Abstract
People with diabetes mellitus have shorter telomeres compared with non-diabetic subjects. The aim of this study was to investigate an in-vitro model of telomere shortening under diabetes metabolic conditions. The mechanisms of the accelerated telomere length attrition and the potential telomere protective action of fenofibrate with related cellular mechanisms were also examined. Human dermal fibroblasts were passaged and cultured in normal (5.5 mM) or high (25 mM) D-glucose, across 7 days with hydrogen peroxide (H2O2), glucosamine (GA), or glycated albumin (AGEs-BSA). Relative telomere length (RTL) was determined by qPCR. The expression of shelterin complex members which regulate telomere stability were measured by qRT-PCR and Western immunoblot. Culture in high glucose decreased RTL compared with normal glucose: H2O2 and GA lowered the RTL after 7 days (each P < 0.05 vs untreated control), whereas AGEs-BSA had no effect compared with control-BSA. At day 7 the mRNA levels of most shelterin complex members, were induced by H2O2 and to a lesser extent by GA. Trf1 and Trf2 protein were induced by H2O2. Co-treatment with fenofibrate (100 μM) significantly attenuated the reduction in RTL caused by H2O2 and GA and prevented Trf induction by H2O2. However knockdown of Trf1 and Trf2 expression using specific siRNA did not prevent H2O2 effects to lower RTL, thus implicating factors other than these Trfs alone in the fenofibrate protection against the H2O2 induction of RTL lowering. These in vitro findings demonstrate that diabetic conditions can induce telomere shortening and that fenofibrate has protective effects on telomere attrition, through as yet undefined mechanisms.
Electronic supplementary material
The online version of this article (10.1007/s12079-019-00521-x) contains supplementary material, which is available to authorized users.
Keywords: Diabetes, Glucosamine, Oxidative-stress, Premature-aging, Telomere
Introduction
Diabetes mellitus is known to cause injury to organs including as a premature form of aging, which adversely affects tissue function and induces structural and molecular changes (Tamura et al. 2016). Telomeres are repeated base pair repeat DNA sequences of TTAGGG, sited on the free ends of chromosomes, and telomere length attrition is widely accepted to be a marker of biological aging (Tamura et al. 2016). Telomere shortening occurs in both type 1 and type 2 diabetes (Tamura et al. 2016), but how this occurs is not certain. Telomere length is maintained by multiple mechanisms. The reverse transcriptase enzyme, telomerase, is an important regulator in cancer and immortalized cells, but is only minimally active in primary cultures of eukaryotic cells (Greider 1996). The shelterin complex, whose main purpose appears to be in protection of the telomere from attrition protection, is present in eukaryotic cells (Smogorzewska et al. 2000; van Steensel and de Lange 1997). The complex consists of six members: TRF1, TRF2, TPP1, TINF2, POT1 and RAP1, of these, only TRF1 and TRF2, can bind to telomere double strand sequences (de Lange 2005). TRF1 and TRF2 are in some systems negative regulators of telomere length (Smogorzewska et al. 2000). The role of shelterin complex members in telomere length homeostasis under many pathological conditions, including in diabetes, is not well understood.
One standing hypothesis about cellular mechanisms of diabetes induced injury to organs places reactive oxidative species (ROS) at the centre of this paradigm (Brownlee 2005). A proposed unifying mechanism suggests that hyperglycemia induces cellular ROS, which further activates nutrient sensing overflow metabolic pathways including the polyol pathway (influx of NADP+ and NADH), the glucosamine pathway, activation of protein kinase C and induction of advanced glycation end products (AGEs) (Brownlee 2005). Fenofibrate is a fibric acid derivative that can correct common dyslipidemia present in diabetes mellitus. Two independent clinical trials, FIELD and ACCORD-lipid, each reported that fenofibrate is beneficial for diabetes subjects, ameliorated end organ damage (Shipman et al. 2016), the mechanism is thought to be via its potent antioxidant actions (Noonan et al. 2013). Whether fenofibrate is able to regulate telomere length in the presence of diabetes, or otherwise is not known. Nor is it known if fenofibrate would prevent the injury caused by ROS under diabetes conditions.
Some studies investigating telomere length have reported that it is negatively impacted by DNA damage due to UV-irradiation and by ROS (hydrogen peroxide) (Ludlow et al. 2014; Ma et al. 2012; Maeda et al. 2013). In this study, we developed an in-vitro system to firstly investigate the impact of metabolic pathways implicated in organ damage in diabetes on telomere length, Secondly, to assess whether telomere length may associate with regulation of TRF1 and TRF2, and finally whether fenofibrate (FF) may regulate telomere length and TRFs.
Materials and methods
Cell culture
Human dermal fibroblasts (CCD-1079Sk, ATCC® CRL-2097™) were used to examine the impact of diabetes conditions on telomere length. Fibroblasts were maintained in 10% fetal bovine serum (FBS, AusgeneX, Australia) in DMEM, (Gibco® Life Technologies) containing 5.5 mM or 25.0 mM D-Glucose and L-glutamine. Fibroblasts were cultured at 37 °C and sub-cultured every seven days. Cells were seeded at 7,400cells/cm2 or as described.
Cell culture treatments
Advanced glycation end-products (AGEs) were prepared in-house as previously described (Yamagishi et al. 1997). The AGEs-BSA presence and bioactivity was confirmed by measurement of fluorescence at 390 nM and the ability to induce fibronectin and CTGF mRNA. (Twigg et al. 2001). The concentration of AGEs-BSA and control-BSA used was 250 μg/mL (~3.8 mM) (Twigg et al. 2001). Glucosamine (GA: Sigma-Aldrich, USA) was reconstituted in sterile deionized water, diluted in media and added to 5 mM. The ROS generator, hydrogen peroxide (H2O2: Merck), was prepared fresh and diluted in sterile DMEM media to deliver a final concentration of 25 μM. Fenofibrate (FF: Sigma-Aldrich) was reconstituted in DMSO and added at 100 μM. The above treatments were added to cells after seeding and media containing treatments was re-applied every 3 days. Cells were harvested, from day 0 as baseline, then at day 7 to day 28.
Determination of relative telomere length
The adapted qPCR method (Cawthon 2002) amplifies the telomeric region (T) of genes using degenerate primers. The single copy gene 36B4 (S) was used as a loading control (for primer details see Table 1). The telomere (T) mastermix contained SensiMix SYBr™ (0.75x:Bioline), MgCl2 (2.8 mM:Bioline) and TelAFa forward (150 nM) and RTel2 reverse (300 nM). Samples were amplified on the Corbett Rotor Gene RG-3000 (Corbett Life Science, Australia) as follows: 95 °C for 10 min, 30 cycles of 95 °C for 15 s and 56 °C for 60s. The S mastermix contained SensiMix SYBr (0.75x), MgCl2 (3.0 mM), 36B4U forward (150 mM) and 36B4D reverse (250 mM). Samples were amplified at 95 °C for 10 min, then 35 cycles of 95 °C for 15 s, 60 °C for 30s and 72 °C for 30s. The cycle threshold was obtained and the relative telomere length (RTL) was expressed as a ratio, T/S, normalised to the gDNA control (Cawthon 2002).
Table 1.
Primer sequences used for RTL measurement and mRNA expression
| qPCR primers for RTL | Sequences (5′ – 3′) |
|---|---|
| Telomere† | |
| TelAFa | CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT |
| RTel2 | GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT |
| 36B4 single copy gene‡ | |
| 36B4(F) | CAGCAAGTGGGAAGGTGTAATCC |
| 36B4(R) | CCCATTCTATCATCAACGGGTACAA |
| Real time qPCR primers | Sequences (5′ – 3′) |
| Trf1 | |
| (F) | TCTAATGAAGGCAGCGGCAA |
| (R) | ACCGCAGACTGTTTGTCACTA |
| Trf2 | |
| (F) | AGAGAAGAATCCCAAAGTACCCA |
| (R) | TACTGTCTTCATCTGGTGCTG |
| Tpp1 | |
| (F) | CGGGTGTTGGTCTGTCTCTG |
| (R) | AGGTTCCTGGAAGGATGTGC |
| Tinf2 | |
| (F) | CTAAAGGCCAAGGTGGTGGT |
| (R) | CCTGCTTTGTAGCCTTGGGA |
| Rap1 | |
| (F) | CGGGAGGGGGTAGCTATTCT |
| (R) | GTCCCTCACGAACAGAGTCG |
| Pot1 | |
| (F) | TTGAGGGAACTTTGGGAGCC |
| (R) | GATGCCCAAACACGTAAGGC |
| 18S | |
| (F) | CGGCTACCACATCCAAGGAA |
| (R) | GCTGGAATTACCGCGGCT |
Gene expression of Shelterin complex members
Total RNA was extracted from ~9 × 104 cells using TRI Reagent (Sigma Aldrich) as per the manufacturers’ instructions. The RNA quantity and quality was determined by NanoDrop (ND-1000, ThermoFisher Scientific) and all samples had A260:A280 ratio between 1.90 and 2.00. Total RNA (1 μg) was reversed transcribed using 50 pmol of Oligo(dT)12–18 and 0.4 pmol of Random Hexamers (Life Technology). Shelterin complex mRNA levels were determined using SensiMix™ SYBr® (Bioline) and 200 nM of each primer (Table 1). Their expression was calculated using the delta/delta method with ribosomal S18 RNA (S18) as the house-keeper.
Trf1 and Trf2 siRNA treatment
To investigate the role of Trf1 and Trf2 in maintenance of telomere length, fibroblasts were treated with small interference RNA (siRNA). The Trf1 siRNA and Trf2 siRNA and each scrambled siRNA sequences were computer-generated and verified using primer BLAST (Ye et al. 2012) and purchased from Sigma-Aldrich (Table 2). Cells were seeded at 10,000cells/cm2, next day they were washed twice in PBS and the siRNAs to Trf1 and Trf2, were added (10.0 nM final) in the presence of N-TER™ (Sigma-Aldrich) in serum free conditions in 25 mM D-glucose DMEM for 4 h. FBS (10%) was added and next day the cells were washed with PBS and the different treatments (GA or H2O2) were added, with or without FF. Four days later the media was changed and the cells were collected at day 8 post transfection.
Table 2.
The siRNA and scramble sequences used
| siRNA | Sequences (5′ – 3′) |
|---|---|
| Trf1 siRNA† | |
| Trf1 siRNA sense | CAAACAGUCUGCGGUAACU |
| Trf1 siRNA anti-sense | AGUUACCGCAGACUGUUUG |
| Trf2 siRNA‡ | |
| Trf2 siRNA sense | CCCAAAGUACCCAAAGGCA |
| Trf2 siRNA anti-sense | UGCCUUUGGGUACUUUGGG |
| scramble Trf1 control | |
| Scr1 siRNA sense | ACGCAUCGACGAAUAUCUG |
| Scr1 siRNA anti-sense | AGCUCUAUACGUGUGGCUA |
| scramble Trf2 control | |
| Scr2 siRNA sense | AGAUCGAACCGAACCACAC |
| Scr2 siRNA anti-sense | AUUGGUGUGCGUUGCGUCU |
†Trf1 siRNA (Hs01 00051035) and ‡Trf2 (Hs01 00188701) siRNA were supplied from Sigma-Aldrich, NSW
Protein level ofTRF1andTRF2
Protein was extracted from ~9 × 104 cells using RIPA buffer containing cOmplete™ Protease Inhibitor Cocktail (Roche) and the concentration was determined using the DC™ Protein Assay (BioRad). For TRF1, samples were reduced under non-denaturing conditions; while for TRF2 samples were reduced and denatured. Samples containing 25 μg protein were run on 12% Mini-Protean® TGX Stain-Free™ Gel (BioRad), transferred to 0.2μΜ nitrocellulose membranes by TransTurbo Blot (BioRad). Stain-Free trihalocompound (BioRad) was used as an immuno-blot loading control (Gurtler et al. 2013). The membranes were blocked in skim milk (5%) for TRF1 and BSA (1%) for TRF2 prior to incubation in anti-TRF1 or anti-TRF2 antibodies (AbCam) respectively. Membranes were then incubated in goat anti-rabbit IgG-peroxidase antibody (Sigma-Aldrich) and signal detected with Clarity™ ECL Substrate (BioRad) using the ChemiDoc MP™ (BioRad). The area of the protein band(s) of interest, was performed by ImageLab™ Software V4.1 (BioRad).
Senescence biomarker measurement
The senescence associated beta-galactosidase (SA-βGal) enzyme activity was measured according to Dimri et al. (Dimri et al. 1995). Cells were seeded in Nunc™ Lab-Tek™ Chamber Slides at ~1.3 × 104 cells/chamber and treated with GA or H2O2, with or without FF. As before media was changed at day 3 post seeding. At day 7, cells were fixed in 3% formaldehyde (Sigma-Aldrich) and incubated at 37 °C for 16 h in freshly prepared SA-βGal stain solution. Cells were washed with PBS and rinsed twice with methanol (100%, Sigma-Aldrich). Cell uptake of SA-βGal was imaged on an Olympus BX53 microscope (Olympus Life Science) at 250x magnification. SA-βGal blue activity was quantified in 10 images/chamber using ImageJ v1.47. The SA-βGal activity was expressed as an average of the percentage blue positive area for each chamber.
Statistical analysis
Prism GraphPad v6.1 (GraphPad Software Inc) was used to assemble data and to perform statistics analysis. Data in this manuscript is from n = 3–5 independent experiments and is presented as mean ± SEM. One way ANOVA and t-tests (2 tailed) were undertaken as described.
Results
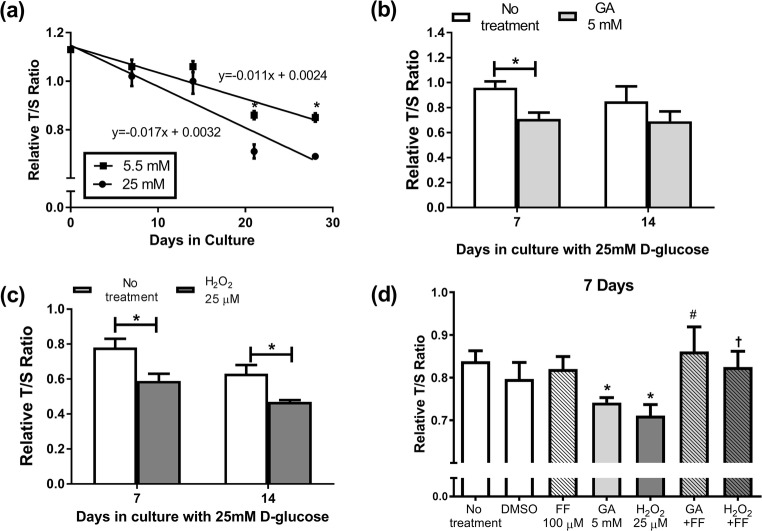
Primary cultures of s CRL-2097™ fibroblasts were used to examine the impact of a diabetic environment on telomeres. Cells were grown and sub-cultured in normal (5.5 mM) and high (25 mM) glucose to mimic the hyperglycaemia present in diabetes. High glucose (25 mM) had no significant impact on RTL at day 7 or day 14. However. from day 21 onwards, the RTL of cells grown in high 25 mM D-glucose was significantly lower than cells grown in 5.5 mM D-glucose (P < 0.05 for days 21 and 28) (Fig. 1a). The decrease in RTL by cells cultured in 25 mM D-glucose was on average 4% faster than cells in 5.5 mM D-glucose, but the overall rate was not significantly different between them. In order to mimic the diabetic environment, cells were cultured in 25 mM D-glucose throughout all future experiments.
Fig. 1.
Impact of the diabetes milieu on RTL in fibroblasts: (a) cells exposed to 5.5 mM or 25 mM D-glucose for up to 28 days. In cells grown in 25 mM D-glucose media, the impact of 7 or 14 days culture in (b) 5 mM GA, (c) 25 μM H2O2; (d) GA, H2O2 and 100μΜ FF combined for 7 days. Data is mean ± SEM. Students’ t-test *P < 0.05 compared to control of the same cell passage, #P < 0.05 vs. 7 days GA, †P < 0.05 t-test vs. 7 days H2O2
GA, H2O2, and AGEs-BSA were used to in-vitro simulate diabetes related metabolic insults (Brownlee 2005). GA at 5 mM reduced the RTL by 20.5% at day 7 (P < 0.05), this effect was not significant at day 14 (Fig. 1b). In contrast, H2O2 at 25 μM accelerated RTL decline, at both 7 and 14 days of treatment, by 24.9% overall (each P < 0.05) (Fig. 1c). The in-house AGEs-BSA treatment had no effect on RTL at either day and there was no synergistic effect of GA and H2O2 treated cells (data not shown). As the most prominent changes were observed at day7 in cells at passage 10 or greater, we concentrated our further studies on 7 days’ treatment. In subsequent studies, cells were treated with fenofibrate (FF), alone and in combination with GA or H2O2 over the 7 day period. The co-incident culture of 100μΜ FF prevented the RTL reduction seen by 5 mM GA and by 25μΜ H2O2 (P < 0.05 compared to GA, or H2O2 alone) (Fig. 1d). Compared with untreated cells, FF alone had no effect on RTL.
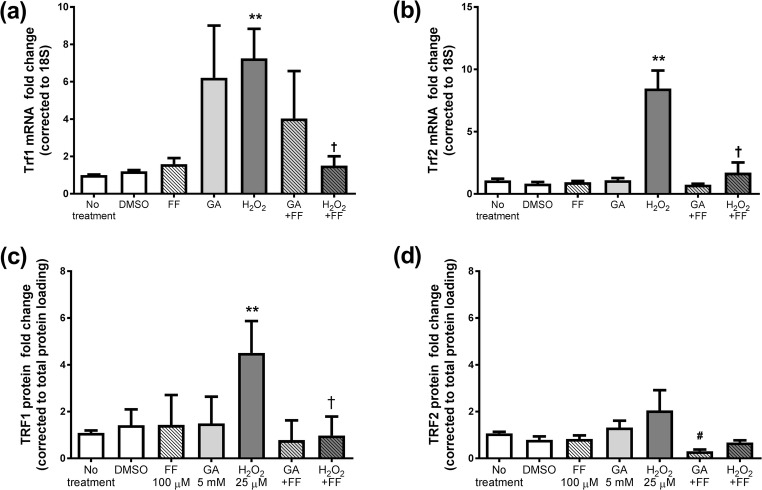
The mRNA levels of Trf1 and Trf2, members of the shelterin complex, were then measured in fibroblasts treated with GA, H2O2, with and without FF (Fig. 2). The most consistent change was seen for H2O2 which increased the expression of shelterin complex members, Trf1 and Trf2, as well as Tpp1, Tinf2 and Rap1 (supplementary data). Addition of GA resulted in small increases in Trf1 and Pot1; significantly increased expression of Tinf2 and Rap1 (supplementary data), whilst the expression of Trf2 was unaltered (Fig. 2b). FF addition prevented the significant induction of shelterin complex members by H2O2 and GA (P < 0.05) (Fig. 2a, b).
Fig. 2.
The mRNA levels of (a) Trf1, (b) Trf2 and protein on average for (c) TRF1, (d) TRF2 after 7 days treatment with 5 mM GA or 25μΜ H2O2 in the presence or absence of 100μΜ FF. Data is mean ± SEM fold change vs. control cells. Students’ t-test *P < 0.05, **P < 0.005 vs. no treatment, †P < 0.05 t-test vs. H2O2
As the mRNA levels of Trf1 and Trf2 were highly regulated by H2O2, the impact on TRF1 and TRF2 protein was examined by Western immunoblot. Cellular TRF1 but not TRF2 were elevated in H2O2 (by 4.5 ± 0.7 fold, P < 0.05), but not GA. FF prevented the induction of TRF1 protein by H2O2 treatment (P < 0.05 compared with H2O2 treatment alone, Fig. 2c). Interestingly FF lowered TRF2 protein compared with GA alone, P < 0.05 (Fig. 2d).
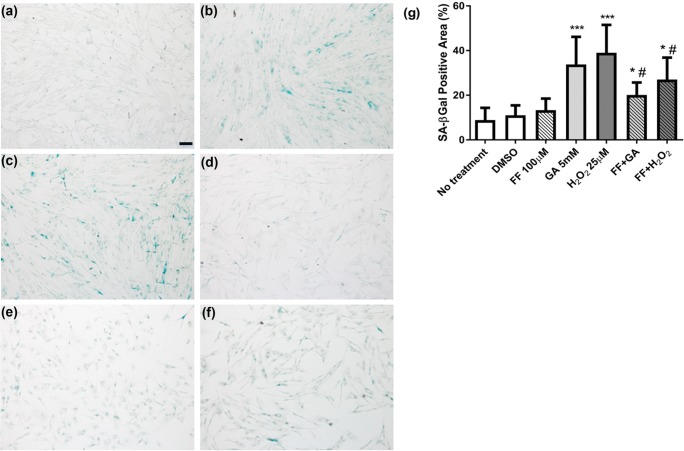
Considering telomere changes predict and links to cell senescence, the impact on senescence was then examined, by measurement of SA-βGal enzyme activity. Representative images of SA-βGal activity in the different treatments are shown in Fig. 3 (a-f) and graphically in Fig. 3g. Notably in Fig. 3b and c, GA and H2O2 treatments increased the signal for SA-βGal. Both GA and H2O2 treatment significantly increased the percentage of cells expressing SA-βGal compared with untreated cells (33.8 ± 2.0%, and 34.8 ± 2.1%, respectively vs. no treatment 8.9 ± 0.86%, both P < 0.0001). The FF treatment partially prevented the increase in SA-βGal in the presence of either GA or H2O2 treatment (Figs. 3e–g), each P < 0.05, compared with GA or H2O2 alone.
Fig. 3.
SA-βGal enzyme activity in cells grown in 25 mM D-glucose after 7 days treatment. Representative images of (a) no treatment, (b) 5 mM GA, (c) 25μMH2O2 (d) 100 μM FF, (e) GA + FF, (f) H2O2 + FF. Quantitative data from 10 fields are shown in (g). Results were analysed by Students’ t-test, *P < 0.05, ***P < 0.001 vs. no treatment, #P < 0.05 vs. control treatment
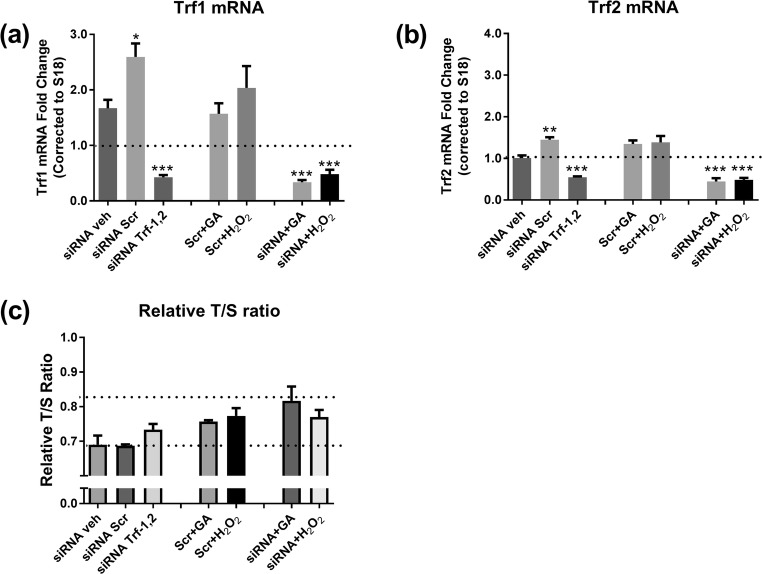
A Trf1 and Trf2 siRNA treatment protocol was then utilized to investigate roles of shelterin complex members, specifically TRF1 and TRF2, in regulation of RTL by H2O2 and GA. Compared with vehicle alone, and with the scramble control, the siRNA mixture lowered Trf1 and Trf2 mRNA by 70% and 50% respectively, P < 0.0005 (Fig. 4a, b). The siRNAs had a mild effect on basal Tpp1 and Tinf2 mRNA, but no demonstrable effect on the Rap1 and Pot1 mRNAs (not shown). The TRF1 and TRF2 protein levels were also confirmed to be reduced compared to vehicle or scramble at 24 h after siRNA transfection (not shown).
Fig. 4.
Effect of Trf1 and Trf2 siRNA on (a) Trf1 mRNA expression compared with cells treated with vehicle (veh), scramble (scr) in the presence or absence of GA or H2O2; (b) Trf2 mRNA; (c) RTL. Data is mean ± SEM. Results were analysed by Students’ t-test, *P < 0.05, **P < 0.005, ***P < 0.0005 vs. siRNA vehicle
The siRNA mixture also prevented the Trf1 and Trf2 mRNA induction seen by GA and by H2O2. Whether these changes in Trf1 and Trf2 were casually linked to the reduction in RTL was then examined. In the basal state, the siRNA treatment had no effect on RTL compared with the scramble or the vehicle control (Fig. 4c). Compared with no treatment, the mild but significant reduction in RTL by GA and by H2O2 in the presence of the scramble control (P < 0.05 each), was not clearly prevented or exacerbated, by the siRNA treatment (Fig. 4c). Thus, in this cell system, targeting the shelterin complex members, Trf1 and Trf2, was not found to affect RTL in either the basal state or after GA or H2O2 treatments.
Discussion
As telomere attrition occurs in diabetes, factors that may mediate this process are of great importance. Yet to date, to our knowledge, no cell culture system has been reported in the diabetic metabolic environment which uses agents which target telomere attrition. This current report describes series of key findings in human primary cell fibroblast cells cultures in-vitro. In summary, cells maintained in 25 mM for 21 days or more were observed to have lower RTL compared with cells maintained in 5.5 mM D-glucose. Under conditions of diabetes range elevated glucose, the agents GA and H2O2, but not AGEs-BSA treatment, accelerated RTL loss in this model after 7 days’ treatment. Furthermore, fenofibrate, FF, prevented these effects of GA and H2O2 (Fig. 1). The expression of the shelterin complex mRNA species, especially Trf1, was induced by GA and H2O2, and the shelterin complex mRNA abnormalities due to GA or H2O2 were normalized by FF treatment (Fig. 2). The Trf1, Trf2 mRNA data was confirmed at the protein level by immuno-blotting and TRF1 protein showed similar regulation as observed for the mRNA results (Fig. 2). In further experiments, the low relative RTL occurred with cell senescence, as shown by the SA-βGal activity staining, in which GA and H2O2 increased SA-βGal positive cells, again in each case prevented by FF (Fig. 3). Finally, the siRNA targeting Trf1 and Trf2 did not show any significant change in RTL regulation despite establishing a clear reduction in Trf1 and Trf2 mRNA levels in this system (Fig. 4).
This series of experiments provides mechanistic information related to factors in the diabetes metabolic environment which reduce telomere levels. In this model system elevated extracellular D-glucose, glucosamine as an agent related to activating the hexosamine pathway, and hydrogen peroxide each reduced telomere length. In contrast, the synthetic AGEs with adducts attached to BSA backbone, had no demonstrable effect, even up to 400 μg/mL (not shown). These results collectively indicate some degree of reagent specificity studied on telomere attrition. As described in the methods, the AGEs-BSA preparation used induced mRNA levels of fibronectin compared with control-BSA, and we have previously published that fibronectin induction is a useful positive control, including in the cells studied in this current series of experiments (Twigg et al. 2001). Notably, we did not study the more reactive AGEs intermediates, including methylglyoxal (Brownlee 2005), and it remains possible that AGEs will cause telomere attrition if these more potent AGEs adducts were applied to the culture system.
While others have reported the effect of ROS/ DNA damage on telomere length and telomerase activity, to the best of our knowledge our report is the first discussing potential glucosamine and AGEs effects on telomere length. Compared to other cell based models (Ludlow et al. 2014; Maeda et al. 2013), chronic effects were seen in the current system in low H2O2 concentrations and we did not need to UV-irradiate our cells to simulate oxidative effect and DNA damage (Ma et al. 2012). Interestingly, the acceleration of telomere length attrition was only well demonstrated in later passage cell number, from p10 onwards, indicating that cell ageing in culture is a determinant effect. We maintained cells under conditions recommended by the supplier, ATCC, which includes the high glucose conditions. This passage dependent data suggests that older primary cultures may be more susceptible to effects of the metabolic agents. Indeed, more prolonged cultures of fibroblastic cells results in progressive senescence (Goldstein et al. 1991), which implies that some greater baseline senescence is more likely to potentiate effects of diabetes metabolic insults to induce telomere attrition.
Unifying hypotheses have been developed to integrate biochemical pathways thought to mediate elevated glucose effects on diabetes complications. These include overflow pathways from glycolysis including the hexosamine biosynthetic pathway, advanced glycation, protein kinase C isoforms, and the polyol pathway (Brownlee 2005). The current series of experiments identified two of these four pathways as being able to cause telomere attrition in-vitro. Of particular note in these hypotheses is the central role identified for reactive oxygen species in exacerbating cellular injury, including the compound hydrogen peroxide; initial hypotheses implicated effects of mitochondrial derived oxidative stress (Brownlee 2005), and more recent hypotheses have suggested cellular oxidant stress may be key (Sedeek et al. 2012). Thus the findings in the current work that hydrogen peroxide in the presence of high glucose causes telomere attrition has direct relevance to diabetes complications research and it may link diabetes to concepts of accelerated diabetes complications with ageing (Tamura et al. 2016), as well as with other chronological age related conditions, such as cardiovascular disease, stroke and Alzheimer (Barzilai et al. 2012).
FF had been shown in 2 independent clinical trials, by pre-defined secondary end point analysis, both FIELD and ACCORD-lipid, to reduce diabetes complications related risk in type 2 diabetes (Shipman et al. 2016). FF therapy has not previously been shown to be associated with telomere length. In this study, we found that telomere length was able to be preserved by FF in the presence of the GA or H2O2. While the exact mechanisms by which FF preserves the RTL in-vitro are not known it is possible that this occurred due to the anti-oxidant properties of FF. FF may also be anti-inflammatory, possibly also linked to its antioxidant actions (Noonan et al. 2013). Whether FF may prevent telomere attrition in-vivo, pre-clinically or clinically, remains to be reported.
The shelterin complex members, especially Trf1 and Trf2 mRNA and protein expression levels were increased by H2O2 and to a lesser extent by GA. These results are in agreement with those described by Maeda et al. for H2O2 (Maeda et al. 2013). We believe that the induction of the shelterin complex, especially the TRF1 and TRF2 were in response to a counter-regulation inducing protection in the cell cycle checkpoint (Maeda et al. 2013). We observed that the high expression of Trf1 and Trf2 mRNA was negatively associated with lower RTL. That FF treatment was able to normalize the increase of the shelterin mRNA species, is consistent with the finding that FF was able to mitigate the negative impact of GA and H2O2. However, in this current model we were unable to demonstrate an effect of regulating Trf1 and Trf2 on telomere length, either in the basal state or after GA and H2O2 treatments. This finding suggests that the shelterin complex members are markers of the telomeric insult, and that at least for Trf1 and Trf2, they are not critically required protective factors against the telomere shortening observed.
The current work has both limitations and strengths. The reporting of a cell culture system to examine mechanism of effect of telomere attrition by diabetes will be a valuable model to define cellular mechanism. In the current work only some of the potential metabolic and cellular mediators in diabetes were examined and only one mesenchymal cell type. While such cells may mediate diabetes complications (Twigg et al. 2001), especially those associated with fibrosis, it will be relevant in future work to examine cells such as endothelial primary cultures, glomerular podocytes, and renal tubular epithelial cells, to explore the potential value of the cell conditions studied in those clinically relevant cell types.
Diabetes is a complex metabolic condition, with multiple potential mediators inducing end-organ complications and accelerated ageing. We have established an in-vitro model that considers chronological cellular hyperglycemia, and different metabolic pathway stressors combined. Short telomere length under diabetes conditions likely contributes to limited longevity and this model system can now further explore and refine mechanisms of effect of diabetes on telomere shortening. Fenofibrate may be able to prevent or reduce the impact of these factors in this in-vitro setting, reflects that it may have value in-vivo in diabetes, which requires further study.
Electronic supplementary material
The mRNA levels of shelterin complex members after 7 days treatment with 5 mM GA or 25μΜ H2O2 in the presence or absence of 100μΜ Fenofibrate (FF). Data is presented as fold change vs untreated cells, after normalization with ribosomal S18 RNA and presented as mean ± SEM. Results were analysed by Students’ t-test, 2 tails *P < 0.05 compared with no treatment, **P < 0.005 compared with no treatment, †P < 0.05 t-test 2 tails compared to H2O2 treatment. (PNG 203 kb)
The protein expression levels after 7 days treatment with 5 mM GA or 25μΜ H2O2 in the presence or absence of 100μΜ Fenofibrate (FF): (a) TRF1 and (b) TRF2 representative Western blot images; (c) TRF1 protein and (d) TRF2 quantification histograms from 3 independent experiments. Results were analysed by Students’ t-test, 2 tails **P < 0.05 compared with no treatment, #P < 0.05 compared with GA treatment, †P < 0.05 compared with H2O2 treatment. (PNG 418 kb)
Acknowledgements
This study is supported by the Endocrinology & Diabetes Research Foundation, The University of Sydney. SS had been supported by the Endocrinology & Diabetes Research Foundation, The University of Sydney, a NHMRC Dora Lush Postgraduate Scholarship and NHMRC CTC Scholarship for Diabetes Research. Rebecca L. Cooper Medical Research Foundation had contributed towards equipment grant used in this study. Ms. Shanaz Maleakhi (Histopathology Laboratory, Department of Pathology, The University of Sydney) has provided support for the SA-βGal enzyme activity staining optimisation and microscope image acquirement. Dr. Ali Reza had been instrumental in optimizing ImageJ analysis for the SA-βGal staining.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Surya Shin Ichi Sutanto, Email: surya.sutanto@sydney.edu.au.
Susan Virginia McLennan, Email: sue.mclennan@sydney.edu.au.
Anthony C. Keech, Email: tony@ctc.usyd.edu.au
Stephen Morris Twigg, Email: stephen.twigg@sydney.edu.au.
References
- Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S, Moerman EJ, Jones RA, Baxter RC. Insulin-like growth factor binding protein 3 accumulates to high levels in culture medium of senescent and quiescent human fibroblasts. Proc Natl Acad Sci U S A. 1991;88:9680–9684. doi: 10.1073/pnas.88.21.9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider CW. Telomere length regulation. Annu Rev Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- Gurtler A, et al. Stain-free technology as a normalization tool in Western blot analysis. Anal Biochem. 2013;433:105–111. doi: 10.1016/j.ab.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Ksiazek K, Passos JF, Olijslagers S, Saretzki G, Martin-Ruiz C, von Zglinicki T. Premature senescence of mesothelial cells is associated with non-telomeric DNA damage. Biochem Biophys Res Commun. 2007;362:707–711. doi: 10.1016/j.bbrc.2007.08.047. [DOI] [PubMed] [Google Scholar]
- Ludlow AT, Spangenburg EE, Chin ER, Cheng WH, Roth SM. Telomeres shorten in response to oxidative stress in mouse skeletal muscle fibers. J Gerontol A Biol Sci Med Sci. 2014;69:821–830. doi: 10.1093/gerona/glt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HM, Liu W, Zhang P, Yuan XY. Human skin fibroblast telomeres are shortened after ultraviolet irradiation. J Int Med Res. 2012;40:1871–1877. doi: 10.1177/030006051204000526. [DOI] [PubMed] [Google Scholar]
- Maeda T, Guan JZ, Koyanagi M, Makino N. Telomerase activity and telomere length distribution in vascular endothelial cells in a short-term culture under the presence of hydrogen peroxide. Geriatr Gerontol Int. 2013;13:774–782. doi: 10.1111/j.1447-0594.2012.00936.x. [DOI] [PubMed] [Google Scholar]
- Noonan JE, Jenkins AJ, Ma JX, Keech AC, Wang JJ, Lamoureux EL. An update on the molecular actions of fenofibrate and its clinical effects on diabetic retinopathy and other microvascular end points in patients with diabetes. Diabetes. 2013;62:3968–3975. doi: 10.2337/db13-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedeek M, et al. Oxidative stress, Nox isoforms and complications of diabetes--potential targets for novel therapies. J Cardiovasc Transl Res. 2012;5:509–518. doi: 10.1007/s12265-012-9387-2. [DOI] [PubMed] [Google Scholar]
- Shipman KE, Strange RC, Ramachandran S. Use of fibrates in the metabolic syndrome: a review. World J Diabetes. 2016;7:74–88. doi: 10.4239/wjd.v7.i5.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer MR, Schnapp G, de Lange T. Control of human telomere length by TRF1 and TRF2. Mol Cell Biol. 2000;20:1659–1668. doi: 10.1128/MCB.20.5.1659-1668.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y, Takubo K, Aida J, Araki A, Ito H. Telomere attrition and diabetes mellitus. Geriatr Gerontol Int. 2016;16(Suppl 1):66–74. doi: 10.1111/ggi.12738. [DOI] [PubMed] [Google Scholar]
- Twigg SM, et al. Advanced glycosylation end products up-regulate connective tissue growth factor (insulin-like growth factor-binding protein-related protein 2) in human fibroblasts: a potential mechanism for expansion of extracellular matrix in diabetes mellitus. Endocrinology. 2001;142:1760–1769. doi: 10.1210/endo.142.5.8141. [DOI] [PubMed] [Google Scholar]
- van Steensel B, de Lange T. Control of telomere length by the human telomeric protein TRF1. Nature. 1997;385:740–743. doi: 10.1038/385740a0. [DOI] [PubMed] [Google Scholar]
- Yamagishi S-i, et al. Advanced glycation end products-driven angiogenesis in vitro induction of the growth and tube formation of human microvascular endothelial cells through autocrine vascular endothelial growth factor. J Biol Chem. 1997;272:8723–8730. doi: 10.1074/jbc.272.13.8723. [DOI] [PubMed] [Google Scholar]
- Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL (2012) Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. Bmc Bioinformatics 13. 10.1186/1471-2105-13-134 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The mRNA levels of shelterin complex members after 7 days treatment with 5 mM GA or 25μΜ H2O2 in the presence or absence of 100μΜ Fenofibrate (FF). Data is presented as fold change vs untreated cells, after normalization with ribosomal S18 RNA and presented as mean ± SEM. Results were analysed by Students’ t-test, 2 tails *P < 0.05 compared with no treatment, **P < 0.005 compared with no treatment, †P < 0.05 t-test 2 tails compared to H2O2 treatment. (PNG 203 kb)
The protein expression levels after 7 days treatment with 5 mM GA or 25μΜ H2O2 in the presence or absence of 100μΜ Fenofibrate (FF): (a) TRF1 and (b) TRF2 representative Western blot images; (c) TRF1 protein and (d) TRF2 quantification histograms from 3 independent experiments. Results were analysed by Students’ t-test, 2 tails **P < 0.05 compared with no treatment, #P < 0.05 compared with GA treatment, †P < 0.05 compared with H2O2 treatment. (PNG 418 kb)