Abstract
Patients with chronic kidney disease (CKD) are more likely to experience falls and fractures due to renal osteodystrophy and the high prevalence of risk factors for falls. However, it is not well established how great the risk is for falls and fractures for the different stages of CKD compared to the general population. The objective of this systematic review and meta-analysis was to assess whether, and in which degree, CKD was associated with falls and fractures in adults. A systematic search in PubMed, Embase, CINAHL, and The Cochrane Library was performed on 7 September 2018. All retrospective, cross-sectional, and longitudinal studies of adults (18 years of older) that studied the association between CKD, fractures, and falls were included. Additional studies were identified by cross-referencing. A total of 39 publications were included, of which two publications assessed three types of outcome and four publications assessed two types of outcome. Ten studies focused on accidental falling; seventeen studies focused on hip, femur, and pelvis fractures; seven studies focused on vertebral fractures; and thirteen studies focused on any type of fracture without further specification. Generally, the risk of fractures increased when kidney function worsened, with the highest risks in the patients with stage 5 CKD or dialysis. This effect was most pronounced for hip fractures and any type of fractures. Furthermore, results on the association between CKD and accidental falling were contradictory. Compared to the general population, fractures are highly prevalent in patients with CKD. Besides more awareness of timely fracture risk assessment, there also should be more focus on fall prevention.
Electronic supplementary material
The online version of this article (10.1007/s00198-019-05190-5) contains supplementary material, which is available to authorized users.
Keywords: Accidental falls, Chronic kidney disease, Dialysis, Fracture
Introduction
Worldwide, chronic kidney disease (CKD) is highly prevalent, with an estimated prevalence of 7% in stages 3 to 5 and with even higher rates in the elderly population [1]. Patients with CKD are prone to fractures due to renal osteodystrophy. This is a complex disease which is caused by a disturbance in metabolic and hormone levels (e.g., altered levels of calcium, phosphorus, parathyroid hormone, and vitamin D) that impairs bone quality and is characterized by abnormal bone remodeling [2, 3]. These bone abnormalities are seen in a majority of patients with CKD stage 3–5 and in all patients requiring dialysis [4]. Therefore, it is likely that patients with mild to moderate CKD already have a higher risk of fractures and that risk of fracture increases when kidney function decreases. Fractures in patients with CKD are a serious complication and are associated with a high morbidity, mortality [5], and economic burden [6, 7].
An important risk factor for fractures are falls [8]. Falls are a result of a complex interaction of factors such as muscle weakness, neuropathy, polypharmacy, chronic illnesses, cognitive decline, impaired mobility, and frailty [9], of which are all highly prevalent in patients with CKD [10]. Therefore, it is likely that patients with CKD are also more prone to falls than patients without CKD. In addition to a high morbidity, mortality, and economic burden, falls can also lead to fear of falling, which can cause a decrease in physical activity and social isolation [11] and could thereby even further increase the risk of falling. Hence, although both falls and fractures seem to be important problems for patients with CKD, it is not well established how great the risk is for falls and fractures for the different stages of CKD compared to the general population.
More knowledge about the risk of falls and fractures could lead to better risk stratification, which could lead to better prevention strategies. Therefore, the objective of this systematic review is to assess whether, and in what degree, chronic kidney disease is associated with falls and fractures in adults.
Methods
Search strategy and selection criteria
We aimed to identify cross-sectional or cohort studies that investigated the association between chronic kidney disease, falls, and fractures, through a comprehensive search (from conception to September 7, 2018) of PubMed, Embase, CINAHL, and The Cochrane Library. We used the search terms chronic kidney disease (dialysis patients included), fracture, and falling, with relevant synonyms. The complete search strategy is shown in Appendix 1. No limits were applied in the search.
Two authors (NG, GW) independently screened title and abstract, removed duplicate publications, and selected studies that assessed the association of CKD and fractures or falling. Studies were also included if association estimates could be calculated from prevalence/incidence from a CKD population compared to a non-CKD population. Animal studies, studies in children, studies in very specific populations (e.g., only patients with systemic lupus erythematosus, aluminum related bone disease), case reports, systematic reviews, conference abstracts, opinion papers, and studies not published in English were excluded. Considering intervention studies could possibly influence the outcome of falls or fractures, only intervention studies in which the placebo group was assessed were included in the study. The publication retrieval was completed by cross-reference checking in Web of Science for selected articles; citations of retrieved reviews, meta-analysis, and guidelines were also screened for potentially omitted studies. A similar selection procedure as described above was followed to check for eligibility of articles that were thereby retrieved. Initial disagreements on eligibility and selection of articles were resolved by discussion and their inclusion is based on full consensus.
Data extraction
Data regarding study design and results were independently extracted by two investigators (NG and FO) for each eligible study. Items that were extracted are study design, patient selection, number of participants (dialysis, CKD), demographics (age, sex), method for estimated glomerular filtration rate (eGFR) calculation, as well as the outcomes in terms of association between CKD falling and fractures. If a study provided various measurements of eGFR, first choice was to extract data of the CKD-EPI based on serum creatinine. When this was not available, second choice was the MDRD based on serum creatinine, followed by the Cockcroft Gault (CG) (based on serum creatinine) and other measurements. Measurements of eGFR based on urinary creatinine were not included, as these made our studies less comparable. Furthermore, baseline characteristics were extracted for the whole population.
Quality assessment
The methodological quality of each of the studies was assessed independently by two reviewers (NG and FO), using the Newcastle-Ottawa quality assessment scale. This scale was adapted to create one scale for quality assessment of longitudinal studies, case-control studies, and cross-sectional studies (Appendix 2). Disagreements among the reviewers were discussed during a consensus meeting, and in case of persisting disagreement, the assistance of a third reviewer (MH) was enlisted.
Data synthesis and analysis
If baseline characteristics were not available for the whole population, these were calculated when possible. To increase comparability, we estimated unadjusted odds ratio (OR) or rate ratios using the reported number of participants or reported incidence rates for the studies in which only this information and no adjusted results were published. When multiple incidences were provided in the course of the study, the most recent incidence was used to calculate a rate ratio. Furthermore, to keep the studies as comparable as possible, when data was stratified by age, only data of all age categories of ≥ 65 years were included (n = 2 [12, 13]). To visualize the risks of falls and the various fracture types, the calculated and given association estimates were visualized in a graph. To enhance the clarity of this graph, we only included eGFR categories 60–89, 45–59, 30–44, 15–29, and < 15.
For the meta-analysis, we summarized results for the studies that provided a hazard ratio (HR) or rate ratio for the hip fracture and any type of fracture group using a random-effects model using the generic inverse variance method expressed as HR with 95% confidence intervals (95%CI). Heterogeneity was quantified by the I2 statistic. All analyses were conducted using Review Manager 5.3. For the group of vertebral fractures and accidental falls the number of studies per different stage of CKD was considered too small and the studies too heterogeneous (different association estimates, different outcomes, e.g., all falls vs. only serious hospitalized fall incidents) to perform a meta-analysis.
Results
Characteristics of included studies
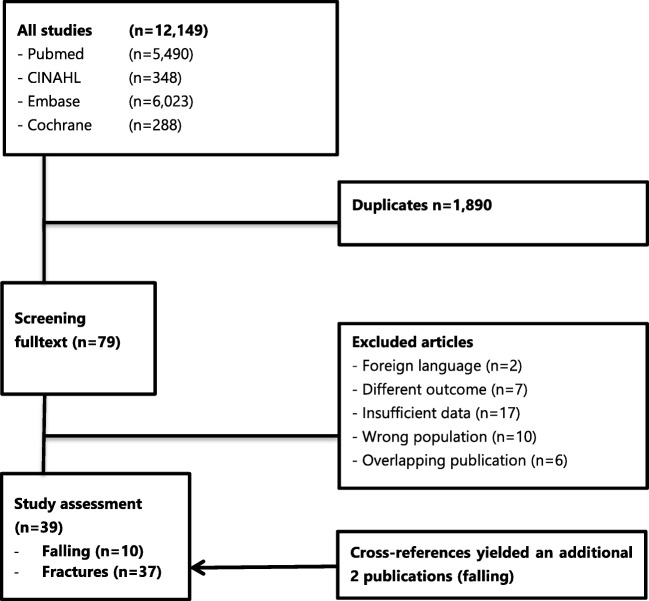
Our search identified 12,149 potential publications (6023 from Embase, 5490 from PubMed, 348 from CINAHL, and 288 from Cochrane). After removing 1890 duplicates and 10,220 studies for other reasons (Fig. 1), a total of 37 unique publications were included in this review. Cross reference checking yielded two additional publications.
Fig. 1.
Flowchart diagram
The characteristics of the 39 included studies are summarized in Table 1. The first publication is from 2000 and the most recent from 2018. Most studies were conducted in the USA. The size of the study populations ranged from 173 to 4,099,342 (median 5601). Most studies included elderly patients, with a me(di)an age over 65 years in most studies. Eight studies included only dialysis patients [13–20], all other studies included various stages of CKD. Ten studies focused on accidental falling [19, 21–29]; seventeen studies focused on hip, femur, and pelvis fractures [12–14, 17, 18, 20, 24, 30–39]; seven studies on vertebral fractures [12, 15, 22, 24, 31, 40, 41]; and thirteen studies focused on any type of fracture without further specification [12, 16, 22, 29, 42–50].
Table 1.
Characteristics of the included studies
| Study | Study design | Patient selection | Number of participants | Number of patients with eGFR < 60/dialysis** | Age (me(di)an (± range) | % male | Me(di)an follow-up time in years (range) | Overall score quality assessment (…/9) | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accidental falls | Hip/femur +pelvis fractures | Vertebral fracture | Any type of fracture | |||||||||
| Alem, 2000 [14] | RCS | US Renal Data System (USRDS) | ? | 326,464** | NR | 56% | ? (?–7) | 4 | X | |||
| Arneson, 2013 [13] | RCS | Medicare, USA | 1,267,416* | 101,995** | ≥ 66 | 46%* | ? (?–1) | 7 | X | |||
| Atteritano, 2017 [15] | CS | Population-based cohort, USA | 192 | 92** | 65.9* | 78%* | NA | 8 | X | |||
| Bowling, 2016 [21] | PCS | Population-based cohort, USA (REGARDS) | 8744 | 1604 | ≥ 65 | 51% | 5.9 (?–9.9) | 7 | X | |||
| Chen, 2018 [22] | PCS | Population-based cohort, The Netherlands (LASA) | 1477 | 560* | 75.8 ± 6.6 | 48% | ? (?–6) | 7/8 | X | X | X | |
| Coco, 2000 [18] | RCS | Outpatient dialysis unit (monocenter) | NR | 1272 | 58 ± 0.4 | 49% | 3.2 (?–10) | 7 | X | |||
| Daya, 2016 [42] | PCS | Population-based (ARIC), USA | 10,955 | 693 | 63.3* | 44%* | 13 (?–15) | 7 | X | |||
| Dooley, 2008 [30] | RS | Multicenter Veteran clinic, USA | 33,091 | 13,632 | 67.5 (?) | 100% | 3 (?–7) | 8 | X | |||
| Dukas, 2005 [23] | PCS | Multicenter study, Germany | 186 | NR | 75.0 ± 4.1 | 48%* | ? (?–0.7) | 6 | X | |||
| Dukas, 2005 [24] | CS | Post hoc subanalysis of RCT | 5313 | NR | 74.0 (?) | 20% | NA | 6/5 | X | X | X | |
| Elliott, 2013 [12] | PCS | Population-based cohort, Canada | 1,815,943 | 128,957 | ≥ 65 | 44% | 4.4 (?–6) | 5 | X | X | X | |
| Ensrud, 2007 [31] | CC | Population-based cohort, USA (SOF) | 396 | 186 | ≥ 65 | 0% | 5.9 (?) | 6 | X | X | ||
| Ensrud, 2012 [43] | CC | Multicenter cohort study, USA (WHI-OS) | 2190 | NR | 64.3* | 0% | 8.6 (?–12) | 8 | X | |||
| Ensrud, 2014 [32] | CC | Population-based cohort, USA (MrOS) | 1602 | 388 | 73.8* | 100% | 7.9 (?–8) | 8 | X | |||
| Fried, 2007 [33] | PCS | Population-based cohort, USA | 5888 | 1190* | 74.8* | 42%* | 7.1 (?) | 7 | X | |||
| Hall, 2015 [25] | RCS | Multiple nursing homes, USA (RCT CONNECT for quality) | 510 | 179* | 77.2 ± 11.5 | 73% | 0.2 (?–0.5) | 7 | X | |||
| Hall, 2018 [44] | PCS | Multicenter Veteran clinic, USA | 712,918 | 356,459 | 73.0* | 100% | 5.2 (?–10) | 6 | X | |||
| Hansen, 2016 [16] | RCS | Danish population + all patients receiving dialysis in Denmark | 4,099,342 | 7566** | 46* | 49% | ? (?–2) | 6 | X | |||
| Iwagami, 2018 [34] | RCS | Population-based cohort, England | 484,698 | 242,349 | 75.4 ± 9.7 | 39% | 4.2 (?–10) | 7 | X | |||
| Kaji, 2010 [40] | CS | Outpatient clinic for metabolic bone disorders, Japan | 659 | 85 | 64.5 ± 8.2 | 0% | NA | 5 | X | |||
| Kim, 2016 [35] | RCS | Multicenter cohort, USA (NIS) | 278,018 | 38,932 | NR | 31%* | ? (?–1) | 5 | X | |||
| Kinsella, 2010 [45] | CS | Monocenter study, Ireland | 1702 | 347 | 61.7 ± 10.8 | 0% | 4 | X | ||||
| Kistler, 2018 [26] | CS | Behavioral risk factor surveillance system (BRFSS), USA | 157,753 | 9116 | ≥ 65 | 44% | NA | 6 | X | |||
| Kurajoh, 2018 [46] | CS | Multicenter study, Japan | 555 | 181 | 76.8* | 0% | NA | 6 | X | |||
| LaCroix, 2008 [39] | CC | Multicenter cohort study, USA (WHI-OS) | 794 | 144 | 71 (?) | 0% | 7 (0.7–9.3) | 9 | X | |||
| Liao, 2016 [47] | RCS | Population-based cohort, Taiwan (LHID2005) | 11,312 | 1427 | ≥ 40 | 68% | 2.6 (?–10) | 6 | X | |||
| Maravic, 2014 [17] | RCS | French national database | 68,953 | 29,487** | 82.1* | 24%* | ? (?–1) | 5 | X | |||
| McCarthy, 2008 [50] | PCS | Multicenter cohort + population-based cohort, USA | 427 | 85 | 68 ± 13.5 | 0% | 14 (?–25) | 7 | X | |||
| Mishima, 2015 [41] | CS | Tertiary center, Japan | 173 | 68 | 62.3 ± 12.2 | 57% | NA | 4 | X | |||
| Naylor, 2014 [29] | PCS | Population-based cohort, Canada | 679,114 | 107,841 | > 65 | 45%* | ? (?–3) | 7 | X | X | ||
| Naylor, 2015 [49] | PCS | Population-based cohort, Canada (CaMos) | 2107 | 320 | 67.2* | 29% | 4.8 (?–5) | 4 | X | |||
| Nickolas, 2006 [36] | CS | Population-based cohort, USA (NHANES III) | 6270 | 875 | 64.9* | 48%* | NA | 6 | X | |||
| Pérez-Sáez, 2015 [37] | RCS | Population-based cohort, Spain (SIDIAPQ) | 873,073 | 32,934 | 67.6 | 47% | 3 (?–3) | 6 | X | |||
| Račić, 2015 [19] | CS | Multiple HD centers, Bosnia and Herzegovina and Serbia + primary care center | 406 | 106** | 77.6* | 61% | NA | 4 | X | |||
| Rafiq, 2014 [27] | RS | Multiple GP databases, UK (QICKD) | 135,433 | NR | 75.4 ± 7.6 | 44% | 2,5 (?–5) | 6 | X | |||
| Robertson, 2018 [38] | RCS | Single health region Scotland | 39,630 | 19,882 | 63.3* | 41%* | ? (5.5–?) | 7 | X | |||
| Rothenbacher, 2014 [28] | PCS | Population-based cohort, Ulm + Germany (ActiFE) | 1385 | 196 | 75.6 ± 6.5 | 57% | 1 (?–1) | 8 | X | |||
| Wakasugi, 2013 [20] | RCS | All dialysis facilities in Japan | NR | 128,141 | NR | 62% | ? (?–1) | 4 | X | |||
| Yenchek, 2012 [48] | PCS | Population-based cohort, USA (Health, aging, and body composition study) | 2754 | 587 | 73.6 ± 2.9 | 49%* | 11.3 (?) | 7 | X | |||
PCS prospective cohort study, CS cross-sectional study, RCS retrospective cohort study, CC case-control study, NR not reported, NA not applicable, ? not reported
*Calculated
**Only dialysis patients included
Quality assessment
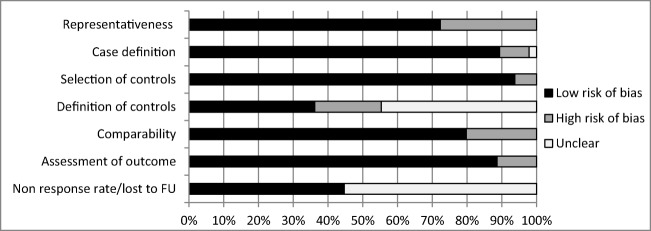
Results of quality assessment can be found in Fig. 2 and Table 1. Reviewer agreement was over 95% for all aspects. The overall quality of included articles was good with a mean score of 6.1 out of 9 (standard deviation (SD) 1.2). Especially for the studies that assessed different types of fractures, many studies did not specify if they included or excluded patients with a previous fracture [13–15, 17, 18, 22, 24, 35–37, 40, 41, 46, 48, 50], and so risk of bias was often unclear regarding the definition of controls. This was also a concern with the non-response rate and rate of lost to follow-up: almost half of the studies did not report data on this. Furthermore, for the any type of fracture group, almost all studies used ICD codes without radiographic confirmation. Full details of the quality assessment can be found in Appendix 3.
Fig. 2.
Quality assessment
Accidental falling
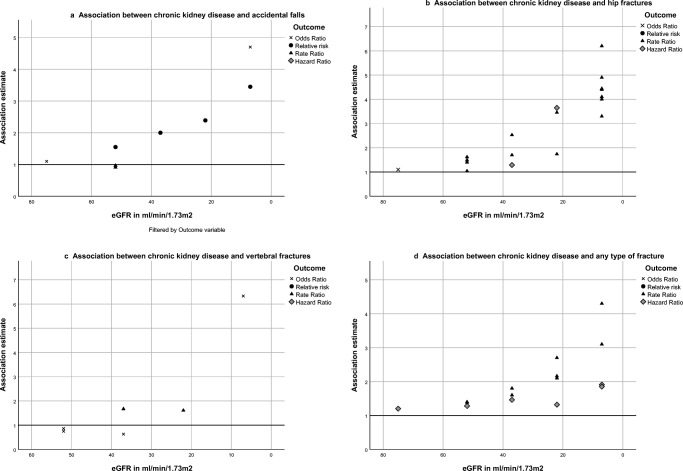
Results for accidental falling are shown in Table 2 and Fig. 3a. Ten studies assessed the association between CKD and accidental falling [19, 21–29]. Of these, five studies used an eGFR ≥ 60 as a reference category [19, 21, 25, 28], two studies an eGFR ≥ 65 [23, 24], one study an eGFR > 90 [27], one study the highest quartile (eGFR ≥ 74) [22], and one study compared used self-reported medical history of CKD [26]. Half of the included studies did not find an association between CKD and accidental falling [21, 22, 25, 27, 28], irrespective of CKD stage, reference category, and/or adjustment for potential confounders. The two studies that used an eGFR of ≥ 65 as reference both showed a significant association between a lower eGFR and falls with adjusted odds ratios ranging from 1.69 to 4.01 [23, 24]. An increasing risk of accidental falling was seen with decreasing kidney function in the two studies where risk ratios were calculated from prevalence/incidence (stage 3a risk ratio 1.55, stage 3b risk ratio 2.00, stage 4 risk ratio 2.39, stage 5 risk ratio 3.45 [29], and hemodialysis risk ratio 4.7 [19], Fig. 3a). In addition, one study addressed the association between self-reported medical history of CKD and falls and found a significant association (ORadj 1.26, 95% CI 1.13–1.47) [26].
Table 2.
Study results for the association between accidental falling and chronic kidney disease
| Accidental falls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Degree of kidney impairment | Adjusted (+/−) | Reference group | |||||||
| eGFR method | < 15 | 15–29 | 30–44 | < 45 | 45–59 | < 60 | Other | |||
| Bowling, 2016 [21] | CKD-EPI (creatinine) | HR 1.09 (0.86–1.37)‡ | HR 0.91 (0.76–1.09) | +a,b,c,d,e,f | ≥ 60 | |||||
| Chen, 2018 [22] | MDRD (creatinine) |
eGFR 9–57 HR 0.999 (0.995–1.002) |
+a,b,c,d | ≥ 74 | ||||||
| Dukas, 2005 [23] | CG (creatinine) |
eGFR < 65 OR 4.01 (1.48–10.89)‡ |
+a,c,d,e | ≥ 65 | ||||||
| Dukas, 2005 [24] | CG (creatinine) |
eGFR < 65 OR 1.69 (1.50-1.91)‡ |
+a,c,e | ≥ 65 | ||||||
| Hall, 2015 [25] | MDRD (creatinine) | Rate ratio 1.06 (0.85–1.32) | Rate ratio 0.97 (0.76–1.23) | +a,f | ≥ 60 | |||||
| Kistler, 2018 [26] | Self-report |
CKD OR 1.26 (1.13-1.47) |
+a,d,f | Non-CKD | ||||||
| Naylor, 2014 [29] | CKD-EPI (creatinine) | ♀ Risk ratio 3.45* | ♀Risk ratio 2.39* | ♀Risk ratio 2.00* | ♀Risk ratio 1.55* | − | ≥ 60 | |||
| Račić, 2015 [19] | Hemodialysis | Risk ratio 4.7* | − | Non-CKD | ||||||
| Rafiq, 2014 [27] | NHS codes | OR 0.9 (0.9–0.9) |
eGFR 60–89 OR 1.1 (1.1–1.2) |
+a,b,d,e | ≥ 90 | |||||
| Rothenbacher, 2014 [28] | CKD-EPI (cystatin C) | Risk ratio 1.03 (0.83-1.28)* | − | ≥ 60 | ||||||
CG Cockgroft-Gault formula, CKD-EPI Chronic Kidney Disease Epidemiology Collaboration, MDRD Modification of Diet in Renal Disease, OR odds ratio, HR hazard ratio
*Calculated from available data
‡Dialysis patients and/or stage 5 excluded
Adjustment
aDemographics
bIntoxications (e.g., alcohol, smoking status)
cBMI, weight
dComorbidity
eUse of antihypertensive medication, psychoactive medication, antidepressants, sedatives, polypharmacy
fImpaired mobility
Fig. 3.
a–d Association between chronic kidney disease, accidental falls (a), hip fractures (b), vertebral fractures (c), and all fractures (d)
Hip fractures
Results for hip fractures are shown in Table 3 and Fig. 3b. Seventeen studies reported on the association between CKD and hip fractures [12–14, 17, 18, 20, 24, 30–39]. Fifteen studies used an eGFR ≥ 60 as a reference category [12–14, 17, 18, 20, 30, 32–38, 51], one study an eGFR ≥ 65 [24], and one study an eGFR > 90 [39]. Eleven out of seventeen studies found a higher risk of hip fractures for the different stages of CKD [12–14, 17, 18, 20, 34–38]. Three studies found an association for only the higher stages of CKD (eGFR < 30 [30], eGFR < 45 [31], and < 60 [39]) and hip fractures. Furthermore, three out of seventeen studies did not find an association between CKD an hip fractures [12, 32, 33]; although one study did show an increasing rate ratio when kidney function decreases, no association was seen between CKD and hip fracture when adjusted for potential confounders [12]. Generally, risks were increased when kidney function decreased [12, 31, 38], with the highest fracture risks in stage 5/dialysis [13, 14, 17, 18, 20, 35] (Fig. 3b).
Table 3.
Study results for the association between hip/femur fractures and chronic kidney disease
| Hip/femur fractures | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Degree of kidney impairment | Adjusted (+/−) | Reference group | ||||||||
| eGFR method | < 15 | 15–29 | < 30 | 30–44 | < 45 | 45–59 | < 60 | Other | |||
| Alem, 2000 [14] | Dialysis |
♂Rate ratio 4.44 (4.16–4.75) ♀Rate ratio 4.40 (4.16–4.64) |
+a | General population | |||||||
| Arneson, 2013 [13] | Hemodialysis | Rate ratio 4.0* | +a,d | Non-ESKD | |||||||
| Coco, 2000 [18] | Dialysis | Rate ratio 17.4 (12.4–34.0) | − | General population | |||||||
| Dooley, 2008 [30] | MDRD (creatinine) | ♂HR 3.65 (1.87–7.13)‡ | +a,b,c,f | General population | |||||||
| Dukas, 2005 [24] | CG (creatinine) | OR 1.57 (1.18–2.09)‡ | +a,b,c,e,f | ≥ 65 | |||||||
| Elliott, 2013 [12] | CKD-EPI (creatinine) | Rate ratio 3.46 (3.13–3.83)* | Rate ratio 2.53 (2.38–2.69)* | Rate ratio 1.62 (1.54–1.71)* | − | ≥ 60 | |||||
| Ensrud, 2007 [31] | MDRD (creatinine) | ♀HR 1.58 (0.59–4.23) | ♀HR 1.49 (0.93–2.38) | +a,c,h | ≥ 60 | ||||||
| Ensrud, 2014 [32] | CKD-EPI (creatinine) | ♂HR 0.92 (0.58–1.47) | +a,c,d,g,h | ≥ 60 | |||||||
| Fried, 2007 [33] | MDRD (creatinine) |
♂ HR 0.97 (0.58–1.62) ♀ HR 1.38 (0.99-1.94) |
+a,b,c,d | ≥ 60 | |||||||
| Iwagami, 2018 [34] | CKD-EPI (creatinine) | HR 1.72 (1.59–1.85)‡ | HR 1.29 (1.24–1.36) | HR 1.04 (1.00–1.08) | HR 1.11 (1.07–1.15)‡ | +a,d,e | ≥ 60 | ||||
| Kim, 2016 [35] | ICD | Rate ratio 3.30* | Rate ratio 1.53*‡ | +a,b,e,f,h | Normal kidney function | ||||||
| LaCroix, 2008 [39] | Cystatin C^-1*76.7 | ♀OR 2.64 (1.41–4.97) |
GFR 60-89: ♀OR 1.10 (0.71–1.73) |
+a,b,c, | ≥ 90 | ||||||
| Maravic, 2014 [17] | Dialysis | Rate ratio 4.1* | − | Non-dialysis | |||||||
| Nickolas, 2006 [36] | MDRD (creatinine) | OR 2.32 (1.13–4.74)‡ | +a,b,c,d,g,h | ≥ 60 | |||||||
| Pérez-Sáez, 2015 [37] | ICD | HR 1.16 (1.06–1.27) | +a,b,c,d,f | ≥ 60 | |||||||
| Robertson, 2018 [38] | MDRD (creatinine) | Rate ratio 1.74 (1.30–2.33) | Rate ratio 1.70 (1.38–2.09) | Rate ratio 1.40 (1.16–1.70) | Rate ratio 1.49 (1.24–1.79) | +a,d | ≥ 60 | ||||
| Wakasugi, 2013 [20] | Dialysis |
♂Rate ratio 6.2 (5.7–6.8) ♀Rate ratio 4.9 (4.6–5.3) |
+a | General population | |||||||
CG Cockgroft-Gault formula, CKD-EPI Chronic Kidney Disease Epidemiology Collaboration, MDRD Modification of Diet in Renal Disease, ESKD end-stage kidney disease, OR odds ratio, HR hazard ratio
*Calculated from available data
‡Dialysis patients and/or stage 5 excluded
Adjustment
aDemographics (e.g., age, sex, race)
bIntoxications (e.g., alcohol use, smoking status)
cBMI, weight
dComorbidity (e.g., diabetes, osteoporosis)
eAdjusted for antiosteoporotic treatment (e.g., bisphosphonates) or exclusion of patients with antiosteoporotic drug use
fUse of steroids, or exclusion of patients with steroid use
gLaboratory values (e.g., calcium, phosphorus, PTH, 25-OHD)
hBMD
Vertebral fractures
Results for vertebral fractures are shown in Table 4 and Fig. 3c. Seven studies reported on vertebral fractures and CKD [12, 15, 22, 24, 31, 40, 41]. All but one study [24] used an eGFR of ≥ 60 as the reference category. Four out of seven studies found a higher risk of patients with CKD of developing vertebral fractures, compared to the non-CKD population [15, 24, 40, 41]. Furthermore, two other studies found a higher risk of vertebral fractures for patients with CKD, but when adjusted for potential confounders this risk was fully attenuated [12, 31]. This effect was not seen in the remaining study that did not found an association at all [22].
Table 4.
Study results for the association between vertebral fractures and chronic kidney disease
| Vertebral fractures | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Degree of kidney impairment | Adjusted (+/−) | Reference group | ||||||
| eGFR method | < 15 | 15–29 | 30–44 | < 45 | 45–59 | < 60 | |||
| Atteritano, 2017 [15] | Hemodialysis | OR 6.33 (2.92–13.73)* | +a | Normal kidney function | |||||
| Chen, 2018 [22] | MDRD (creatinine) | OR 0.63 (0.53–1.24) | OR 0.86 (0.56–1.32) | +a,b,c,d | ≥ 60 | ||||
| Dukas, 2005 [24] | CG (creatinine) | OR 1.31 (1.10–1.55)‡ | +a,b,c,e,f | ≥ 65 | |||||
| Elliott, 2013 [12] | CKD-EPI (creatinine) | Rate ratio 1.61 (1.37–1.89)* | Rate ratio 1.67 (1.54–1.81)* | Rate ratio 1.35 (1.27–1.44)* | − | ≥ 60 | |||
| Ensrud, 2007 [31] | MDRD (creatinine) | ♀ OR 0.73 (0.24–2.24) | ♀ OR 0.75 (0.45–1.23) | +a,c,h | ≥ 60 | ||||
| Kaji, 2010 [40] | MDRD (creatinine) | ♀ OR 2.32 (1.45–3.71)* | −e,f | ≥ 60 | |||||
| Mishima, 2015 [41] | Formula proposed by the Japanese Society of Nephrology | OR 2.48 (1.20–5.12)*‡ | −e,f | ≥ 60 | |||||
CG Cockgroft-Gault formula, CKD-EPI Chronic Kidney Disease Epidemiology Collaboration, MDRD Modification of Diet in Renal Disease, OR odds ratio
*Calculated from available data
‡Dialysis patients and/or stage 5 excluded
Adjustment
aDemographics (e.g., age, sex, race)
bIntoxications (e.g., alcohol use, smoking status)
cBMI, weight
dComorbidity (e.g., diabetes, osteoporosis)
eAdjusted for anti-osteoporotic treatment (e.g., bisphosphonates) or exclusion of patients with antiosteoporotic drug use
fUse of steroids, or exclusion of patients with steroid use
gLaboratory values (e.g., calcium, phosphorus, PTH, 25-OHD)
hBMD
Any type of fracture
Results for any type of fracture are shown in Table 5 and Fig. 3d. Thirteen studies reported on incident fractures of any type and CKD [12, 16, 22, 29, 42–50]. Six studies used an eGFR ≥ 60 as reference category [12, 22, 29, 44, 48, 49], two studies a reference category of ≥ 90 [42, 43], one study a reference category of 75–89 [45], two studies did not specify their reference category (no CKD/general population) [16, 47], and two studies assessed the association between fractures in a continuous way [46, 50]. Eight out of thirteen studies found a higher risk of fractures when eGFR decreased < 60 ml/min/1.73 m2 [16, 22, 29, 43–45, 48, 49]. Two studies found an increasing rate ratio, but when adjusted for potential confounders, this was fully attenuated [12, 42]. The three remaining studies that did not find an association studied very mild CKD (eGFR 60–90) [50], assessed eGFR in a continuous way [46] or did not specify their reference group [47]. In all included studies where multiple CKD stages were included, the risk of fractures increased when eGFR worsens [12, 22, 29, 43–45] (Fig. 3d).
Table 5.
Study results for the association between any type of fracture and chronic kidney disease
| Any type of fracture | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Degree of kidney impairment | Adjusted (+/−) | Reference group | ||||||
| eGFR method | < 15 | 15–29 | 30–44 | 45–59 | < 60 | Other | |||
| Chen, 2018 [22] | MDRD (creatinine) | HR 1.46 (1.12–1.91) | HR 1.28 (1.12–1.46) | +a,b,c,d | ≥ 60 | ||||
| Daya, 2016 [42] | CKD-EPI (creatinine) | HR 0.89 (0.56–1.41) | +a,b,c,d,e,f | ≥ 90 | |||||
| Elliott, 2013 [12] | CKD-EPI (creatinine) | Rate ratio 2.16 (2.00–2.34)* | Rate ratio 1.80 (1.72–1.88)* | Rate ratio 1.38 (1.33–1.42)* | − | ≥ 60 | |||
| Ensrud, 2012 [43] | CKD-EPI (cystatin C) | ♀HR 2.46 (1.16–5.21) |
GFR 60–89 ♀HR 1.16 (0.85–1.58) |
+a,e | ≥ 90 | ||||
| Hall, 2018 [44] | MDRD | ♂HR 1.91 (1.45–2.50) | ♂HR 1.32 (1.16–1.49) | +a,b,c,d,e | ≥ 60 | ||||
| Hansen, 2016 [16] | Dialysis | HR 1.85 (1.75–1.95) | +a,d,e | General population | |||||
| Kinsella, 2010 [45] | MDRD (creatinine) | ♀OR 1.37 (1.0–1.89) |
GFR < 75 ♀OR 1.20 (0.93–1.55) |
+a,e,f | 75–89 | ||||
| Kurajoh, 2018 [46] | Japanese formula for eGFRcr |
Per 10 ml/min/1.73 m2decrease: OR 0.96 (0.85–1.01) |
+a,b,c,d | NA | |||||
| Liao, 2016 [47] | ? |
CKD HR 1.10 (0.95–1.27) |
+a,d,f | No CKD | |||||
| McCarthy, 2008 [50] | MDRD |
Per 10 ml/min/1.73 m2decrease: HR 0.92 (0.84–1.01) |
+a,c | NA | |||||
| Naylor, 2014 [29] | CKD-EPI |
♂ Rate ratio 4.3 (3.70–5.00) ♀Rate ratio 3.1 (2.80–3.50) |
♂Rate ratio 2.7 (2.20–3.30) ♀Rate ratio 2.1 (1.90–2.30) |
♂Rate ratio 1.8 (1.60–2.00) ♀Rate ratio 1.6 (1.50–1.70) |
♂Rate ratio 1.3 (1.20–1.40) ♀Rate ratio 1.40 (1.30–1.50) |
− | ≥ 60 | ||
| Naylor, 2015 [49] | CKD-EPI (creatinine) | Risk ratio 1.86 (1.07–3.24)* | − | ≥ 60 | |||||
| Yenchek, 2012 [48] | MDRD (creatinine) | Risk Ratio 1.26 (1.02–1.56)* | − | ≥ 60 | |||||
CG Cockgroft-Gault formula, CKD-EPI Chronic Kidney Disease Epidemiology Collaboration, MDRD Modification of Diet in Renal Disease, CKD chronic kidney disease
*Calculated from available data
‡Dialysis patients and/or stage 5 excluded
Adjustment
aDemographics (e.g., age, sex, race)
bIntoxications (e.g., alcohol use, smoking status)
cBMI, weight
dComorbidity (e.g., diabetes, osteoporosis)
eAdjusted for antiosteoporotic treatment (e.g., bisphosphonates) or exclusion of patients with antiosteoporotic drug use
fUse of steroids, or exclusion of patients with steroid use
gLaboratory values (e.g., calcium, phosphorus, PTH, 25-OHD)
hBMD
Meta-analysis
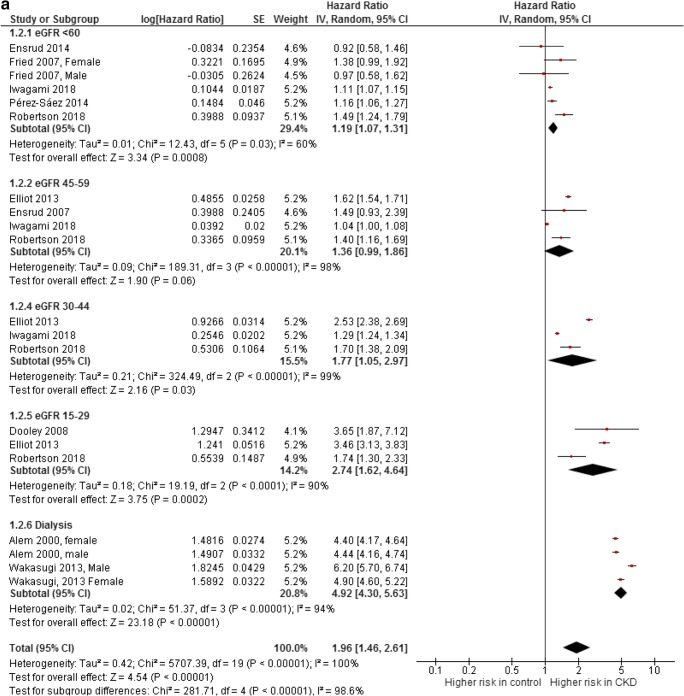
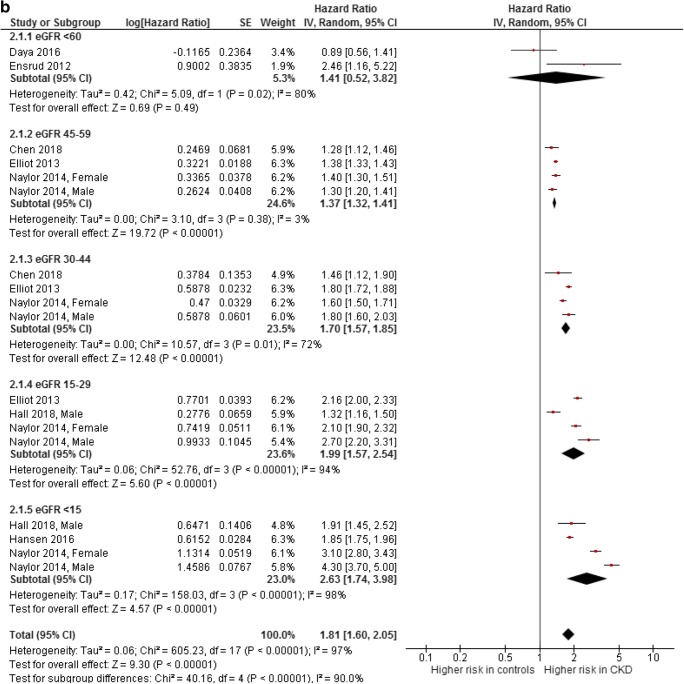
For the hip fracture outcome, all studies that provided a hazard ratio or from which a rate ratio with 95%CI could be calculated were summarized in a meta-analysis (Fig. 4a). Subsequently, three studies were excluded because they only provided an odds ratio [24, 36, 39] and three studies were excluded because no 95%CI could be calculated [13, 17, 35]. All excluded studies found an association between CKD and hip fractures [13, 17, 24, 35, 36, 39]. All studies that were included into the meta-analysis assessed older adults (mean age of 63 years and older) [12, 14, 20, 30, 32–34, 37, 38]. Only in the largest study, which consisted of 1,815,943 patients, adjusted rate ratios were not reported [12]. There was a significant association between fractures and eGFR category < 60, 30–44, 15–29, and < 15. For the eGFR category of 45–59, there was a borderline association with a pooled HR of 1.36 (95%CI 0.99–1.86). There was a graded risk, with higher risk among the more severe stages of CKD. However, the heterogeneity among the estimates was large (in most subgroups I2 ≥ 90%).
Fig. 4.
a Associations of chronic kidney disease with incidence of hip fractures. b Associations of chronic kidney disease with any type of fracture
Also for the any type of fracture outcome, all studies that provided a hazard ratio or from which a rate ratios with 95%CI could be calculated are summarized in a meta-analysis (Fig. 4b). Two studies were excluded because they only provided odds ratios [45, 46], and two studies were excluded because they only provided risk ratios [48, 49]. As only one study assessed CKD in a continuous way with a hazard ratio as outcome [50], a meta-analysis for this outcome was not considered feasible. Half of the excluded studies did not find an association between mild stages of CKD and fractures [45, 46, 50]. In the studies that were included into the meta-analysis, mean age ranged from 46 [16] to 75.8 years [22]. Furthermore, two of the included studies rate ratios could not be adjusted for potential confounders [12, 29]. In the meta-analysis, there was a significant association between fractures and eGFR categories 45–59, 30–44, 15–29, and < 15. The risk was higher in more severe stages of CKD, with the highest risk in patients with an eGFR < 15 (pooled HR of 2.63 (95%CI 1.74–3.98). However, the heterogeneity was large, especially in the more severe stages of CKD (stage 4 and 5, I2 94% and I2 98%, respectively).
Discussion
In this systematic review, we found that a lower eGFR is associated with a higher fracture risk. This effect was the most pronounced for the hip fractures and the any type of fracture group. Furthermore, the risk is higher when kidney function worsens, and starts approximately at an eGFR of < 60 (Fig. 3a–d and Fig. 4). For the association between a decreased eGFR and accidental falling, the evidence is contradictory.
The findings that we report on fractures support our hypothesis that a decreasing eGFR is associated with a higher fracture risk. Moreover, almost all studies that assessed patients with stage 5 found that CKD is an independent risk factor for fractures with pooled hazard ratios of 4.9 for hip fractures and 2.6 for the any type of fracture group. This is in line with previous studies that showed that even in early stages of CKD, and in almost all patients with stage 5, an abnormal bone histology was found [52]. Although there were only a limited number of studies that assessed vertebral fractures, it is interesting that this risk seems to be lower compared to the hip and any type of fracture group. One possible explanation for these lower relative risks could be that half of the studies used ICD codes or medical history to diagnose vertebral fractures [12, 24, 31]. Prior research showed that approximately two thirds of vertebral fractures remain unnoticed as they are frequently asymptomatic [53]. Thus, it is likely that fractures are missed in studies that used ICD codes and/or medical history to diagnose vertebral fractures, and therefore, a potential difference between vertebral fractures in patients with CKD and patients without CKD.
In general, there was a graded risk for falls when kidney function worsens (Fig. 3a). However, half of our studies did not find an association between CKD and accidental falling. One possible reason that some studies did not found an association between falls and CKD is that they adjusted their results for multiple confounders. There are several reasons why patients with CKD could fall more often compared to patients without CKD. First, CKD is frequently caused by hypertension and diabetes, which are both associated with falls [54, 55]. Second, CKD and treatment for optimization of CKD can lead to risk factors of falling. For example, due to inflammation and malnutrition, patients with CKD are more prone for muscle degeneration [56], which could lead to instability and falls. Furthermore, medication (e.g., ACE inhibitors) that is frequently administrated to patients with CKD could lead to postural hypotension, which is also a risk factor for falls. Third, CKD is more common in the elderly population, which is also an important risk factor [9]. Subsequently, this could mean that patients with CKD fall more often because of their risk profile, and not primarily because they have CKD.
Risks could also have been influenced by the type of measurement of eGFR. To keep our study results as comparable as possible, we chose to use the most frequently used measurements to estimate GFR (CKD-EPI, MDRD, and CG based on serum creatinine). However, serum creatinine is dependent on muscle mass, which could lead to a false-negatively low serum creatinine due to low muscle mass and therefore relatively “good” eGFR in the frail elderly [42]. This could potentially have led to a higher fracture rate in the better eGFR ranges. Another method to estimated eGFR in this population could be the use of cystatin C which is independent of muscle mass. For example, two studies did not found an association for eGFR based on creatinine, but did found an association between eGFR based on cystatin C and any type of fracture [42, 46]. On the other hand, another study that compared both methods did not find any difference for the association between CKD and hip fractures [32]. More research is needed to explore the differences in outcome when using cystatin C compared with serum creatinine.
As patients with CKD have much higher rates of fractures compared to the non-CKD population, it is very important to screen these patients timely for potential risk factors. For fractures, the updated Kidney Disease: Improving Global Outcomes (KDIGO) guideline recommends “BMD testing to assess fracture risk in patients with CKD stage 3a to 5 with evidence of CKD-BMD and/or risk factors for osteoporosis, if results will impact treatment decisions [57].” This could mean that in clinical practice, much more frequent BMD measurements should be done in patients with CKD, especially in the elderly with previous falls. Furthermore, as treatment of renal osteodystrophy is very complicated in the advanced stages of CKD due to the heterogeneity of the illness and the limited experience with different treatments, and most of the low-energetic fractures in patients older than 50 years are caused by a fall [22, 58], it is also very important to prevent and lower the risks of falling as much as possible. Prior research has shown that most patients who experienced a fall did not mention this to their healthcare provider [59]; therefore, it is necessary for nephrologists and general practitioners to actively ask about previous falls in patients with CKD. At this moment, there is no clear recommendation from the KDIGO guidelines to screen for accidental falling or to start interventions in patients with high risk of fracture (or falls). This could be important as it can potentially prevent morbidity and even mortality, as various studies showed that multiple interventions are able to lower the risk of falling in patients with CKD [60–62].
This systematic review provides valuable information about the fracture and fall risk of patients with CKD, but it has several limitations. Included studies were heterogeneous, assessing different CKD stages, different eGFR methods, different definitions for falls and fractures, and different methods to measure the outcome. Therefore, a meta-analysis could not be performed for the outcome of vertebral fractures and accidental falls. Second, most studies were performed in elderly patients. Although we also presented some evidence for the younger patients, this evidence was scarce and our findings can possibly not be fully extrapolated to the younger CKD population. However, considering falls and fractures, elderly are most at risk and therefore identification in this population could lead to the highest benefit. Third, considering non-English manuscripts were excluded what could potentially have led to publication bias.
In conclusion, fractures are very common in the CKD population and the risk increases when kidney function worsens. Besides more awareness of timely fracture risk assessment, there also should be more focus on fall prevention.
Electronic supplementary material
(PDF 201 kb)
Compliance with ethical standards
Conflicts of interest
None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang Q-L, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8(1):117. doi: 10.1186/1471-2458-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moorthi RN, Moe SM. Recent advances in the noninvasive diagnosis of renal osteodystrophy. Kidney Int. 2013;84(5):886–894. doi: 10.1038/ki.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damasiewicz MJ, Nickolas TL. Rethinking bone disease in kidney disease. JBMR Plus. 2018;2(6):309–322. doi: 10.1002/jbm4.10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coen G, Ballanti P, Bonucci E, Calabria S, Costantini S, Ferrannini M, Giustini M, Giordano R, Nicolai G, Manni M, Sardella D, Taggi F. Renal osteodystrophy in predialysis and hemodialysis patients: comparison of histologic patterns and diagnostic predictivity of intact PTH. Nephron. 2002;91(1):103–111. doi: 10.1159/000057611. [DOI] [PubMed] [Google Scholar]
- 5.Nitsch D, Mylne A, Roderick PJ, Smeeth L, Hubbard R, Fletcher A. Chronic kidney disease and hip fracture-related mortality in older people in the UK. Nephrol Dial Transplant. 2009;24(5):1539–1544. doi: 10.1093/ndt/gfn678. [DOI] [PubMed] [Google Scholar]
- 6.Schumock GT, Sprague SM. Clinical and economic burden of fractures in patients with renal osteodystrophy. Clin Nephrol. 2007;67(4):201–208. doi: 10.5414/cnp67201. [DOI] [PubMed] [Google Scholar]
- 7.Doan QV, Gleeson M, Kim J, Borker R, Griffiths R, Dubois RW. Economic burden of cardiovascular events and fractures among patients with end-stage renal disease. Curr Med Res Opin. 2007;23(7):1561–1569. doi: 10.1185/030079907x199790. [DOI] [PubMed] [Google Scholar]
- 8.Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, Mason N, Prutz KG, Young EW, Pisoni RL. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006;70(7):1358–1366. doi: 10.1038/sj.ki.5001754. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. WHO global report on falls prevention in older age. Community Health (Bristol) [Internet]. 2007;53. Available from: http://www.who.int/ageing/publications/Falls_prevention7March.pdf. Accessed 1/3/2018
- 10.Bowling C, Booth JN, Gutierrez OM, Kurella Tamura M, Judd S, Warnock D, et al. Nondisease-specific problems and all-cause mortality among older adults with chronic kidney disease: findings from the REasons for geographic and racial differences in stroke (REGARDS) study. J Am Geriatr Soc. 2014;62:S149. [Google Scholar]
- 11.Desmet C, Beguin C, Swine C, Jadoul M. Falls in hemodialysis patients: prospective study of incidence, risk factors, and complications. Am J Kidney Dis. 2005;45(1):148–153. doi: 10.1053/j.ajkd.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Elliott MJ, James MT, Quinn RR, Ravani P, Tonelli M, Palacios-Derflingher L, et al. Estimated GFR and fracture risk: a population-based study. Clin J Am Soc Nephrol. 2013;8(8):1367–1376. doi: 10.2215/CJN.09130912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arneson TJ, Li S, Liu J, Kilpatrick RD, Newsome BB, St Peter WL. Trends in hip fracture rates in US hemodialysis patients, 1993-2010. Am J Kidney Dis. 2013;62(4):747–754. doi: 10.1053/j.ajkd.2013.02.368. [DOI] [PubMed] [Google Scholar]
- 14.Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, Wong C, Stehman-Breen C. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58(1):396–399. doi: 10.1046/j.1523-1755.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 15.Atteritano M, Di Mauro E, Canale V, Bruzzese AM, Ricciardi CA, Cernaro V, et al. Higher serum sclerostin levels and insufficiency of vitamin D are strongly associated with vertebral fractures in hemodialysis patients: a case control study. Osteoporos Int. 2017;28(2):577–584. doi: 10.1007/s00198-016-3770-9. [DOI] [PubMed] [Google Scholar]
- 16.Hansen D, Olesen JB, Gislason GH, Abrahamsen B, Hommel K. Risk of fracture in adults on renal replacement therapy: a Danish national cohort study. Nephrol Dial Transplant. 2016;31(10):1654–1662. doi: 10.1093/ndt/gfw073. [DOI] [PubMed] [Google Scholar]
- 17.Maravic M, Ostertag A, Torres PU, Cohen-Solal M. Incidence and risk factors for hip fractures in dialysis patients. Osteoporos Int. 2014;25(1):159–165. doi: 10.1007/s00198-013-2435-1. [DOI] [PubMed] [Google Scholar]
- 18.Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115–1121. doi: 10.1053/ajkd.2000.19812. [DOI] [PubMed] [Google Scholar]
- 19.Racic M, Petkovic N, Bogicevic K, Maric I, Matovic J, Pejovic V, et al. Comprehensive geriatric assessment: comparison of elderly hemodialysis patients and primary care patients. Ren Fail. 2015;37(7):1126–1131. doi: 10.3109/0886022X.2015.1057459. [DOI] [PubMed] [Google Scholar]
- 20.Wakasugi M, Kazama JJ, Taniguchi M, Wada A, Iseki K, Tsubakihara Y, Narita I. Increased risk of hip fracture among Japanese hemodialysis patients. J Bone Miner Metab. 2013;31(3):315–321. doi: 10.1007/s00774-012-0411-z. [DOI] [PubMed] [Google Scholar]
- 21.Bowling CB, Bromfield SG, Colantonio LD, Gutierrez OM, Shimbo D, Reynolds K, et al. Association of reduced eGFR and Albuminuria with serious fall injuries among older adults. Clin J Am Soc Nephrol. 2016;11(7):1236–1243. doi: 10.2215/CJN.11111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, Lips P, Vervloet MG, van Schoor NM, de Jongh RT. Association of renal function with bone mineral density and fracture risk in the Longitudinal Aging Study Amsterdam. Osteoporos Int. 2018;29(9):2129–2138. doi: 10.1007/s00198-018-4592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dukas LC, Schacht E, Mazor Z, Stähelin HB. A new significant and independent risk factor for falls in elderly men and women: A low creatinine clearance of less than 65 ml/min. Osteoporos Int. 2005;16(3):332–338. doi: 10.1007/s00198-004-1690-6. [DOI] [PubMed] [Google Scholar]
- 24.Dukas L, Schacht E, Stähelin HB. In elderly men and women treated for osteoporosis a low creatinine clearance of <65 ml/min is a risk factor for falls and fractures. Osteoporos Int. 2005;16(12):1683–1690. doi: 10.1007/s00198-005-1903-7. [DOI] [PubMed] [Google Scholar]
- 25.Hall RK, Landerman LR, O’Hare AM, Anderson RA, Colon-Emeric CS, Erman LR, et al. Chronic kidney disease and recurrent falls in nursing home residents: a retrospective cohort study. Geriatr Nurs. 2015;36(2):136–141. doi: 10.1016/j.gerinurse.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kistler BM, Khubchandani J, Jakubowicz G, Wilund K, Sosnoff J. Falls and fall-related injuries among US adults aged 65 or older with chronic kidney disease. Prev Chronic Dis. 2018;15(6):1–9. doi: 10.5888/pcd15.170518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rafiq M, McGovern A, Jones S, Harris K, Tomson C, Gallagher H, de Lusignan S. Falls in the elderly were predicted opportunistically using a decision tree and systematically using a database-driven screening tool. J Clin Epidemiol. 2014;67(8):877–886. doi: 10.1016/j.jclinepi.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Rothenbacher D, Klenk J, Denkinger MD, Herbolsheimer F, Nikolaus T, Peter R, et al. Prospective evaluation of renal function, serum vitamin D level, and risk of fall and fracture in community-dwelling elderly subjects. Osteoporos Int. 2014;25(3):923–932. doi: 10.1007/s00198-013-2565-5. [DOI] [PubMed] [Google Scholar]
- 29.Naylor KL, McArthur E, Leslie WD, Fraser L-AA, Jamal SA, Cadarette SM, et al. The three-year incidence of fracture in chronic kidney disease. Kidney Int. 2014;86(4):810–818. doi: 10.1038/ki.2013.547. [DOI] [PubMed] [Google Scholar]
- 30.Dooley AC, Weiss NS, Kestenbaum B. Increased risk of hip fracture among men with CKD. Am J Kidney Dis. 2008;51(1):38–44. doi: 10.1053/j.ajkd.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Ensrud KE, Lui LY, Taylor BC, Ishani A, Shlipak MG, Stone KL, et al. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007;167(2):133–139. doi: 10.1001/archinte.167.2.133. [DOI] [PubMed] [Google Scholar]
- 32.Ensrud KE, Parimi N, Fink HA, Ishani A, Taylor BC, Steffes M, et al. Estimated GFR and risk of hip fracture in older men: comparison of associations using cystatin C and creatinine. Am J Kidney Dis. 2014;63(1):31–39. doi: 10.1053/j.ajkd.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fried LF, Biggs ML, Shlipak MG, Seliger S, Kestenbaum B, Stehman-Breen C, et al. Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol. 2007;18(1):282–286. doi: 10.1681/ASN.2006050546. [DOI] [PubMed] [Google Scholar]
- 34.Iwagami M, Caplin B, Smeeth L, Tomlinson LA, Nitsch D. Chronic kidney disease and cause-specific hospitalisation: a matched cohort study using primary and secondary care patient data. Br J Gen Pract. 2018;68:e512–e523. doi: 10.3399/bjgp18X697973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SM, Long J, Montez-Rath M, Leonard M, Chertow GM. Hip fracture in patients with non-dialysis-requiring chronic kidney disease. J Bone Miner Res. 2016;31(10):1803–1809. doi: 10.1002/jbmr.2862. [DOI] [PubMed] [Google Scholar]
- 36.Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol. 2006;17(11):3223–3232. doi: 10.1681/ASN.2005111194. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Saez MJ, Prieto-Alhambra D, Barrios C, Crespo M, Redondo D, Nogues X, et al. Increased hip fracture and mortality in chronic kidney disease individuals: the importance of competing risks. Bone. 2015;73:154–159. doi: 10.1016/j.bone.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 38.Robertson L, Black C, Fluck N, Gordon S, Hollick R, Nguyen H, et al. Hip fracture incidence and mortality in chronic kidney disease: the GLOMMS-II record linkage cohort study. BMJ Open. 2018;8(4):e020312. doi: 10.1136/bmjopen-2017-020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaCroix AZA, Lee JSJ, Wu L, Cauley JA, Shlipak MMG, Ott SM, et al. Cystatin-C, renal function, and incidence of hip fracture in postmenopausal women. J Am Geriatr Soc. 2008;56(8):1434–1441. doi: 10.1111/j.1532-5415.2008.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaji H, Yamauchi M, Yamaguchi T, Shigematsu T, Sugimoto T. Mild renal dysfunction is a risk factor for a decrease in bone mineral density and vertebral fractures in Japanese postmenopausal women. J Clin Endocrinol Metab. 2010;95(10):4635–4642. doi: 10.1210/jc.2010-0099. [DOI] [PubMed] [Google Scholar]
- 41.Mishima T, Motoyama K, Imanishi Y, Hamamoto K, Nagata Y, Yamada S, Kuriyama N, Watanabe Y, Emoto M, Inaba M. Decreased cortical thickness, as estimated by a newly developed ultrasound device, as a risk for vertebral fracture in type 2 diabetes mellitus patients with eGFR of less than 60 mL/min/1.73 m2. Osteoporos Int. 2014;26(1):229–236. doi: 10.1007/s00198-014-2843-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daya N, Voskertchian A, Schneider ALC, Ballew S, McAdams DeMarco M, Coresh J, Appel LJ, Selvin E, Grams ME. Kidney function and fracture risk: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2016;67(2):218–226. doi: 10.1053/j.ajkd.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ensrud KE, Barbour K, Canales MT, Danielson ME, Boudreau RM, Bauer DC, Lacroix AZ, Ishani A, Jackson RD, Robbins JA, Cauley JA. Renal function and nonvertebral fracture risk in multiethnic women: the Women’s Health Initiative (WHI) Osteoporos Int. 2012;23(3):887–899. doi: 10.1007/s00198-011-1667-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall RK, Sloane R, Pieper C, Van Houtven C, LaFleur J, Adler R, et al. Competing risks of fracture and death in older adults with chronic kidney disease. J Am Geriatr Soc. 2018;66(3):532–538. doi: 10.1111/jgs.15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinsella S, Chavrimootoo S, Molloy MG, Eustace JA. Moderate chronic kidney disease in women is associated with fracture occurrence independently of osteoporosis. Nephron Clin Pract. 2010;116(3):c256–c262. doi: 10.1159/000317207. [DOI] [PubMed] [Google Scholar]
- 46.Kurajoh M, Inaba M, Nagata Y, Yamada S, Imanishi Y, Emoto M. Association of cystatin C- and creatinine-based eGFR with osteoporotic fracture in Japanese postmenopausal women with osteoporosis: sarcopenia as risk for fracture. J Bone Miner Metab. 2018;123456789:1–10. doi: 10.1007/s00774-018-0913-4. [DOI] [PubMed] [Google Scholar]
- 47.Liao Kuang-Ming, Liang Fu-Wen, Li Chung-Yi. Risks of all-cause and site-specific fractures among hospitalized patients with COPD. Medicine. 2016;95(40):e5070. doi: 10.1097/MD.0000000000005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yenchek RH, Ix JH, Shlipak MG, Bauer DC, Rianon NJ, Kritchevsky SB, Harris TB, Newman AB, Cauley JA, Fried LF, Health, Aging, and Body Composition Study Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol. 2012;7(7):1130–1136. doi: 10.2215/CJN.12871211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naylor KL, Garg AX, Zou G, Langsetmo L, Leslie WD, Fraser LA, Adachi JD, Morin S, Goltzman D, Lentle B, Jackson SA, Josse RG, Jamal SA. Comparison of fracture risk prediction among individuals with reduced and normal kidney function. Clin J Am Soc Nephrol. 2015;10(4):646–653. doi: 10.2215/CJN.06040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCarthy JT, Rule AD, Achenbach SJ, Bergstralh EJ, Khosla S, Melton LJ., 3rd Use of renal function measurements for assessing fracture risk in postmenopausal women. Mayo Clin Proc. 2008;83(11):1231–1239. doi: 10.4065/83.11.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ensrud KE. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007;167(2):133–139. doi: 10.1001/archinte.167.2.133. [DOI] [PubMed] [Google Scholar]
- 52.Elder G. Pathophysiology and recent advances in the management of renal osteodystrophy. J Bone Miner Res. 2002;17(12):2094–2105. doi: 10.1359/jbmr.2002.17.12.2094. [DOI] [PubMed] [Google Scholar]
- 53.Kendler DL, Bauer DC, Davison KS, Dian L, Hanley DA, Harris ST, et al. Vertebral fractures: clinical importance and management. Am J Med. 2016;129(2):221.e1–221.e10. doi: 10.1016/j.amjmed.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 54.Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk factors for falls in community-dwelling older people. Epidemiology. 2010;21(5):658–668. doi: 10.1097/EDE.0b013e3181e89905. [DOI] [PubMed] [Google Scholar]
- 55.Jansen S, Bhangu J, de Rooij S, Daams J, Kenny RA, van der Velde N. The association of cardiovascular disorders and falls: a systematic review. J Am Med Dir Assoc. 2016;17(3):193–199. doi: 10.1016/j.jamda.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 56.Moorthi RN, Avin KG. Clinical relevance of sarcopenia in chronic kidney disease. Curr Opin Nephrol Hypertens. 2017;26(3):219–228. doi: 10.1097/MNH.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, Moe SM, Shroff R, Tonelli MA, Toussaint ND, Vervloet MG, Leonard MB. Diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder: synopsis of the kidney disease: Improving global outcomes 2017 clinical practice guideline update. Ann Intern Med. 2018;168(6):422–430. doi: 10.7326/M17-2640. [DOI] [PubMed] [Google Scholar]
- 58.CBO (2011) Richtlijn Osteoporose en fractuurpreventie. https://www.volksgezondheidenzorg.info/bestanden/documenten/cbo-richtlijn-osteoporose-enfractuurpreventie-2011. Published 2011. Accessed 27 May 2019
- 59.Stevens JA, Ballesteros MF, Mack KA, Rudd RA, DeCaro E, Adler G. Gender differences in seeking care for falls in the aged medicare population. Am J Prev Med. 2012;43(1):59–62. doi: 10.1016/j.amepre.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 60.Nussbaum J. Fall prevention, reduced morbidity, and improved functional outcome measures in frail patients with end stage kidney disease undergoing a skilled physical therapy program: The prohealth experience. Blood Purif. 2013;35(1):157–158. [Google Scholar]
- 61.Chang JT, Morton SC, Rubenstein LZ, Mojica WA, Maglione M, Suttorp MJ, et al. Interventions for the prevention of falls in older adults: systematic review and meta-analysis of randomised clinical trials. Br Med J. 2004;328(March):680–687. doi: 10.1136/bmj.328.7441.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heung M, Adamowski T, Segal JH, Malani PN. A successful approach to fall prevention in an outpatient hemodialysis center. Clin J Am Soc Nephrol. 2010;5(10):1775–1779. doi: 10.2215/CJN.01610210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 201 kb)