Abstract
Diabetic retinopathy (DR) is considered as a diabetes-related complication that can render severe visual impairments and is also a risk factor for acquired blindness in both developed as well as developing countries. Through fibrovascular epiretinal membranes (ERMs), this condition can similarly lead to tractional retinal detachment. Laboratory efforts evaluating the DR pathogenesis can be provided by ocular vitreous fluid and ERMs resulting from vitrectomy. The clinical stages of DR are significantly associated with expression levels of certain chemokines, including monocyte chemotactic protein-1 (MCP-1) in the intraocular fluid. The MCP-1 is also a known potent chemotactic factor for monocytes and macrophages that can stimulate them to produce superoxide and other mediators. Following hyperglycemia, retinal pigmented epithelial (RPE) cells, endothelial cells, and Müller’s glial cells are of utmost importance for MCP-1 production, and vitreous MCP-1 levels rise in patients with DR. Increased expression of the MCP-1 in the eyes can also play a significant role in the pathogenesis of DR. In this review, current clinical and laboratory progress achieved on the MCP-1 and the DR concerning neovascularization and inflammatory responses in vitreous and/or aqueous humor of DR patients was summarized. It was suggested that further exploration of the MCP-1/CCR2 axis association between clinical stages of DR and expression levels of inflammatory and angiogenic cytokines and chemokines, principally the MCP-1 might lead to potential therapies aiming at neutralizing antibodies and viral vectors.
Keywords: Retinopathy, CCL2, MCP-1, DR, PDR
Introduction
Vascular disorders induced by diabetes mellitus can be divided into two major groups, microvascular and macrovascular, stemmed from impairments in small and large blood vessels; respectively. Diabetic retinopathy (DR) constitutes one of the most frequent microvascular complications (Semeraro et al. 2015), whereas proliferative diabetic retinopathy (PDR) represents a significant microvascular complication related to long-standing type I (T1D) and type II diabetes (T2D) mellitus (Klein et al. 2017). The report by Rogers et al. also rendered DR as one of the most important causes of blindness in the working-age population of the Western world (Yau et al. 2012). Moreover, numerous studies have demonstrated elevated levels of several inflammatory cytokines and chemokines in the ocular fluid of diabetic individuals (Funatsu et al. 2005a, b; Hernandez et al. 2005; Patel et al. 2008; Goldberg 2009; Tang and Kern 2011; Abcouwer 2013), (Zhang et al. 2011a, b; Semeraro et al. 2015). The MCP-1, also called CCL2, is a member of the CC chemokine family that plays a vital role in retinal inflammation induced by diabetes, as the MCP-1 further stimulates recruitment and activation of monocytes and macrophages (Behfar et al. 2017; Vakilian et al. 2017). As well, this chemokine has a potent contribution to fibrosis and angiogenesis induction (Gharaee-Kermani et al. 1996), increasing the production of vascular endothelial growth factor (VEGF) (Hong et al. 2005). Evidence showed that the MCP-1 expression is regulated by the transcription factor nuclear factor-kappa B (NF-κB) (Ueda et al. 1994). Moreover; hyperglycemia is another important mediator of oxidative stress, reactive oxygen species, and retinal inflammation (Cui et al. 2006) in the secondary ERMs in diabetic patients (El-Asrar et al. 2006; Zhang et al. 2011a, b). In patients with hyperglycemia, RPE cells, glial cells, and endothelial cells regulate the MCP1 production (Bian et al. 1999; Harkness et al. 2003; Harada et al. 2006a, b). Monocytes and macrophages expressing the C-C chemokine receptor type 2 (CCR2) are further involved in the pathogenesis of DR (Dong et al. 2013). Indeed, an experimental study revealed a likely relationship between activation of macrophages and retinal angiogenesis through the Notch pathway (Outtz et al. 2011). It is also possible that the adhesion of macrophages to the capillary endothelium leads to capillary occlusion and consequently to retinal ischemia (Murugeswari et al. 2008; Ghasemi et al. 2012). Macrophages also accumulate in the ERMs and thus increased MCP-1 levels have been found in the vitreous fluid of diabetic patients. Several studies have demonstrated higher MCP-1 levels in the vitreous fluid compared to the serum in DR patients (Mitamura et al. 2001), highly supporting a local vitreous fluid production based on the MCP-1 expression pattern (Mitamura et al. 2001; Murugeswari et al. 2008). Furthermore, there is a significant association between the vitreous levels of the MCP-1 and the severity of the DR (Tashimo et al. 2004). Reviewing the existing literature, the inflammatory impact of the MCP-1 on the pathogenic mechanisms of DR was elucidated in this study.
MCP-1 biology
The human MCP-1 gene, located on chromosome 17 (17q11.2), encodes a protein of 76 amino acids with a size of 13 kd size (Zhang et al. 2012). Initially, the MCP-1/CCL2 structure was recognized in a purified natural material. Due to O-glycosylation, different MCP-1 molecular masses were revealed, further contributing to chemotactic potency reduction (Kim and Tam 2011). The MCP-1 to MCP-4, consists of members of the MCP family, with a high homology sequence (Van Coillie et al. 1999).
As mentioned before, the MCP-1 constitutes a powerful chemotactic factor of macrophages and monocytes, representing the first discovered human CC chemokine with monocyte chemoattractant properties (Deshmane et al. 2009). Numerous cell types; including monocytes, macrophages, fibroblasts, astrocytes as well as endothelial, epithelial, smooth muscle, mesangial, and microglial cells are also involved in the MCP-1 production and secretion, either directly or following stimulation by growth factors, cytokines, or oxidative stress (Cushing et al. 1990; Standiford et al. 1991; Brown et al. 1992; Lockwood et al. 2006). However, monocytes and macrophages have been identified as the main sources of the MCP-1 (Yoshimura et al. 1989). The major MCP-1 function is also the regulation of monocytes, macrophages, natural killer (NK) cells, and memory T lymphocytes migration and accumulation (Conti and Rollins 2004).
MCP-1 receptors
Various genes located in the 3p21–22 chromosome are involved in the encoding of CCR proteins, comprised of 360 amino acids (Wells et al. 1996). Chemokine receptors can be also divided into two main subtypes based on their functioning: G protein-coupled chemokine receptors, contributing to effective leukocyte recruitment, and atypical chemokine receptors, that might signal via non-G protein-coupled mechanisms (Bachelerie et al. 2013). Basically, these CC receptors consist of three regions: seven hydrophobic transmembranes, a short extracellular N-terminus, and a C-terminal intracellular region domain. All human MCP members can be attached to at least two CCRs, expressed by leukocytes. It is very interesting that various CCRs, such as the CCR2 might be attached to diverse ligands. Therefore, understanding these ligand/receptor interactions and their impact on specific and unique chemokine functions can be challenging (Mantovani 1999). Moreover, the ligation of the MCP-1/CCR2 mediates its effects, while the CCR2 is expressed by specific cell types (O’Connor et al. 2015).
The CCR2A and CCR2B are known as two alternatively spliced isoforms of the CCR2. Vascular smooth muscle cells and mononuclear ones which mainly express the CCR2A isoform, while the CCR2B isoform is expressed on the surface of activated NK cells and monocytes (Bartoli et al. 2001). Several studies have shown that these isoforms differ only in their C-terminal tails and might further activate different signaling pathways exerting different actions (Charo et al. 1994). For instance, the MCP-1 could attract cells expressing the CCR2A receptors in the absence of Ca2+, while the presence of Ca2+ is necessary for the CCR2B+ cells (Sanders et al. 2000; Yoon et al. 2007). In this respect, Cho et al. reported that, the MCP-1 may have a synergistic effect on the CCR2A expression, but not that of the CCR2B in patients with rheumatoid arthritis (RA) (Yoon et al. 2007). However, the CCR2 disposes both pro-inflammatory (i.e., dependent on antigen-presenting cells and T cells) and anti-inflammatory properties (i.e., dependent on expression of CCR2 by regulatory T cells). Seven single nucleotide polymorphisms (SNPs) have been also recognized regarding the CCR2, whereas it is not clarified if these SNPs may have an impact on clinical outcomes in case of the CCR2-associated diseases or not (Yeo et al. 2006). According to an in vitro study, the CCR2 binding with a heterodimeric compound formed by a seven-amino acid truncated (7ND) protein and the wild-type CCL2 could block the MCP-1-mediated monocyte chemotaxis (Zhang and Rollins 1995). In the primary stages of the DR, the effect of monocytes and neutrophils has been previously reported in diabetes-induced vascular lesions of the retina (Schröder et al. 1991; Adamis 2002). Moreover, Veenstra et al. demonstrated no subclass of inflammatory monocytes in the blood samples of the CCR2-deficient mice, further disposing a monocyte deficient phenotype. Retinal capillary degeneration, leukostasis, and superoxide production also decreased significantly in the diabetic CCR2−/− animals. Moreover, reduced superoxide production and inhibition of retinal endothelial cell dysfunction were observed in the presence of in vitro co-cultured leukocytes isolated from the CCR2−/− mice with diabetes. Therefore, it could be concluded that the leukocyte-mediated degeneration of retinal capillaries had occurred due to inflammatory monocytes in diabetes, indicating the potential role of the MCP-1/CCR2 axis in DR (Veenstra and Kern 2014).
MCP-1 and monocyte recruitment into retinal tissues
Previous studies have suggested an association between the DR pathogenesis and retinal inflammation (Adamis 2002; Kern 2007). Increased levels of inflammatory mediators may also lead to chronic inflammation in the diabetic retina, leading to the leukocyte activation, adherence to the vascular walls, and migration into the retinal tissues (Goldberg 2009; Semeraro et al. 2013). Specific chemokines and related receptors such as the MCP-1/CCR2 are also responsible for the entrance of leukocytes into the retina (Feng et al. 2017). In this respect, Rangasamy et al. revealed that MCP-1 increase in the diabetic retina could change blood-retinal barrier (BRB) affecting vascular cell permeability and leukocytes’ recruitment in an animal model of DR. It was also stated that post-diabetes induction of the gene expression of the MCP-1 was significantly up-regulated in the retinas, accompanied by high levels of perivascular monocytes into the retinal tissues (Rangasamy et al. 2014). It should be highlighted that high levels of the MCP-1 are produced in retinal endothelial cells in response to high glucose levels (Rajamani and Jialal 2014) as the MCP-1 in normal conditions cannot lead to high endothelial cell permeability. The MCP-1 up-regulation may alter the retinal vascular permeability via monocyte recruitment, resulting in the absence of lesions in the diabetic MCP-1−/− mice compared with wild-type animals (Rangasamy et al. 2014).
Inflammatory biomarkers for DR
Previous studies revealed that Müller’s cells could be responsible for production and secretion of several inflammatory mediators including VEGF, MCP-1, TNF-α, IL-1β, IL-6, pigment epithelium-derived factor, matrix metalloproteinase, prostaglandin E2, tumor growth factor-β, cyclooxygenase (COX) 2, AGE receptor (RAGE), calcium binding protein B (S100B), nitric oxide (NO), and inducible NO synthase (iNOS) (Vujosevic and Simó 2017). However, in more progressive phases of DR; for instance, in PDR and diabetic macular edema aqueous, humor levels of platelet-derived growth factor (PDGF), VEGF, MCP-1, and intercellular adhesion molecule (ICAM)-1, IP-10, IL-12, IL-6, and IL-8 were remarkably elevated (Funatsu et al. 2002; Antunica et al. 2012; Cheung et al. 2012; Jonas et al. 2012; Vujosevic et al. 2016). Moreover, in vitreous fluid, concentrations of VEGF, MCP-1, IL-1β, IL-6, TNF-α, IL-8, ICAM-1, and complement components were increased (Funatsu et al. 2005a, b; Hernandez et al. 2005; Demircan et al. 2006; Funatsu et al. 2009). Evidence showed that, analysis of vitreous fluid biomarkers compared with aqueous humor might provide more reliable findings from the pathophysiological status of retinal, but it was not suitable for clinical practice except for vitrectomy because of the invasiveness of the sampling method (Vujosevic and Simó 2017). Among the inflammatory biomarkers of DR, the VEGF is more common because this mediator, having angiogenic properties, plays a significant role in the progression of DR and PDR (Boulton et al. 1998; Simó et al. 2014). However, the results of a study showed that 38% of diabetic patients were suffering from PDR and the VEGF concentrations were not detectable in vitreous fluid (Aiello et al. 1994). Such results are the reason why intravitreal anti-VEGF treatment has not been successful in a considerable part of patients with DR. These findings suggested that VEGF-independent pathways might contribute to the pathogenesis of DR (Adamis and Berman 2008; Bromberg-White et al. 2013). Therefore, improvement of therapeutic approaches for blocking other growth factors, pro-inflammatory cytokines, and involved chemokines such as MCP-1 seems to be crucial.
MCP-1 in DR
In this section, human and animal studies conducted to determine the role of the MCP-1 in the pathogenesis of DR and PDR were discussed.
Clinical studies
Long-term cohort and population-based incidence studies have determined that both hyperglycemia and hypertension are connected to an increased risk of DR development (Klein et al. 1998). Several studies have also demonstrated that enhancement of leucocyte and macrophage adhesion to the endothelium result in capillary occlusion, playing a critical role in retinal ischemia progression in DR. In addition, a relationship between macrophage infiltration, angiogenesis, and pathogenesis of the DR have reported (Esser et al. 1993, Knott et al. 1999) findings that highlight the important role of leucocytes and macrophages in the pathogenesis of the DR. The presence of intraocular macrophages at inflammation sites can be further elucidated by the intraocular amounts and biological features of the MCP-1 (Tashimo et al. 2004). Additionally, the high glucose levels in vascular cells constitute an important MCP-1 regulatory factor that is further involved in inflammatory processes related to the diabetes pathogenesis (Dragomir and Simionescu 2006). Therefore, the MCP-1 may influence the infiltration of intraocular macrophages in patients with different stages of DR.
In this respect, Matsumoto et al. revealed that the vitreous MCP-1 levels in patients with DR were significantly higher compared to those in controls. They speculated that additional investigations were required to determine the underlying mechanism but there might be a direct association between the MCP-1 levels and degree of DR progression (Matsumoto et al. 2002). In another study, Tashimo et al. examined the relationship between aqueous humor levels of MCP-1 and macrophage migration inhibitory factor (MIF) in diabetic and non-diabetic patients, demonstrating a significant correlation between the MCP-1 and the MIF levels. Furthermore, levels of these chemokines were connected to the clinical stage of DR (Tashimo et al. 2004). Ozturk et al. also conducted a study to investigate the role of the MCP-1 and the VEGF in DR. Their findings revealed elevated serum levels of the VEGF and the MCP-1 in DR patients compared to those in controls, indicating a potential regulatory effect of MCP-1 in DR that might be further used as a biomarker to evaluate diabetic patients’ risk (Ozturk et al. 2009). Wakabayshi et al. also reported elevated vitreous levels of the MCP-1 and the CXCL8 in DR patients with hypertension (Wakabayashi et al. 2011).
Moreover, a study by El-Asrar et al. showed that vascular endothelial cells and myofibroblasts in PDR patients might be able to express the MCP-1 in epiretinal membranes (El-Asrar et al. 2006). As well, Murugeswari et al. investigated the mechanism of angiogenesis in PDR based on the levels of angiogenic growth factor, pro-inflammatory cytokines, and anti-angiogenic factor in the vitreous humor. They suggested that, the elevated levels of the MCP-1 in PDR and other pro-inflammatory cytokines, such as the IL-6 and the CXCL8, were involved in the pathogenesis of induced retinal neovascularization (Murugeswari et al. 2008). Hernández et al. also conducted a study on 22 diabetic patients with PDR so as to determine the intravitreous levels of the MCP-1 in patients with PDR. The results of this study also revealed increased levels of inflammatory chemokines, including the CXCL8 and the MCP-1, in the vitreous fluid of the PDR patients and found that the intravitreous levels of both CXCL8 and MCP-1 were positively correlated with the severity of the PDR (Hernandez et al. 2005). Another study showed that the MCP-1 and the CXCL10 levels were significantly higher in samples of vitreous humor than in serum samples in PDR patients. However, there was no positive correlation between serum and vitreous humor levels. These results indicated that the myofibroblasts in PDR could express the MCP-1 and the CXCL10, while the vascular endothelial cells in the PDR membranes were able to express the MCP-1 and the CXCL12 (El-Asrar et al. 2006).
Retinopathies can induce the production of inflammatory chemokines via activation of Müller’s cells, microglial cells, and RPE ones (Rutar et al. 2015). The NF-κB is also activated by the high level of glucose, up-regulating the activity of the MCP-1 promoter and expressing the protein in glial cells (Harada et al. 2006a, b). Müller’s cells in DR patients can also secrete significant levels of the NF-κB-induced MCP-1 into the anterior chamber and vitreous cavity (Eastlake et al. 2016). In this respect, Harada et al. showed that the expression of the MCP-1 was significantly higher in patients with PDR than that in the control group, supporting that the MCP-1 protein could be colocalized with the active form of the NF-κB p50 (Harada et al. 2006a, b). Another study reported that there was a significant increase of MCP-1 in PDR patients compared to patients with ERM and macular hole (as control groups) (Suzuki et al. 2011).
As previously discussed, an in vivo angiogenesis analysis indicated that both VEGF and MCP-1 could be potent inducers of angiogenesis (Hong et al. 2005; Suzuki et al. 2016). In this respect, El-Asrar et al. reported that the levels of the MCP-1 were higher in vitreous samples obtained from PDR patients in comparison to those in controls (El-Asrar et al. 2011), supporting the pivotal role of these immune response mediators in the pathogenesis and development of PDR, as a subclinical chronic inflammation disorder. Zhou et al. also reported that inflammatory cytokines and angiogenic factors such as MCP-1, CXCL8, IL-6, IL-1, VEGF, and tumor necrosis factor had increased in the vitreous fluid of PDR patients (Zhou et al. 2012). Furthermore; highlighting the role of these inflammatory mediators in the PDR pathogenesis, another study found higher vitreous levels of the MCP-1, IL-6, and CXCL8 prior to vitrectomy in the PDR patients compared to those in controls (Yoshida et al. 2015). The MCP-1 and IL-6 levels in the collected samples were significantly higher during intraocular lens implantation compared to those collected before vitrectomy. The elevated levels of the MCP-1 and the IL-6 might also contribute to prolonged inflammation even after successful vitrectomy, which could further cause postoperative diabetic macular edema. In this respect, Reddy et al. suggested that the use of the MCP-1 as a potential biomarker may predict the development of retinopathy in patients with young-onset T2D (Reddy et al. 2017).
Previous studies have also confirmed that angiogenic factors play a crucial role in DR pathogenesis. Within these factors, cysteine-rich 61 (Cyr61) as another angiogenic mediator, could also induce the expression of the MCP-1 (You et al. 2014). However, the mechanism behind this phenomenon has not been proven yet. Consistent with previous investigations, findings by Nawaz et al. revealed that the MCP-1 levels in vitreous fluid from PDR patients were significantly higher than those in controls (Nawaz et al. 2013). Recently, El-Asrar showed a remarkably increased expression of osteoprotegerin, VEGF, and MCP-1 in vitreous samples obtained from PDR patients compared with non-diabetic subjects. Additionally, there was a significantly positive correlation between levels of the MCP-1, osteoprotegerin, and VEGF (El-Asrar et al. 2017). Taken together, over-expression of these molecules in the ocular microenvironment might be connected to angiogenesis development in patients with PDR. Conversely, few studies reported lower levels of the MCP-1 in the undiluted aqueous humor samples obtained from DR and PDR patients in comparison to non-diabetic subjects (Chen et al. 2017a, b; Yu et al. 2017). It is probable that several factors; including sample size, measurement techniques, and other unknown sources were responsible for this discrepancy.
Other studies also reported that the CD40 might constitute an important factor in retinal inflammation (Willermain et al. 2000). In this regard, Portillo et al. indicated that the CD40 in Müller’s cells was responsible for the production of retinal inflammatory factors such as MCP-1 (Portillo et al. 2014a, b). Hence, it could be concluded that down-stream signals of the CD40 and the MCP-1 expressions were involved in angiogenesis as well as recruitment of monocytes and macrophages into the retina. In this domain, another study showed that CD40 ligand (CD40L, CD154) could induce IL-1β production via activation of inflammasomes, while the CD40L-induced IL-1β production in an autocrine/paracrine manner could be responsible for MCP-1 secretion by RPE cells (Bian et al. 2018). Sassa et al. also found that, the levels of the MCP-1 had increased in the vitreous fluid of PDR patients following vitreous surgery. They revealed that the MCP-1 levels had a tendency to instantly rise subsequent to surgery and then decline with time. This study also showed that in case of a second tractional retinal detachment operation, the levels of MCP-1 were considerably higher than those with other retinal disorders; including pars plana vitrectomy, neovascular glaucoma, macular pucker, and secondary intraocular lens implant (Sassa et al. 2016).
Association between MCP-1 polymorphisms and DR pathogenesis
Genetic investigations have revealed that polymorphisms might have a serious impact on the pathogenesis of DR. For instance, a case-control study in 2013 assessed the relationship between the SNP in the MCP-1 gene and the PDR in a Korean population with T2D. The findings of this study revealed that the c.2518A/A genotype in the MCP-1 could be used as a susceptibility gene in T2D patients with an increased risk of PDR (Jeon et al. 2013). In another study, Jiang et al. reported that the 2518 GG genotype and the G allele of MCP-1 might be related to an increased risk of PDR in a Chinese Han population. It is possible that the polymorphism affects the expression of the MCP-1 gene, that may play an essential role in the pathogenesis of DR (Jiang et al. 2016). Furthermore, the association between the MCP-1 2518 A/G polymorphism and DR has attracted scholar’s interest. A meta-analysis of existing data conducted by Wang et al. in this respect proposed that the MCP-1 2518 A/G polymorphism had influenced the presence and the development of DR in T2D patients. This meta-analysis revealed that individuals with G allele and GG genotypes were more susceptible to DR; as well, a connection was reported between the MCP-1 polymorphism and presence or progression of the DR (Wang et al. 2016). It was concluded that additional studies evaluating the MCP-1 and other environmental factors as well as gene polymorphisms are further required to elucidate the mechanisms of DR, as it constitutes a multi-genetic marker.
Animal studies and basic foundations
Almost three decades ago, Schroder et al. revealed that monocytes and macrophages in a rat model could play a crucial role in the pathogenesis of DR. Particularly, they showed that the levels of blood monocytes in short-term diabetic rats had been elevated accompanied by a respective increase in retinal vessel circulation (Schröder et al. 1991). Accordingly, it was concluded that monocytes were the first leukocytes that could appear extravascularly and mediate early capillary occlusions. There is also evidence that monocytes and macrophages are involved in the release of angiogenic factors contributing to further neovascularization. In addition, macrophages might be able to discharge chemokines, inducing migration of leukocytes (Schröder et al. 1991). For example, Dong et al. in a rodent animal model study reported that up-regulation of the MCP-1 might be initiated throughout the primary stage of the DR and further elevated along with the progression of the disease. Their results also demonstrated that retinal neurons consisted of the main sources of the MCP-1 activating retinal microglial, which was associated with the pathogenesis of DR (Dong et al. 2012). As well, Das et al. in another animal study on B6.129P2-Cx3cr16-GFP mice observed increased infiltration of monocytes and amplified expression of the F4/80 gene (a marker for monocytes and macrophages) in the retinal tissue and vessels following intravitreal injection of recombinant MCP-1 (Das et al. 2012). Recently, Chen et al. in an animal model study on diabetic rats treated with quercetin observed no significant differences in the mRNA and protein expression levels of the MCP-1 between animals affected with DR and the quercetin treated group (Chen et al. 2017a, b). Müller’s cells, endothelial cells, and microglial ones in the retina of diabetic mice could also express the CD40 (Portillo et al. 2014a, b).
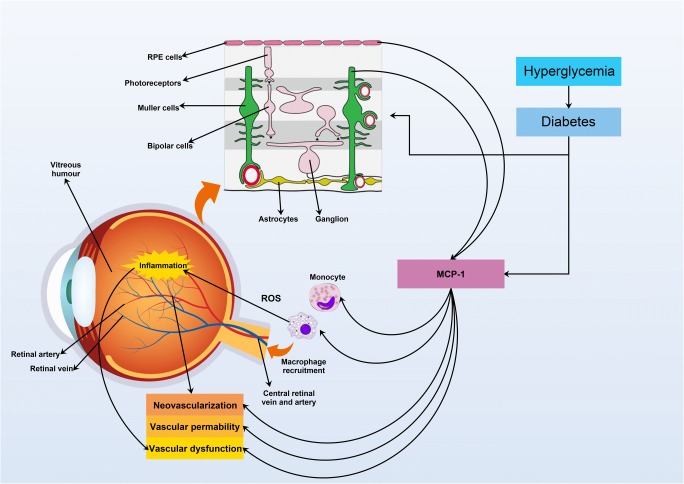
Based on the available clinical and experimental data, secretion of the MCP-1, as a potent chemotactic factor, by different types of retinal cells and through attachment to its receptor (CCR2), plays a significant role in recruitment and accumulation of monocyte/macrophage, alteration in the retinal vascular permeability, ROS formation, cell injury, inflammation and angiogenesis, as well as contribution to DR pathogenesis (Fig. 1 and Table 1).
Fig. 1.
Involvement of RPE cells, Müller cells in the production of MCP-1 in the retinal inflammation, vascular permeability, and neovascularization following hyperglycemia and diabetes in DR patients. In addition, MCP-1 is the main factor in the recruitment of monocytes and macrophages into the retina, and these type of cells are responsible for ROS production and inflammation. ROS: reactive oxygen species; MCP-1: monocyte chemotactic protein-1; RPE cell: retinal pigment epithelium cell
Table 1.
Demonstrates some clinical studies and their findings determining the association between MCP-1 and diabetic retinopathy
| Authors/Year | Type of retinopathy | Type of Specimen | Main techniques | Main results |
|---|---|---|---|---|
| Elner et al. 1995 | PDR (n = 30) | Vitreous | ELISA | • A significant increase of MCP-1 in PDR patients |
| Capeans et al. 1998 | PDR (n = 15) | Vitreous | ELISA | • A significant increase of MCP-1 in PDR patients |
| Matsumoto et al. 2002 | DR (n = 43) | Vitreous | ELISA | • A significant increase of MCP-1 in DR patients |
| Tashimo et al. 2004 | PDR (n = 24) | Aqueous humour | ELISA | • Aqueous MCP-1 levels in PDR patients were higher than DR patients |
| Hernandez et al. 2005 | PDR (n = 22) | Vitreous | ELISA | • MCP-1 was strikingly higher in diabetic patients with PDR in compare to the control group |
| El-Asrar et al. 2006 | PDR (n = 88), RD(n = 57) | Vitreous humor and serum | ELISA | • MCP-1 levels in vitreous humor samples from patients with active PDR were significantly higher than in rhegmatogenous retinal detachment (RD) |
| • MCP-1 levels in vitreous humor samples were significantly higher than in serum samples | ||||
| Harada et al., 2006a, b | PDR (n = 19), ERM (n = 16) | Epiretinal membrane and cultured Muller cells | IHC | • A significant increase of MCP-1 protein expression in PDR compared with idiopathic ERMs (control group) |
| RT-PCR | ||||
| ELISA | • Immunohistochemical analysis showed that MCP-1 protein is colocalized with active form of NF-kB p50 | |||
| Murugeswari et al. 2008 | PDR (n = 25), macular hole (MH)(n = 25) | Vitreous | ELISA | • A significant increase of MCP-1 in PDR patients than in MH patients (control group) |
| Ozturk et al. 2009 | PDR (n = 46) and DR (n = 49) | Serum | multiplex bead immunoassay | • A significant increase of MCP-1 in DR and PDR patients than in control |
| • Serum levels of MCP-1 presented a statistically significant increase with the development of DR | ||||
| El-Asrar et al. 2011 | PDR (n = 29) | Vitreous | ELISA | • MPC-1 levels were around twofold higher in active PDR patients in compare to inactive PDR patients |
| Wakabayashi et al. 2011 | DR (n = 41) | Vitreous | Flow cytometry (Cytometric Bead) Array Flex immunoassay | • A significant increase of MCP-1 in active DR patients compared with inactive diabetic patients |
| Suzuki et al. 2011 | PDR (n = 76), ERM and macular hole (n = 23) | Vitreous | array system (Bio-Plex) | • A significant increase of MCP-1 in PDR group than in ERM and macular hole (control group) |
| Zhou et al. 2012 | PDR (n = 62) | Vitreous | ELISA | • A significant increase of MCP-1 in PDR patients |
| Nawaz et al. 2013 | PDR (n = 40) | Vitreous | ELISA | • A significant increase of MCP-1 in PDR patients |
| Yoshida et al. 2015 | PDR (n = 36) | Vitreous | Flow cytometry (cytometric bead array technology) | • A significant increase of MCP-1 in PDR patients |
| Sassa et al. 2016 | PDR (n = 21) | Vitreous | ELISA | • The MCP-1 level was significantly elevated at the time of second surgery compared with the first vitrectomy. |
| Reddy et al. 2017 | PDR (n = 35) and DR (n = 35) | Serum | ELISA | • A significant increase of MCP-1 in DR and PDR patients than in control |
| El-Asrar et al. 2017 | PDR (n = 47) | Vitreous | ELISA | • A significant increase of MCP-1 in PDR patients |
| Chen et al., 2017a, b | PDR (n = 52) | Aqueous humor, serum | Multiplex bead immunoassay | • There was no significant differences between patients groups (DR and PDR) and non diabetic patients |
| DR (n = 49) | ||||
| Yu et al. 2017 | PDR (n = 10) | Undiluted vitreous humor | ELISA | • MCP-1 level was significantly lower in PDR eyes compared to non diabetic patients |
PDR; proliferative diabetic retinopathy; DR: diabetic retinopathy; ERM: epiretinal membrane; ELISA: enzyme-linked immunosorbent assay; IHC: Immunohistochemistry; RT-PCR: Real time PCR
Therapeutic targets and clinical outcomes
Previous studies reported that the levels of chemokines had significantly increased in patients with DR and the MCP-1 was the most frequently added chemokine in serum and vitreous levels (Elner et al. 1995; El-Asrar et al. 1997; Capeans et al. 1998). It should be noted that the MCP-1 plays a pivotal role in vascular inflammation through induction, activation, and recruitment of monocytes and macrophages (Yap et al. 2017). In this regard, Rangasamy et al. showed that the knockout of the MCP-1 gene in diabetic mice had an inhibitory effect on modifications of the BRB (Rangasamy et al. 2012). Then, it could be possible that the MCP-1 gene inhibition might prevent BRB alterations in patients suffering from diabetes. Additionally, transfection of the IκB mutant as a blocker of the NF-κB activation (Van Antwerp et al. 1996), could serve as an angiogenesis inhibitor (Huang et al. 2000; Oitzinger et al. 2001). Accordingly, the possible synergistic inhibition of the PDR and thus the formation of the ERM could be succeeded through mixing angiogenic factor blockers and the NF-κB-specific blocker and/or antisense oligonucleotide (Harada et al. 2006a, b). Based on a study by Harada et al. (Harada et al. 2006a, b), the MCP-1 or the CCR2 inhibition could treat DR in several clinical trials. The CCR2 inhibition also contributed to the treatment of inflammatory diseases; such as RA, multiple sclerosis, systemic lupus erythematosus, and atherosclerosis (Xia and Sui 2009).
Furthermore, previous studies showed that vitreous levels of the VEGF and the MCP-1 had considerably decreased following intravitreal injection of ranibizumab (IVR) (Murugeswari et al. 2014; Yin et al. 2016). However, the mechanism of ranibizumab in the alteration of vitreous cytokines’ profile has still remained unclear in patients with PDR (Zou et al. 2018). Different trophic factors secreted by glial cells (Harada et al. 2000, Harada, Harada et al. 2002) might also trigger the enlargement of ERMs (Mitamura et al. 2005; Harada et al. 2006a, b). Therefore, glial cells and vascular endothelial cells in ERMs can be used as novel therapeutic targets in DR. However, further investigations are required to exactly determine the activity of the NFκB, the CCR2, and the MCP-1 in different ERMs and retina resident cells to draw a conclusion on clinical purposes .
Concluding remarks
Based on the latest data; retinal resident cells including endothelial cells, Müller’s cells, microglial cells, and RPE ones with their own stimulatory effects may be involved in the neovascularization and inflammatory responses in vitreous and/or aqueous humor of DR patients through the production of MCP-1. Moreover, there might be a significant association between the clinical stages of DR and the expression levels of inflammatory and angiogenic cytokines and chemokines, principally the MCP-1. Potential therapies employing specific blockers and also neutralizing antibodies and viral vectors might be similarly developed via further explorations of the MCP-1/CCR2 axis. Likewise, understanding the mechanisms of diabetes pathogenesis in detail is required for the possible control of the DR.
Acknowledgments
This research project was supported by Rafsanjan University of Medical Sciences, Iran.
Compliance with ethical standards
Conflict of interest
All of the authors declared no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abcouwer SF (2013) Angiogenic factors and cytokines in diabetic retinopathy. J Clin Cell Immunol 1(11):1–12 [DOI] [PMC free article] [PubMed]
- Adamis A P. Is diabetic retinopathy an inflammatory disease? British Journal of Ophthalmology. 2002;86(4):363–365. doi: 10.1136/bjo.86.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamis AP, Berman AJ (2008) Immunological mechanisms in the pathogenesis of diabetic retinopathy. Seminars in immunopathology. Springer [DOI] [PubMed]
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Antunica AG, Karaman K, Znaor L, Sapunar A, Buško V, Puzović V. IL-12 concentrations in the aqueous humor and serum of diabetic retinopathy patients. Graefes Arch Clin Exp Ophthalmol. 2012;250(6):815–821. doi: 10.1007/s00417-011-1905-4. [DOI] [PubMed] [Google Scholar]
- Bachelerie Francoise, Ben-Baruch Adit, Burkhardt Amanda M., Combadiere Christophe, Farber Joshua M., Graham Gerard J., Horuk Richard, Sparre-Ulrich Alexander Hovard, Locati Massimo, Luster Andrew D., Mantovani Alberto, Matsushima Kouji, Murphy Philip M., Nibbs Robert, Nomiyama Hisayuki, Power Christine A., Proudfoot Amanda E. I., Rosenkilde Mette M., Rot Antal, Sozzani Silvano, Thelen Marcus, Yoshie Osamu, Zlotnik Albert. International Union of Basic and Clinical Pharmacology. LXXXIX. Update on the Extended Family of Chemokine Receptors and Introducing a New Nomenclature for Atypical Chemokine Receptors. Pharmacological Reviews. 2013;66(1):1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli C, Civatte M, Pellissier J, Figarella-Branger D. CCR2A and CCR2B, the two isoforms of the monocyte chemoattractant protein-1 receptor are up-regulated and expressed by different cell subsets in idiopathic inflammatory myopathies. Acta Neuropathol. 2001;102(4):385–392. doi: 10.1007/s004010100394. [DOI] [PubMed] [Google Scholar]
- Behfar Shadi, Hassanshahi Gholamhossein, Nazari Alireza, Khorramdelazad Hossein. A brief look at the role of monocyte chemoattractant protein-1 (CCL2) in the pathophysiology of psoriasis. Cytokine. 2018;110:226–231. doi: 10.1016/j.cyto.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Bian Z-M, Elner SG, Strieter RM, Kunkel SL, Lukacs NW, Elner VM. IL-4 potentiates IL-1ß-and TNF-a-stimulated IL-8 and MCP-1 protein production in human retinal pigment epithelial cells. Curr Eye Res. 1999;18(5):349–357. doi: 10.1076/ceyr.18.5.349.5353. [DOI] [PubMed] [Google Scholar]
- Bian Z-M, Field MG, Elner SG, Kahlenberg JM, Elner VM. Distinct Cd40l receptors mediate inflammasome activation and secretion of Il-1β and Mcp-1 in cultured human retinal pigment epithelial cells. Exp Eye Res. 2018;170:29–39. doi: 10.1016/j.exer.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton M, Foreman D, Williams G, McLeod D. VEGF localisation in diabetic retinopathy. Br J Ophthalmol. 1998;82(5):561–568. doi: 10.1136/bjo.82.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-White JL, Glazer L, Downer R, Furge K, Boguslawski E, Duesbery NS. Identification of VEGF-independent cytokines in proliferative diabetic retinopathy vitreous. Invest Ophthalmol Vis Sci. 2013;54(10):6472–6480. doi: 10.1167/iovs.13-12518. [DOI] [PubMed] [Google Scholar]
- Brown Z, Strieter RM, Neild GH, Thompson RC, Kunkel SL, Westwick J. IL-1 receptor antagonist inhibits monocyte chemotactic peptide 1 generation by human mesangial cells. Kidney Int. 1992;42(1):95–101. doi: 10.1038/ki.1992.266. [DOI] [PubMed] [Google Scholar]
- Capeans C, De MR, Lojo S, Salorio MS. CC chemokines in the vitreous of patients with proliferative vitreoretinopathy and proliferative diabetic retinopathy. Retina (Philadelphia, PA) 1998;18(6):546–550. [PubMed] [Google Scholar]
- Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci. 1994;91(7):2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, He T, Xing Y, Cao T. Effects of quercetin on the expression of MCP-1, MMP-9 and VEGF in rats with diabetic retinopathy. Exp Ther Med. 2017;14(6):6022–6026. doi: 10.3892/etm.2017.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhang X, Liao N, Wen F. Assessment of biomarkers using multiplex assays in aqueous humor of patients with diabetic retinopathy. BMC Ophthalmol. 2017;17(1):176. doi: 10.1186/s12886-017-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CMG, Vania M, Ang M, Chee SP, Li J. Comparison of aqueous humor cytokine and chemokine levels in diabetic patients with and without retinopathy. Mol Vis. 2012;18:830. [PMC free article] [PubMed] [Google Scholar]
- Conti, I. and B. J. Rollins (2004). CCL2 (monocyte chemoattractant protein-1) and cancer. Semin Cancer Biol, Elsevier, 14, 149, 154 [DOI] [PubMed]
- Cui Y, Xu X, Bi H, Zhu Q, Wu J, Xia X, Ren Q, Ho PC. Expression modification of uncoupling proteins and MnSOD in retinal endothelial cells and pericytes induced by high glucose: the role of reactive oxygen species in diabetic retinopathy. Exp Eye Res. 2006;83(4):807–816. doi: 10.1016/j.exer.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M, Parhami F, Gerrity R, Schwartz CJ, Fogelman AM. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci. 1990;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Rangasamy S, McGuire P. Chemokine mediated monocyte trafficking into the retina: role of inflammation in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2012;53(14):5768–5768. [Google Scholar]
- Demircan N, Safran B, Soylu M, Ozcan A, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye. 2006;20(12):1366–1369. doi: 10.1038/sj.eye.6702138. [DOI] [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interf Cytokine Res. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N, Li X, Xiao L, Yu W, Wang B, Chu L. Upregulation of retinal neuronal MCP-1 in the rodent model of diabetic retinopathy and its function in vitro. Invest Ophthalmol Vis Sci. 2012;53(12):7567–7575. doi: 10.1167/iovs.12-9446. [DOI] [PubMed] [Google Scholar]
- Dong N, Xu B, Wang B, Chu L. Study of 27 aqueous humor cytokines in patients with type 2 diabetes with or without retinopathy. Mol Vis. 2013;19:1734. [PMC free article] [PubMed] [Google Scholar]
- Dragomir E, Simionescu M. Monocyte chemoattractant protein-1–a major contributor to the inflammatory process associated with diabetes. Arch Physiol Biochem. 2006;112(4–5):239–244. doi: 10.1080/13813450601094672. [DOI] [PubMed] [Google Scholar]
- Eastlake K, Banerjee P, Angbohang A, Charteris D, Khaw P, Limb G. Müller glia as an important source of cytokines and inflammatory factors present in the gliotic retina during proliferative vitreoretinopathy. Glia. 2016;64(4):495–506. doi: 10.1002/glia.22942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Asrar AMA, Van Damme J, Put W, Veckeneer M, Dralands L, Billiau A, Missotten L. Monocyte chemotactic protein-1 in proliferative vitreoretinal disorders. Am J Ophthalmol. 1997;123(5):599–606. doi: 10.1016/s0002-9394(14)71072-4. [DOI] [PubMed] [Google Scholar]
- El-Asrar AMA, Struyf S, Kangave D, Geboes K, Van Damme J. Chemokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Eur Cytokine Netw. 2006;17(3):155–165. [PubMed] [Google Scholar]
- El-Asrar AMA, Nawaz MI, Kangave D, Geboes K, Ola MS, Ahmad S, Al-Shabrawey M. High-mobility group box-1 and biomarkers of inflammation in the vitreous from patients with proliferative diabetic retinopathy. Mol Vis. 2011;17:1829. [PMC free article] [PubMed] [Google Scholar]
- El-Asrar AMA, Struyf S, Mohammad G, Gouwy M, Rytinx P, Siddiquei MM, Hernández C, Alam K, Mousa A, De Hertogh G. Osteoprotegerin is a new regulator of inflammation and angiogenesis in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017;58(7):3189–3201. doi: 10.1167/iovs.16-20993. [DOI] [PubMed] [Google Scholar]
- Elner SG, Elner VM, Jaffe GJ, Stuart A, Kunkel SL, Strieter RM. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res. 1995;14(11):1045–1053. doi: 10.3109/02713689508998529. [DOI] [PubMed] [Google Scholar]
- Esser P, Heimann K, Wiedemann P. Macrophages in proliferative vitreoretinopathy and proliferative diabetic retinopathy: differentiation of subpopulations. Br J Ophthalmol. 1993;77(11):731–733. doi: 10.1136/bjo.77.11.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Wang X, Liu T, Zhang M, Xu G, Ni Y. Expression of CCL2 and its receptor in activation and migration of microglia and monocytes induced by photoreceptor apoptosis. Mol Vis. 2017;23:765. [PMC free article] [PubMed] [Google Scholar]
- Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002;133(1):70–77. doi: 10.1016/s0002-9394(01)01269-7. [DOI] [PubMed] [Google Scholar]
- Funatsu H, Yamashita H, Noma H, Mimura T, Nakamura S, Sakata K, Hori S. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005;243(1):3–8. doi: 10.1007/s00417-004-0950-7. [DOI] [PubMed] [Google Scholar]
- Funatsu H, Yamashita H, Sakata K, Noma H, Mimura T, Suzuki M, Eguchi S, Hori S. Vitreous levels of vascular endothelial growth factor and intercellular adhesion molecule 1 are related to diabetic macular edema. Ophthalmology. 2005;112(5):806–816. doi: 10.1016/j.ophtha.2004.11.045. [DOI] [PubMed] [Google Scholar]
- Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116(1):73–79. doi: 10.1016/j.ophtha.2008.09.037. [DOI] [PubMed] [Google Scholar]
- Gharaee-Kermani M, Denholm EM, Phan SH. Costimulation of fibroblast collagen and transforming growth factor β1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem. 1996;271(30):17779–17784. doi: 10.1074/jbc.271.30.17779. [DOI] [PubMed] [Google Scholar]
- Ghasemi H, Ghazanfari T, Yaraee R, Owlia P, Hassan ZM, Faghihzadeh S. Roles of IL-10 in ocular inflammations: a review. Ocul Immunol Inflamm. 2012;20(6):406–418. doi: 10.3109/09273948.2012.723109. [DOI] [PubMed] [Google Scholar]
- Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metabol. 2009;94(9):3171–3182. doi: 10.1210/jc.2008-2534. [DOI] [PubMed] [Google Scholar]
- Harada T, Harada C, Nakayama N, Okuyama S, Yoshida K, Kohsaka S, Matsuda H, Wada K. Modification of glial–neuronal cell interactions prevents photoreceptor apoptosis during light-induced retinal degeneration. Neuron. 2000;26(2):533–541. doi: 10.1016/s0896-6273(00)81185-x. [DOI] [PubMed] [Google Scholar]
- Harada T, Harada C, Mitamura Y, Akazawa C, Ohtsuka K, Ohno S, Takeuchi S, Wada K. Neurotrophic factor receptors in epiretinal membranes after human diabetic retinopathy. Diabetes Care. 2002;25(6):1060–1065. doi: 10.2337/diacare.25.6.1060. [DOI] [PubMed] [Google Scholar]
- Harada C, Mitamura Y, Harada T. The role of cytokines and trophic factors in epiretinal membranes: involvement of signal transduction in glial cells. Prog Retin Eye Res. 2006;25(2):149–164. doi: 10.1016/j.preteyeres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Harada C, Okumura A, Namekata K, Nakamura K, Mitamura Y, Ohguro H, Harada T. Role of monocyte chemotactic protein-1 and nuclear factor kappa B in the pathogenesis of proliferative diabetic retinopathy. Diabetes Res Clin Pract. 2006;74(3):249–256. doi: 10.1016/j.diabres.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Harkness K, Sussman J, Davies-Jones G, Greenwood J, Woodroofe M. Cytokine regulation of MCP-1 expression in brain and retinal microvascular endothelial cells. J Neuroimmunol. 2003;142(1):1–9. doi: 10.1016/s0165-5728(03)00251-0. [DOI] [PubMed] [Google Scholar]
- Hernandez C, Segura R, Fonollosa A, Carrasco E, Francisco G, Simo R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet Med. 2005;22(6):719–722. doi: 10.1111/j.1464-5491.2005.01538.x. [DOI] [PubMed] [Google Scholar]
- Hong KH, Ryu J, Han KH. Monocyte chemoattractant protein-1–induced angiogenesis is mediated by vascular endothelial growth factor-a. Blood. 2005;105(4):1405–1407. doi: 10.1182/blood-2004-08-3178. [DOI] [PubMed] [Google Scholar]
- Huang S, Robinson JB, DeGuzman A, Bucana CD, Fidler IJ. Blockade of nuclear factor-κB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Res. 2000;60(19):5334–5339. [PubMed] [Google Scholar]
- Jeon HJ, Choi HJ, Park BH, Lee YH, Oh T. Association of monocyte chemoattractant protein-1 (MCP-1) 2518A/G polymorphism with proliferative diabetic retinopathy in Korean type 2 diabetes. Yonsei Med J. 2013;54(3):621–625. doi: 10.3349/ymj.2013.54.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Hennein L, Xu Y, Bao N, Coh P, Tao L. Elevated serum monocyte chemoattractant protein-1 levels and its genetic polymorphism is associated with diabetic retinopathy in Chinese patients with type 2 diabetes. Diabet Med. 2016;33(1):84–90. doi: 10.1111/dme.12804. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Jonas RA, Neumaier M, Findeisen P. Cytokine concentration in aqueous humor of eyes with diabetic macular edema. Retina. 2012;32(10):2150–2157. doi: 10.1097/IAE.0b013e3182576d07. [DOI] [PubMed] [Google Scholar]
- Kern TS (2007) Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. J Diabetes Res 2007 [DOI] [PMC free article] [PubMed]
- Kim MJ, Tam FW. Urinary monocyte chemoattractant protein-1 in renal disease. Clin Chim Acta. 2011;412(23):2022–2030. doi: 10.1016/j.cca.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin epidemiologic study of diabetic retinopathy: XVII: the 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes11Proprietary interest: none. Ophthalmology. 1998;105(10):1801–1815. doi: 10.1016/S0161-6420(98)91020-X. [DOI] [PubMed] [Google Scholar]
- Klein BE, Horak KL, Maynard JD, Lee KE, Klein R. Association of Skin Intrinsic Fluorescence with retinal microvascular complications of long term type 1 diabetes in the Wisconsin epidemiologic study of diabetic retinopathy. Ophthalmic Epidemiol. 2017;24(4):211–216. doi: 10.1080/09286586.2016.1269934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott R, Robertson M, Muckersie E, Folefac V, Fairhurst F, Wileman S, Forrester J. A model system for the study of human retinal angiogenesis: activation of monocytes and endothelial cells and the association with the expression of the monocarboxylate transporter type 1 (MCT-1) Diabetologia. 1999;42(7):870–877. doi: 10.1007/s001250051240. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Matta P, Krikun G, Koopman LA, Masch R, Toti P, Arcuri F, Huang S-TJ, Funai EF, Schatz F. Regulation of monocyte chemoattractant protein-1 expression by tumor necrosis factor-α and interleukin-1β in first trimester human decidual cells: implications for preeclampsia. Am J Pathol. 2006;168(2):445–452. doi: 10.2353/ajpath.2006.050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A. The chemokine system: redundancy for robust outputs. Immunol Today. 1999;20(6):254–257. doi: 10.1016/s0167-5699(99)01469-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Takahashi M, Ogata M. Relationship between glycoxidation and cytokines in the vitreous of eyes with diabetic retinopathy. Jpn J Ophthalmol. 2002;46(4):406–412. doi: 10.1016/s0021-5155(02)00508-7. [DOI] [PubMed] [Google Scholar]
- Mitamura Y, Takeuchi S, Matsuda A, Tagawa Y, Mizue Y, Nishihira J. Monocyte chemotactic protein-1 in the vitreous of patients with proliferative diabetic retinopathy. Ophthalmologica. 2001;215(6):415–418. doi: 10.1159/000050900. [DOI] [PubMed] [Google Scholar]
- Mitamura Y, Harada C, Harada T. Role of cytokines and trophic factors in the pathogenesis of diabetic retinopathy. Curr Diabetes Rev. 2005;1(1):73–81. doi: 10.2174/1573399052952596. [DOI] [PubMed] [Google Scholar]
- Murugeswari P, Shukla D, Rajendran A, Kim R, Namperumalsamy P, Muthukkaruppan V. PROINFLAMMATORY CYTOKINES AND ANGIOGENIC AND ANTI-ANGIOGENIC FACTORS IN VITREOUS OF PATIENTS WITH PROLIFERATIVE DIABETIC RETINOPATHY AND EALES’DISEASE. Retina. 2008;28(6):817–824. doi: 10.1097/IAE.0b013e31816576d5. [DOI] [PubMed] [Google Scholar]
- Murugeswari P, Shukla D, Kim R, Namperumalsamy P, Stitt AW, Muthukkaruppan V. Angiogenic potential of vitreous from proliferative diabetic retinopathy and Eales' disease patients. PLoS One. 2014;9(10):e107551. doi: 10.1371/journal.pone.0107551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz M, Van Raemdonck K, Mohammad G, Kangave D, Van Damme J, El-Asrar AA, Struyf S. Autocrine CCL2, CXCL4, CXCL9 and CXCL10 signal in retinal endothelial cells and are enhanced in diabetic retinopathy. Exp Eye Res. 2013;109:67–76. doi: 10.1016/j.exer.2013.01.008. [DOI] [PubMed] [Google Scholar]
- O’Connor T, Borsig L, Heikenwalder M. CCL2-CCR2 signaling in disease pathogenesis. Endocr Metab Immune Disord Drug Targets. 2015;15(2):105–118. doi: 10.2174/1871530315666150316120920. [DOI] [PubMed] [Google Scholar]
- Oitzinger W, Hofer-Warbinek R, Schmid JA, Koshelnick Y, Binder BR, de Martin R. Adenovirus-mediated expression of a mutant IκB kinase 2 inhibits the response of endothelial cells to inflammatory stimuli. Blood. 2001;97(6):1611–1617. doi: 10.1182/blood.v97.6.1611. [DOI] [PubMed] [Google Scholar]
- Outtz HH, Tattersall IW, Kofler NM, Steinbach N, Kitajewski J. Notch1 controls macrophage recruitment and notch signaling is activated at sites of endothelial cell anastomosis during retinal angiogenesis in mice. Blood. 2011;118(12):3436–3439. doi: 10.1182/blood-2010-12-327015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk BT, Bozkurt B, Kerimoglu H, Okka M, Kamis U, Gunduz K. Effect of serum cytokines and VEGF levels on diabetic retinopathy and macular thickness. Mol Vis. 2009;15:1906. [PMC free article] [PubMed] [Google Scholar]
- Patel J, Saleh G, Hykin P, Gregor Z, Cree I. Concentration of haemodynamic and inflammatory related cytokines in diabetic retinopathy. Eye. 2008;22(2):223–228. doi: 10.1038/sj.eye.6702584. [DOI] [PubMed] [Google Scholar]
- Portillo J-AC, Greene JA, Okenka G, Miao Y, Sheibani N, Kern TS, Subauste CS. CD40 promotes the development of early diabetic retinopathy in mice. Diabetologia. 2014;57(10):2222–2231. doi: 10.1007/s00125-014-3321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo J-AC, Schwartz I, Zarini S, Bapputty R, Kern TS, Gubitosi-Klug RA, Murphy RC, Subauste MC, Subauste CS. Proinflammatory responses induced by CD40 in retinal endothelial and Müller cells are inhibited by blocking CD40-Traf2, 3 or CD40-Traf6 signaling. Invest Ophthalmol Vis Sci. 2014;55(12):8590–8597. doi: 10.1167/iovs.14-15340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamani U, Jialal I. Hyperglycemia induces toll-like receptor-2 and-4 expression and activity in human microvascular retinal endothelial cells: implications for diabetic retinopathy. J Diabetes Res. 2014;2014:1–15. doi: 10.1155/2014/790902. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Das Arup, Rangasamy Sampathkumar, McGuire PaulG. Diabetic retinopathy and inflammation: Novel therapeutic targets. Middle East African Journal of Ophthalmology. 2012;19(1):52. doi: 10.4103/0974-9233.92116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy S, McGuire PG, Nitta CF, Monickaraj F, Oruganti SR, Das A. Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS One. 2014;9(10):e108508. doi: 10.1371/journal.pone.0108508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S, Amutha A, Rajalakshmi R, Bhaskaran R, Monickaraj F, Rangasamy S, Anjana RM, Abhijit S, Gokulakrishnan K, Das A, Mohan V, Balasubramanyam M. Association of increased levels of MCP-1 and cathepsin-D in young onset type 2 diabetes patients (T2DM-Y) with severity of diabetic retinopathy. J Diabetes Complicat. 2017;31(5):804–809. doi: 10.1016/j.jdiacomp.2017.02.017. [DOI] [PubMed] [Google Scholar]
- Rutar M, Natoli R, Chia R, Valter K, Provis JM. Chemokine-mediated inflammation in the degenerating retina is coordinated by Müller cells, activated microglia, and retinal pigment epithelium. J Neuroinflammation. 2015;12(1):8. doi: 10.1186/s12974-014-0224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SK, Crean SM, Boxer PA, Kellner D, LaRosa GJ, Hunt SW. Functional differences between monocyte chemotactic protein-1 receptor a and monocyte chemotactic protein-1 receptor B expressed in a Jurkat T cell. J Immunol. 2000;165(9):4877–4883. doi: 10.4049/jimmunol.165.9.4877. [DOI] [PubMed] [Google Scholar]
- Sassa Y, Yoshida S, Ishikawa K, Asato R, Ishibashi T, Kono T. The kinetics of VEGF and MCP-1 in the second vitrectomy cases with proliferative diabetic retinopathy. Eye. 2016;30(5):746–753. doi: 10.1038/eye.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder S, Palinski W, Schmid-Schönbein G. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol. 1991;139(1):81. [PMC free article] [PubMed] [Google Scholar]
- Semeraro F, Bamonte G, Cifariello F, Romano MR, Costagliola C (2013) Vitreous mediators in retinal hypoxic diseases. Mediat Inflamm 2013 [DOI] [PMC free article] [PubMed]
- Semeraro F, Cancarini A, Rezzola S, Romano M, Costagliola C. Diabetic retinopathy: vascular and inflammatory disease. J Diabetes Res. 2015;2015:1–16. doi: 10.1155/2015/582060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simó R, Sundstrom JM, Antonetti DA. Ocular anti-VEGF therapy for diabetic retinopathy: the role of VEGF in the pathogenesis of diabetic retinopathy. Diabetes Care. 2014;37(4):893–899. doi: 10.2337/dc13-2002. [DOI] [PubMed] [Google Scholar]
- Standiford TJ, Kunkel S, Phan S, Rollins B, Strieter R. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J Biol Chem. 1991;266(15):9912–9918. [PubMed] [Google Scholar]
- Suzuki Y, Nakazawa M, Suzuki K, Yamazaki H, Miyagawa Y. Expression profiles of cytokines and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. Jpn J Ophthalmol. 2011;55(3):256–263. doi: 10.1007/s10384-011-0004-8. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Suzuki K, Kudo T, Metoki T, Nakazawa M. Level of vascular endothelial growth factor in the vitreous fluid of proliferative diabetic retinopathy patients and prognosis after vitrectomy. Ophthalmologica. 2016;236(3):133–138. doi: 10.1159/000449261. [DOI] [PubMed] [Google Scholar]
- Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30(5):343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashimo A, Mitamura Y, Nagai S, Nakamura Y, Ohtsuka K, Mizue Y, Nishihira J. Aqueous levels of macrophage migration inhibitory factor and monocyte chemotactic protein-1 in patients with diabetic retinopathy. Diabet Med. 2004;21(12):1292–1297. doi: 10.1111/j.1464-5491.2004.01334.x. [DOI] [PubMed] [Google Scholar]
- Ueda A, Okuda K, Ohno S, Shirai A, Igarashi T, Matsunaga K, Fukushima J, Kawamoto S, Ishigatsubo Y, Okubo T. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol. 1994;153(5):2052–2063. [PubMed] [Google Scholar]
- Vakilian A, Khorramdelazad H, Heidari P, Rezaei ZS, Hassanshahi G. CCL2/CCR2 signaling pathway in glioblastoma multiforme. Neurochem Int. 2017;103(1–7):1–7. doi: 10.1016/j.neuint.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274(5288):787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- Van Coillie E, Van Damme J, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999;10(1):61–86. doi: 10.1016/s1359-6101(99)00005-2. [DOI] [PubMed] [Google Scholar]
- Veenstra AA, Kern T. Role of inflammatory CCR2+ monocytes in early stage diabetic retinopathy. Invest Ophthalmol Vis Sci. 2014;55(13):1050–1050. [Google Scholar]
- Vujosevic S, Simó R. Local and systemic inflammatory biomarkers of diabetic retinopathy: an integrative approach. Invest Ophthalmol Vis Sci. 2017;58(6):BIO68–BIO75. doi: 10.1167/iovs.17-21769. [DOI] [PubMed] [Google Scholar]
- Vujosevic S, Micera A, Bini S, Berton M, Esposito G, Midena E. Proteome analysis of retinal glia cells-related inflammatory cytokines in the aqueous humour of diabetic patients. Acta Ophthalmol. 2016;94(1):56–64. doi: 10.1111/aos.12812. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Usui Y, Okunuki Y, Kezuka T, Takeuchi M, Iwasaki T, Ohno A, Goto H. Increases of vitreous monocyte chemotactic protein 1 and interleukin 8 levels in patients with concurrent hypertension and diabetic retinopathy. Retina. 2011;31(9):1951–1957. doi: 10.1097/IAE.0b013e31820d3cee. [DOI] [PubMed] [Google Scholar]
- Wang W, He M, Huang W. Association of monocyte chemoattractant protein-1 gene 2518A/G polymorphism with diabetic retinopathy in type 2 diabetes mellitus: a meta-analysis. Diabetes Res Clin Pract. 2016;120:40–46. doi: 10.1016/j.diabres.2016.07.016. [DOI] [PubMed] [Google Scholar]
- Wells T, Power CA, Lusti-Narasimhan M, Hoogewerf AJ, Cooke RM, Chung C, Peitsch M, Proudfoot A. Selectivity and antagonism of chemokine receptors. J Leukoc Biol. 1996;59(1):53–60. doi: 10.1002/jlb.59.1.53. [DOI] [PubMed] [Google Scholar]
- Willermain F, Caspers-Velu L, Baudson N, Dubois C, Hamdane M, Willems F, Velu T, Bruyns C. Role and expression of CD40 on human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2000;41(11):3485–3491. [PubMed] [Google Scholar]
- Xia M, Sui Z. Recent developments in CCR2 antagonists. Expert Opin Ther Pat. 2009;19(3):295–303. doi: 10.1517/13543770902755129. [DOI] [PubMed] [Google Scholar]
- Yap H, Frankel A, Tam F (2017) Review article-MCP-1: a potential target for diabetic microvascular complications. Urol Nephrol Open Access J 5(3). 10.15406/unoaj.2017.05.00171
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen S-J, Dekker JM, Fletcher A, Grauslund J. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo TK, Ahad MA, Kuo N-w, Spagnolo P, Menezo V, Lympany P, Lightman S. Chemokine gene polymorphisms in idiopathic anterior uveitis. Cytokine. 2006;35(1):29–35. doi: 10.1016/j.cyto.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Yin H, Fang X, Ma J, Chen M, Yang Y, Guo S, Chen Z, Su Z, Feng L, Ye P. Idiopathic choroidal neovascularization: intraocular inflammatory cytokines and the effect of intravitreal ranibizumab treatment. Sci Rep. 2016;6:31880. doi: 10.1038/srep31880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon B-y, Ju J-h, Jung YO, Jhun J-y, Park M-k, Park S-h, Cho C-s, Kim H-y. Expression of CCR2A, an isoform of MCP-1 receptor, is increased by MCP-1, CD40 ligand and TGF-[beta] in fibroblast like synoviocytes of patients with RA. Exp Mol Med. 2007;39(4):499. doi: 10.1038/emm.2007.55. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Kubo Y, Kobayashi Y, Zhou Y, Nakama T, Yamaguchi M, Tachibana T, Ishikawa K, Arita R, Nakao S. British Journal of ophthalmology: bjophthalmol-2014-306366. 2015. Increased vitreous concentrations of MCP-1 and IL-6 after vitrectomy in patients with proliferative diabetic retinopathy: possible association with postoperative macular oedema; pp. 960–966. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Robinson E, Tanaka S, Appella E, Leonard E. Purification and amino acid analysis of two human monocyte chemoattractants produced by phytohemagglutinin-stimulated human blood mononuclear leukocytes. J Immunol. 1989;142(6):1956–1962. [PubMed] [Google Scholar]
- You J-J, Yang C-H, Yang C-M, Chen M-S. Cyr61 induces the expression of monocyte chemoattractant protein-1 via the integrin ανβ3, FAK, PI3K/Akt, and NF-κB pathways in retinal vascular endothelial cells. Cell Signal. 2014;26(1):133–140. doi: 10.1016/j.cellsig.2013.08.026. [DOI] [PubMed] [Google Scholar]
- Yu Y, Zhang J, Zhu R, Zhao R, Chen J, Jin J, Tian Y, Su S. The profile of Angiogenic factors in vitreous humor of the patients with proliferative diabetic retinopathy. Curr Mol Med. 2017;17(4):280–286. doi: 10.2174/1566524017666171106111440. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Rollins BJ. A dominant negative inhibitor indicates that monocyte chemoattractant protein 1 functions as a dimer. Mol Cell Biol. 1995;15(9):4851–4855. doi: 10.1128/mcb.15.9.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Liu H, Al-Shabrawey M, Caldwell RW, Caldwell RB. Inflammation and diabetic retinal microvascular complications. J Cardiovasc Dis Res. 2011;2(2):96–103. doi: 10.4103/0975-3583.83035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Wenbo, Liu Hua, Al-Shabrawey Mohamed, Caldwell Robert W., Caldwell Ruth B. Inflammation and diabetic retinal microvascular complications. Journal of Cardiovascular Disease Research. 2011;2(2):96–103. doi: 10.4103/0975-3583.83035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang J, Zeng L, Huang H, Yang M, Fu X, Tian C, Xiang Z, Huang J, Fan H. The-2518A/G polymorphism in the MCP-1 gene and tuberculosis risk: a meta-analysis. PLoS One. 2012;7(7):e38918. doi: 10.1371/journal.pone.0038918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang S, Xia X. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr Eye Res. 2012;37(5):416–420. doi: 10.3109/02713683.2012.661114. [DOI] [PubMed] [Google Scholar]
- Zou C, Han C, Zhao M, Yu J, Bai L, Yao Y, Gao S, Cao H, Zheng Z. Change of ranibizumab-induced human vitreous protein profile in patients with proliferative diabetic retinopathy based on proteomics analysis. Clin Proteomics. 2018;15(1):12. doi: 10.1186/s12014-018-9187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]