatic vagotomy significantly reduced cisplatin-induced kaolin intake (1.1±0.3 vs. 2.2±0.3g, p=0.014) and also blocked the inhibitory effect of EA on kaolin intake in the first day. 3) Cisplatin significantly increased the expression of c-fos in the NTS and AP. Vagotomy greatly reduced c-fos expression in both NTS and AP. EA reduced the cisplatin-induced c-fos expression in the AP but not the NTS.
Conclusions
EA at PC6 with appropriate parameters has an inhibitory effect on cisplatin-induced nausea. The anti-emetic effect of the EA is centrally medicated involving the AP via the vagal pathway as well as the potential effect on AP by reducing the release of hormones.
Keywords: Eelectroacupuncture, Area postrema, Cisplatin, Chemotherapy-induced nausea and vomiting, Pica
Introduction
Nausea and vomiting are common symptoms resulting from surgery, opiates, radiotherapy and chemotherapy 1. Chemotherapy-induced nausea and vomiting (CINV) are two of the most distressing and feared side effects of cancer treatment 2. Cisplatin is one of the most widely used anticancer agents since the discovery of its antitumor effect in 1969. However, its multiple adverse side effects such as nausea, vomiting and anorexia impose a severe physical and emotional burden on cancer patients 3. Anti-emetic agents that have been developed were based on the neurochemical control of vomiting and are the most common interventions for patients undergoing chemotherapy. However, about 25% to 50% of patients undergoing highly emetic chemotherapies still experience nausea and vomiting while using standard anti-emetics 4. Thus, there is an urgent need to develop efficacious anti-emetic strategies to deal with the intense and protracted nausea and vomiting induced by chemotherapies.
Acupuncture or electroacupuncture (EA) has been shown to be beneficial for postoperative nausea and vomiting 5, 6. The acupuncture point, pericardium 6 (PC6) or Neiguan, located on the anterior surface of the wrist between the tendons of the flexor carpi radialis and the palmaris longus, is the most commonly used acupuncture point to control nausea and vomiting 7–9. Acupuncture or EA has also been used as an alternative treatment of CINV10 and shown to be effective in reducing acute vomiting in patients as reported in a few meta-analysis studies 11, 12 and in animals13–15. However, there is lack of systematic studies investigating methodologies for EA and mechanisms underlying anti-emetic effect of EA are largely unknown.
CINV result from both direct and indirect stimulation of the vomiting center located in the area postrema (AP), which is stimulated directly by afferent input from the vagal and splanchnic nerves. This nucleus is susceptible to stimulation by substances present in the blood or cerebral spinal fluid. Cisplatin dose-dependently increases c-fos expressions in the rat hind brain such as the nucleus of the solitary tract (NTS) and AP 16, suggesting a central mechanism. However, it is unclear whether the autonomic function plays a role to ameliorate the effect of EA on cisplatin-induced emesis.
As a subjective experience, nausea cannot be directly measured in non-human animals, while some species, such as rats, do not have a vomiting reflex. In addition to conditioned flavor aversion, pica (consumption of a substance without nutritional value such as kaolin, Chinese clay) has been well established as an index of nausea and emesis, and a surrogate marker of CNIV in animal studies 17, 18.
We hypothesized that EA has an anti-emetic effect on CINV in rats and this effect is mediated by altering neuronal activity in the AP via the vagal pathway. Accordingly, the aims of the present study were to study the effect of EA with different parameters on pica and to explore underlying anti-emetic mechanisms involving the vagal nerve, NTS and AP.
Materials and Methods
Animals
Male Sprague–Dawley rats (280 – 350 g, Charles River Laboratories, Greensboro, NC, USA) were used in this study. The housing room was humidity-and temperature-controlled (20~22°C) and maintained on a fixed 12-hour light/dark cycle (lights on 6am). The Institutional Animal Care and Use Committee at the Oklahoma City VA Medical Center approved the surgical and experimental protocol.
Implantation of chronic EA electrodes
All rats in this study were chronically implanted with a pair of temporary myocardial pacing wires (Streamline 6494, Medtronic Inc. Minneapolis, MN) at bilateral acupoint PC6. Briefly, the rat was anesthetized with sodium pentobarbital (60mg/kg) and a 3mm-long exposed wire (serving as an electrode) was inserted into PC6 and affixed with sutures. The electrode connecting wires were firstly fixed in the muscle layer around the acupoints, then tunneled subcutaneously to the back of the neck and externalized. Buprenorphine (0.05 mg/kg) and cefazolin (30 mg/kg) were given for 3 days to control pain and infection, respectively. No local infection was noted during the experiment period.
EA stimulation
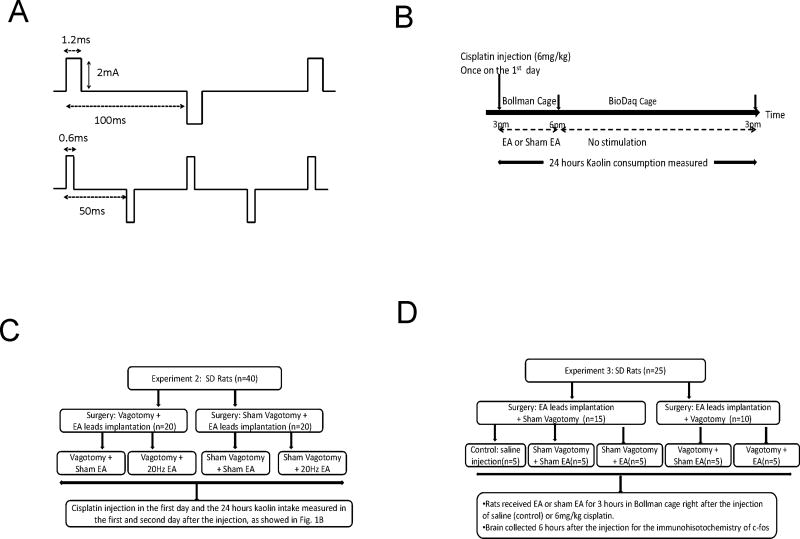
EA was delivered by a constant current pulse generator (World Precision Instruments, Sarasota, FL). Two sets of EA parameters were used in Experiment 1: 1) frequency of 20Hz, pulse width of 0.6ms and amplitude of 2mA; 2) frequency of 10Hz, pulse width of 1.2ms and amplitude of 2mA (Fig.1A). EA of 20Hz was reported to be effective in reducing nausea and vomiting in patients undergoing chemotherapy 10 and reducing kaolin consumption in rats treated with cisplatin 13. With the fixed pulse width, 20Hz was shown to be more effective than 10Hz 13. However, it was unknown whether the better performance with 20Hz was attributed to the increased stimulation energy. To answer this question, we selected a second set of parameters: a reduced frequency of 10Hz but an increased pulse width of 1.2ms. Accordingly, parameter set 2 was of the same stimulation energy as Set 1. The set of EA parameters that resulted in a higher reduction in kaolin intake in Experiment 1 was used in Experiments 2 and 3.
Fig. 1.
Schematic illustration on the setting of stimulation parameters and experimental protocols. A. Two sets of biphasic stimulation parameters: one with 0.6ms pulse width at 20Hz, the other with 1.2ms pulse width at 10 Hz. B. The flow charts of experimental protocol to study the efficacy of EA on cisplatin-induced kaolin intake. C. The flow chart of experimental protocol to study the vagal mechanism involved in the anti-emetic effect of EA. D The flow chart of experimental protocol to study the effect and mechanism of EA on cisplatin-induced c-fos expressions on the AP and NTS. * p < 0.05, ** p < 0.01
Subdiaphragmatic vagotomy
After an overnight fast, rats were anesthetized with sodium pentobarbital (60mg/kg, i.p.). After a midline incision in the abdomen, the liver was retracted cranially and the stomach was retracted caudally to expose the diagram. The ventral and dorsal subdiaphragmatic vagal nerve trunks were isolated from the surrounding connective tissue and a 2–3mm section was removed from each of these trunks for a complete transection. Rats after the vagotomy surgery were given a nutrient drink (Ensure plus, Abbott Laboratories, USA) 2 ml twice a day for three days for better recovery 19. In sham vagotomy surgery, rats had the same surgical procedure without the transection of the vagal nerve trunk.
Measurement of kaolin intake
Rats were allowed to acclimate to Bollman cages in the presence of kaolin for one week before the surgery. The Bollman cage was made with adjustable bars which allowed the rat to move forward and backward but not turn around. Three days after surgery, the rats were transferred to BioDaq home cages equipped with two openings that accommodate two food hoppers: one for regular chow and the other for kaolin. The amount of kaolin consumed by the rat (kaolin intake in 24 hours) was defined as the difference in weight between the total amount of kaolin given and the amount leftover. Any spillage of kaolin was collected and weighed to the nearest 0.1 g and subtracted from the amount of intake.
Experiment 1: Efficacy of EA on cisplatin-induced kaolin intake
In this experiment, 18 rats were divided into a 3×3 Latin Square cross-over design in which each rat received a sequence of 3 treatments: EA of 20Hz, EA of 10Hz and sham EA (no stimulation). In the first day, cisplatin (6mg/kg) was injected intraperitoneal once at 3pm in all rats (Fig.1B). Rats received one of the three treatments for 3 hours from 3 to 6pm while remaining in Bollman cages and were then returned to their home cages with wire bottoms. On the second day, the rats received the same treatment as that of the first day for 3 hours from 3 pm to 6 pm. The rats had free access to kaolin while remaining in Bollman cages and home cages. There was an interval of 2 weeks between two consecutive treatments.
Experiment 2: Vagal mechanism involved in the anti-emetic effect of EA
Twenty rats with vagotomy and 20 rats with sham-vagotomy were used in this experiment (Fig.1C). The vagotomized rats were divided into two groups: Vagotomy + EA and Vagotomy + Sham-EA. The rats with sham-vagotomy were also divided into two groups: Sham-Vagotomy + Sham-EA and Sham-Vagotomy +EA. Seven days after the surgery, all 40 rats were injected with cisplatin (6mg/kg) and then treated with sham-EA or EA for two days, as described in Experiment 1.
Experiment 3: Effect and mechanism of EA on cisplatin induced c-fos expression on AP and NTS
Ten rats with vagotomy and 15 rats with sham vagotomy were used in this study (Fig.1D). The 15 rats with sham-vagotomy were divided into three groups: 1) Control: Sham-Vagotomy + saline injection; 2) Sham-Vagotomy + Sham-EA; 3) Sham-Vagotomy + EA. The 10 rats with vagotomy were randomly divided into two groups: 4) Vagotomy + Sham-EA; and 5) Vagotomy +EA. Three hours after the removal of food and water, the rats were injected (i.p.) with saline (Control group) or 6mg/kg cisplatin (all other groups) at 11am and treated with EA or sham EA immediately afterward for 3 hours in Bollman cages then placed in their home cages from 2 to 5pm. At 5 pm, the rats were deeply anesthetized by sodium pentobarbital (100 mg/kg, i.p.). The thoracic cavity was opened and 0.3 ml of heparin (1000 IU/ml) was given intracardially, followed by a transcardial perfusion with 200 ml of phosphate buffered saline (PBS; pH7.4) and then 250ml of 10% Neutral Buffered Formalin (NBF). Brains were removed and placed in NBF for 16 hours at 4°C. Then brainstems were placed in 10% sucrose followed sequentially by using 20% and 30% sucrose solutions at 4°C each for 24 hours and afterward stored at −80°C for later immunohistochemistry. Coronal sections (20 µm thick) were collected at approximately the same level relative to bregma (NTS: range from −15.0 to −13.0mm and AP: range from −13.7 to −14.1mm). The brain sections were initially rinsed in PBS to remove cryoprotectant. A sequence of incubation steps were done in 1% sodium borohydride in PBS (20 min), 0.3% hydrogen peroxide in PBS (30 min), and 5% normal goat serum (NGS) in PBS containing 0.3% triton X-100 (PBS-TX) for 1 hour, with rinses between each step. Sections were then incubated at 4°C in 1:5000 polyclonal anti-fos antibody (Abcam, San Francisco, CA) containing 5% NGS in PBS-TX for 20 h. Following rinses in PBS-TX, sections were placed in 1:500 biotinylated goat anti-rabbit for 2 hours at room temperature. This was followed by rinses in PBS and one hour incubation in Vector ABC complex. Sections were then rinsed twice in PBS and once in the Tris buffered solution (TBS). The sections were then placed in 3,3′-diaminobenzidine (DAB; 5 mg/ml in TBS) with or without nickel sulfate (25 mg/ml) for 3–5 min for the chromogen reaction. Finally, the sections were rinsed twice in PBS. After dehydration, the sections were mounted on slides and cover-slipped. The tissue sections were viewed with an Olympus microscope (CX41) equipped with a digital camera. Brain regions and cells expressing c-fos were imaged, and the numbers of cells expressing c-fos as a black or brown reaction product in the cell nuclei were counted. According to the stereotaxic coordinates of rat brain 20, c-fos expressions in NTS caudal (−14.7 bregma, NTSc) and rostral (−13.3, NTSr) were averaged as the fos expression in NTS and the expression at AP (−13.9 to bregma) was assessed 19.
Statistical Analysis
In experiment 1, kaolin intake was analyzed with two way ANOVA repeated-measures including fixed effects for treatment and time period. Post hoc Fisher least significant difference test for multiple comparisons was used. In experiments 2 and 3, one-way ANOVA followed by post hoc Fisher least significant difference test was used. Comparison of data were considered statistically significant if p < 0.05.
Results
Inhibitory effects of EA on kaolin intake
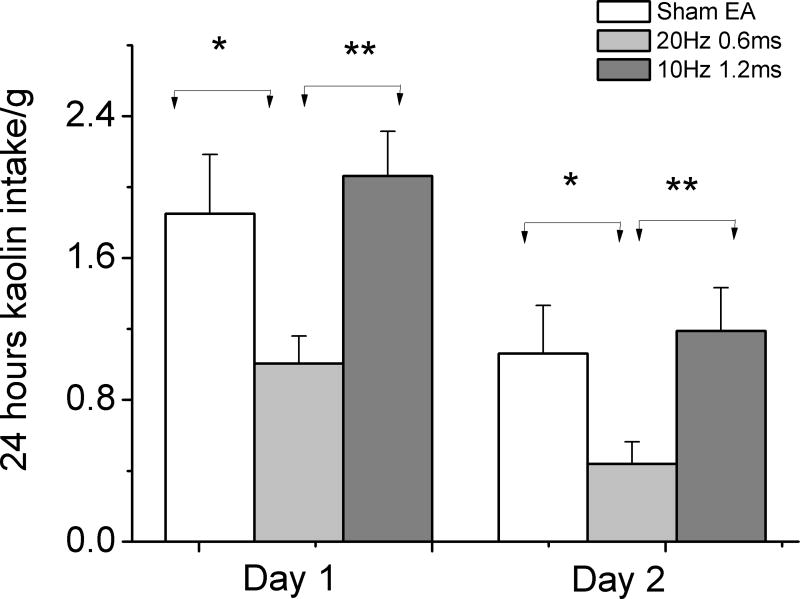
During the acclimation, only three rats chewed a small amount of kaolin when in the home cage and Bollman cage. None of them consumed more than 0.2g kaolin in 24 hours. Cisplatin induced substantial kaolin intake ranging from 0.6 to 4.1g on the first day after cisplatin injection. Two rats treated with sham EA died after the second cisplatin injection, possibly attributed to the loss of appetite. Treatment with EA of 20Hz significantly reduced cisplatin-induced kaolin intake on the first and second day after cisplatin injection compared to Sham-EA group (the first day, 20Hz vs. Sham-EA, 1.0±0.2 vs.1.9±0.3g p=0.017; the second day, 0.4±0.2 vs.1.1±0.3g p=0.010) and EA of 10Hz treatment (the first day, 20Hz vs. 10Hz, 2.1±0.3g, p=0.005; the second day, 1.2±0.2g, p=0.002). The treatment with EA of 10Hz showed no effects on kaolin intake in the first or second day when compared to that of Sham-EA treatment (the first day, sham vs. 10Hz, 1.9±0.3vs.2.1±0.3, p=0.650; the second day, 1.1±0.3vs. 1.2±0.2, p=0.573) (Fig.2)
Fig. 2.
24 hour kaolin intake in the first and second day after 6mg/kg cisplatin injection. Cisplatin significant induced kaolin intake in rats. EA of 20Hz/0.6ms but not EA of 10Hz/1.2ms reduced kaolin intake in the first and second day after injection. Control: no stimulation.
Vagal mechanism involved in the anti-emetic effect of EA
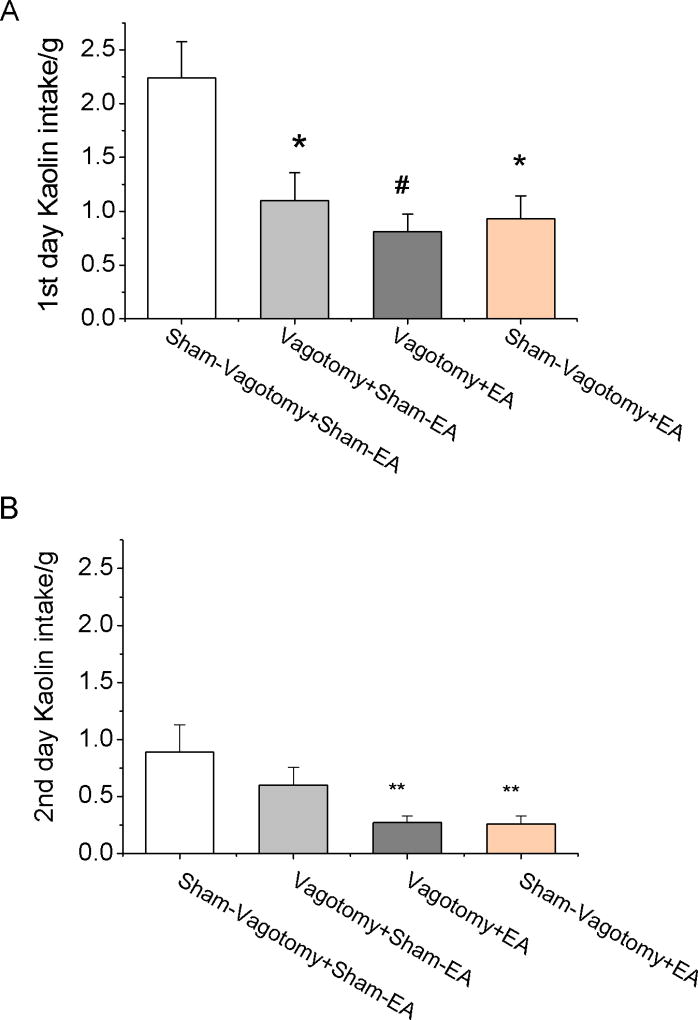
Subdiaphragmatic vagotomy significantly reduced cisplatin-induced kaolin intake on the first day (Vagotomy + Sham-EA 1.1±0.3 vs. Sham-Vagotomy + Sham-EA 2.2±0.3g, p=0.014) but not on the second day (Vagotomy + Sham-EA 0.6±0.2 vs. Sham-Vagotomy + Sham-EA 0.9±0.2g, p=0.518) (Fig. 3). EA of 20Hz showed no effects on the cisplatin-induced kaolin intake of the first day (0.8±0.2g) in Vagotomy + EA rats, compared to Vagotomy + Sham-EA rats (p=0.844). However, EA reduced kaolin intake on the second day in Vagotomy + EA rats (0.3±0.1g, p=0.024) and Sham-Vagotomy + EA rats (0.3±0.1g, p=0.027), compared to Vagotomy + Sham-EA.
Fig. 3.
Vagotomy reduced cisplatin-induced kaolin intake. A. Twenty four hours kaolin intake in the first day after cisplatin (6mg/kg) injection. EA (20Hz, 0.6ms, continuous) could reduce kaolin intake in the first day after injection. Subdiaphragmatic vagotomy significantly reduced rat 24 hours kaolin intake. After vagotomy, EA has no further effect on kaolin intake. Compared with Cisplatin: ** < 0.01, # < 0.001. B. EA reduces delayed emesis (the second day kaolin intake), independent of vagal pathway. Vagotomy had no effect on the second day kaolin intake. Compared with Cisplatin * < 0.05.
EA reduced cisplatin-induced c-fos expression on the AP and NTS
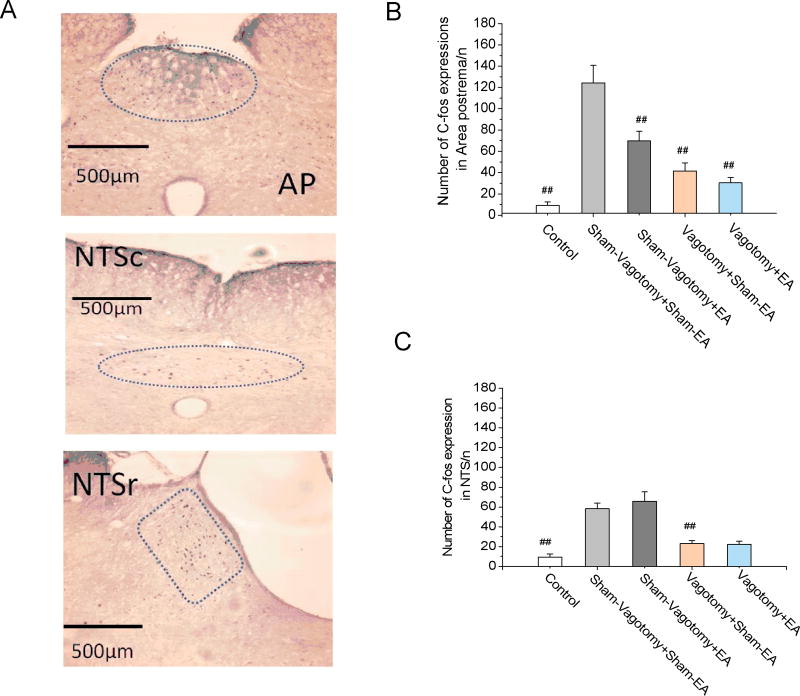
Cisplatin increased but vagotomy decreased c-fos expression in the NTS; EA showed no effects on the cisplatin-induced increase in c-fos expression in NTS. The expression of c-fos in the NTS was significantly increased from 9.4±3.2 in the saline control group to 58.2±5.8 (P < 0.001) in the cisplatin treated Sham-Vagotomy + Sham-EA group. Vagotomy significantly reduced c-fos expression in the NTS (23.0±3.0 vs. 58.2±5.8, p < 0.001, Vagotomy + Sham-EA vs. Sham-Vagotomy + Sham-EA group). EA showed no effects in the cisplatin-induced c-fos expression in the NTS (65.8±9.4, p=0.341, Fig 4A &C) in the rats with sham-vagotmoy, compared to sham-EA.
Fig. 4.
Immunohistochemical staining for cisplatin-induced c-fos activation in area postrema (AP) and nucleus tractus solitarii (NTS). A. Location (enclosed by dashed line) of c-fos positive cells in nucleus of the solitary tract (NTS; r, rostral; c, caudal) and area postrema (AP) in rats injected with cisplatin (6 mg/kg, ip). Compared with saline, cisplatin increased c-fos expressions in both AP (3B) and NTS (3C) in rats with sham vagotomy surgery. Electroacupuncture (EA) reduced cisplatin-induced c-fos expressions in AP but not in NTS. Subdiaphragmatic vagotomy reduced c-fos expressions in the AP and NTS in both EA and sham-EA groups. #: p < 0.001 compared to Sham-Vagotomy + Sham-EA.
Cisplatin increased c-fos expression in the AP; EA reduced the cisplatin-induced increase in the c-fos expression and the inhibitory effect was blocked by vagotomy. The expression of c-fos in the AP was significantly increased from 9.2±3.3 in the saline control group to 124.2±16.6 (p < 0.001) in the cisplatin treated group (Sham-Vagotomy + Sham-EA). Vagotomy greatly reduced c-fos expression in AP to 41.2±7.7, compared to Sham-Vagotomy + Sham-EA group (p < 0.001). EA significantly reduced the cisplatin-induced c-fos expression in AP (69.8±9.2 of Sham-Vagotmoy + EA vs. 124.2±16.6 of Sham-Vagotomy + Sham-EA, p < 0.001, Fig 4A &B). In the vagotomized rats, EA showed no effects in reducing cisplatin-induced c-fos expression compared to Vagotomy + Sham-EA (p=0.415).
Discussion
This study showed: 1) Cisplatin substantially induced kaolin intake and c-fos expression in NTS and AP neurons. 2) EA at 20 Hz but not 10 Hz significantly reduced cisplatin-induced kaolin intake. 3) Subdiaphragmatic vagotomy significantly reduced cisplatin-induced kaolin intake and c-fos expression in NTS and AP neurons, suggesting a vagally mediated mechanism of cisplatin. 4) Vagotomy blocked the inhibitory effect of EA on cisplatin-induced kaolin intake on the first day but not the second day, suggesting a vagally mediated mechanism of EA on acute nausea and non-vagal mechanisms of EA on delayed nausea.
Acupuncture or EA was reported to be effective in reducing chemotherapy-induced acute vomiting11, 12. EA was reported to be more effective than manual acupuncture in reducing acute vomiting 21–23, suggesting the therapeutic role of electrical stimulation. In the clinic, EA also was reported to have more consistent and reproducible results than manual acupuncture24, 25. In this study, EA (20Hz, 0.6ms, continuous) significantly reduced cisplatin-induced kaolin intake and c-fos expression in AP. The ineffectiveness of EA at 10Hz (same stimulation energy with 20Hz) demonstrated the importance of stimulation parameters. Transcutaneous electrical nerve stimulation at 2~20 Hz at the PC-6 and PC-5 suppresses cardiovascular sympathetic activity and induces hypotension while at 40Hz significantly sustained blood pressure in patients 26. High frequency electrical stimulation of other organs and tissue may have superior effect and act on different mechanisms, compared to low frequency stimulation 27. It will be of interest to test the efficacy of EA using a wide range of frequencies for reducing the cisplatin-induced kaolin consumption in rats.
We confirmed that pica was a good surrogate for nausea and vomiting in rats. Clinically, nausea and vomiting are common side effects for a wide range of medicines. However, the lack of an emetic reflex in common laboratory animals, such as rats and mice limits studies on mechanisms of emesis and identification of novel anti-emetics. Rats consume non-nutritive kaolin that they would not normally ingest when injected with toxins such as ampomorphine and cisplatin or subjected to motion exposure, which can induce emesis in a species endowed with an emetic28. Cisplatin-induced pica is a robust response in rats and used as a reliable index in the assessment of emesis severity in rats17. In the present study, the i.p. administration of cisplatin at a dose of 6 mg/kg induced robust kaolin consumption with an average of nearly 2g in the first 24 hours, which was comparable to that reported in a previous study using the same dosage (6mg/kg) 28 or higher dose (8mg/kg)13.
Our findings suggested different mechanisms involved in the ameliorating effects of EA on acute and delayed emesis. Typically chemotherapy-induced nausea and vomiting are divided into acute (less than 24 h) and delayed (above 24 h) phases which have distinct mechanisms19. Cisplatin-induced emesis is inhibited by common antiemetic drugs, such as 5-HT3 and NK1 receptor antagonists, as well as corticosteroids29. The treatment with 5-HT3 receptor antagonists such as ondansetron substantially reduced cisplatin-induced kaolin intake in rats 17, 30. Cisplatin elicits acute emesis primarily through the stimulation of 5-HT release from enteroendocrine cells that activate gastrointestinal vagal afferent fibers 31, 32. Ablation of the vagus nerve substantially suppressed cisplatin-induced vomiting in dogs and house musk shrews 33, 34, indicating the important role of an intact vagus in the emetic response to cisplatin. Accordingly, subdiaphragmatic vagotomy significantly reduces cisplatin-induced kaolin intake in rats 35, suggesting the involvement of the vagal nerve in pica. In the present study, subdiaphragmatic vagotomy significantly reduced cisplatin-induced kaolin intake in the first and second day, which was in agreement with a previous report 19. The ineffectiveness on reducing the first day kaolin intake by EA in the vagotomized rats suggested a vagal mechanism; that is, the inhibitory effect of EA on kaolin intake in the first day in the rats with intact vagal nerve was mediated via the vagal pathway. Whereas, the effectiveness on reducing the second day kaolin intake by EA in the vagotomized rats indicated that mechanisms other than vagal nerve are involved. Both 5-HT and substance P levels were reported to increase in blood 36–38 and the ileum38 after cisplatin treatment, which might directly activate AP to produce pica. Transcutaneous EA at 20Hz was previously reported reduce nausea and vomiting, and the chemotherapy-induced increase in serum levels of 5-HT and dopamine in patients with chemotherapy 10. Further investigations are needed to study whether 5-HT and dopamine are involved in the inhibitory effect of EA on the second day kaolin intake (delayed emesis). In addition, greater splanchnic nerves were noted to be partially involved in cisplatin-induced emesis39 and EA was found to modulate the blood pressure response induced by splanchnic nerve afferent stimulation 40. Therefore, possible involvement of the splanchnic nerve in the inhibitory effect of EA on second day kaolin intake should also be investigated in future studies.
A central mechanism involving AP was noted with cisplatin and EA in this study. Cisplatin was reported to increase c-fos expression in the NTS and AP in species with vomiting reflexes, such as the cat and ferret 41–43. In the present rodent study, cisplatin was also found to increase c-fos expression in the NTS and AP, which was consistent with previous findings 17, 35, and the cisplatin-induced increases were reduced by vagotomy, suggesting a vagal mediated mechanism 16, 35. However, a few previous studies speculated a direct action on the AP via the cisplatin-induced release of serotonin and substance P in blood 36–38, 43. 5-HT3 receptor antagonists were reported to greatly reduce cisplatin-induced c-fos expression in the NTS but not in AP, and abdominal vagotomy exerted no effects on c-fos mRNA expression in the NTS or AP, also suggesting a direct 5-HT3 action without the involvement of the vagal mechanism44. Similarly, cisplatin induces c-fos gene expression in NTS which was blocked by 5-HT3 receptor antagonist and in AP by a vagal independent, direct action43. In the present study, EA showed no effects on the cisplatin-induced c-fos expression in the NTS but reduced the cisplatin-induced c-fos expression in the AP in a vagal-dependent way as its effect was blocked by vagotomy.
EA at PC6 with appropriate parameters via chronically implanted electrodes has an inhibitory effect on cisplatin-induced nausea. The anti-emetic effect of the EA is centrally mediated involving the area postrema via the vagal pathway as well as the potential effect on AP by reducing the release of hormones.
Acknowledgments
The authors thank Dr. Robert Foreman and Dr. Jay Farber for helpful revision and comments.
Source of funding: This work was supported by a grant, 1R21CA149956-01, from National Cancer Institute, National Institute of Health to JC.
Footnotes
Authorship Statement: All authors were involved with the design of the study and helped with the data analysis. Dr. Li and Dr. Lei conducted the research, collected the data, and analyzed the results presented in the manuscript with important intellectual input from Dr. Chen. All authors approved the final manuscript.
Conflict of Interest: No conflict of interest
References
- 1.Navari RM, Reinhardt RR, Gralla RJ, et al. Reduction of cisplatin-induced emesis by a selective neurokinin-1-receptor antagonist. L-754,030 Antiemetic Trials Group. N Engl J Med. 1999 Jan 21;340(3):190–195. doi: 10.1056/NEJM199901213400304. [DOI] [PubMed] [Google Scholar]
- 2.Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients' quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006 Sep 20;24(27):4472–4478. doi: 10.1200/JCO.2006.05.6382. [DOI] [PubMed] [Google Scholar]
- 3.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008 Jun 5;358(23):2482–2494. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- 4.Warr DG, Grunberg SM, Gralla RJ, et al. The oral NK(1) antagonist aprepitant for the prevention of acute and delayed chemotherapy-induced nausea and vomiting: Pooled data from 2 randomised, double-blind, placebo controlled trials. Eur J Cancer. 2005 Jun;41(9):1278–1285. doi: 10.1016/j.ejca.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Lee MS, Choi DH, Lee SK. Electroacupuncture on PC6 prevents opioid-induced nausea and vomiting after laparoscopic surgery. Chin J Integr Med. 2013 Apr;19(4):277–281. doi: 10.1007/s11655-013-1425-7. [DOI] [PubMed] [Google Scholar]
- 6.Tang W, Ma W, Fu GQ, Yuan L, Shen WD. [Impacts of electroacupuncture at different frequencies on the postoperative nausea and vomiting of patients with laparoscopic surgery] Zhongguo Zhen Jiu. 2013 Feb;33(2):159–162. [PubMed] [Google Scholar]
- 7.Ezzo J, Vickers A, Richardson MA, et al. Acupuncture-point stimulation for chemotherapy-induced nausea and vomiting. J Clin Oncol. 2005 Oct 1;23(28):7188–7198. doi: 10.1200/JCO.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 8.Lv JQ, Feng RZ, Li N. P6 acupoint stimulation for prevention of postoperative nausea and vomiting in patients undergoing craniotomy: study protocol for a randomized controlled trial. Trials. 2103;14:153. doi: 10.1186/1745-6215-14-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatewaki M, Strickland C, Fukuda H, et al. Effects of acupuncture on vasopressin-induced emesis in conscious dogs. Am J Physiol Regul Integr Comp Physiol. 2005 Feb;288(2):R401–408. doi: 10.1152/ajpregu.00344.2004. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Jin HF, Fan YH, Lu B, Meng LN, Chen JD. Effects and mechanisms of transcutaneous electroacupuncture on chemotherapy-induced nausea and vomiting. Evid Based Complement Alternat Med. 2014;2014:860631. doi: 10.1155/2014/860631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ezzo JM, Richardson MA, Vickers A, et al. Acupuncture-point stimulation for chemotherapy-induced nausea or vomiting. Cochrane Database Syst Rev. 2006;(2):CD002285. doi: 10.1002/14651858.CD002285.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Garcia MK, McQuade J, Haddad R, et al. Systematic review of acupuncture in cancer care: a synthesis of the evidence. J Clin Oncol. 2013 Mar 1;31(7):952–960. doi: 10.1200/JCO.2012.43.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallam HS, Song J, Chen JZ. Electroacupuncture via Chronically Implanted Electrodes: Potential Treatment for Chemotherapy-Induced Delayed Emesis. Neuromodulation. 2015 Aug;18(6):494–498. doi: 10.1111/ner.12303. discussion 498. [DOI] [PubMed] [Google Scholar]
- 14.Cui Y, Wang L, Shi G, Liu L, Pei P, Guo J. Electroacupuncture alleviates cisplatin-induced nausea in rats. Acupunct Med. 2015 Sep 17; doi: 10.1136/acupmed-2015-010833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie J, Chen LH, Ning ZY, et al. Effect of transcutaneous electrical acupoint stimulation combined with palonosetron on chemotherapy-induced nausea and vomiting: a single-blind, randomized, controlled trial. Chin J Cancer. 2017 Jan 10;36(1):6. doi: 10.1186/s40880-016-0176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horn CC, Ciucci M, Chaudhury A. Brain Fos expression during 48 h after cisplatin treatment: neural pathways for acute and delayed visceral sickness. Auton Neurosci. 2007 Mar 30;132(1–2):44–51. doi: 10.1016/j.autneu.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeda N, Hasegawa S, Morita M, Matsunaga T. Pica in rats is analogous to emesis: an animal model in emesis research. Pharmacol Biochem Behav. 1993 Aug;45(4):817–821. doi: 10.1016/0091-3057(93)90126-e. [DOI] [PubMed] [Google Scholar]
- 18.Saeki M, Sakai M, Saito R, et al. Effects of HSP-117, a novel tachykinin NK1-receptor antagonist, on cisplatin-induced pica as a new evaluation of delayed emesis in rats. Jpn J Pharmacol. 2001 Jul;86(3):359–362. doi: 10.1254/jjp.86.359. [DOI] [PubMed] [Google Scholar]
- 19.Horn CC. Brain Fos expression induced by the chemotherapy agent cisplatin in the rat is partially dependent on an intact abdominal vagus. Auton Neurosci. 2009 Jun 15;148(1–2):76–82. doi: 10.1016/j.autneu.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5. Elsevier Academic Press; Amsterdam Boston: 2005. [Google Scholar]
- 21.Dundee JW, Ghaly RG, Fitzpatrick KT, Lynch GA, Abram WP. Acupuncture to prevent cisplatin-associated vomiting. Lancet. 1987 May 9;1(8541):1083. doi: 10.1016/s0140-6736(87)90501-0. [DOI] [PubMed] [Google Scholar]
- 22.Shen J, Wenger N, Glaspy J, et al. Electroacupuncture for control of myeloablative chemotherapy-induced emesis: A randomized controlled trial. JAMA. 2000 Dec 6;284(21):2755–2761. doi: 10.1001/jama.284.21.2755. [DOI] [PubMed] [Google Scholar]
- 23.Streitberger K, Friedrich-Rust M, Bardenheuer H, et al. Effect of acupuncture compared with placebo-acupuncture at P6 as additional antiemetic prophylaxis in high-dose chemotherapy and autologous peripheral blood stem cell transplantation: a randomized controlled single-blind trial. Clin Cancer Res. 2003 Jul;9(7):2538–2544. [PubMed] [Google Scholar]
- 24.Li Y, Tougas G, Chiverton SG, Hunt RH. The effect of acupuncture on gastrointestinal function and disorders. Am J Gastroenterol. 1992 Oct;87(10):1372–1381. [PubMed] [Google Scholar]
- 25.Lux G, Hagel J, Backer P, et al. Acupuncture inhibits vagal gastric acid secretion stimulated by sham feeding in healthy subjects. Gut. 1994 Aug;35(8):1026–1029. doi: 10.1136/gut.35.8.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arai YC, Ito A, Ohshima K, et al. Transcutaneous Electrical Nerve Stimulation on the PC-5 and PC-6 Points Alleviated Hypotension after Epidural Anaesthesia, Depending on the Stimulus Frequency. Evid Based Complement Alternat Med. 2012;2012:727121. doi: 10.1155/2012/727121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapural L, Yu C, Doust MW, et al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) Is Superior to Traditional Low-frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: The SENZA-RCT Randomized Controlled Trial. Anesthesiology. 2015 Oct;123(4):851–860. doi: 10.1097/ALN.0000000000000774. [DOI] [PubMed] [Google Scholar]
- 28.Liu YL, Malik N, Sanger GJ, Friedman MI, Andrews PL. Pica--a model of nausea? Species differences in response to cisplatin. Physiol Behav. 2005 Jun 30;85(3):271–277. doi: 10.1016/j.physbeh.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Andrews PL, Davis CJ, Bingham S, Davidson HI, Hawthorn J, Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can J Physiol Pharmacol. 1990 Feb;68(2):325–345. doi: 10.1139/y90-047. [DOI] [PubMed] [Google Scholar]
- 30.Malik NM, Liu YL, Cole N, Sanger GJ, Andrews PL. Differential effects of dexamethasone, ondansetron and a tachykinin NK1 receptor antagonist (GR205171) on cisplatin-induced changes in behaviour, food intake, pica and gastric function in rats. Eur J Pharmacol. 2007 Jan 26;555(2–3):164–173. doi: 10.1016/j.ejphar.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 31.Horn CC, Richardson EJ, Andrews PL, Friedman MI. Differential effects on gastrointestinal and hepatic vagal afferent fibers in the rat by the anti-cancer agent cisplatin. Auton Neurosci. 2004 Sep 30;115(1–2):74–81. doi: 10.1016/j.autneu.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Minami M, Endo T, Hirafuji M, et al. Pharmacological aspects of anticancer drug-induced emesis with emphasis on serotonin release and vagal nerve activity. Pharmacol Ther. 2003 Aug;99(2):149–165. doi: 10.1016/s0163-7258(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 33.Fukui H, Yamamoto M, Sato S. Vagal afferent fibers and peripheral 5-HT3 receptors mediate cisplatin-induced emesis in dogs. Jpn J Pharmacol. 1992 Jun;59(2):221–226. doi: 10.1254/jjp.59.221. [DOI] [PubMed] [Google Scholar]
- 34.Sam TS, Cheng JT, Johnston KD, et al. Action of 5-HT3 receptor antagonists and dexamethasone to modify cisplatin-induced emesis in Suncus murinus (house musk shrew) Eur J Pharmacol. 2003 Jul 4;472(1–2):135–145. doi: 10.1016/s0014-2999(03)01863-6. [DOI] [PubMed] [Google Scholar]
- 35.De Jonghe BC, Horn CC. Chemotherapy-induced pica and anorexia are reduced by common hepatic branch vagotomy in the rat. Am J Physiol Regul Integr Comp Physiol. 2008 Mar;294(3):R756–765. doi: 10.1152/ajpregu.00820.2007. [DOI] [PubMed] [Google Scholar]
- 36.Cubeddu LX, Hoffmann IS, Fuenmayor NT, Malave JJ. Changes in serotonin metabolism in cancer patients: its relationship to nausea and vomiting induced by chemotherapeutic drugs. Br J Cancer. 1992 Jul;66(1):198–203. doi: 10.1038/bjc.1992.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higa GM, Auber ML, Altaha R, et al. 5-Hydroxyindoleacetic acid and substance P profiles in patients receiving emetogenic chemotherapy. J Oncol Pharm Pract. 2006 Dec;12(4):201–209. doi: 10.1177/1078155206072080. [DOI] [PubMed] [Google Scholar]
- 38.Fukui H, Yamamoto M, Ando T, Sasaki S, Sato S. Increase in serotonin levels in the dog ileum and blood by cisplatin as measured by microdialysis. Neuropharmacology. 1993 Oct;32(10):959–968. doi: 10.1016/0028-3908(93)90060-g. [DOI] [PubMed] [Google Scholar]
- 39.Fukui H, Yamamoto M, Sasaki S, Sato S. Emetic effects of anticancer drugs and involvement of visceral afferent fibers and 5-HT3 receptors in dogs. Eur J Pharmacol. 1993 Dec 7;250(2):281–287. doi: 10.1016/0014-2999(93)90392-u. [DOI] [PubMed] [Google Scholar]
- 40.Li P, Tjen ALSC, Longhurst JC. Excitatory projections from arcuate nucleus to ventrolateral periaqueductal gray in electroacupuncture inhibition of cardiovascular reflexes. Am J Physiol Heart Circ Physiol. 2006 Jun;290(6):H2535–2542. doi: 10.1152/ajpheart.00972.2005. [DOI] [PubMed] [Google Scholar]
- 41.Ariumi H, Saito R, Nago S, Hyakusoku M, Takano Y, Kamiya H. The role of tachykinin NK-1 receptors in the area postrema of ferrets in emesis. Neurosci Lett. 2000 Jun 2;286(2):123–126. doi: 10.1016/s0304-3940(00)01113-7. [DOI] [PubMed] [Google Scholar]
- 42.Miller AD, Ruggiero DA. Emetic reflex arc revealed by expression of the immediate-early gene c-fos in the cat. J Neurosci. 1994 Feb;14(2):871–888. doi: 10.1523/JNEUROSCI.14-02-00871.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reynolds DJ, Barber NA, Grahame-Smith DG, Leslie RA. Cisplatin-evoked induction of c-fos protein in the brainstem of the ferret: the effect of cervical vagotomy and the anti-emetic 5-HT3 receptor antagonist granisetron (BRL 43694) Brain Res. 1991 Nov 29;565(2):231–236. doi: 10.1016/0006-8993(91)91654-j. [DOI] [PubMed] [Google Scholar]
- 44.Endo T, Minami M, Nakayasu M, Hirafuji M, Hamaue N, Omae N, Kang Y, Iwanaga T. Effects of granisetron and vagotomy on c-fos mRNA expression in the rat medulla oblongata as assessed by in situ hybridization. Biomed. Res. 2004;25:229–235. [Google Scholar]