Abstract
Background
Staple-line leaks following laparoscopic sleeve gastrectomy (LSG) remain a concerning complication. Staple-line buttressing is largely adopted as an acceptable reinforcement but data regarding leaks have been equivocal. This study compared staple-line leaks in five reinforcement options during LSG: no reinforcement (NO-SLR), oversewing (suture), nonabsorbable bovine pericardial strips (BPS), tissue sealant or fibrin glue (Seal), or absorbable polymer membrane (APM).
Methods
This systematic review study of articles published between 2012 and 2016 regarding LSG leak rates aligned with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Variables of interest included leak rates, bleeding, and complications in addition to surgical and population parameters. An independent Fisher’s exact test was used to compare the number of patients with and without leaks for the different reinforcement options.
Results
Of the 1633 articles identified, 148 met inclusion criteria and represented 40,653 patients. Differences in age (older in APM; p = 0.001), starting body mass index (lower in Suture; p = 0.008), and distance from pylorus (closer in BPS; p = 0.04) were observed between groups, but mean bougie size was equivalent. The overall leak rate of 1.5% (607 leaks) ranged from 0.7% for APM (significantly lower than all groups; p ≤ 0.007 for next lowest leak rate) to 2.7% (BPS).
Conclusions
This systematic review of staple-line leaks following LSG demonstrated a significantly lower rate using APM staple-line reinforcement as compared to oversewing, use of sealants, BPS reinforcement, or no reinforcement. Variation in surgical technique may also contribute to leak rates.
Electronic supplementary material
The online version of this article (10.1007/s00464-019-06782-2) contains supplementary material, which is available to authorized users.
Keywords: Bariatric, Metabolic, Laparoscopic sleeve gastrectomy, LSG, Leak, Staple line, Reinforcement, Systematic review
Laparoscopic sleeve gastrectomy (LSG) has become the most commonly performed primary bariatric procedure performed in the United States (US) and worldwide [1, 2]. Since its early days of adoption, the complication of staple-line leak remains the greatest concern with the reported leak rates averaging 2.4% and ranging from 1.1 to 4.7% [3–8]. Over the past 10 years, multiple studies have attempted to identify parameters associated with decreasing the risk of leaks, which have included: varying bougie size, distance from the pylorus, surgeon experience, and reinforcement of the staple line [6, 9–12]. In regards to staple-line reinforcement, expert opinion from the International Sleeve Gastrectomy Consensus Conference in 2011 demonstrated that 77% of experienced LSG surgeons deemed staple-line buttressing as “acceptable” [7]. Multiple retrospective studies have further evaluated staple-line reinforcement, with the largest study published to date utilizing the Metabolic and Bariatric Surgery Accreditation Quality Improvement Program data base [10]. This study suggested that reinforcement of the staple line may actually be associated with increased leak rates, but the study results were limited by the lack of granular data to separate outcomes based on actual type of reinforcement utilized and the inclusion of discontinued material (i.e. glycolide diaxonone trimethylene carbonate, Duet TRS, Covidien, Norwalk, CT) [10].
In an attempt to provide buttressing-specific data, we previously reported the results of a systematic review of 88 articles published up to March 2012 with the purpose of comparing staple-line leak rates of 4 prevalent surgical staple-line reinforcement methods in 8279 LSG procedures. In that review, the overall leak rate was 2.1%, with the lowest rate in absorbable permeable membrane (APM) reinforced staple lines of 1.09% [13]. Our follow-up to this study included an additional 3416 APM-reinforced LSG patients and demonstrated that overall leak rates decreased to 0.67% from 2012 to 2015, perhaps suggesting a “learning curve” associated with the procedure [14].
Since the cutoff date for these previous reviews, the use of tissue sealants has become more prevalent. In this current systematic review, relevant articles of LSG and the use of staple-line reinforcement methods published from 2012 to 2016 are evaluated. The leak rates from 5 reinforcement methods of no reinforcement (NO-SLR), over sewing (Suture), bovine pericardium membrane (BPM), tissue sealant (Seal), and APM are evaluated.
Methods and materials
Search strategy, inclusion criteria, variables of interest
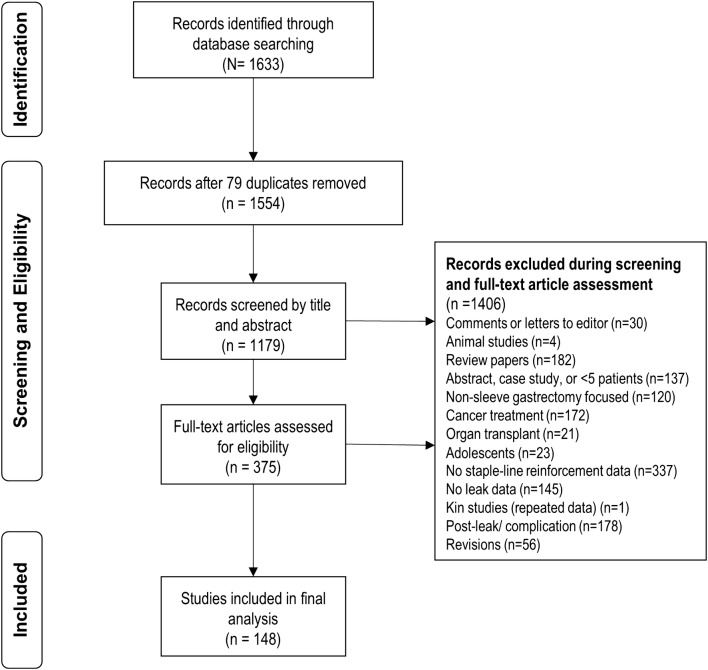
The search strategy used for this current review was consistent with our systematic review reported in 2014 and was aligned with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) [13, 15]. Briefly, the electronic literature search of the BIOSIS Previews®, Embase®, Embase®Alert, and MEDLINE® databases with the keywords: “sleeve gastrectomy,” laparoscopic sleeve gastrectomy,” “vertical gastrectomy,” “leak,” “complication,” “morbidity,” or “fistula” limited to human patients and reports in English. The search period started from March 2012 through June 2016 (published or e-published ahead of print). Electronic results were screened by title to exclude duplicate studies and the remaining records were screened by reading abstracts. Full-text articles were included only if an LSG procedure, leak data, and type of staple-line reinforcement were reported. Of note, articles may have reported data for more than 1 reinforcement method of interest. As summarized in Fig. 1, excluded from eligibility were: Comments, Letters to the Editor, case reports series or studies with sample sizes of ≤ 5 patients, animal studies, review articles without accompanying data, and kin studies (i.e., reports with overlapping data or an author group that reported outcomes for similar periods of time). Analysis objectives centered on 5 reinforcement methods NO–SLR, suture, BPM, tissue sealant seal, APM and the number of patients with leak and without leak; bleeding, overall complications, and mortality were collected as text fields but not categorically summarized. Additionally, population and surgical variables of gender, age, body mass index (BMI), calibrating bougie size, and distance between the pylorus and gastric transection line were collected. Stapler types, staple heights, port type, number and placement, and other procedural characteristics were not included, as these details were not consistently reported.
Fig. 1.
Search strategy
Abbreviated terms
No reinforcement = “NO-SLR”. Reinforcement by over sewing alone = “suture”. Reinforcement with nonabsorbable bovine pericardial strips (Peri-Strips Dry, Baxter® Healthcare, St. Paul, MN) = “BPS”. Reinforcement with tissue sealant or fibrin glue (FloSeal or Tisseel fibrin sealant [Baxter® Deerfield, IL, USA], Ifabond® glue [Ifamedical, France], or Evicel® glue [Ethicon™ Biosurgery, Inc., Somerville, NJ, USA] = “Seal”. Reinforcement with absorbable polymer membrane (GORE® SEAMGUARD®, W. L. Gore & Associates, Elkton, MD, USA) = “APM”.
Statistical analysis
Data were extracted by an individual from original sources to fields within an Excel (Microsoft, Redmond, WA, USA) database. Data manipulation and analysis was conducted using JMP statistical software, version 13.2.0 (SAS Institute Inc., Cary, NC). Criteria-based data were aggregated from selected studies representative of the 5 LSG reinforcement options of interest. Select demographic variables of age, % females, and body mass index (BMI, kg/m2) and the surgical technique variables of bougie size and distance from pylorus were summarized using mean, standard deviation, range, and the percentage of studies reporting on each variable. The overall leak rate for LSG patients, as well as, patient leak rates within each of the 5 reinforcement categories were calculated. An independent Fisher’s exact tests was used to compare the number of patients with and without leaks for the different reinforcement options [16]. All statistical tests were 2-tailed and alpha was set at p < 0.05.
Results
Study characteristics
A total of 1633 articles were identified in the initial search. Figure 1 illustrates the identification, screening, and eligibility selection process. After removing duplicates (n = 79), the 1554 records were screened by title and abstract after which 1179 were excluded and the full-text articles for the remaining 375 records were assessed for eligibility. A total of 148 papers were included in the final analysis and the number of studies per reinforcement method were: 69 for NO-SLR [9, 17–84], 70 for suture [9, 19, 20, 22, 26, 30, 46, 51, 53, 75, 76, 78, 83, 85–140], 9 for BPS [9, 72, 78, 83, 86, 89, 141–143], 9 for Seal [9, 30, 61, 74, 78, 103, 144–146], and 24 for APM [9, 39, 52, 53, 63, 89, 103, 131, 147–162]. Studies included in the analysis were comprised of 11 case series, 22 prospective randomized studies, 29 prospective studies, 1 randomized clinical trial, and 85 retrospective reviews and were conducted in Western Europe (n = 58), the US (n = 33), and other regions (n = 57; i.e. Asia and Middle East). Table 1 describes the study characteristics by reinforcement method and the associated article reference which reflects “double-counting” of an article in cases when more than 1 reinforcement method was reported for an article.
Table 1.
Characteristics of accepted studies by reinforcement method groups
| Reinforcement method | |||||
|---|---|---|---|---|---|
| NO-SLR | Suture | BPS | Seal | APM | |
| Variables | |||||
| Publication date range | 2012–2016 | 2012–2016 | 2012–2015 | 2012–2015 | 2012– 2016 |
| Study design typea | |||||
| Case series | 2 | 7 | 2 | 1 | 2 |
| Prospective randomized | 13 | 12 | 2 | 4 | 1 |
| Prospective | 11 | 17 | 1 | 2 | 5 |
| Randomized clinical trial | 0 | 1 | 0 | 0 | 0 |
| Retrospective review | 43 | 33 | 4 | 2 | 16 |
| Total | N = 69 | N = 70 | N = 9 | N = 9 | N = 24 |
| Region, n (%)a | |||||
| Other | 26 (36.1) | 38 (52.8) | 4 (0.06) | 3 (0.46) | 1 (0.01) |
| United States | 10 (27.0) | 15 (40.5) | 1 (0.03) | 1 (0.03) | 10 (27.0) |
| Western Europe | 33 (45.8) | 17 (23.6) | 4 (0.06) | 5 (0.07) | 13 (18.1) |
APM absorbable polymer membrane, BPS bovine pericardial strips, NO-SLR no staple-line reinforcement, n number of studies per reinforcement type, N number of studies overall, NR not reported, P prospective, R retrospective, RCT randomized controlled trial, seal tissue sealant, suture oversewing alone
aN = 148 for total number of citations included in analysis and N = 181 for total reinforcement outcome results and reflects some articles that were double counted for report of more than 1 reinforcement method
Patient characteristics
The final analysis consisted of 40,653 patients from the 148 papers. At least one of the three patient characteristics variables (age, gender, or starting BMI) were reported in all but the following eight studies representing 12,473 patients [24, 28, 49, 63, 76, 97, 144]. In 8 additional studies, only one of the three patient characteristic variables were reported. Age was not reported for 40 patients in one study [43]; gender was missing for 1103 patients in 5 studies [42, 52, 143, 159, 161]; and starting BMI was not reported for 45 patients in two studies [25, 162]. Overall, patients had a mean age of 41 years, a mean starting BMI of 46.1 kg/m2, and 74% were female. Among studies reporting characteristics, differences were noted among the five reinforcement method groups in that patients in the APM group were older (45.6 ± 3.2 kg/m2; p = 0.001) and the starting BMI was lower for the suture group (43.7 ± 4.3 kg/m2; p = 0.008). The gender ratio in this analysis data set was similar across the reinforcement groups (Table 2).
Table 2.
Characteristics of patients reported in accepted studies by reinforcement method
| Reinforcement method | p value | |||||
|---|---|---|---|---|---|---|
| NO-SLR | Suture | BPS | Seal | APM | ||
| Variables mean ± SD (rangea) [% reportedb] | ||||||
| Age, years | 39.9 ± 5.2 (29.9–54.3) [90%] | 41.1 ± 5.4 (27.0–64.1) [96%] | 38.6 ± 5.5 (31.5–45.6) [100%] | 39.8 ± 3.9 (32.3–44.1) [89%] | 45.6 ± 3.2 (41.0–54.5) [92%] | 0.0009 |
| Female, % | 75.9 ± 8.8 (47.0–100.0) [88%] | 75.2 ± 8.6 (43.0–95.0) [96%] | 73.2 ± 8.3 (40.0–86.0) [89%] | 71.3 ± 14.9 (39.0–92.0) [89%] | 73.2 ± 11.5 (10.0–100.0) [79%] | 0.7608 |
| Starting BMI, kg/m2 | 44.5 ± 4.9 (32.6–66.0) [90%] | 43.7 ± 4.3 (34.9–68.4) [96%] | 47.0 ± 3.4 (42.0–51.0) [100%] | 47.9 ± 7.7 (42.1–65.0) [89%] | 47.4 ± 3.3 (40.1–55.5) [88%] | 0.0079 |
APM absorbable polymer membrane, BPS bovine pericardial strips, N number of patients, NO-SLR no staple-line reinforcement, seal tissue sealant, suture oversewing alone
aMinimum to maximum
bPercentage of studies that reported variables
Surgical technique
The mean bougie size ranged from 36 Fr (NO-SLR and suture groups) to 34.6 Fr (Seal group); the differences between reinforcement type groups were not significantly different. The mean distance from pylorus ranged from 3.2 cm (BPS group) to 5.0 cm (suture group) and the difference was significantly different (p = 0.04) (Table 3).
Table 3.
Bougie size and distance from pylorus by reinforcement method
| Reinforcement method | ||||||
|---|---|---|---|---|---|---|
| NO-SLR | Suture | BPS | Seal | APM | p value | |
| Variables mean ± SD (rangea) [% reportedb] | ||||||
| Bougie size (Fr) | 36.1 ± 2.1 (30.0–50.0) [97%] | 36.2 ± 7.2 (27.0–60.0) [93%] | 35.1 ± 3.1 (32.0–40.0) [89%] | 34.6 ± 4.7 (26.4–40.0) [78%] | 35.7 ± 2.4 (29.0–42.0) [92%] | 0.9834 |
| Distance from pylorus (cm) | 4.8 ± 1.1 (1.5–6.5) [90%] | 5.0 ± 1.6 (1.5–10.5) [89%] | 3.2 ± 0.4 (3.0–4.0) [67%] | 3.9 ± 1.1 (3.0–5.5) [67%] | 4.8 ± 0.8 (3.0–6.0) [79%] | 0.0362 |
APM absorbable polymer membrane, BPS bovine pericardial strips, max maximum, min minimum, N number of studies reporting variables, NO-SLR no staple-line reinforcement, seal tissue sealant, suture oversewing alone
aMinimum to maximum
bPercentage of studies that reported variables
Staple-line leak rate
A total of 607 leaks were reported in 40,653 patients yielding an overall leak rate of 1.49% (Table 4). The percentage of leaks was significantly lower for the APM reinforcement method (0.73%) compared with and in ranking order, suture (1.21%; p = 0.007), NO-SLR (1.89%; p < 0.0001), Seal (1.89%; p = 0.027), and BPS (2.73%; p < 0.0001) (Table 4). The leak rate for the tissue sealant reinforcement method was comparable to that of no staple-line reinforcement (p = 0.271). When looking at only studies conducted in the US, the APM reinforcement method continues to have the lowest leak rate (0.39%) among the reinforcement methods evaluated (Table 4).
Table 4.
Leak rate by reinforcement method
| Reinforcement Type | ||||||
|---|---|---|---|---|---|---|
| NO-SLR | Suture | BPS | Seal | APM | TOTAL N = 40,653 |
|
| Study overall | ||||||
| Leaks, n | 314 | 222 | 34 | 7 | 30 | 607 |
| Patients without leaks, n | 16,318 | 18,092 | 1210 | 356 | 4070 | 40,046 |
| Leaks, % | 1.9 | 1.2 | 2.7 | 1.9 | 0.7 | 1.5 |
| P value compared to APMa | < 0.0001 | 0.007 | < 0.0001 | 0.0271 | – | – |
| United States only | ||||||
| Leaks, n | 14 | 23 | 4 | 1 | 9 | |
| Patients without leaks, n | 1059 | 3175 | 265 | 54 | 2302 | |
| Leaks, % | 1.30% | 0.72% | 1.49% | 1.82% | 0.39% | |
APM absorbable polymer membrane, BPS bovine pericardial strips, NO-SLR no staple-line reinforcement, seal tissue sealant, suture oversewing alone
aTwo-tailed Fisher’s exact test
Discussion
Laparoscopic sleeve gastrostomy is a popular operation, and in the US, LSG has surpassed Roux-en-Y gastric bypass because of more favorable outcomes of lower mortality and overall morbidity, similar weight loss, and resolution of health comorbidities at 5 years [163–166]. Further supporting LSG as a preferred procedure is the lower leak rates, the twofold lower complication rate, and a mortality rate that is half that of Roux–en–Y gastric bypass [167]. Our current meta-analysis of 148 articles gathering data on 40,653 LSG patients, demonstrates an overall leak rate of 1.5% among the 5 staple-line reinforcement methods evaluated. Reinforcement with APM had the lowest statistically significant leak rate at 0.7% (p ≤ 0.007) despite a patient population that was older (p = 0.0009) and with a higher BMI [suture alone group had lower starting BMI (p = 0.0079)], both notorious as factors contributing to higher leak rates [168].
The variability in staple-line leak rates among the five reinforcement types indicates that the type of reinforcement material is an important factor related to this complication. When comparing the leak rates from the current analysis to the previous review, it is interesting to note the reliability of the data between both studies [13]. Although leak rates have decreased among all reinforcement types, the overall propensity is the same: APM had the lowest (0.73% vs 1.09%) followed by suture (1.21% vs 2.04%), NO-SLR (1.89% vs 2.60%), and BPS (2.73% vs 3.30%) [13]. Though tissue sealants were not evaluated in the previous review, it should be noted in the current study that Seal and NO-SLR methods had similar leak rates and that the addition of tissue sealants in the analysis did not alter the trend of lower leak rates. We speculate that the temporal reduction in leak complications in LSG is most likely related to surgical experience since there have been minimal-to-no-changes in the buttressing material from the previous to the current review. Two studies have demonstrated that surgeon technique and skill is associated with improved outcomes following bariatric surgery [11, 169]. Improvements in surgical techniques include: improved dissection with preservation of healthier and more vascular tissue by reducing thermal injury and tissue trauma, selection of appropriate staple height to accommodate tissue thickness, avoidance of narrowing near the angularis incisura, choice of adequate bougie sizes, and avoidance of stapling along the esophagus. If this is indeed the case, there is the possibility that further reduction in leak complications could be gained by improving intraoperative strategies. It has previously been reported by the Michigan Bariatric Surgery Collaborative that more experienced and higher volume surgeons use intracorporeal suturing more frequently [11]. This trend may have occurred in this current study and resulted in lower leak rates in the suture group. Indeed, a recent randomized study comparing the use of a running suture with invagination to no reinforcement demonstrated a reduction in leak rates for the suturing approach, although this came at a cost of higher operative time by 18 min [170]. Increased operative time and cost with intracorporeal suturing is supported in other studies that reported an additional 13 to 24 min per case. Additionally, there is evidence that staple-line buttressing with APM may actually be more cost effective at 6-months post-surgery [39, 171]. As APM and Suture were the two reinforcements with the lowest leak rates, this comparison warrants further study.
This current review highlights that the leak rate for studies conducted in the US were lower than the overall average leak rate of all studies evaluated. The APM was associated with the lowest leak rate when looking only at studies conducted in the US (0.39%) versus all studies in all geographic locations reported (0.73%). Indeed, every reinforcement method had a substantially lower leak rate in the US studies compared to the overall publications, with the exception for the Seal group (1.8% versus 1.9%, respectively).
The present study had many limitations. Inherently, the nature of the review method itself is a limitation as it relies solely on data provided within the publication. Further, this systematic review included only one randomized-controlled trial that met our review criteria. The collection of granular data such as the use of reinforcement on the entire staple-line versus selective areas, the use of buttressing material on both the cartridge and anvil side versus one side or the other, stapler type, and staple height would have been beneficial. Additionally, this study did not include a discontinued variety of 100% PGA APM or a recently available variety of 100% PGA APM due to a lack of sufficient publications.
This study was not designed to evaluate costs in relation to leak or bleeding complication. It is known that leaks are extremely costly, and for example, can result in prolonged hospitalization within an intensive care unit as well as additional outpatient costs [172]. Since bleeding complications can be associated with leaks, data regarding bleeding would have been an asset. Unfortunately, these data were inconsistently reported and thus were collected as free text which could not be categorically summarized. As mentioned previously, staple height selection was not uniformly collected, but might be a significant factor associated with staple-line leaks. Thick gastric tissue (i.e. antrum) is at risk of crush injury with too short a staple load, with incomplete staple formation which would fail to close the gastric resection margin, and thin gastric tissue (i.e. cardia) is at risk of loose staple-line formation with too tall staple load. With most leaks occurring on the proximal staple line near the gastroesophageal junction, it is possible that the thinner wall is at risk of injury related to uneven staple compression or inadequate compression to approximate the tissues. Other elements may be responsible, like ischemia and morphology. Buttressing material has been shown to more evenly distribute the staple pressure over a wider surface area thus resulting in higher burst pressures and lower bleed rates [173–179]. As such, we hypothesize that the lower leak rate associated with the use of a thin buttressing material, such as APM (0.5 mm maximum total thickness), is related to improved staple compression, given, of course, appropriate staple height selection. Conversely, we speculate that the variable thicker BPS reinforcement (0.4 mm - 1.2 mm) could result in variations of tissue compression, potentially resulting in a segment of staple line that is either too tight or too loose.
Conclusion
Systematic review of 148 included studies representing 40,653 patients found that the leak rate in LSG was significantly lower using APM staple-line reinforcement than oversewing, BPS reinforcement, or no reinforcement. Selected operative strategies can result in lower leak rates after sleeve gastrectomy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Disclosures
This study was supported by WL Gore & Associates, more specifically for providing partial funding assistance. Dr Michel Gagner has received honoraria for speaking engagements from Medtronic, Ethicon Endo-Surgery, W.L. Gore, & Associates and Valeant. Also, he has received research honoraria from Lexington Medical and W.L. Gore, & Associates. Dr Paul Kemmeter has received honoraria for speaking engagements from W.L. Gore, & Associates, and also research honoraria from W.L. Gore, & Associates. The authors thank Ms. Millie Hollandbeck, for writing assistance, and Mr. Joshua Washburn, for biostatistical assistance.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Michel Gagner, Email: gagner.michel@gmail.com.
Paul Kemmeter, Email: pkemmeter@grandhealthpartners.com.
References
- 1.Varela JE, Nguyen NT. Laparoscopic sleeve gastrectomy leads the US utilization of bariatric surgery at academic medical centers. Surg Obes Relat Dis. 2015;11(5):987–990. doi: 10.1016/j.soard.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 2.International Federation for the Surgery of Obesity and Metabolic Disorders website; http://www.ifso.com/sleeve-gastrectomy/. Accessed 06 Mar 2018
- 3.Mui WL, Ng EK, Tsung BY, Lam CC, Yung MY. Laparoscopic sleeve gastrectomy in ethnic obese Chinese. Obes Surg. 2008;18(12):1571–1574. doi: 10.1007/s11695-008-9538-3. [DOI] [PubMed] [Google Scholar]
- 4.Burgos AM, Braghetto I, Csendes A, et al. Gastric leak after laparoscopic-sleeve gastrectomy for obesity. Obes Surg. 2009;19(12):1672–1677. doi: 10.1007/s11695-009-9884-9. [DOI] [PubMed] [Google Scholar]
- 5.Csendes A, Braghetto I, León P, Burgos AM. Management of leaks after laparoscopic sleeve gastrectomy in patients with obesity. J Gastrointest Surg. 2010;14(9):1343–1348. doi: 10.1007/s11605-010-1249-0. [DOI] [PubMed] [Google Scholar]
- 6.Aurora A, Khaitan L, Saber A. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc. 2012;26(6):1509–1515. doi: 10.1007/s00464-011-2085-3. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal RJ, Diaz AA, Arvidsson D, et al. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of > 12,000 cases. Surg Obes Relat Dis. 2012;8(1):8–19. doi: 10.1016/j.soard.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Sakran N, Goitein D, Raziel A, et al. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27(1):240–245. doi: 10.1007/s00464-012-2426-x. [DOI] [PubMed] [Google Scholar]
- 9.D’Ugo S, Gentileschi P, Benavoli D, et al. Comparative use of different techniques for leak and bleeding prevention during laparoscopic sleeve gastrectomy: a multicenter study. Surg Obes Relat Dis. 2014;10(3):450–454. doi: 10.1016/j.soard.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Berger ER, Clements RH, Morton JM, et al. The impact of different surgical techniques on outcomes in laparoscopic sleeve gastrectomies: the first report from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) Ann Surg. 2016;264(3):464–473. doi: 10.1097/SLA.0000000000001851. [DOI] [PubMed] [Google Scholar]
- 11.Varban OA, Sheetz KH, Cassidy RB, et al. Evaluating the effect of operative technique on leaks after laparoscopic sleeve gastrectomy: a case-control study. Surg Obes Relat Dis. 2017;13(4):560–567. doi: 10.1016/j.soard.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Cesana G, Cioffi S, Giorgia R, et al. Proximal leakage after laparoscopic sleeve gastrectomy: an analysis of preoperative and operative predictors on 1738 consecutive procedures. Obes Surg. 2018;28(3):627–635. doi: 10.1007/s11695-017-2907-z. [DOI] [PubMed] [Google Scholar]
- 13.Gagner M, Buchwald JN. Comparison of laparoscopic sleeve gastrectomy leak rates in four staple-line reinforcement options: a systematic review. Surg Obes Relat Dis. 2014;10(4):713–723. doi: 10.1016/j.soard.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Gagner M, Brown M. Update on sleeve gastrectomy leak rate with the use of reinforcement. Obes Surg. 2016;26(1):146–150. doi: 10.1007/s11695-015-1899-9. [DOI] [PubMed] [Google Scholar]
- 15.Moher David, Liberati Alessandro, Tetzlaff Jennifer, Altman Douglas G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agresti A. A survey of exact inference for contingency tables. Statist Sci. 1992;27(1):131–153. [Google Scholar]
- 17.Abd Ellatif ME, Abdallah E, Askar W, et al. Long term predictors of success after laparoscopic sleeve gastrectomy. Int J Surg. 2014;12(5):504–508. doi: 10.1016/j.ijsu.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Abdallah E, El Nakeeb A, Youssef T, et al. Impact of extent of antral resection on surgical outcomes of sleeve gastrectomy for morbid obesity (a prospective randomized study) Obes Surg. 2014;24(10):1587–1594. doi: 10.1007/s11695-014-1242-x. [DOI] [PubMed] [Google Scholar]
- 19.Abdallah E, Emile SH, Elfeki H. Laparoscopic sleeve gastrectomy with or without staple line inversion and distal fixation to the transverse mesocolon: impact on early postoperative outcomes. Obes Surg. 2017;27(2):323–329. doi: 10.1007/s11695-016-2277-y. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal S, Sharma AP, Ramaswamy N. Outcome of laparoscopic sleeve gastrectomy with and without staple line oversewing in morbidly obese patients: a randomized study. J Laparoendosc Adv Surg Tech A. 2013;23(11):895–899. doi: 10.1089/lap.2013.0137. [DOI] [PubMed] [Google Scholar]
- 21.Al Sharqawi N, Al Sabah S, Al Mulla A, Al Anezi K, Jumaa T. Conversional surgery: single-step conversion of laparoscopic adjustable gastric band to laparoscopic sleeve gastrectomy. Obes Surg. 2014;24(10):1808–1811. doi: 10.1007/s11695-014-1358-z. [DOI] [PubMed] [Google Scholar]
- 22.Albanopoulos K, Tsamis D, Arapaki A, Kleidi E, Zografos G, Leandros E. Staple line reinforcement with stitch in laparoscopic sleeve gastrectomies. Is it useful or harmful? J Laparoendosc Adv Surg Tech A. 2015;25(7):561–565. doi: 10.1089/lap.2014.0433. [DOI] [PubMed] [Google Scholar]
- 23.Albeladi B, Bourbao-Tournois C, Huten N. Short- and midterm results between laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy for the treatment of morbid obesity. J Obes. 2013;1:1–10. doi: 10.1155/2013/934653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alqahtani A, Almari H, Elahmedi M, Mohammed R. Laparoscopic sleeve gastrectomy in adult and pediatric obese patients: a comparative study. Surg Endosc. 2012;26(11):3094–3100. doi: 10.1007/s00464-012-2345-x. [DOI] [PubMed] [Google Scholar]
- 25.Bekheit M, Katri KM, Nabil W, Sharaan MA, El Said A. Earliest signs and management of leakage after bariatric surgeries: single institute experience. Alexandria J Med. 2013;49(1):29–33. [Google Scholar]
- 26.Bellows CF, Gauthier JM, Webber LS. Bariatric aftercare and outcomes in the Medicaid population following sleeve gastrectomy. JSLS. 2014 doi: 10.4293/JSLS.2014.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Billing PS, Crouthamel MR, Oling S, Landerholm RW. Outpatient laparoscopic sleeve gastrectomy in a free-standing ambulatory surgery center: first 250 cases. Surg Obes Relat Dis. 2014;10(1):101–115. doi: 10.1016/j.soard.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Bransen J, Gilissen LP, van Rutte PW, Nienhuijs SW. Costs of leaks and bleeding after sleeve gastrectomies. Obes Surg. 2015;25(10):1767–1771. doi: 10.1007/s11695-015-1584-z. [DOI] [PubMed] [Google Scholar]
- 29.Brockmeyer JR, Simon TE, Jacob RK, Husain F, Choi Y. Upper gastrointestinal swallow study following bariatric surgery: institutional review and review of the literature. Obes Surg. 2012;22(7):1039–1043. doi: 10.1007/s11695-012-0658-4. [DOI] [PubMed] [Google Scholar]
- 30.Bülbüller N, Aslaner A, Oner OZ, et al. Comparison of four different methods in staple line reinforcement during laparascopic sleeve gastrectomy. Int J Clin Exp Med. 2013;6(10):985–990. [PMC free article] [PubMed] [Google Scholar]
- 31.Chakravartty S, Sarma DR, Chang A, Patel AG. Staple line bleeding in sleeve gastrectomy - a simple and cost-effective solution. Obes Surg. 2016;26(7):1422–1428. doi: 10.1007/s11695-015-1986-y. [DOI] [PubMed] [Google Scholar]
- 32.Desiderio J, Trastulli S, Scalercio V, et al. Effects of laparoscopic sleeve gastrectomy in patients with morbid obesity and metabolic disorders. Diabetes Technol Ther. 2013;15(12):1004–1009. doi: 10.1089/dia.2013.0162. [DOI] [PubMed] [Google Scholar]
- 33.Dogan K, Gadiot RP, Aarts EO, et al. Effectiveness and safety of sleeve gastrectomy, gastric bypass, and adjustable gastric banding in morbidly obese patients: a multicenter, retrospective, matched cohort study. Obes Surg. 2015;25(7):1110–1118. doi: 10.1007/s11695-014-1503-8. [DOI] [PubMed] [Google Scholar]
- 34.Eid GM, Brethauer S, Mattar SG, Titchner RL, Gourash W, Schauer PR. Laparoscopic sleeve gastrectomy for super obese patients: forty-eight percent excess weight loss after 6 to 8 years with 93% follow-up. Ann Surg. 2012;256(2):262–265. doi: 10.1097/SLA.0b013e31825fe905. [DOI] [PubMed] [Google Scholar]
- 35.El Chaar M, Claros L, Ezeji GC, Miletics M, Stoltzfus J. Improving outcome of bariatric surgery: best practices in an accredited surgical center. Obes Surg. 2014;24(7):1057–1063. doi: 10.1007/s11695-014-1209-y. [DOI] [PubMed] [Google Scholar]
- 36.ElGeidie A, ElHemaly M, Hamdy E, El Sorogy M, AbdelGawad M, GadElHak N. The effect of residual gastric antrum size on the outcome of laparoscopic sleeve gastrectomy: a prospective randomized trial. Surg Obes Relat Dis. 2015;11(5):997–1003. doi: 10.1016/j.soard.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 37.Fernández JI, Farías CO, Ovalle CL, Cabrera CS, de la Maza JC. Transumbilical single-incision laparoscopic sleeve gastrectomy. Obes Surg. 2015;25(3):430–435. doi: 10.1007/s11695-014-1414-8. [DOI] [PubMed] [Google Scholar]
- 38.Frattini F, Lavazza M, Mangano A, et al. Indocyanine green-enhanced fluorescence in laparoscopic sleeve gastrectomy. Obes Surg. 2015;25(5):949–950. doi: 10.1007/s11695-015-1640-8. [DOI] [PubMed] [Google Scholar]
- 39.Gayrel X, Loureiro M, Skalli EM, Dutot C, Mercier G, Nocca D. Clinical and economic evaluation of absorbable staple line buttressing in sleeve gastrectomy in high-risk patients. Obes Surg. 2016;26(8):1710–1716. doi: 10.1007/s11695-015-1991-1. [DOI] [PubMed] [Google Scholar]
- 40.Gerin O, Rebibo L, Dhahri A, Regimbeau JM. The safety of laparoscopic sleeve gastrectomy in patients receiving chronic anticoagulation therapy: a case-matched study. Obes Surg. 2015;25(9):1686–1692. doi: 10.1007/s11695-015-1590-1. [DOI] [PubMed] [Google Scholar]
- 41.Gumbau V, Bruna M, Canelles E, et al. A prospective study on inflammatory parameters in obese patients after sleeve gastrectomy. Obes Surg. 2014;24(6):903–908. doi: 10.1007/s11695-014-1186-1. [DOI] [PubMed] [Google Scholar]
- 42.Hawasli A, Jacquish B, Almahmeed T, et al. Early effects of bougie size on sleeve gastrectomy outcome. Am J Surg. 2015;209(3):473–477. doi: 10.1016/j.amjsurg.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Hawasli A, Reyes M, Hare B, et al. Can morbidly obese patients with reflux be offered laparoscopic sleeve gastrectomy? A case report of 40 patients. Am J Surg. 2016;211(3):571–576. doi: 10.1016/j.amjsurg.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Hirth DA, Jones EL, Rothchild KB, Mitchell BC, Schoen JA. Laparoscopic sleeve gastrectomy: long-term weight loss outcomes. Surg Obes Relat Dis. 2015;11(5):1004–1007. doi: 10.1016/j.soard.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 45.Hoogerboord M, Wiebe S, Klassen D, Ransom T, Lawlor D, Ellsmere J. Laparoscopic sleeve gastrectomy: perioperative outcomes, weight loss and impact on type 2 diabetes mellitus over 2 years. Can J Surg. 2014;57(2):101–105. doi: 10.1503/cjs.024212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karakoyun R, Gündüz U, Bülbüller N, et al. The effect of serosal suture reinforcement on burst pressure in sleeve gastrectomy specimens. Surg Laparosc Endosc Percutan Tech. 2014;24(5):424–428. doi: 10.1097/SLE.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 47.Kasalicky M, Dolezel R, Vernerova E, Haluzik M. Laparoscopic sleeve gastrectomy without over-sewing of the staple line is effective and safe. Wideochir Inne Tech Maloinwazyjne. 2014;9(1):46–52. doi: 10.5114/wiitm.2014.40387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kruger RS, Pricolo VE, Streeter TT, Colacchio DA, Andrade UA. A bariatric surgery center of excellence: operative trends and long-term outcomes. J Am Coll Surg. 2014;218(6):1163–1174. doi: 10.1016/j.jamcollsurg.2014.01.056. [DOI] [PubMed] [Google Scholar]
- 49.Kular KS, Manchanda N, Rutledge R. Analysis of the five-year outcomes of sleeve gastrectomy and mini gastric bypass: a report from the Indian sub-continent. Obes Surg. 2014;24(10):1724–1728. doi: 10.1007/s11695-014-1264-4. [DOI] [PubMed] [Google Scholar]
- 50.Lainas P, Tranchart H, Gaillard M, Ferretti S, Donatelli G, Dagher I. Prospective evaluation of routine early computed tomography scanner in laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2016;12(8):1483–1490. doi: 10.1016/j.soard.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 51.Lakdawala M, Agarwal A, Dhar S, Dhulla N, Remedios C, Bhasker AG. Single-incision sleeve gastrectomy versus laparoscopic sleeve gastrectomy. A 2-year comparative analysis of 600 patients. Obes Surg. 2015;25(4):607–614. doi: 10.1007/s11695-014-1461-1. [DOI] [PubMed] [Google Scholar]
- 52.Lemaître F, Léger P, Nedelcu M, Nocca D. Laparoscopic sleeve gastrectomy in the South Pacific. Retrospective evaluation of 510 patients in a single institution. Int J Surg. 2016;30:1–6. doi: 10.1016/j.ijsu.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Lopez J, Vilallonga R, Targarona EM, et al. Can LigaSure™ be used to perform sleeve gastrectomy? - Tensile strength and histological changes. Minim Invasive Ther Allied Technol. 2014;23(3):144–151. doi: 10.3109/13645706.2013.875924. [DOI] [PubMed] [Google Scholar]
- 54.Maluenda F, León J, Csendes A, Burdiles P, Giordano J, Molina M. Single-incision laparoscopic sleeve gastrectomy: initial experience in 20 patients and 2-year follow-up. Eur Surg. 2014;46:32–37. doi: 10.1007/s10353-013-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michalsky D, Dvorak P, Belacek J, Kasalicky M. Radical resection of the pyloric antrum and its effect on gastric emptying after sleeve gastrectomy. Obes Surg. 2013;23(4):567–573. doi: 10.1007/s11695-012-0850-6. [DOI] [PubMed] [Google Scholar]
- 56.Mittermair R, Pratschke J, Sucher R. Single-incision laparoscopic sleeve gastrectomy. Am Surg. 2013;79(4):393–397. [PubMed] [Google Scholar]
- 57.Mittermair R, Sucher R, Perathoner A, Wykypiel H. Routine upper gastrointestinal swallow studies after laparoscopic sleeve gastrectomy are unnecessary. Am J Surg. 2014;207(6):897–901. doi: 10.1016/j.amjsurg.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 58.Mittermair R, Sucher R, Perathoner A. Results and complications after laparoscopic sleeve gastrectomy. Surg Today. 2014;44(7):1307–1312. doi: 10.1007/s00595-013-0688-0. [DOI] [PubMed] [Google Scholar]
- 59.Mittermair R. Transumbilical single-incision laparoscopic sleeve gastrectomy: short-term results and technical considerations. J Minim Access Surg. 2013;9(3):104–108. doi: 10.4103/0972-9941.115367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mukherjee S, Devalia K, Rahman MG, Mannur KR. Sleeve gastrectomy as a bridge to a second bariatric procedure in superobese patients-a single institution experience. Surg Obes Relat Dis. 2012;8(2):140–144. doi: 10.1016/j.soard.2011.04.232. [DOI] [PubMed] [Google Scholar]
- 61.Musella M, Milone M, Maietta P, Bianco P, Pisapia A, Gaudioso D. Laparoscopic sleeve gastrectomy: efficacy of fibrin sealant in reducing postoperative bleeding. A randomized controlled trial. Updates Surg. 2014;66(3):197–201. doi: 10.1007/s13304-014-0257-0. [DOI] [PubMed] [Google Scholar]
- 62.Noel P, Iannelli A, Sejor E, Schneck AS, Gugenheim J. Laparoscopic sleeve gastrectomy: how I do it. Surg Laparosc Endosc Percutan Tech. 2013;23(1):e14–e16. doi: 10.1097/SLE.0b013e318274b8cf. [DOI] [PubMed] [Google Scholar]
- 63.Noel P, Nedelcu M, Gagner M. Impact of the surgical experience on leak rate after laparoscopic sleeve gastrectomy. Obes Surg. 2016;26(8):1782–1787. doi: 10.1007/s11695-015-2003-1. [DOI] [PubMed] [Google Scholar]
- 64.Noun R, Chakhtoura G, Nasr M, et al. Laparoscopic sleeve gastrectomy for mildly obese patients (body mass index of 30 < 35 kg/m2): operative outcome and short-term results. J Obes. 2012 doi: 10.1155/2012/813650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noun R, Slim R, Nasr M, et al. Results of laparoscopic sleeve gastrectomy in 541 consecutive patients with low baseline body mass index (30-35 kg/m2) Obes Surg. 2016;26(12):2824–2828. doi: 10.1007/s11695-016-2224-y. [DOI] [PubMed] [Google Scholar]
- 66.Pequignot A, Dhahri A, Verhaeghe P, Desailoud R, Lalau JD, Regimbeau JM. Efficiency of laparoscopic sleeve gastrectomy on metabolic syndrome disorders: two-year results. J Visc Surg. 2012;149:e350–e355. doi: 10.1016/j.jviscsurg.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 67.Pequignot A, Prevot F, Dhahri A, Rebibo L, Badaoui R, Regimbeau JM. Is sleeve gastrectomy still contraindicated for patients aged ≥ 60 years? A case-matched study with 24 months of follow-up. Surg Obes Relat Dis. 2015;11(5):1008–1013. doi: 10.1016/j.soard.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 68.Laimer E, Pratschke J, Mittermair R. Significant weight loss and rapid resolution of diabetes and dyslipidemia during short-term follow-up after laparoscopic sleeve gastrectomy. Obes Surg. 2013;23(12):1966–1972. doi: 10.1007/s11695-013-1038-4. [DOI] [PubMed] [Google Scholar]
- 69.Rossetti G, Fei L, Docimo L, et al. Is nasogastric decompression useful in prevention of leaks after laparoscopic sleeve gastrectomy? A randomized trial. J Invest Surg. 2014;27(4):234–239. doi: 10.3109/08941939.2013.875606. [DOI] [PubMed] [Google Scholar]
- 70.Rossetti G, Moccia F, Marra T, et al. Does helicobacter pylori infection have influence on outcome of laparoscopic sleeve gastrectomy for morbid obesity? Int J Surg. 2014;12(Suppl 1):S68–S71. doi: 10.1016/j.ijsu.2014.05.051. [DOI] [PubMed] [Google Scholar]
- 71.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shah SS, Todkar JS, Shah PS. Buttressing the staple line: a randomized comparison between staple-line reinforcement versus no reinforcement during sleeve gastrectomy. Obes Surg. 2014;24(12):2014–2020. doi: 10.1007/s11695-014-1374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spivak H, Rubin M, Sadot E, Pollak E, Feygin A, Goitein D. Laparoscopic sleeve gastrectomy using 42-French versus 32-French bougie: the first-year outcome. Obes Surg. 2014;24(7):1090–1093. doi: 10.1007/s11695-014-1199-9. [DOI] [PubMed] [Google Scholar]
- 74.Spivak H, Segev L, Meydan C, Yosef R, Ronen I, Heller L. Achieving the “minimal scarring” concept in bariatrics by port-site relocation. Obes Surg. 2016;26(3):683–687. doi: 10.1007/s11695-015-2019-6. [DOI] [PubMed] [Google Scholar]
- 75.Sroka G, Milevski D, Shteinberg D, Mady H, Matter I. Minimizing hemorrhagic complications in laparoscopic sleeve gastrectomy - a randomized controlled trial. Obes Surg. 2015;25(9):1577–1583. doi: 10.1007/s11695-015-1580-3. [DOI] [PubMed] [Google Scholar]
- 76.Stroh C, Köckerling F, Volker L, et al. Results of more than 11,800 sleeve gastrectomies: data analysis of the German Bariatric Surgery Registry. Ann Surg. 2016;263(5):949–955. doi: 10.1097/SLA.0000000000001559. [DOI] [PubMed] [Google Scholar]
- 77.Sucher R, Resch T, Mohr E, et al. Single-incision laparoscopic sleeve gastrectomy versus multiport laparoscopic sleeve gastrectomy: analysis of 80 cases in a single center. J Laparoendosc Adv Surg Tech A. 2014;24(2):83–88. doi: 10.1089/lap.2013.0250. [DOI] [PubMed] [Google Scholar]
- 78.Timucin Aydin M, Aras O, Karip B, Memisoglu K. Staple line reinforcement methods in laparoscopic sleeve gastrectomy: comparison of burst pressures and leaks. JSLS. 2015;19(3):e2015.00040. doi: 10.4293/JSLS.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Topart P, Becouarn G, Ritz P. Comparative early outcomes of three laparoscopic bariatric procedures: sleeve gastrectomy, Roux-en-Y gastric bypass, and biliopancreatic diversion with duodenal switch. Surg Obes Relat Dis. 2012;8(3):250–254. doi: 10.1016/j.soard.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 80.Tsamis D, Natoudi M, Arapaki A, et al. Using Ligasure™ or Harmonic Ace® in laparoscopic sleeve gastrectomies? A prospective randomized study. Obes Surg. 2015;25(8):1454–1457. doi: 10.1007/s11695-014-1551-0. [DOI] [PubMed] [Google Scholar]
- 81.van Rutte P, Nienhuijs SW, Jakimowicz JJ, van Montfort G. Identification of technical errors and hazard zones in sleeve gastrectomy using OCHRA: “OCHRA for sleeve gastrectomy”. Surg Endosc. 2017;31(2):561–566. doi: 10.1007/s00464-016-4997-4. [DOI] [PubMed] [Google Scholar]
- 82.van Rutte PW, Smulders JF, de Zoete JP, Nienhuijs SW. Outcome of sleeve gastrectomy as a primary bariatric procedure. Br J Surg. 2014;101(6):661–668. doi: 10.1002/bjs.9447. [DOI] [PubMed] [Google Scholar]
- 83.Wahby M, Salama AF, Elezaby AF, et al. Is routine postoperative gastrografin study needed after laparoscopic sleeve gastrectomy? Experience of 712 cases. Obes Surg. 2013;23(11):1711–1717. doi: 10.1007/s11695-013-1013-0. [DOI] [PubMed] [Google Scholar]
- 84.Zachariah SK, Chang PC, Ooi AS, Hsin MC, Kin Wat JY, Huang CK. Laparoscopic sleeve gastrectomy for morbid obesity: 5 years experience from an Asian center of excellence. Obes Surg. 2013;23(7):939–946. doi: 10.1007/s11695-013-0887-1. [DOI] [PubMed] [Google Scholar]
- 85.Abdelbaki TN, Sharaan M, Abdel-Baki NA, Katri K. Laparoscopic gastric greater curvature plication versus laparoscopic sleeve gastrectomy: early outcome in 140 patients. Surg Obes Relat Dis. 2014;10(6):1141–1146. doi: 10.1016/j.soard.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 86.Al Hajj GN, Haddad J. Preventing staple-line leak in sleeve gastrectomy: reinforcement with bovine pericardium vs oversewing. Obes Surg. 2013;23(11):1915–1921. doi: 10.1007/s11695-013-1062-4. [DOI] [PubMed] [Google Scholar]
- 87.Alsina E, Ruiz-Tovar J, Alpera MR, et al. Incidence of deep vein thrombosis and thrombosis of the portal-mesenteric axis after laparoscopic sleeve gastrectomy. J Laparoendosc Adv Surg Tech A. 2014;24(9):601–605. doi: 10.1089/lap.2014.0125. [DOI] [PubMed] [Google Scholar]
- 88.Alvarenga ES, Lo Menzo E, Szomstein S, Rosenthal RJ. Safety and efficacy of 1020 consecutive laparoscopic sleeve gastrectomies performed as a primary treatment modality for morbid obesity. A single-center experience from the metabolic and bariatric surgical accreditation quality and improvement program. Surg Endosc. 2016;30(7):2673–2678. doi: 10.1007/s00464-015-4548-4. [DOI] [PubMed] [Google Scholar]
- 89.Barreto TW, Kemmeter PR, Paletta MP, Davis AT. A comparison of a single center’s experience with three staple line reinforcement techniques in 1,502 laparoscopic sleeve gastrectomy patients. Obes Surg. 2015;25(3):418–422. doi: 10.1007/s11695-014-1432-6. [DOI] [PubMed] [Google Scholar]
- 90.Belgaumkar AP, Carswell KA, Hughes RD, et al. The effect of intraoperative N-acetylcysteine on hepatocellular injury during laparoscopic bariatric surgery. A randomised controlled trial. Obes Surg. 2016;26(6):1254–1265. doi: 10.1007/s11695-015-1904-3. [DOI] [PubMed] [Google Scholar]
- 91.Bhatia P, Bindal V, Singh R, et al. Robot-assisted sleeve gastrectomy in morbidly obese versus super obese patients. JSLS. 2014;18(3):e2014.00099. doi: 10.4293/JSLS.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boza C, Daroch D, Barros D, León F, Funke R, Crovari F. Long-term outcomes of laparoscopic sleeve gastrectomy as a primary bariatric procedure. Surg Obes Relat Dis. 2014;10(6):1129–1133. doi: 10.1016/j.soard.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 93.Boza C, Salinas J, Salgado N, et al. Laparoscopic sleeve gastrectomy as a stand-alone procedure for morbid obesity: report of 1,000 cases and 3-year follow-up. Obes Surg. 2012;22(6):866–871. doi: 10.1007/s11695-012-0591-6. [DOI] [PubMed] [Google Scholar]
- 94.Cal P, Deluca L, Jakob T, Fernández E. Laparoscopic sleeve gastrectomy with 27 versus 39 Fr bougie calibration: a randomized controlled trial. Surg Endosc. 2016;30(5):1812–1815. doi: 10.1007/s00464-015-4450-0. [DOI] [PubMed] [Google Scholar]
- 95.Catheline JM, Fysekidis M, Dbouk R, et al. Weight loss after sleeve gastrectomy in super superobesity. J Obes. 2012 doi: 10.1155/2012/959260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Corcelles R, Boules M, Froylich D, et al. Laparoscopic three-port sleeve gastrectomy: a single institution case series. J Laparoendosc Adv Surg Tech A. 2016;26(5):361–365. doi: 10.1089/lap.2015.0532. [DOI] [PubMed] [Google Scholar]
- 97.Daes J, Jimenez M, Said N, Daza J, Dennis R. Laparoscopic sleeve gastrectomy: symptoms of gastroesophageal reflux can be reduced by changes in surgical technique. Obes Surg. 2012;22:1874–1879. doi: 10.1007/s11695-012-0746-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Daes J, Jimenez ME, Said N, Dennis R. Improvement of gastroesophageal reflux symptoms after standardized laparoscopic sleeve gastrectomy. Obes Surg. 2014;24(4):536–540. doi: 10.1007/s11695-013-1117-6. [DOI] [PubMed] [Google Scholar]
- 99.Dakour Aridi H, Alami R, Tamim H, Shamseddine G, Fouani T, Safadi B. Long-term outcomes of laparoscopic sleeve gastrectomy: a Lebanese center experience. Surg Obes Relat Dis. 2016;12(9):1689–1696. doi: 10.1016/j.soard.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 100.Dotai T, Coker AM, Antozzi L, et al. Transgastric large-organ extraction: the initial human experience. Surg Endosc. 2013;27(2):394–399. doi: 10.1007/s00464-012-2473-3. [DOI] [PubMed] [Google Scholar]
- 101.Fridman A, Moon R, Cozacov Y, et al. Procedure-related morbidity in bariatric surgery: a retrospective short- and mid-term follow-up of a single institution of the American College of Surgeons Bariatric Surgery Centers of Excellence. J Am Coll Surg. 2013;217(4):614–620. doi: 10.1016/j.jamcollsurg.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 102.Garofalo F, Denis R, Abouzahr O, Garneau P, Pescarus R, Atlas H. Fully ambulatory laparoscopic sleeve gastrectomy: 328 consecutive patients in a single tertiary bariatric center. Obes Surg. 2016;26(7):1429–1435. doi: 10.1007/s11695-015-1984-0. [DOI] [PubMed] [Google Scholar]
- 103.Gentileschi P, Camperchioli I, D’Ugo S, Benavoli D, Gaspari AL. Staple-line reinforcement during laparoscopic sleeve gastrectomy using three different techniques: a randomized trial. Surg Endosc. 2012;26:2623–2629. doi: 10.1007/s00464-012-2243-2. [DOI] [PubMed] [Google Scholar]
- 104.Grueneberger JM, Karcz-Socha I, Marjanovic G, et al. Pylorus preserving loop duodeno-enterostomy with sleeve gastrectomy—preliminary results. BMC Surg. 2014;14:20. doi: 10.1186/1471-2482-14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gunkova P, Gunka I, Zonca P, Dostalik J, Ihnat P. Laparoscopic sleeve gastrectomy for morbid obesity with natural orifice specimen extraction (NOSE) Bratisl Lek Listy. 2015;116(7):422–425. doi: 10.4149/bll_2015_080. [DOI] [PubMed] [Google Scholar]
- 106.Huang CK, Chhabra N, Goel R, Hung CM, Chang PC, Chen YS. Laparoscopic adjustable gastric banded plication: a case-matched comparative study with laparoscopic sleeve gastrectomy. Obes Surg. 2013;23(8):1319–1323. doi: 10.1007/s11695-013-0951-x. [DOI] [PubMed] [Google Scholar]
- 107.Jakob T, Cal P, Deluca L, Fernández E. Shorter than 24-h hospital stay for sleeve gastrectomy is safe and feasible. Surg Endosc. 2016;30(12):5596–5600. doi: 10.1007/s00464-016-4933-7. [DOI] [PubMed] [Google Scholar]
- 108.Ji Y, Ye H, Wang Y, Zhan X, Zhu J. Laparoscopic plicated sleeve gastrectomy: a technical report. Obes Surg. 2016;26(1):234–237. doi: 10.1007/s11695-015-1946-6. [DOI] [PubMed] [Google Scholar]
- 109.Keidar A, Hazan D, Sadot E, Kashtan H, Wasserberg N. The role of bariatric surgery in morbidly obese patients with inflammatory bowel disease. Surg Obes Relat Dis. 2015;11(1):132–136. doi: 10.1016/j.soard.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 110.Lee WJ, Lee KT, Ser KH, Chen JC, Tsou JJ, Lee YC. Laparoscopic adjustable gastric banding (LAGB) with gastric plication: short-term results and comparison with LAGB alone and sleeve gastrectomy. Surg Obes Relat Dis. 2015;11(1):125–130. doi: 10.1016/j.soard.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 111.Lee WJ, Pok EH, Almulaifi A, Tsou JJ, Ser KH, Lee YC. Medium-term results of laparoscopic sleeve gastrectomy: a matched comparison with gastric bypass. Obes Surg. 2015;25(8):1431–1438. doi: 10.1007/s11695-015-1582-1. [DOI] [PubMed] [Google Scholar]
- 112.Lin MY, Tavakol MM, Sarin A, et al. Laparoscopic sleeve gastrectomy is safe and efficacious for pretransplant candidates. Surg Obes Relat Dis. 2013;9(5):653–658. doi: 10.1016/j.soard.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 113.Moon RC, Kreimer F, Teixeira AF, Campos JM, Ferraz A, Jawad MA. Morbidity rates and weight loss after Roux-en-Y gastgric bypass, sleeve gastrectomy, and adjustable gastric banding in patients older than 60 years old: which procedure to choose? Obes Surg. 2016;26(4):730–736. doi: 10.1007/s11695-015-1824-2. [DOI] [PubMed] [Google Scholar]
- 114.Moon RC, Stephenson D, Royall NA, Teixeira AF, Jawad MA. Robot-assisted versus laparoscopic sleeve gastrectomy: learning curve, perioperative, and short-term outcomes. Obes Surg. 2016;26(10):2463–2468. doi: 10.1007/s11695-016-2131-2. [DOI] [PubMed] [Google Scholar]
- 115.Nimeri A, Maasher A, Salim E, Ibrahim M, Al Hadad M. The use of intraoperative endoscopy may decrease postoperative stenosis in laparoscopic sleeve gastrectomy. Obes Surg. 2016;26(7):1398–1401. doi: 10.1007/s11695-015-1958-2. [DOI] [PubMed] [Google Scholar]
- 116.Obeidat F, Shanti H, Mismar A, Albsoul N, Al-Qudah M. The magnitude of antral resection in laparoscopic sleeve gastrectomy and its relationship to excess weight loss. Obes Surg. 2015;25(10):1928–1932. doi: 10.1007/s11695-015-1642-6. [DOI] [PubMed] [Google Scholar]
- 117.Paluszkiewicz R, Kalinowski P, Wróblewski T, et al. Prospective randomized clinical trial of laparoscopic sleeve gastrectomy versus open Roux-en-Y gastric bypass for the management of patients with morbid obesity. Wideochir Inne Tech Maloinwazyjne. 2012;7(4):225–232. doi: 10.5114/wiitm.2012.32384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Park JY, Kim YJ. Laparoscopic gastric bypass vs sleeve gastrectomy in obese Korean patients. World J Gastroenterol. 2015;21(44):12612–12619. doi: 10.3748/wjg.v21.i44.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peterli R, Borbély Y, Kern B, et al. Early results of the Swiss Multicentre Bypass or Sleeve Study (SM-BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann Surg. 2013;258(5):690–694. doi: 10.1097/SLA.0b013e3182a67426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rogula T, Daigle C, Dua M, et al. Laparoscopic bariatric surgery can be performed through a single incision: a comparative study. Obes Surg. 2014;24(7):1102–1108. doi: 10.1007/s11695-014-1291-1. [DOI] [PubMed] [Google Scholar]
- 121.Rogula T, Khorgami Z, Bazan M, et al. Comparison of reinforcement techniques using suture on staple-line in sleeve gastrectomy. Obes Surg. 2015;25(11):2219–2224. doi: 10.1007/s11695-015-1864-7. [DOI] [PubMed] [Google Scholar]
- 122.Ruiz-Tovar J, Martínez R, Bonete JM, et al. Long-term weight and metabolic effects of laparoscopic sleeve gastrectomy calibrated with a 50-Fr bougie. Obes Surg. 2016;26(1):32–37. doi: 10.1007/s11695-015-1731-6. [DOI] [PubMed] [Google Scholar]
- 123.Ruiz-Tovar J, Muñoz JL, Gonzalez J, et al. Postoperative pain after laparoscopic sleeve gastrectomy: comparison of three analgesic schemes (isolated intravenous analgesia, epidural analgesia associated with intravenous analgesia and port-sites infiltration with bupivacaine associated with intravenous analgesia) Surg Endosc. 2017;31(1):231–236. doi: 10.1007/s00464-016-4961-3. [DOI] [PubMed] [Google Scholar]
- 124.Ruiz-Tovar J, Sola-Vera J, Miranda E, et al. Laparoscopic sleeve gastrectomy with endoscopic versus bougie calibration: results of a prospective study. J Laparoendosc Adv Surg Tech A. 2014;24(10):671–675. doi: 10.1089/lap.2014.0104. [DOI] [PubMed] [Google Scholar]
- 125.Ruiz-Tovar J, Zubiaga L, Diez M, et al. Preoperative regular diet of 900 kcal/day vs balanced energy high-protein formula vs immunonutrition formula: effect on preoperative weight loss and postoperative pain, complications and analytical acute phase reactants after laparoscopic sleeve gastrectomy. Obes Surg. 2016;26(6):1221–1227. doi: 10.1007/s11695-015-1880-7. [DOI] [PubMed] [Google Scholar]
- 126.Seki Y, Kasama K, Hashimoto K. Long-term outcome of laparoscopic sleeve gastrectomy in morbidly obese Japanese patients. Obes Surg. 2016;26(1):138–145. doi: 10.1007/s11695-015-1728-1. [DOI] [PubMed] [Google Scholar]
- 127.Serrano OK, Tannebaum JE, Cumella L, et al. Weight loss outcomes and complications from bariatric surgery in the super super obese. Surg Endosc. 2016;30(6):2505–2511. doi: 10.1007/s00464-015-4509-y. [DOI] [PubMed] [Google Scholar]
- 128.Shen D, Ye H, Wang Y, Ji Y, Zhan X, Zhu J, Li W. Comparison of short-term outcomes between laparoscopic greater curvature plication and laparoscopic sleeve gastrectomy. Surg Endosc. 2013;27(8):2768–2774. doi: 10.1007/s00464-013-2805-y. [DOI] [PubMed] [Google Scholar]
- 129.Shimizu H, Phuong V, Maia M, et al. Bariatric surgery in patients with liver cirrhosis. Surg Obes Relat Dis. 2013;9(1):1–6. doi: 10.1016/j.soard.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 130.Soliman AM, Lasheen M. Effect of banded laparoscopic sleeve gastrectomy on weight loss maintenance: comparative study between banded and non-banded sleeve on weight loss. Bariatr Surg Pract Patient Care. 2015;10(3):99–104. [Google Scholar]
- 131.Sucandy I, Antanavicius G, Bonanni F., Jr Outcome analysis of early laparoscopic sleeve gastrectomy experience. JSLS. 2013;17(4):602–606. doi: 10.4293/108680813X13693422520963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Szewczyk T, Janczak P, Janiak A, Gaszyński T, Modzelewski B. Laparoscopic sleeve gastrectomy - 7 years of own experience. Wideochir Inne Tech Maloinwazyjne. 2014;9(3):427–435. doi: 10.5114/wiitm.2014.44167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Thereaux J, Corigliano N, Poitou C, Oppert JM, Czernichow S, Bouillot JL. Comparison of results after one year between sleeve gastrectomy and gastric bypass in patients with BMI ≥ 50 kg/m2. Surg Obes Relat Dis. 2015;11(4):785–790. doi: 10.1016/j.soard.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 134.Våge V, Sande VA, Mellgren G, Laukeland C, Behme J, Andersen JR. Changes in obesity-related diseases and biochemical variables after laparoscopic sleeve gastrectomy: a two-year follow-up study. BMC Surg. 2014;14:8. doi: 10.1186/1471-2482-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Verdi D, Prevedello L, Albanese A, Lobba A, Foletto M. Laparoscopic gastric plication (LGCP) vs sleeve gastrectomy (LSG): a single institution experience. Obes Surg. 2015;25(9):1653–1657. doi: 10.1007/s11695-015-1600-3. [DOI] [PubMed] [Google Scholar]
- 136.Vix M, Diana M, Marx L, et al. Management of staple line leaks after sleeve gastrectomy in a consecutive series of 378 patients. Surg Laparosc Endosc Percutan Tech. 2015;25(1):89–93. doi: 10.1097/SLE.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 137.Yang JJ, Wang B, Liang YK, Song ZC, Gu Y. Early clinical efficacy of laparoscopic sleeve gastrectomy as a bariatric surgery for obese patients: a uni-center report in China. Biomed Environ Sci. 2013;26(7):539–545. doi: 10.3967/0895-3988.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 138.Youssef T, Abdalla E, El-Alfy K, Dawoud I, Morshed M, Farid M. Impact of botulinum neurotoxin pyloric injection during laparoscopic sleeve gastrectomy on postoperative gastric leak: a clinical randomized study. Obes Surg. 2016;26(3):494–504. doi: 10.1007/s11695-015-1794-4. [DOI] [PubMed] [Google Scholar]
- 139.Zerrweck C, Sepúlveda EM, Maydón HG, et al. Laparoscopic gastric bypass vs sleeve gastrectomy in the super obese patient: early outcomes of an observational study. Obes Surg. 2014;24(5):712–717. doi: 10.1007/s11695-013-1157-y. [DOI] [PubMed] [Google Scholar]
- 140.Zhang Y, Zhao H, Cao Z, et al. A randomized clinical trial of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy for the treatment of morbid obesity in China: a 5-year outcome. Obes Surg. 2014;24(10):1617–1624. doi: 10.1007/s11695-014-1258-2. [DOI] [PubMed] [Google Scholar]
- 141.Kehagias I, Spyropoulos C, Karamanakos S, Kalfarentzos F. Efficacy of sleeve gastrectomy as sole procedure in patients with clinically severe obesity (BMI ≤ 50 kg/m2) Surg Obes Relat Dis. 2013;9(3):363–369. doi: 10.1016/j.soard.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 142.Sdralis E, Argentou M, Mead N, Kehagias I, Alexandridis T, Kalfarentzos F. A prospective randomized study comparing patients with morbid obesity submitted to sleeve gastrectomy with or without omentectomy. Obes Surg. 2013;23(7):965–971. doi: 10.1007/s11695-013-0925-z. [DOI] [PubMed] [Google Scholar]
- 143.Spyropoulos C, Argentou MI, Petsas T, Thomopoulos K, Kehagias I, Kalfarentzos F. Management of gastrointestinal leaks after surgery for clinically severe obesity. Surg Obes Relat Dis. 2012;8(5):609–615. doi: 10.1016/j.soard.2011.04.222. [DOI] [PubMed] [Google Scholar]
- 144.Cerci M, Bellini MI, Russo F, et al. Bariatric surgery in moderately obese patients: a prospective study. Gastroenterol Res Pract. 2013;1:1–10. doi: 10.1155/2013/276183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kassir R, Blanc P, Bruna Tibalbo LM, Breton C, Lointier P. C-Reactive protein and procalcitonin for the early detection of postoperative complications after sleeve gastrectomy: preliminary study in 97 patients. Surg Endosc. 2015;29(6):1439–1444. doi: 10.1007/s00464-014-3821-2. [DOI] [PubMed] [Google Scholar]
- 146.Rawlins L, Rawlins MP, Brown CC, Schumacher DL. Sleeve gastrectomy: 5-year outcomes of a single institution. Surg Obes Relat Dis. 2013;9(1):21–25. doi: 10.1016/j.soard.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 147.Andreas A, Adamantios M, Antonios A, Theofilos R, Christos T, Theodoros D. Laparoscopic sleeve gastrectomy for morbid obesity with intra-operative endoscopy: lessons we learned after 100 consecutive patients. Obes Surg. 2015;25(7):1223–1228. doi: 10.1007/s11695-014-1524-3. [DOI] [PubMed] [Google Scholar]
- 148.El Chaar M, Hammoud N, Ezeji G, Claros L, Miletics M, Stoltzfus J. Laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass: a single center experience with 2 years follow-up. Obes Surg. 2015;25(2):254–262. doi: 10.1007/s11695-014-1388-6. [DOI] [PubMed] [Google Scholar]
- 149.Elli E, Gonzalez-Heredia R, Sarvepalli S, Masrur M. Laparoscopic and robotic sleeve gastrectomy: short- and long-term results. Obes Surg. 2015;25(6):967–974. doi: 10.1007/s11695-014-1499-0. [DOI] [PubMed] [Google Scholar]
- 150.Gomberawalla A, Salamat A, Lutfi R. Outcome analysis of single incision vs traditional multiport sleeve gastrectomy: a matched cohort study. Obes Surg. 2014;24(11):1870–1874. doi: 10.1007/s11695-014-1312-0. [DOI] [PubMed] [Google Scholar]
- 151.Iannelli A, Schneck AS, Topart P, Carles M, Hébuterne X, Gugenheim J. Laparoscopic sleeve gastrectomy followed by duodenal switch in selected patients versus single-stage duodenal switch for superobesity: case-control study. Surg Obes Relat Dis. 2013;9(4):531–538. doi: 10.1016/j.soard.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 152.Luppi CR, Balagué C, Targarona EM, et al. Laparoscopic sleeve gastrectomy in patients over 60 years: impact of age on weight loss and co-morbidity improvement. Surg Obes Relat Dis. 2015;11(2):296–301. doi: 10.1016/j.soard.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 153.Nguyen NT, Smith BR, Reavis KM, Nguyen XM, Nguyen B, Stamos MJ. Strategic laparoscopic surgery for improved cosmesis in general and bariatric surgery: analysis of initial 127 cases. J Laparoendosc Adv Surg Tech A. 2012;22(4):355–361. doi: 10.1089/lap.2011.0370. [DOI] [PubMed] [Google Scholar]
- 154.Noel P, Nedelcu M, Gagner M. SPIDER® sleeve gastrectomy–a new concept in single-trocar bariatric surgery: initial experience and technical details. J Visc Surg. 2014;151(2):91–96. doi: 10.1016/j.jviscsurg.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 155.Ruscio S, Abdelgawad M, Badiali D, et al. Simple versus reinforced cruroplasty in patients submitted to concomitant laparoscopic sleeve gastrectomy: prospective evaluation in a bariatric center of excellence. Surg Endosc. 2016;30(6):2374–2381. doi: 10.1007/s00464-015-4487-0. [DOI] [PubMed] [Google Scholar]
- 156.Saul D, Stephens D, Hofstätter Rde C, Ahmed L, Langhoff E, Heimann TM. Preliminary outcomes of laparoscopic sleeve gastrectomy in a Veterans Affairs medical center. Am J Surg. 2012;204(5):e1–e6. doi: 10.1016/j.amjsurg.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 157.Schraibman V, Macedo AL, Epstein MG, et al. Comparison of the morbidity, weight loss, and relative costs between robotic and laparoscopic sleeve gastrectomy for the treatment of obesity in Brazil. Obes Surg. 2014;24(9):1420–1424. doi: 10.1007/s11695-014-1239-5. [DOI] [PubMed] [Google Scholar]
- 158.Toro JP, Patel AD, Lytle NW, et al. Observed variability in sleeve gastrectomy volume and compliance does not correlate to postoperative outcomes. Surg Laparosc Endosc Percutan Tech. 2015;25(4):324–330. doi: 10.1097/SLE.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 159.Uffort E, Nease B, Canterbury T. Laparoscopic sleeve gastrectomy with comparable weight loss in all obese groups: a VA hospital experience. Am Surg. 2013;79(7):739–742. [PubMed] [Google Scholar]
- 160.Vidal P, Ramón JM, Goday A, et al. Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy as a definitive surgical procedure for morbid obesity. Mid-term results. Obes Surg. 2013;23(3):292–299. doi: 10.1007/s11695-012-0828-4. [DOI] [PubMed] [Google Scholar]
- 161.Yaghoubian A, Tolan A, Stabile B, et al. Laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy achieve comparable weight loss at 1 year. Am Surg. 2012;78(12):1325–1328. [PubMed] [Google Scholar]
- 162.Young JA, Alijani A, Patil PV, Shimi SM. Safety and feasibility of retrieval of intragastric balloon followed by antiobesity surgery on the same day. Surg Laparosc Endosc Percutan Tech. 2014;24(4):e137–e139. doi: 10.1097/SLE.0b013e3182901544. [DOI] [PubMed] [Google Scholar]
- 163.Chaar ME, Lundberg P, Stoltzfus J. Thirty-day outcomes of sleeve gastrectomy versus Roux-en-Y gastric bypass: first report based on metabolic and bariatric surgery accreditation and quality improvement program database. Surg Obes Relat Dis. 2018;14(5):545–551. doi: 10.1016/j.soard.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 164.Inaba CS, Koh CY, Sujatha-Bhaskar S, et al. One-year mortality after contemporary laparoscopic bariatric surgery: an analysis of the bariatric outcomes longitudinal database. J Am Coll Surg. 2018;226(6):1166–1174. doi: 10.1016/j.jamcollsurg.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Peterli R, Wölnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319(3):255–265. doi: 10.1001/jama.2017.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. 2018;319(3):241–254. doi: 10.1001/jama.2017.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Alizadeh RF, Li S, Inaba C, et al. Risk factors for gastrointestinal leak after bariatric surgery: MBSAQIP Analysis. J Am Cull Surg. 2018;227(1):135–141. doi: 10.1016/j.jamcollsurg.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 168.Kumar SB, Hamilton BC, Wood SG, Rogers SJ, Carter JT, Lin MY. Is laparoscopic sleeve gastrectomy safer than laparoscopic gastric bypass? a comparison of 30-day complications using the MBSAQIP data registry. Surg Obes Relat Dis. 2018;14(3):264–269. doi: 10.1016/j.soard.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 169.Birkmeyer JD, Finks JF, O’Reilly A, et al. Surgical skill and complication rates after bariatric surgery. N Engl J Med. 2013;369(15):1434–1442. doi: 10.1056/NEJMsa1300625. [DOI] [PubMed] [Google Scholar]
- 170.Hany M, Ibrahim M. Comparison between stable line reinforcement by barbed suture and non-reinforcement in sleeve gastrectomy: a randomized prospective controlled study. Obes Surg. 2018;28:2157–2164. doi: 10.1007/s11695-018-3175-2. [DOI] [PubMed] [Google Scholar]
- 171.Durmush EK, Ermerak G, Durmush D. Short-term outcomes of sleeve gastrectomy for morbid obesity: does staple line reinforcement matter? Obes Surg. 2014;24(7):1109–1116. doi: 10.1007/s11695-014-1251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nedelcu M, Manos T, Gagner M, Eddbali I, Ahmed A, Noel P. Cost analysis of leak after sleeve gastrectomy. Surg Endosc. 2017;31(11):4446–4450. doi: 10.1007/s00464-017-5495-z. [DOI] [PubMed] [Google Scholar]
- 173.Baker RS, Foote J, Kemmeter P, Brady R, Vroegop T, Serveld M. The science of stapling and leaks. Obes Surg. 2004;14(10):1290–1298. doi: 10.1381/0960892042583888. [DOI] [PubMed] [Google Scholar]
- 174.Elariny H, Gonzalez H, Wang B. Tissue thickness of human stomach measured on excised gastric specimens from obese patients. Surg Technol Int. 2005;14:119–124. [PubMed] [Google Scholar]
- 175.Nguyen NT, Longoria M, Welbourne S, Sabio A, Wilson SE. Glycolide copolymer staple-line reinforcement reduces staple site bleeding during laparoscopic gastric bypass: a prospective randomized trial. Arch Surg. 2005;140(8):773–778. doi: 10.1001/archsurg.140.8.773. [DOI] [PubMed] [Google Scholar]
- 176.Rawlins L, Rawlins M, Teel D. Human tissue thickness measurements from excised sleeve gastrectomy specimens. Surg Endosc. 2014;28(3):811–814. doi: 10.1007/s00464-013-3264-1. [DOI] [PubMed] [Google Scholar]
- 177.van Rutte PW, Naagen BJ, Spek M, Jakimowicz JJ, Nienhuijs SW. Gastric wall thickness in sleeve gastrectomy patients: thickness variation of the gastric wall. Surg Technol Int. 2015;27:123–128. [PubMed] [Google Scholar]
- 178.Huang R, Gagner M. A thickness calibration device is needed to determine staple height and avoid leaks in laparoscopic sleeve gastrectomy. Obes Surg. 2015;25:2360–2367. doi: 10.1007/s11695-015-1705-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Barski K, Binda A, Kudlicka E, Jaworski P, Tarnowski W. Gastric wall thickness and stapling in laparoscopic sleeve gastrectomy—a literature review. Wideochir Inne Tech Maloinwazyjne. 2018;13(1):122–127. doi: 10.5114/wiitm.2018.73362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.