Abstract
Background
The open-label, randomized, active-controlled KEYNOTE-045 study (NCT02256436) showed that second-line pembrolizumab significantly improved overall survival (OS) of patients with advanced/metastatic urothelial cancer (UC) that progressed after first-line platinum-containing chemotherapy, compared with standard chemotherapy (paclitaxel, docetaxel, or vinflunine). Pembrolizumab is approved for patients with bladder cancer in Japan.
Patients and methods
Analysis was performed in the subgroup of Japanese patients enrolled in the KEYNOTE-045 study. Coprimary end points were OS and progression-free survival (PFS). Objective response rate (ORR) and safety were secondary end points.
Results
Fifty-two Japanese patients (pembrolizumab, n = 30; chemotherapy, n = 22) were followed up for a median of 26.1 months. Patients who received pembrolizumab compared with chemotherapy had a 19% lower risk for death (hazard ratio [HR] 0.81, 95% CI 0.44–1.50); after adjusting for baseline covariates, the HR for OS was 0.61 (95% CI 0.32–1.15). The 24-month OS rate was higher with pembrolizumab (26.9% vs 14.3%). PFS was 2.0 and 4.9 months for pembrolizumab and chemotherapy, respectively (HR 1.71, 95% CI 0.95–3.08). ORR was similar for pembrolizumab and chemotherapy (20.0% vs 18.2%); durability of response was higher with pembrolizumab: 67% and 33% of patients, respectively, maintained a response for > 12 months. Treatment-related adverse events, including grade 3–5 events, occurred less frequently with pembrolizumab.
Conclusions
Pembrolizumab provided durable antitumor activity in patients with locally advanced/metastatic UC that progressed after platinum-containing chemotherapy in the overall population and in the Japanese subgroup; safety profile was consistent with that previously observed for pembrolizumab.
Electronic supplementary material
The online version of this article (10.1007/s10147-019-01545-4) contains supplementary material, which is available to authorized users.
Keywords: Pembrolizumab, Advanced urothelial cancer, Japanese, KEYNOTE-045, Immune checkpoint blockade
Introduction
Urothelial cancers (UCs) include a variety of tumors from the urinary tract, renal pelvis, ureter, and urethra, but > 90% of all UCs originate in the bladder [1]. Bladder cancer is the tenth most common cancer worldwide, accounting for approximately 550,000 new cancer diagnoses annually, and the sixth most common cancer in men [2]. In Japan, bladder cancer is the 13th most common cancer overall, and the eighth most common cancer in men [3]. Although the incidence of bladder cancer is lower in Asia than in Western countries [2], its incidence in Japan (5.6 per 100,000) is among the highest in Asia [3, 4]. This might be because smoking is a major risk factor for bladder cancer; however, the prevalence of smoking among Japanese men decreased from 84% in 1965 to 30% in 2014 [5]. It was expected that more than 20,000 cases would be diagnosed in 2018, with approximately three-quarters of those cases in men [6]. The mortality rate for Japanese patients is 25%, and 5-year survival rates for patients diagnosed with bladder cancer are 76.5% in men and 64.4% in women [4, 7].
Development of new antitumor agents for metastatic bladder cancer is an urgent priority in Japan [7]. In 2009, the Japanese Urological Association issued guidelines for first-line treatment of bladder cancer, recommending methotrexate, vinblastine, doxorubicin, and cisplatin or gemcitabine plus cisplatin as systemic chemotherapy for metastatic bladder cancer, because these compounds have showed comparable efficacy in clinical trials [8]. However, the gemcitabine plus cisplatin regimen is preferred because of a more favorable tolerability profile [8]. No recommendation has been made regarding second-line treatment or treatment of patients ineligible for platinum-based therapy, and a variety of combination and single-agent treatments are used in the management of UC in clinical practice in Japan [8–10].
Bladder cancer is associated with a high mutation burden and is highly antigenic, which has been correlated with higher immunotherapy response rates in UC and in other cancers, such as melanoma and lung cancer [11]. The potential of immunotherapy in bladder cancer treatment has been known for approximately 30 years, since the efficacy of using intravesical bacillus Calmette–Guérin to provoke an immune response to treat certain types of bladder cancer was identified [11]. Programmed death ligand 1 (PD-L1) expression on tumor cells can suppress immune responses against tumors by binding to programmed death 1 (PD-1) receptors on T cells [11, 12]. Monoclonal antibodies against this pathway have been developed to promote antitumor immune responses in patients with advanced or metastatic cancers; results of phase 1/2 studies showed that monoclonal antibodies have promising efficacy and manageable tolerability in management of UC [13–17]. As a result, 3 anti–PD-L1 and 2 anti–PD-1 antibodies have been approved by the US Food and Drug Administration for the treatment of patients with platinum-refractory bladder cancer [11, 12].
The randomized phase 3 KEYNOTE-045 study is the only study that shows an overall survival (OS) benefit with pembrolizumab compared with chemotherapy. Use of pembrolizumab (vs chemotherapy) significantly improved OS and objective response rates (ORR) of patients with recurrent, advanced UC that progressed during or after platinum-based chemotherapy [18]. This outcome was consistently observed independent of PD-L1 expression, presence of liver metastases, or type of chemotherapy comparator, and it has been confirmed after more than 2 years of follow-up [18, 19]. Compared with chemotherapy, pembrolizumab had a better safety profile and was associated with improved quality-of-life outcomes [18–20].
Pembrolizumab is currently the only immune checkpoint inhibitor approved in Japan for treating platinum-refractory UC [12]. A subgroup analysis of the KEYNOTE-045 study was performed to compare pembrolizumab with chemotherapy in Japanese patients with progressive UC. The results of this subgroup analysis are presented herein.
Patients and methods
Study design and patient population
A full description of the KEYNOTE-045 study has been published [18, 19]. Patients included in this subgroup analysis were enrolled in 20 study centers in Japan and had been diagnosed with transitional cell–predominant UC of the bladder, renal pelvis, ureter, or urethra, and experienced progressive disease after first- or second-line platinum-based chemotherapy or recurrence < 12 months after perioperative platinum-based chemotherapy. Patients had an Eastern Cooperative Group performance status (ECOG PS) of 0, 1, or 2 and a tumor sample available for biomarker assessment.
This study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki, and the study protocol was approved by institutional review boards or ethics committees of all participating sites. All patients provided written informed consent to participate before enrollment, and the study was registered on ClinicalTrials.gov before enrollment of the first patient (ClinicalTrials.gov, NCT02256436).
Treatment and assessments
Patients were randomly assigned 1:1 to receive either intravenous (IV) pembrolizumab 200 mg every 3 weeks (Q3W) or investigator’s choice of paclitaxel (175 mg/m2 IV Q3W), docetaxel (75 mg/m2 IV Q3W), or vinflunine (320 mg/m2 IV Q3W). However, vinflunine was not an approved second-line treatment for bladder cancer in Japan at the time of this study; therefore, none of the patients in Japan received that treatment. Patients were stratified by ECOG PS score (0/1 vs 2), presence of liver metastases (yes vs no), hemoglobin concentration (< 10 g/dL vs ≥ 10 g/dL), and time since the last dose of chemotherapy (< 3 vs ≥ 3 months) before randomization. Patients were treated until disease progression, unacceptable toxicity, patient or investigator decision to discontinue treatment, or completion of 2 years of pembrolizumab treatment, whichever occurred first.
Tumor response was assessed by blinded, independent, central radiologic review per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 [21]. The full assessment schedule is provided elsewhere [18]. Adverse events (AEs) were assessed and graded per National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 [22]. All treatment-emergent AEs were evaluated for potential immunologic etiology.
Patients were followed up for at least 30 days after the last dose of study treatment or until initiation of a new line of treatment. Radiologic imaging for disease status continued per study post-treatment follow-up schedule for patients who discontinued therapy without documented disease progression.
Study end points
Study end points have been previously published [18, 19]. Coprimary end points included OS and progression-free survival (PFS). Additional coprimary end points assessed OS and PFS for patients with PD-L1–positive tumors (combined positive score [CPS] ≥ 10). Key secondary end points included ORR, duration of response (DOR), and safety and tolerability. Prespecified health-related quality-of-life (HRQOL) analysis was conducted using end points of time to traditional deterioration (TTD), defined as time to first onset of a ≥ 10-point decrease from baseline without confirmation under right-censoring rule (the last observation) and change from baseline in the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) global health status/QOL score at week 15.
Statistical analyses
Primary efficacy end points and safety end points were analyzed using data from the intention-to-treat (ITT) population and all-patients-as-treated population (all randomly assigned patients who received at least 1 dose of study treatment), respectively. Median OS and PFS were estimated using the Kaplan–Meier method. Multivariate Cox proportional hazards models were used to adjust the hazard ratio (HR) for OS, taking into consideration the following baseline covariates: ECOG PS (0 vs 1/2), visceral metastases (presence vs absence), hemoglobin level (≥ 10 g/dL vs < 10 g/dL), and time from completion of most recent chemotherapy (< 3 months vs ≥ 3 months). Median DOR was summarized descriptively using Kaplan–Meier analysis of the subset of patients who experience complete response or partial response. Additional details of the statistical analyses have been described previously [18, 19]. The stratified Cox model was used and the log-rank test was performed in the overall population; however, corresponding unstratified analyses were performed in the Japanese population because of the small sample size.
Change in baseline EORTC QLQ-C30 score at week 15 was assessed using pairwise comparisons. HRs for TTD were calculated using a Cox regression model with treatment as a covariate.
Results
Patients
Enrolled Japanese patients (n = 52) were randomly assigned to receive either pembrolizumab (n = 30) or chemotherapy (n = 22; paclitaxel, n = 11; docetaxel, n = 11) (see Supplementary Fig. 1). Key differences in baseline characteristics between the pembrolizumab arm and the chemotherapy arm were the percentages of patients with lymph-node metastases only (17.7% vs 36.4%) or visceral metastases (83.3% vs 63.6%) at baseline (Table 1). Twelve patients had tumors with PD-L1 CPS ≥ 10 (pembrolizumab, n = 4; chemotherapy, n = 8). The low number of patients and the imbalance between the arms precluded the inclusion of any meaningful analysis based on PD-L1 expression.
Table 1.
Patient baseline characteristics
| Characteristic | Pembrolizumab N = 30 |
Chemotherapy N = 22 |
|---|---|---|
| Age (years), median (range) | 72 (51–83) | 70.5 (56–84) |
| Sex, n (%) | ||
| Male | 24 (80.0) | 16 (72.7) |
| Geographic location of disease, n (%) | ||
| Upper tract | 12 (40.0) | 5 (22.7) |
| Lower tract | 18 (60.0) | 17 (77.3) |
| ECOG PS, n (%) | ||
| 0 | 23 (76.7) | 14 (63.6) |
| 1 | 7 (23.3) | 7 (31.8) |
| 2 | 0 (0) | 1 (4.5) |
| Visceral disease, n (%) | 25 (83.3) | 14 (63.6) |
| Disease in lymph node only, n (%) | 5 (17.7) | 8 (36.4) |
| Liver metastases, n (%) | 7 (23.3) | 4 (18.2) |
| Hemoglobin < 10 g/dL,an (%) | 8 (26.7) | 4 (18.2) |
| Time since completion of most recent prior therapy, n (%) | ||
| ≥ 3 months | 17 (56.7) | 10 (45.5) |
| < 3 months | 13 (43.3) | 12 (54.5) |
| Setting of most recent prior therapy, n (%) | ||
| Neoadjuvant | 1 (3.3) | 2 (9.1) |
| Adjuvant | 1 (3.3) | 4 (18.2) |
| First line | 24 (80.0) | 12 (54.5) |
| Second line | 4 (13.3) | 4 (18.2) |
| Prior platinum-based therapy, n (%) | ||
| Cisplatin | 24 (80.0) | 18 (81.8) |
| Carboplatin | 6 (20.0) | 2 (9.1) |
| Otherb | 0 (0) | 2 (9.1) |
| Smoking status, n (%) | ||
| Never | 13 (43.3) | 8 (36.4) |
| Former | 15 (50.0) | 12 (54.5) |
| Current | 2 (6.7) | 2 (9.1) |
| PD-L1 CPS ≥ 10, n (%) | 4 (13.3) | 8 (36.4) |
| Risk factors,cn (%) | ||
| 0 | 8 (26.7) | 4 (18.2) |
| 1 | 13 (43.3) | 10 (45.5) |
| 2 | 5 (16.7) | 6 (27.3) |
| 3 or 4 | 4 (13.3) | 2 (9.1) |
| EORTC QLQ-C30 global health status/QOL score | 57.8 (25.4) | 54.9 (27.4) |
CPS combined positive score, ECOG PS Eastern Cooperative Oncology Group performance status, EORTC QLQ-C30 European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30, PD-L1 programmed death ligand-1, QOL quality of life
aLatest hemoglobin test value before or on randomization date
bOxaliplatin, nedaplatin
cBellmunt risk factors of ECOG PS > 0, hemoglobin level < 10 g/dL, and liver metastases [31] + time from prior chemotherapy < 3 months [32]
Median follow-up was 26.1 months (range 0.9–29.5 months) at data cutoff on October 26, 2017. At the time of analysis, all patients administered chemotherapy and 28 (93.3%) patients administered pembrolizumab had discontinued treatment. Two patients had completed the maximum 2 years of pembrolizumab treatment. Median (range) duration of treatment was 3.04 months (1 day–23.92 months) for pembrolizumab and 1.76 months (1 day–24.44 months) for chemotherapy.
Efficacy
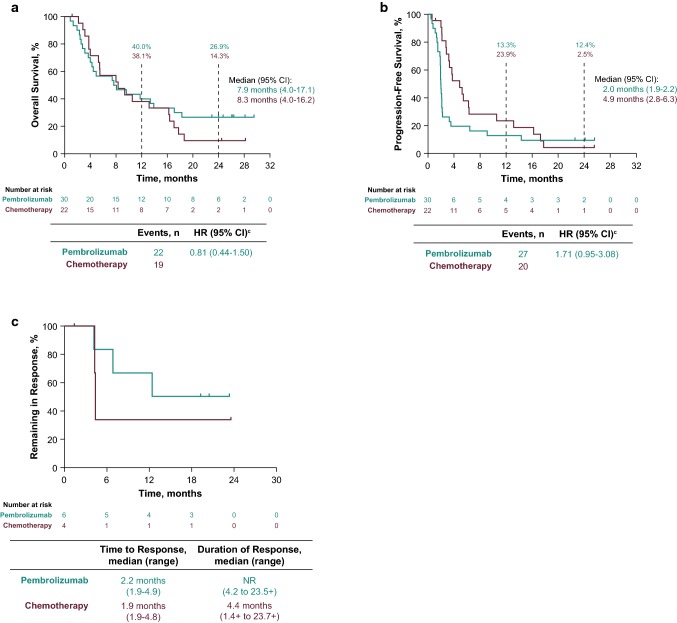
Median OS was 7.9 months (95% CI 4.0–17.1) with pembrolizumab and 8.3 months (95% CI 4.0–16.2) with chemotherapy (HR 0.81, 95% CI 0.44–1.50, Fig. 1a); patients who received pembrolizumab had a 19% lower risk for death than patients who received chemotherapy. Results of multivariate analysis showed that the HR for OS was 0.61 (95% CI 0.32–1.15) after adjusting for additional covariates. OS at 6 and 12 months was comparable between patient groups; 24-month OS rates were higher in the pembrolizumab group (26.9%) than in the chemotherapy group (14.3%). Median PFS was longer in the chemotherapy arm than in the pembrolizumab arm (4.9 months [95% CI 2.8–6.3] and 2.0 months [95% CI 1.9–2.2], respectively; HR 1.71, 95% CI 0.95–3.08, Fig. 1b).
Fig. 1.
Kaplan–Meier estimates of a OS, b PFS, and c DORa in Japanese patients (ITT population) with advanced or metastatic urothelial carcinoma treated with pembrolizumab (n = 30) or chemotherapy (n = 22). aDOR was assessed in patients who achieved an objective response of CR or PR. CI confidence interval, CR complete response, DOR duration of response, HR hazard ratio, ITT intention-to-treat, NR not reached, OS overall survival, PFS progression-free survival, PR partial response
ORRs for pembrolizumab and chemotherapy were similar (20.0% vs 18.2%; Table 2). The disease control rate was 26.7% (stable disease, 6.7%) in the pembrolizumab arm and 63.6% (stable disease, 45.5%) in the chemotherapy arm. At data cutoff, median DOR had not been reached (range 4.2 to 23.5+ months) for responders in the pembrolizumab arm and was 4.4 months (range 1.4+ to 23.7+ months) for responders in the chemotherapy arm (Fig. 1c). Responses were maintained for at least 12 months by 67% of responders to pembrolizumab therapy and by 33% of responders to chemotherapy.
Table 2.
Best overall response (ITT population)
| Best overall response | Pembrolizumab N = 30 |
Chemotherapy N = 22 |
||
|---|---|---|---|---|
| n | % (95% CI)a | n | % (95% CI)a | |
| Objective response rate (CR + PR) | 6 | 20.0 (7.7–38.6) | 4 | 18.2 (5.2–40.3) |
| CR | 3 | 10.0 (2.1–26.5) | 1 | 4.5 (0.1–22.8) |
| PR | 3 | 10.0 (2.1–26.5) | 3 | 13.6 (2.9–34.9) |
| SD | 2 | 6.7 (0.8–22.1) | 10 | 45.5 (24.4–67.8) |
| Disease control rate (CR + PR + SD) | 8 | 26.7 (12.3–45.9) | 14 | 63.6 (40.7–82.8) |
| Progressive disease | 20 | 66.7 (47.2–82.7) | 3 | 13.6 (2.9–34.9) |
| No assessment | 0 | 6.7 (0.8–22.1) | 3 | 9.1 (1.1–29.2) |
| Nonevaluable | 2 | 0.0 (0.0–11.6) | 2 | 13.6 (2.9–34.9) |
CI confidence interval, CR complete response, ITT intention-to-treat, PR partial response, SD stable disease
aBased on binomial exact CI method
Safety
Treatment-related AEs (TRAEs) of any grade and of grade 3–5 were reported in 56.7% and 16.7%, respectively, of patients in the pembrolizumab arm and in 95.5% and 77.3%, respectively, of patients in the chemotherapy arm. Three patients in the pembrolizumab arm (interstitial lung disease [n = 2]; pneumonitis [n = 1]) and 2 patients in the chemotherapy arm (interstitial lung disease [n = 1]; cardiac failure [n = 1]) discontinued treatment because of TRAEs.
TRAEs that occurred in > 15% of patients in the chemotherapy arm were alopecia, decreased white blood cell count, decreased appetite, decreased neutrophil count, nausea, peripheral sensory neuropathy, anemia, neutropenia, fatigue, malaise, stomatitis, diarrhea, and dysgeusia (Table 3). The most common TRAEs in the pembrolizumab arm were pruritus (n = 6 [20.0%] patients), hypothyroidism (n = 4 [13.3%]), rash (n = 4 [13.3%]), nausea (n = 3 [10.0%]), and malaise (n = 3 [10.0%]).
Table 3.
TRAEs of any grade and grade 3 or 4 occurring in > 5% of patients in either treatment group (APaT population)
| TRAEs, n (%) | Pembrolizumab N = 30 |
Chemotherapy N = 22 |
||
|---|---|---|---|---|
| Any grade | Grade 3–5 | Any grade | Grade 3–5 | |
| Any | 17 (56.7) | 5 (16.7) | 21 (95.5) | 17 (77.3) |
| Alopecia | – | – | 12 (54.5) | – |
| Decreased white blood cell count | – | – | 9 (40.9) | 8 (36.4) |
| Decreased appetite | 1 (3.3) | 9 (40.9) | 1 (4.5) | |
| Decreased neutrophil count | – | – | 8 (36.4) | 7 (31.8) |
| Nausea | 3 (10.0) | – | 8 (36.4) | – |
| Peripheral sensory neuropathy | – | – | 8 (36.4) | – |
| Anemia | – | – | 7 (31.8) | 4 (18.2) |
| Neutropenia | – | – | 7 (31.8) | 6 (27.3) |
| Fatigue | 2 (6.7) | – | 8 (36.4) | 2 (9.1) |
| Malaise | 3 (10.0) | – | 5 (22.7) | – |
| Pruritus | 6 (20.0) | – | 2 (9.1) | – |
| Stomatitis | 1 (3.3) | 1 (3.3) | 4 (18.2) | – |
| Diarrhea | 1 (3.3) | – | 4 (18.2) | – |
| Dysgeusia | – | – | 4 (18.2) | – |
| Decreased lymphocyte count | 1 (3.3) | 1 (3.3) | 3 (13.6) | 3 (13.6) |
| Hiccups | – | – | 3 (13.6) | – |
| Rash | 4 (13.3) | 1 (3.3) | 1 (4.5) | – |
| Hypothyroidism | 4 (13.3) | – | – | – |
| Upper abdominal pain | 1 (3.3) | – | 2 (9.1) | – |
| Febrile neutropenia | – | – | 2 (9.1) | 2 (9.1) |
| Hyperkalemia | – | – | 2 (9.1) | 2 (9.1) |
| Hyponatremia | – | – | 2 (9.1) | 2 (9.1) |
| Leukopenia | – | – | 2 (9.1) | 2 (9.1) |
| Nail discoloration | – | – | 2 (9.1) | – |
| Constipation | – | – | 2 (9.1) | – |
| Peripheral edema | – | – | 2 (9.1) | – |
| Interstitial lung disease | 2 (6.7) | 1 (3.3) | 1 (4.5) | – |
| Hyperthyroidism | 2 (6.7) | – | – | – |
| Pyrexia | 2 (6.7) | – | 1 (4.5) | – |
APaT all patients as treated, TRAE treatment-related adverse event
Seven treatment-related serious AEs (SAEs) were reported among 5 (16.7%) patients administered pembrolizumab and 2 events were reported in 2 (9.1%) patients administered chemotherapy. No treatment-related SAEs were reported by more than 1 patient, except for interstitial lung disease in 2 patients in the pembrolizumab arm.
12 immune-mediated AEs were reported in 9 (30.0%) patients administered pembrolizumab; the most common were hypothyroidism (n = 4), hyperthyroidism (n = 2), and interstitial lung disease (n = 2). One patient with grade 1 or 2 and 4 patients with grade 3–5 immune-mediated AEs in the pembrolizumab arm received systemic corticosteroids. Single events were also reported for colitis, pneumonitis, rash, and type 1 diabetes. One immune-mediated AE of interstitial lung disease was reported in the chemotherapy arm. No deaths from TRAEs were reported.
HRQOL
No significant difference in EORTC QLQ-C30 scores was observed at week 15 of treatment between the pembrolizumab (n = 18) and chemotherapy arms (n = 10; least-squares mean change from baseline: + 2.14 vs – 0.66 points). However, a trend toward a delay in TTD was observed for patients randomly assigned to receive pembrolizumab versus chemotherapy (20 vs 15 events; HR, 0.58, 95% CI, 0.29–1.16; Fig. 2).
Fig. 2.
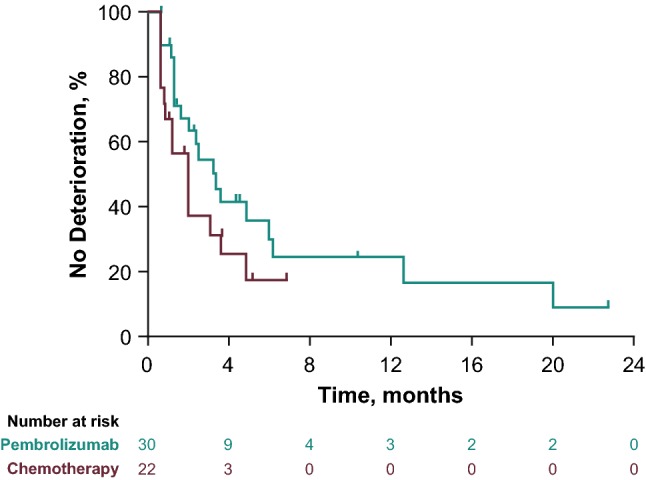
TTD in HRQoL in Japanese patients (ITT population) with advanced or metastatic urothelial carcinoma treated with pembrolizumab (n = 30) or chemotherapy (n = 22). HRQoL health-related quality of life, ITT intent-to-treat, TTD time to traditional deterioration
Discussion
There is an unmet need for a safe and effective second-line treatment recommendation for Japanese patients with platinum-refractory advanced UC. Current second-line chemotherapy options include single agents docetaxel and paclitaxel; both have been associated with modest activity and hematologic AEs [23]. The current subgroup analysis of KEYNOTE-045 is the first investigation of the safety and efficacy of pembrolizumab in Japanese patients with locally advanced/metastatic UC that progressed after platinum-based chemotherapy compared with standard chemotherapy.
Overall results of the ITT population in the KEYNOTE-045 study showed that pembrolizumab resulted in significantly longer OS—by approximately 3 months—than investigator’s choice of docetaxel or vinflunine (10.1 vs 7.3 months; HR 0.70, 95% CI 0.57–0.85, p < 0.001) [18, 19]. The benefit of pembrolizumab compared with chemotherapy was observed in the total population and among patients with tumors that expressed PD-L1 CPS ≥ 10 (HR 0.55, 95% CI 0.37–0.81, p = 0.001) [19]. ORR was significantly higher in the pembrolizumab arm than in the chemotherapy arm (21.1 vs 11.0%) [18, 19]. In the overall population, pembrolizumab also had a better safety profile than chemotherapy.
The current analysis was conducted to assess the safety and efficacy of pembrolizumab and chemotherapy among Japanese patients enrolled in the KEYNOTE-045 study. Even with a higher percentage of Japanese patients with visceral disease in the pembrolizumab arm than in the control arm (83.3% vs 63.6%) at baseline, results showed that the risk for death was reduced by 19% in the pembrolizumab arm (HR 0.81). After adjusting for baseline covariates in the multivariate Cox model, the HR for OS improved to 0.61. With additional follow-up, there was a trend toward more favorable median OS in the pembrolizumab arm than in the chemotherapy arm (data not shown), and 24-month OS rates were higher with pembrolizumab (26.9%) than with chemotherapy (14.3%). In the Japanese subgroup, the proportions of patients with objective responses were similar between the pembrolizumab and chemotherapy arms but trended toward a more favorable response with pembrolizumab. At the time of analysis, 67% of patients in the pembrolizumab arm and 33% of patients in the chemotherapy arm maintained a response for > 12 months. Because the presence of visceral disease is a negative prognostic factor for UC, greater proportions of patients with visceral disease in the pembrolizumab may contribute to the longer PFS observed in the chemotherapy arm.
A lack of improvement in PFS with simultaneous improvement in OS was also shown in the ITT population of KEYNOTE-045, which remains the only randomized trial of UC to show a survival benefit of immunotherapy compared with chemotherapy. This pattern of response, OS improvement but not PFS improvement, has also been reported in randomized trials with checkpoint inhibitors in other tumor types, continuing to suggest that PFS may not be a reliable surrogate end point for the clinical benefit of immunotherapy [24–27].
In the current analysis, pembrolizumab was generally well tolerated in Japanese patients and had a better safety profile than chemotherapy. In the KEYNOTE-045 primary analysis population and in the Japanese subgroup, a lower percentage of patients who received pembrolizumab, compared with chemotherapy, experienced grade 3–5 TRAEs (15.0% vs 49.4% in the overall population and 16.7% vs 77.3% in the Japanese subgroup). Consistent with the known safety profile of pembrolizumab, immune-mediated AEs occurred more frequently with pembrolizumab than with chemotherapy. No deaths were attributed to TRAEs.
Pembrolizumab may also result in improved QOL compared with chemotherapy. There was a trend toward a delay in TTD for Japanese patients who received pembrolizumab compared with chemotherapy. This outcome is supported by results of the HRQOL analysis of the overall population enrolled in the KEYNOTE-045 study, which showed that patients who received pembrolizumab had significantly longer TTD than patients who received chemotherapy (p = 0.004) [20].
According to the National Comprehensive Cancer Network, pembrolizumab is the preferred second-line therapy for patients with advanced/metastatic bladder cancer and may also be recommended as first-line therapy for patients whose tumors express PD-L1 CPS ≥ 10 or who are ineligible to receive platinum-based therapy [23]. Pembrolizumab is approved for second-line treatment of patients with advanced/metastatic UC in Japan [12]; however, there are no clear guidelines on choosing second-line therapy for these patients. In clinical practice, a variety of combination and single-agent therapies are prescribed [9, 10, 28]. One small study was conducted to evaluate second-line combinations of gemcitabine and taxanes in Japanese patients with metastatic UC that progressed after platinum-based chemotherapy (N = 78) [28]. Among 70 evaluable patients treated with gemcitabine combined with either docetaxel or paclitaxel, ORR was 8.6% and median PFS was 3.5 months. Median OS was 9.6 months, with no significant difference in survival between the regimens. The most commonly reported grade ≥ 3 hematologic AEs in the gemcitabine plus paclitaxel arm and the gemcitabine plus docetaxel arm were neutropenia (80.5% vs 59.5%), anemia (43.9% vs 10.8%), and thrombocytopenia (43.9% vs 29.7%). Although not recommended in international guidelines, some triplet regimens, including paclitaxel, ifosfamide, and nedaplatin [9, 10] or paclitaxel, cisplatin, and gemcitabine [29], are also used as second-line treatment regimens in real-world studies of bladder cancer in Japan. Both triplet regimens have been associated with development of grade 3–4 neutropenia [29, 30]. These studies highlight the need for more tolerable second-line therapies to treat patients with advanced UC.
The small sample size of the subgroup of Japanese patients in the KEYNOTE-045 study limits the comparison of the Japanese population with the overall study population and interpretation of the analyses presented herein. The study was not powered for formal efficacy comparisons in the Japanese subpopulation. Another limitation is that single-agent comparators, including vinflunine (not standard of care in Japan), may not be fully representative of second-line treatment options used in clinical practice in Japan. Regardless, the current analysis offers valuable information about the efficacy and safety of pembrolizumab in Japanese patients. Outcomes will be confirmed in the real-world clinical setting of patients treated in Japan.
In conclusion, the efficacy and safety profile of pembrolizumab as second-line treatment of advanced/metastatic UC in Japanese patients is consistent with the overall results of the international KEYNOTE-045 study. Pembrolizumab is an effective and well-tolerated treatment option that is recommended in international guidelines as the preferred second-line treatment for UC [23].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients and their families and all investigators and site personnel. The authors also thank Eric Rubin and Markus Puhlmann (Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA) and Satoko Miyata and Tomohiro Ubakai (employees of MSD K.K., Tokyo, Japan) for their contributions to the development of the manuscript. Medical writing and/or editorial assistance was provided by Cathy Winter, PhD, and Matthew Grzywacz, PhD, of the ApotheCom pembrolizumab team (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Author contributions
HN: acquisition of the data and interpretation of the results. YY: acquisition of the data and interpretation of the results. NS: acquisition of the data and interpretation of the results. KN: acquisition of the data. KF: acquisition of the data. SF: acquisition of the data and interpretation of the results. MY: acquisition of the data. HE: acquisition of the data. KT: analysis of the data and interpretation of the results. YT: acquisition of the data and interpretation of the results. KI: analysis and acquisition of the data, interpretation of the data, and drafting of the manuscript. TS: acquisition of the data and interpretation of the results. RP: conception, design or planning of the study, interpretation of the results, and drafting of the manuscript. TF: acquisition of the data, interpretation of the results, and drafting of the manuscript. DB: conception, design or planning of the study, and interpretation of the results. JB: conception, design or planning of the study, analysis and acquisition of the data, interpretation of the results, and provision of study materials/patients. All authors reviewed the manuscript for important intellectual content and approved the final version of the manuscript for submission.
Data availability statement
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA’s, data sharing policy, including restrictions, is available at https://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
Compliance with ethical standards
Conflict of interest
HN: No conflicts to disclose. YY: No conflicts to disclose. NS: No conflicts to disclose. KN: Received lecture fees from Astellas and Novartis. KF: No conflicts to disclose. SF: Received research funding from Merck. MY: No conflicts to disclose. HE: No conflicts to disclose. KT: Employee of MSD K.K., Tokyo, Japan. Stockholder of Merck & Co., Inc., Kenilworth, NJ, USA. YT: Employee of MSD K.K., Tokyo, Japan. Stockholder of Merck & Co., Inc., Kenilworth, NJ, USA. KI: Employee of MSD K.K., Tokyo, Japan. Stockholder of Merck & Co., Inc., Kenilworth, NJ, USA. TS: Employee of MSD K.K., Tokyo, Japan. Stockholder of Merck & Co., Inc., Kenilworth, NJ, USA. RP: Employee and stockholder of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. TF: Employee and stockholder of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. DB: Served in an advisory role for Pfizer, AstraZeneca, Bristol Myers Squibb, Genentech, Fidia Farmaceutici, Roche, Merck, Eli Lilly, received fees for promotional materials from Merck, received research funding from Merck and other (eg trips, travel or gifts) from Eli-Lilly. JB: Served in an advisory role for Genentech, Merck, AstraZeneca, and Bristol-Myers Squibb, patent royalties from UpToDate, honoraria from Merck and Bristol-Myers Squibb, research funding from Pfizer, and MSD Serono, and other (trips, travel or gifts) from Pfizer and Ipsen.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Clark PE, Agarwal N, Biagioli MC, et al. Bladder cancer. J Natl Compr Canc Netw. 2013;11(4):446–475. doi: 10.6004/jnccn.2013.0059. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Matsuda T, Okuyama A. Incidence rate for bladder cancer in Japanese in Japan and in the United States from the Cancer Incidence in Five Continents. Jpn J Clin Oncol. 2017;47(3):284–285. doi: 10.1093/jjco/hyx017. [DOI] [PubMed] [Google Scholar]
- 4.Nishiyama H. Asia consensus statement on NCCN clinical practice guideline for bladder cancer. Jpn J Clin Oncol. 2018;48(1):3–6. doi: 10.1093/jjco/hyx130. [DOI] [PubMed] [Google Scholar]
- 5.Nagao M, Tsugane S. Cancer in Japan: prevalence, prevention and the role of heterocyclic amines in human carcinogenesis. Genes Environ. 2016;38:16. doi: 10.1186/s41021-016-0043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Information Service (2018) Projected cancer statistics 2018. Tokyo, Japan: Center for Cancer Control and Information Services, National Cancer Center. https://ganjoho.jp/en/public/statistics/short_pred.html. Accessed 19 Aug 2019
- 7.Akaza H. Urologic cancer in Japan: role of Japan at the frontier of issues in Asia. Jpn J Clin Oncol. 2016;46(1):23–30. doi: 10.1093/jjco/hyv123. [DOI] [PubMed] [Google Scholar]
- 8.Kubota Y, Nakaigawa N, et al. Essential content of evidence-based clinical practice guidelines for bladder cancer: the Japanese Urological Association 2015 update. Int J Urol. 2016;23(8):640–645. doi: 10.1111/iju.13141. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto R, Abe T, Ishizaki J, et al. Outcome and prognostic factors in metastatic urothelial carcinoma patients receiving second-line chemotherapy: an analysis of real-world clinical practice data in Japan. Jpn J Clin Oncol. 2018;48(8):771–776. doi: 10.1093/jjco/hyy094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abe T, Ishizaki J, Kikuchi H, et al. Outcome of metastatic urothelial carcinoma treated by systemic chemotherapy: prognostic factors based on real-world clinical practice in Japan. Urol Oncol. 2017;35(2):38–38. doi: 10.1016/j.urolonc.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Stenehjem DD, Tran D, Nkrumah MA, et al. PD1/PDL1 inhibitors for the treatment of advanced urothelial bladder cancer. OncoTargets Ther. 2018;11:5973–5989. doi: 10.2147/OTT.S135157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuasa T, Urakami S, Yonese J. Recent advances in medical therapy for metastatic urothelial cancer. Int J Clin Oncol. 2018;23(4):599–607. doi: 10.1007/s10147-018-1260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–322. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 14.Powles T, O'Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(9):e172411. doi: 10.1001/jamaoncol.2017.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol. 2017;35(19):2117–2124. doi: 10.1200/JCO.2016.71.6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plimack ER, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol. 2017;18(2):212–220. doi: 10.1016/S1470-2045(17)30007-4. [DOI] [PubMed] [Google Scholar]
- 18.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fradet Y, Bellmunt J, Vaughn DJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of > 2 years of follow-up. Ann Oncol. 2019;30(6):970–976. doi: 10.1093/annonc/mdz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaughn DJ, Bellmunt J, Fradet Y, et al. Health-related quality-of-life analysis from KEYNOTE-045: a phase III study of pembrolizumab versus chemotherapy for previously treated advanced urothelial cancer. J Clin Oncol. 2018;36(16):1579–1587. doi: 10.1200/JCO.2017.76.9562. [DOI] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Can. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute (2010) Common terminology criteria for adverse events (CTCAE) version 4. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 19 Aug 2019
- 23.National Comprehensive Cancer Network (2018) NCCN clinical practice guidelines in oncology: bladder cancer (version 5.2018). https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed 19 Aug 2019
- 24.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 27.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeyama Y, Kato M, Nishihara C, et al. Comparison of efficacy and toxicity of second-line combination chemotherapy regimens in patients with advanced urothelial carcinoma. Int J Clin Oncol. 2018;23(5):944–950. doi: 10.1007/s10147-018-1288-1. [DOI] [PubMed] [Google Scholar]
- 29.Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol. 2012;30(10):1107–1113. doi: 10.1200/JCO.2011.38.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi K, Matsuyama H, Shimizu K, et al. Clinical significance of a second-line chemotherapy regimen with paclitaxel, ifosfamide and nedaplatin for metastatic urothelial carcinoma after failure of cisplatin-based chemotherapy. Jpn J Clin Oncol. 2016;46(8):775–780. doi: 10.1093/jjco/hyw071. [DOI] [PubMed] [Google Scholar]
- 31.Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28(11):1850–1855. doi: 10.1200/JCO.2009.25.4599. [DOI] [PubMed] [Google Scholar]
- 32.Sonpavde G, Pond GR, Fougeray R, et al. Time from prior chemotherapy enhances prognostic risk grouping in the second-line setting of advanced urothelial carcinoma: a retrospective analysis of pooled, prospective phase 2 trials. Eur Urol. 2013;63(4):717–723. doi: 10.1016/j.eururo.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA’s, data sharing policy, including restrictions, is available at https://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.