Abstract
Protease activated receptors (PARs) transactivate both epidermal growth factor receptors (EGFR) and transforming growth factor (TGF)-β receptors (TGFBR1) in vascular smooth muscle leading to the increased expression of genes (CHST11 and CHSY1) which are rate limiting for the enzymes that mediate hyperelongation of glycosaminoglycan (GAG) chains on the lipid-binding proteoglycan, biglycan. This is an excellent model to investigate mechanisms of transactivation as the processes are biochemically distinct. EGFR transactivation is dependent on the classical matrix metalloprotease (MMP) based triple membrane bypass mechanism and TGFBR1 transactivation is dependent on Rho/ROCK signalling and integrins. We have shown that all kinase receptor signalling is targeted towards phosphorylation of the linker region of the transcription factor, Smad2. We investigated the mechanisms of thrombin mediated kinase receptor transactivation signalling using anti-phospho antibodies and Western blotting and gene expression by RT-PCR. Thrombin stimulation of phospho-Smad2 (Ser 245/250/255) and of phospho-Smad2(Thr220) via EGFR transactivation commences quickly and extends out to at least 4 h whereas transactivation via TGFBR1 is delayed for 120 min but also persists for at least 4 h. Signalling of thrombin stimulated Smad linker region phosphorylation is approximately equally inhibited by the MMP inhibitor, GM6001 and the ROCK inhibitor, Y27632, and similarly expression of CHST11 and CHSY1 is approximately equally inhibited by GM6001 and Y27632. The data establishes Smad linker region phosphorylation as a central target of all transactivation signalling of GAG gene expression and thus an upstream kinase may be a target to prevent all transactivation signalling and its pathophysiological consequences.
Keywords: Transactivation signalling, G protein coupled receptors, Smad, Smad linker region, G proteins, Serine/threonine kinase receptors
Introduction
G protein coupled receptors (GPCRs) have long been a major interest of pharmacology studies as they are involved in diverse physiological pathways and functions. GPCRs currently constitute the largest family of proteins targeted by approved drugs (Sriram and Insel 2018) accounting for more than 27% of the therapeutic global market (Hauser et al. 2017). The identification and exploitation of new targets is warranted for diseases with unmet clinical needs. There is a need to elucidate GPCR signalling to its full extent. Classic GPCR signalling involves transactivation independent pathways (Kamato et al. 2013a, 2015) passing from the agonist to the receptor, leading to activation of G proteins and signal propagation via a plethora of downstream mechanisms.
For over two decades it has been known that GPCRs can transactivate cell surface protein tyrosine kinase receptors (PTKR) (Daub et al. 1996) and over this time it was also established that GPCRs can also transactivate serine/threonine kinase receptors (S/TKR) (Burch et al. 2010a; Sharifat et al. 2017). Since then there has been considerable work on the mechanism(s) of transactivation (Kamato et al. 2015). In the context of human vascular smooth muscle cells (VSMCs), protease activated receptor (PAR)-1 agonist thrombin transactivates the PTKR epidermal growth factor receptor (EGFR) via the “triple membrane bypass” pathway which involves activating a membrane bound matrix metalloproteinase (MMP) resulting in the cleavage and release of EGFR ligand which subsequently activates its cognate receptor (Kamato et al. 2016; Burch et al. 2013). The PAR-1 receptor can also transactivate the S/TKR, transforming growth factor (TGF)-β type 1 receptor (TGFBR1) via cytoskeletal rearrangement which activates ROCK signalling, leading to the activation of cell surface integrins and ultimately activation of large latent complex which holds TGF-β near the surface that subsequently is rearranged and activates the TGFBR1 (Kamato et al. 2015, 2013a; Burch et al. 2013; Chaplin et al. 2017).
Transactivation signalling has emerged to be equally important to classic signalling when measured by the ability to stimulate gene expression in array studies (Kamato et al. 2017a). Transactivation dependent signalling accounts for approximately 50% of the total genes regulated by the GPCR, PAR-1 (Kamato et al. 2017a). Thus GPCR transactivation dependent signalling significantly broadens the GPCR signalling paradigm (Kamato et al. 2017a, 2017b). Thrombin transactivation of the EGFR and TGFBR1 lead to enhanced synthesis of extracellular matrix (ECM), proteoglycans (Burch et al. 2010a) and the genes involved in the elongation of the glycosaminoglycan (GAG) chains covalently attached to the proteoglycan core protein (Kamato et al. 2016; Afroz et al. 2018; Kamato et al. 2018). The modification of proteoglycan structure predominantly with the elongation of the GAG chains results in an increased binding to lipids in vitro (Little et al. 2002; Ballinger et al. 2004; Getachew et al. 2010) leading to the progression of atherosclerosis in the vessel wall. Hence exploitation of transactivation dependent signalling is of great interest as the identification of a common intermediate could lead to a potential therapeutic target for atherosclerosis (Kamato et al. 2017a).
The canonical TGF-β signalling pathway involves direct carboxy-terminal phosphorylation of Smad2 by TGFBR1 (Derynck and Zhang 2003). In addition to carboxy-terminal phosphorylation, Smads can also be phosphorylated in the linker region (Matsuzaki 2011; Matsuzaki et al. 2009; Kamato et al. 2013b). Downstream of the TGFBR1, serine/threonine kinases are activated which phosphorylate the Smad2 linker region that drives the synthesis and expression of genes for the enzymes involved in the elongation of the GAG chains (Burch et al. 2010b; Rostam et al. 2016; Rostam et al. 2018; Mohamed et al. 2018). The paradigm for Smad signalling is evolving (Dayati et al. 2018). Recent observations by our lab show that in keratinocytes, the GPCR agonist thrombin transactivates the EGFR to phosphorylate the Smad2 linker region (Talati et al. 2018). Smad2 linker region phosphorylation drives proteoglycan synthesis (Burch et al. 2010b) and GAG gene expression (Rostam et al. 2016, 2018; Mohamed et al. 2018) hence we investigated the mechanisms of Smad2 linker phosphorylation as a central intermediate in thrombin mediated GAG gene expression. A central integrating point for all GPCR mediated transactivation of the two kinase receptors leading to GAG hyperelongation has the potential to be developed as a vessel wall directed therapeutic target to prevent atherosclerosis and cardiovascular disease.
This project investigates the mechanisms involved in Smad2 linker region phosphorylation via thrombin transactivation of both the EGFR and TGFBR1. We report that thrombin stimulates Smad2 linker region phosphorylation which occurs via transactivation of the EGFR and TGFBR1. The combined mechanisms involving these signalling pathways account for all thrombin stimulated gene expression of GAG synthesizing enzymes chondroitin 4-sulfotransferase-1 (CHST11) and chondroitin synthase-1 (CHSY-1). This data points towards the Smad2 linker region as a central integrating point for all GPCR transactivation dependent signalling leading to GAG chain hyperelongation and thus upstream kinases represent a potential vessel wall directed therapeutic target to prevent development of atherosclerosis and cardiovascular disease.
Methods and materials
Materials
Foetal bovine serum (FBS) was purchased from Invitro Technologies Pty. Ltd. (VIC, Australia). Dulbecco’s Modified Eagle Medium (DMEM) (0 and 25 mM glucose), Trypsin-Versene, penicillin and streptomycin were from Thermo Scientific (VIC, Aus). Human TGF-β1, anti-pSmad2(Ser245/250/255), anti-rabbit IgG horseradish peroxidase (HRP) anti-GAPDH antibodies were purchased from Cell Signalling Technology (MA, USA). Amersham ECL Prime chemiluminescence detection reagent was purchased from GE Healthcare (NSW, Aus). The primers (forward and reverse) for CHST11, CHSY1 and 18S, RNeasy Mini Kit, the QuantiTect reverse transcription kit, QuantiFast SYBR green PCR kit and the Rotor Gene Q series software were from Qiagen (VIC, Aus). Anti-phospho-Smad2L(Thr220) rabbit IgG polyclonal was a gift from Professor Koichi Matsuzaki (Kansai Medical University, Osaka, Japan).
Cell culture
Primary cultures of human vascular smooth muscle cells (VSMCs) were obtained from discarded sections of saphenous veins from patients undergoing surgery at the Alfred Hospital (Melbourne, Australia) and the acquisition of the vessels was approved by the Alfred Hospital Ethics Committee. VSMCs were grown in DMEM (5 mM glucose, 10% FBS and 1% antibiotics at 37 °C in 5% CO2). VSMCs were seeded in 60 mm dishes and 6 well-plates. Cells were grown to confluence then rendered quiescent by serum deprivation for 48 h. Cells were pre-incubated with inhibitors for 30 min prior to treatment with agonists. Incubation times and concentrations are given in detail in the figure legends.
Western blotting
Whole cell lysates (30 μg of protein) were resolved on 10% SDS-PAGE and semi-dry transferred onto PVDF. Membranes were blocked with 5% bovine serum albumin and incubated with primary antibody targeting protein of interest followed by HRP-anti-rabbit IgG and ECL detection. The protein of interest was normalised with GAPDH to determine equal loading. Blots were imaged using the Bio-Rad gel documentation system and densitometry analysis was performed with Quantity One imaging software.
Assessing mRNA gene expression
The mRNA level of the GAG enzymes was determined by quantitative real-time polymerase chain reaction (RT-PCR). Total RNA was isolated from VSMCs treated as described. RNA was extracted from 5 × 105 cells using RNeasy Mini kit (Qiagen) according to the instructions of the manufacturer. RNA purity was checked by spectrophotometry (260/280 nm) using Nanodrop2000 spectrophotometer (Thermo Fisher Scientific). First strand cDNA was synthesized from 1 μg RNA using Quantitect reverse transcriptase kit (Qiagen) according to the instructions of the manufacturer. Quantitative RT-PCR was performed using Qiagen Rotor Gene Q and QuantiFast SYBR green PCR master mix kit (Qiagen). Data was normalised to the ribosomal 18S housekeeping gene to adjust for control variations between individual experiments. Relative expression of mRNA levels was quantified using comparative delta delta Ct method.
Statistical analysis
Normalised data is expressed as the mean ± standard error of the mean of three or more independent experiments, unless stated otherwise. A one-way ANOVA was used to calculate statistical significance of normalised data as stated followed by least significant difference post-hoc analysis. Results were considered significant when the probability was less than 0.05 (*p < 0.05) and 0.01 (**p < 0.01).
Results
Thrombin mediated Smad2 linker region phosphorylation occurs through the transactivation of the EGFR
Our earlier work showed that thrombin via its receptor PAR-1 transactivates both the EGFR and TGFBR1 to modify proteoglycan synthesis (Burch et al. 2010a, 2013) and mediate GAG chain elongation (Kamato et al. 2016, 2018) via completely distinct biochemical mechanisms. In the context of TGF-β signalling, Smad2 linker region phosphorylation is associated with GAG gene expression (Rostam et al. 2016, 2018) and GAG hyperelongation (Burch et al. 2011). We investigated the role of Smad2 linker region phosphorylation as a central point for all thrombin mediated GAG chain hyperelongation. To address this question we carried out a concentration-dependent study in VSMCs using the EGFR antagonist, AG1478 (0.5-10 μM) and TGFBR1 antagonist, SB431542 (0.3-30 μM), in the presence of thrombin for 30 min on the phosphorylation of the cluster serine residues (Ser245/250/255) and the threonine site (Thr220) of the Smad2 linker region.
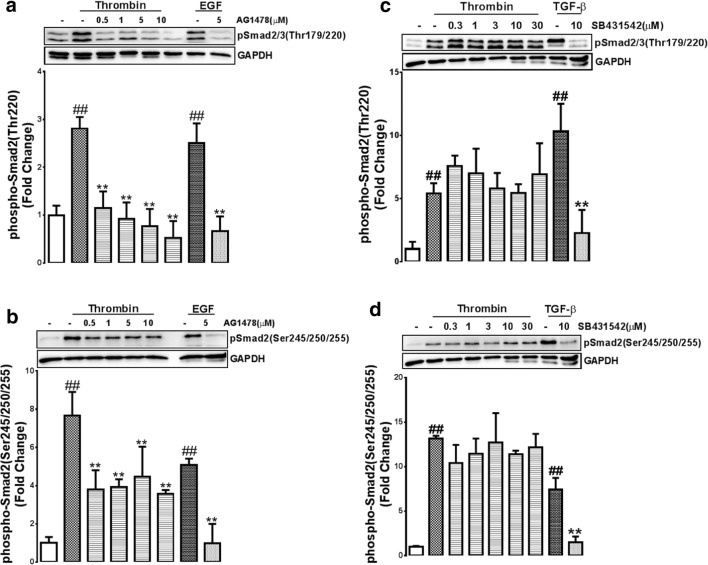
In VSMCs thrombin treatment stimulated phosphorylation of the Thr220 residue which was dose dependently inhibited by AG1478 at concentrations of 0.5-10 μM (p < 0.01) (Fig. 1a). Treatment with EGF (100 nM) showed a 2.5-fold increase in the phosphorylation of Thr220 and as expected this response was abolished in the presence of AG1478 (Fig. 1a). Thrombin treated VSMCs showed a 7.7-fold increase in the phosphorylation of the Ser245/250/255 of the Smad2 linker region (Fig. 1b). In the presence of AG1478 a partial inhibition to approximately 4-fold stimulation (0.1-5 μM)(p < 0.01) was seen (Fig. 1b). EGF treated VSMCs showed a 5-fold increase in the phosphorylation of Ser245/250/255 and as expected this was completely abolished in the presence of AG1478 (p < 0.01) (Fig. 1b). The data demonstrates that at the relatively early time point of 30 min treatment with thrombin transactivates the EGFR to phosphorylate the Ser245/250/255 and Thr220 residues of the Smad2 linker region.
Fig. 1.
Early thrombin mediated linker region phosphorylation is through the transactivation EGFR. VSMCs were treated with thrombin for 30 min, EGF for 15 min or TGF-β for 30 min, in the presence and absence of A-B EGFR antagonist AG1478 (0.5-10 μM) or C-D TGFBR1 antagonist SB431542 (0.3-30 μM). Blots were probed with antibodies specific to A,C. phospho-Smad2/3(Thr220/179) or B,C. phospho-Smad2(Ser245/250/255). Blots are representative of three-independent experiments. Histogram represents band density expressed as fold per basal. Statistical significance was determined by one-way ANOVA, followed by least significant difference post-hoc analysis ##,p < 0.01 versus basal and **, p < 0.01 agonist versus antagonist
We then evaluated the role of TGFBR1 transactivation in thrombin stimulated phosphorylation of Smad linker region sites. Thrombin treated VSMCs showed a 5-fold (p < 0.01) increase in the phosphorylation of the Thr220 residue (Fig. 1c). This response was unaffected by treatment with SB431542 (0.3-30 μM). VSMCs stimulated with TGF-β showed a 10-fold increase in the phosphorylation of the Thr220 residue and this was abolished in the presence of SB431542 (Fig. 1c). Thrombin treated VSMCs showed an increase in the phosphorylation of the Ser245/250/255 by 12-fold (p < 0.01) this was unaffected by treatment with SB431542 (0.3-30 μM) (Fig. 1d). TGF-β treatment increased the phosphorylation of Ser245/250/255 and this was completely inhibited (p < 0.01) by SB431542 (Fig. 1d). These results indicate that at 30 min thrombin stimulated Smad2 linker region phosphorylation is occurring via the transactivation of the EGFR and not the TGFBR1.
Thrombin mediated transactivation of the TGFBR1 leading to phosphorylation of the Smad2 linker region is time dependent
In bovine aortic endothelial cells (BAECs) TGF-β mediated phosphorylation of the Smad2 linker region residues peaked at 60 min (Kamato et al. 2014). Similarly in VSMCs, TGF-β mediated phosphorylation of the Smad2 linker region is maximal at 60 min (Burch et al. 2010b; Rostam et al. 2016, 2018). The hypothesis tested here is if thrombin transactivation of the TGFBR1 leading to Smad2 linker region phosphorylation has a delayed component when compared to thrombin transactivation of the EGFR. VSMCs were treated with thrombin for 60, 120 and 240 min in the presence and absence of SB431542 (10 μM). Thrombin treatment of VSMCs at 60 min caused a 2.2-fold increase in the phosphorylation of Thr220, this was unaffected in the presence of SB431542 (Fig. 2a). However at 120 and 240 min thrombin mediated phosphorylation of Thr220 was increased to approximately 4-fold and this response was inhibited (p < 0.01) by more than 50% in the presence of SB431542 (Fig. 2a). The TGFBR1 antagonist abolished (p < 0.01) TGF-β mediated phosphorylation of the Thr220 in the Smad2 linker region (Fig. 2a).
Fig. 2.
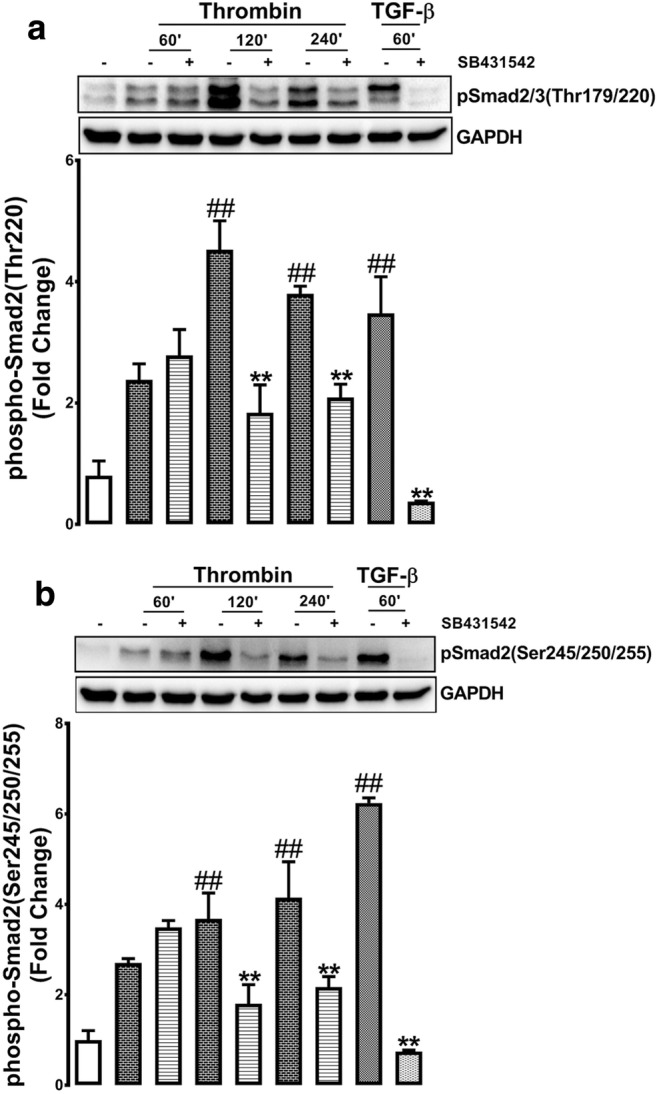
Thrombin mediated Smad2 linker region phosphorylation via the TGFBR1 is time dependent. VSMCs were treated with thrombin over 240 min time course or TGF-β for 60 min in the presence and absence of TGFBR1 antagonist SB431542 (10 μM). Blots were probed with antibodies specific to a. phospho-Smad2/3(Thr220/179) or b. phospho-Smad2(Ser245/250/255). Blots are representative of three-independent experiments. Histogram represents band density expressed as fold per basal. Statistical significance was determined by one-way ANOVA, followed by least significant difference post-hoc analysis ##,p < 0.01 versus basal and **, p < 0.01 agonist versus antagonist
Thrombin mediated phosphorylation of Ser245/250/255 followed a similar pattern of stimulation and inhibition to that observed for the phosphorylation of the Thr220 residue. At 60 min treatment with thrombin there was a 2.2-fold increase in level of the phosphorylation of Ser245/250/255 and this response was unaffected by SB431542 (Fig. 2b). However, VSMCs treated with thrombin for 120 and 240 min showed an increase in the phosphorylation of Ser245/250/255 to 3.8 and 4 fold, respectively (p < 0.01) (Fig. 2b) and treatment with SB431542 inhibited this response by 50–55% (p < 0.01) (Fig. 2b). As the control, TGF-β treated VSMCs caused a large increase in the phosphorylation of the serine residues which was abolished by SB431542 (Fig. 2b). These results indicate that in VSMCs, thrombin stimulation of the phosphorylation of the Smad2 linker region via the transactivation of the TGFBR1 occurs but only after a time delay of 60 min. Thrombin transactivation of the EGFR occurs within 30 min however transactivation of the TGFBR1 leading to Smad2 linker phosphorylation is activated at 120 min and is sustained for 240 min. The implication of these results is consistent with our earlier reports that these two cellular responses occur by distinct biochemical mechanisms.
Thrombin mediated transactivation of the EGFR leading to the phosphorylation of the Smad2 linker region is sustained for 4 h
We have shown that in VSMCs thrombin mediated transactivation of the TGFBR1 leading to Smad2 linker region phosphorylation is time dependent. The onset of GPCR mediated transactivation of EGFR is rapid (Cattaneo et al. 2014; Wang 2016) as compared to transactivation of the TGFBR1. Thrombin stimulates the phosphorylation of the Smad2 linker region via EGFR transactivation within 30 min (Fig. 1c-d), hence we investigated whether this response is sustained for 4 h. To address this question VSMCs were treated with thrombin over a time course (30–240 min) in the presence and absence of AG1478 (5 μM). EGF stimulation at 15 min in the presence of AG1478 was used as a positive control. Thrombin treatment stimulated the phosphorylation of Thr220 at 60, 120 and 240 min (4–6 fold) which was inhibited 50–70% in the presence of AG1478 (Fig. 3a). EGF treatment stimulated the phosphorylation of Thr220 which was abolished in the presence of AG1478 (Fig. 3a). These results show thrombin stimulation of the phosphorylation of the Thr220 residue occurs via transactivation of the EGFR and is sustained for at least 4 h. VSMCs treated with thrombin stimulated the phosphorylation of Ser245/250/255 to 2.5-fold at 60, 120 and 240 min (p < 0.01)(Fig. 3b). At the three time points the phosphorylation was inhibited by 50–60% (p < 0.01) in the presence of AG1478 (Fig. 3b). These results indicate that Smad2 linker region phosphorylation occurring via PAR-1 transactivation of the EGFR occurs within 30 min and is sustained up to 4 h. Thus, mechanistically, both EGFR and TGFBR1 transactivation pathways show sustained stimulation to at least 4 h but the TGFBR1 pathway shows a delayed onset of 60–120 min. These temporal differences in the response profiles most likely arise from the distinct biochemical mechanisms of transactivation so we proceeded to probe deeper into the mechanisms.
Fig. 3.
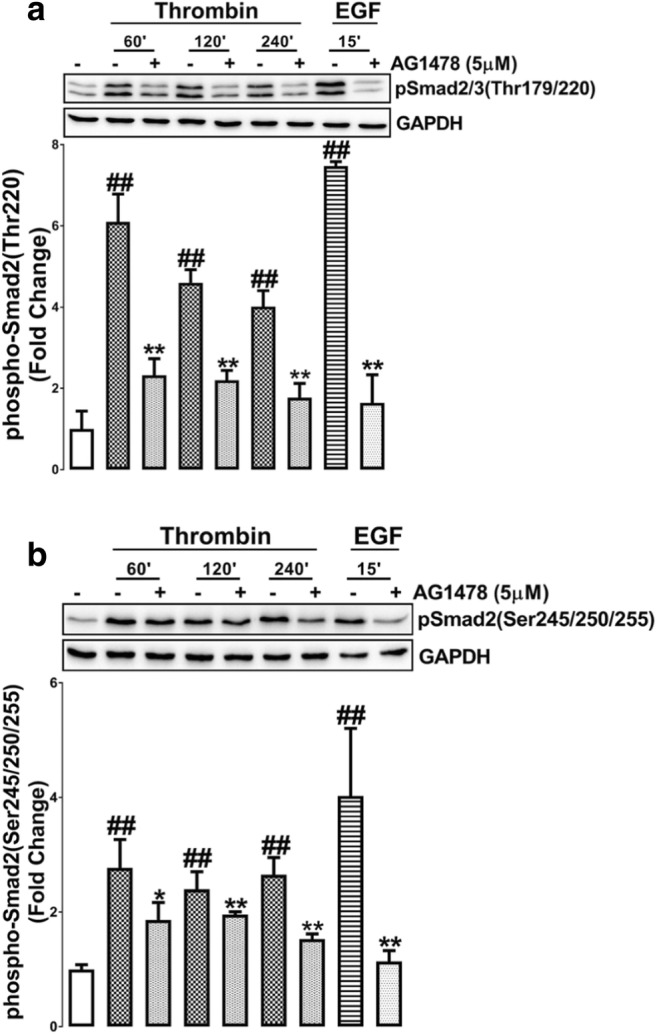
Thrombin mediated Smad2 linker region phosphorylation via the EGFR is sustained for four hr. VSMCs were treated with thrombin over 240 min time course or EGF for 15 min in the presence and absence of EGFR antagonist AG1478. Blots were probed with antibodies specific to a. phospho-Smad2/3(Thr220/179) or b. phospho-Smad2(Ser245/250/255). Blots are representative of three-independent experiments. Histogram represents band density expressed as fold per basal. Statistical significance was determined by one-way ANOVA, followed by least significant difference post-hoc analysis ##,p < 0.01 versus basal and **, p < 0.01 agonist versus antagonist
Thrombin mediated Smad2 linker region requires the activation of matrix metalloproteinase and cytoskeletal rearrangement
The transactivation of PTKR, but not S/TKR, is largely dependent on the triple membrane by-pass system that involves the stimulation of cell surface MMP leading to the generation of the ligand that binds to the EGFR (Cattaneo et al. 2014). In contrast, the transactivation of S/TKRs is achieved by cytoskeletal rearrangement which activates ROCK signalling leading to the activation of cell surface integrins which allow for the latent ligand to bind its receptor (Little et al. 2015). To investigate whether PAR-1 mediated phosphorylation of Smad2 linker region is a common signalling point to both transactivation dependent pathways we used pharmacological inhibitors, GM6001 as a broad spectrum MMP inhibitor and Y27632 as a ROCK antagonist. VSMCs were treated with thrombin for 4 h in the presence and absence of respective antagonists (Fig. 4). Thrombin treatment stimulated the phosphorylation of Thr220 to 1.5-fold (p < 0.01) (Fig. 4a). This response was inhibited by greater than 50% by either MMP (p < 0.05) or ROCK (p < 0.01) antagonists (Fig. 4a). VSMCs treated with thrombin for 4 h increased the phosphorylation of Ser245/250/255 to 2.4 fold (p < 0.01) (Fig. 4b). In presence of MMP and ROCK antagonist this stimulation was by more than 50% inhibited (p < 0.05) (Fig. 4b). Taken together these results indicate that thrombin mediated Smad2 linker region phosphorylation is mediated by transactivation of the EGFR and TGFBR1.
Fig. 4.
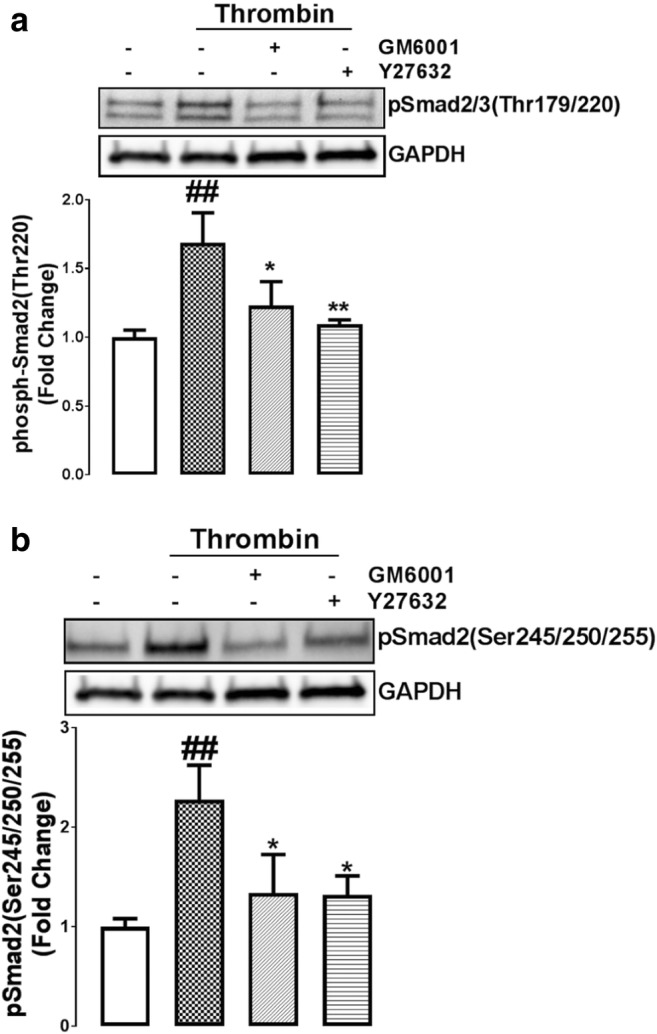
An intact cytoskeleton and MMPs are required for thrombin mediated Smad2 phosphorylation. VSMCs were pre-incubated with treated MMP inhibitor, GM6001 (10 μM) and ROCK inhibitor Y27632 (10 μM) and then exposed to thrombin for 120 min. Blots were probed with antibodies specific to a. phospho-Smad2/3(Thr220/179) or b. phospho-Smad2(Ser245/250/255). Blots are representative of three-independent experiments. Histogram represents band density expressed as fold per basal. Statistical significance was determined by one-way ANOVA, followed by least significant difference post-hoc analysis ##,p < 0.01 versus basal and **, p < 0.01 agonist versus antagonist
Thrombin mediated GAG chain elongation is dependent on matrix metalloproteinase activation and cytoskeletal rearrangement
We explored if the biochemical mechanisms which define EGFR and TGFBR1 transactivation that determine Smad linker region phosphorylation extent to the effects on thrombin stimulated expression of the mRNA of the genes which are rate limiting for GAG elongation on the proteoglycan, biglycan (Kamato et al. 2016; Afroz et al. 2018). Thrombin treatment stimulated the mRNA expression of CHST11 to 4-fold (p < 0.01) (Fig. 5a). In the presence of the MMP inhibitor, GM6001, and ROCK antagonist, Y27632, this response was inhibited by 50% (p < 0.01) (Fig. 5a). VSMCs treated with thrombin showed an increased expression of CHSY-1 mRNA to 3.2-fold (p < 0.01) (Fig. 5b) and treatment with MMP and ROCK antagonists inhibited 60 and 45%, respectively (Fig. 5b). Taken together these results indicate that thrombin mediated Smad2 linker region phosphorylation is mediated by transactivation of EGFR and TGFBR1, these results are consistent with our earlier findings related to hyperelongation of GAG chains (Burch et al. 2013) and the expression of genes for GAG elongation (Kamato et al. 2016).
Fig. 5.

Thrombin mediated mRNA expression of GAG synthesizing genes CHST11 and CHSY1 are dependent on MMP activation and an intact cytoskeleton. VSMCs were pre-incubated with treated MMP inhibitor, GM6001 (10 μM) and ROCK inhibitor Y27632 (10 μM) and then exposed to thrombin for 6 h to study the effects on the mRNA expression of a. CHST11 and b. CHSY1. Data are expressed as the means ± standard error from three experiments. Statistical significance was determined by one-way ANOVA, followed by least significant difference post-hoc analysis. ##p < 0.01 basal versus thrombin and *p < 0.05 thrombin versus inhibitor was used for statistical analysis
Discussion
The exploitation of the signalling pathways which regulate proteoglycan synthesis and specifically the length of the GAG chains on the proteoglycan represents a potential therapeutic target for the prevention of lipid deposition diseases such as atherosclerosis and aortic valve disease (Ballinger et al. 2010; Grande-Allen et al. 2007; Little et al. 2013). In this study we investigated the role of Smad linker region phosphorylation in GPCR transactivation dependent signalling pathways involving both EGFR and TGFBR1. We show that thrombin mediated Smad2 linker region phosphorylation occurs via transactivation of both EGFR and TGFBR1.Transactivation signalling via the TGFBR1 is delayed (by 30-60 min) when compared to transactivation of the EGFR but both pathways are activated for at least 4 h. Thrombin mediated Smad2 linker region phosphorylation is dependent on MMP and ROCK signalling pathways. When examining the genes associated with the elongation of the GAG chains, we observed a partial role for MMPs and ROCK dependent pathways in stimulation of the mRNA expression of CHST11 and CHSY1, MMPs reflect signalling via EGFR and ROCK is involved in TGFBR1 transactivation. Consistent with our earlier results both transactivation dependent pathways mediated GAG elongation (Kamato et al. 2016), however we also demonstrate the role for the Smad linker region as a common intermediate for all signalling occurring via transactivation dependent pathways.
Thrombin transactivation of the EGFR has a rapid 15 min onset and is sustained for at least 4 h, however transactivation of the TGFBR1, mediated Smad2 linker region phosphorylation, requires 120 mins. GPCR agonists such as lysophosphatidic acid (LPA) (Brusevold et al. 2014; Jeong et al. 2013; Daub et al. 1997), thryrotropin releasing hormone (Ohmichi et al. 1994), endothelin-1 (Daub et al. 1996; Cazaubon et al. 1994) and thrombin (Burch et al. 2013; Huang et al. 2012; Smiljanic et al. 2014) rapidly induce the transactivation of the PTKR, EGFR. GPCR-mediated transactivation of the EGFR occurs via the activation of a membrane bound MMP, resulting in the cleavage and release of the EGF ligand which subsequently activates its cognate receptor in an autocrine manner (Prenzel et al. 1999; Fischer et al. 2003; Gschwind et al. 2002). Although a convoluted pathway, surprisingly this occurs very quickly (within minutes). The results presented in this study reveal that thrombin transactivation of the TGFBR1 leading to the phosphorylation of the Smad2 linker region is time dependent in that it occurs after a lag period of about 60 min. GPCR-mediated transactivation of the TGFBR1 is time dependent under various other conditions. For example, in mouse embryonic fibroblasts transfected with β6 integrin, LPA-mediated transactivation of the TGFBR1, assessed by the phosphorylation of the downstream intermediate Smad2, was evident at 2–4 h post treatment (Xu et al. 2009). In human airway smooth muscle cells, LPA and methacholine treated cells resulted in a delayed increase in the phosphorylation of the Smad2 protein signifying a delay in GPCR-mediated transactivation of the TGFBR1 (Tatler et al. 2011). In VSMCs, the protracted time course in thrombin-mediated transactivation of the TGFBR1 is not due to new protein synthesis. The inhibitor of translation and transcription, cycloheximide, has no effect on thrombin-mediated phosphorylation of the Smad2 (Burch et al. 2013). GPCR transactivation of the TGFBR1 occurs via cytoskeletal rearrangement which activates ROCK signalling, resulting in the activation of cell surface integrins which bind to the latent TGF-β complex. This results in the exposure of the TGF-β ligand to the receptor (Munger et al. 1999). The cell surface integrin activation which is involved in the transactivation of TGFBR1 is hypothesized to be the rate limiting event which explains why this transactivation cascade is delayed. Integrins signal via inside out, allowing for the talin and kindlin to bind to the cytoplasmic end of the β subunit (Burch et al. 2013; Munger et al. 1999). Compared to GPCR transactivation of EGFR, signalling via transactivation of TGFBR1 leading to Smad2 linker region phosphorylation is delayed due to the cell surface mechanisms that are involved in transactivation dependent signalling. These cellular processes need to be evaluated in detail to identify the molecular events of PAR-1 mediated transactivation of TGFBR1.
The Smad linker region is associated with a number of pathophysiological conditions, including but not limited to cancer development (Matsuzaki et al. 2009; Suzuki et al. 2015), cardiovascular disease (Burch et al. 2010b; Rostam et al. 2016; Burch et al. 2011; Kamato et al. 2013c) and Alzheimer’s (Ueberham et al. 2006). The work presented here demonstrates that GPCR transactivation of the EGFR and TGFBR1 share Smad2 linker as a common signalling target. A genome wide study published by our group shows that transactivation dependent signalling accounts for 50% of all GPCR mediated signalling (Kamato et al. 2017a). The transactivation dependent signalling pathways are equally important, with approximately the same number of genes regulated by either pathway. GPCR transactivation of a PTKR involves a mechanism that is distinct from transactivation of S/TKR. Together, these findings demonstrate that the Smad2 linker region is a common intermediate between the two pathways, serving as a mediator of biological processes occurring via GPCR transactivation dependent signalling.
In summary, GPCR transactivation of EGFR and TGFBR1 signal via Smad2 linker region to increase the expression of genes associated with GAG chain elongation on proteoglycans. This represents an example and a model of a concurrent signalling cascade and a kinase upstream of the Smad linker region might provide a therapeutic target for various human diseases.
Funding
DK was supported by the NHMRC (APP1160925) and National Heart Foundation Fellowship (102129). Support was received from the University of Queensland through a personal support package to PJL and by the University of Queensland Early Career Grant (DK) (Grant no. 1832825).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Afroz R, Cao Y, Rostam MA, Ta H, Xu S, Zheng W, Osman N, Kamato D, Little PJ. Signalling pathways regulating galactosaminoglycan synthesis and structure in vascular smooth muscle: implications for lipoprotein binding and atherosclerosis. Pharmacol Ther. 2018;187:88–97. doi: 10.1016/j.pharmthera.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Ballinger ML, Nigro J, Frontanilla KV, Dart AM, Little PJ. Regulation of glycosaminoglycan structure and atherogenesis. Cell Mol Life Sci. 2004;61(11):1296–1306. doi: 10.1007/s00018-004-3389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger Mandy L., Osman Narin, Hashimura Kazuhiko, Haan Judy B. de, Jandeleit-Dahm Karin, Allen Terri, Tannock Lisa R., Rutledge John C., Little Peter J. Imatinib inhibits vascular smooth muscle proteoglycan synthesis and reduces LDL binding in vitro and aortic lipid deposition in vivo. Journal of Cellular and Molecular Medicine. 2009;14(6b):1408–1418. doi: 10.1111/j.1582-4934.2009.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusevold IJ, Tveteraas IH, Aasrum M, Ødegård J, Sandnes DL, Christoffersen T. Role of LPAR3, PKC and EGFR in LPA-induced cell migration in oral squamous carcinoma cells. BMC Cancer. 2014;14:432. doi: 10.1186/1471-2407-14-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch ML, Ballinger ML, Yang SNY, Getachew R, Itman C, Loveland K, Osman N, Little PJ. Thrombin stimulation of proteoglycan synthesis in vascular smooth muscle is mediated by protease-activated receptor-1 transactivation of the transforming growth factor beta type I receptor. J Biol Chem. 2010;285(35):26798–26805. doi: 10.1074/jbc.M109.092767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch ML, Yang SNY, Ballinger ML, Getachew R, Osman N, Little PJ. TGF-beta stimulates biglycan synthesis via p38 and ERK phosphorylation of the linker region of Smad2. Cell Mol Life Sci. 2010;67(12):2077–2090. doi: 10.1007/s00018-010-0315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch ML, Zheng W, Little PJ. Smad linker region phosphorylation in the regulation of extracellular matrix synthesis. Cell Mol Life Sci. 2011;68(1):97–107. doi: 10.1007/s00018-010-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch ML, Getachew R, Osman N, Febbraio MA, Little PJ. Thrombin-mediated proteoglycan synthesis utilizes both protein-tyrosine kinase and serine/threonine kinase receptor transactivation in vascular smooth muscle cells. J Biol Chem. 2013;288(10):7410–7419. doi: 10.1074/jbc.M112.400259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo F, Guerra G, Parisi M, de Marinis M, Tafuri D, Cinelli M, Ammendola R. Cell-surface receptors transactivation mediated by g protein-coupled receptors. Int J Mol Sci. 2014;15(11):19700–19728. doi: 10.3390/ijms151119700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazaubon SM, Ramos-Morales F, Fischer S, Schweighoffer F, Strosberg AD, Couraud PO. Endothelin induces tyrosine phosphorylation and GRB2 association of Shc in astrocytes. J Biol Chem. 1994;269(40):24805–24809. [PubMed] [Google Scholar]
- Chaplin R, Thach L, Hollenberg MD, Cao Y, Little PJ, Kamato D. Insights into cellular signalling by G protein coupled receptor transactivation of cell surface protein kinase receptors. J Cell Commun Signal. 2017;11(2):117–125. doi: 10.1007/s12079-017-0375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub H, Ulrich Weiss F, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379(6565):557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- Daub H, Wallasch C, Lankenau A, Herrlich A, Ullrich A. Signal characteristics of G protein-transactivated EGF receptor. EMBO J. 1997;16(23):7032–7044. doi: 10.1093/emboj/16.23.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayati P, Rezaei HB, Sharifat N, Kamato D, Little PJ. G protein coupled receptors can transduce signals through carboxy terminal and linker region phosphorylation of Smad transcription factors. Life Sci. 2018;199:10–15. doi: 10.1016/j.lfs.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Fischer OM, Hart S, Gschwind A, Ullrich A. EGFR signal transactivation in cancer cells. Biochem Soc Trans. 2003;31(Pt 6):1203–1208. doi: 10.1042/bst0311203. [DOI] [PubMed] [Google Scholar]
- Getachew R, Ballinger ML, Burch ML, Reid JJ, Khachigian LM, Wight TN, Little PJ, Osman N. PDGF beta-receptor kinase activity and ERK1/2 mediate glycosaminoglycan elongation on biglycan and increases binding to LDL. Endocrinology. 2010;151(9):4356–4367. doi: 10.1210/en.2010-0027. [DOI] [PubMed] [Google Scholar]
- Grande-Allen KJ, et al. Glycosaminoglycan synthesis and structure as targets for the prevention of calcific aortic valve disease. Cardiovasc Res. 2007;76(1):19–28. doi: 10.1016/j.cardiores.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Gschwind A, Prenzel N, Ullrich A. Lysophosphatidic acid-induced squamous cell carcinoma cell proliferation and motility involves epidermal growth factor receptor signal transactivation. Cancer Res. 2002;62(21):6329–6336. [PubMed] [Google Scholar]
- Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017;16(12):829–842. doi: 10.1038/nrd.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CY, Chen SY, Tsai HC, Hsu HC, Tang CH. Thrombin induces epidermal growth factor receptor transactivation and CCL2 expression in human osteoblasts. Arthritis Rheum. 2012;64(10):3344–3354. doi: 10.1002/art.34557. [DOI] [PubMed] [Google Scholar]
- Jeong KJ, Cho KH, Panupinthu N, Kim H, Kang J, Park CG, Mills GB, Lee HY. EGFR mediates LPA-induced proteolytic enzyme expression and ovarian cancer invasion: inhibition by resveratrol. Mol Oncol. 2013;7(1):121–129. doi: 10.1016/j.molonc.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamato D, Burch ML, Osman N, Zheng W, Little PJ. Therapeutic implications of endothelin and thrombin G-protein-coupled receptor transactivation of tyrosine and serine/threonine kinase cell surface receptors. J Pharm Pharmacol. 2013;65(4):465–473. doi: 10.1111/j.2042-7158.2012.01577.x. [DOI] [PubMed] [Google Scholar]
- Kamato D, Burch ML, Piva TJ, Rezaei HB, Rostam MA, Xu S, Zheng W, Little PJ, Osman N. Transforming growth factor-beta signalling: role and consequences of Smad linker region phosphorylation. Cell Signal. 2013;25(10):2017–2024. doi: 10.1016/j.cellsig.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Kamato D, Babaahmadi Rezaei H, Getachew R, Thach L, Guidone D, Osman N, Roufogalis B, Duke CC, Tran VH, Zheng W, Little PJ. (S)-[6]-Gingerol inhibits TGF-beta-stimulated biglycan synthesis but not glycosaminoglycan hyperelongation in human vascular smooth muscle cells. J Pharm Pharmacol. 2013;65(7):1026–1036. doi: 10.1111/jphp.12060. [DOI] [PubMed] [Google Scholar]
- Kamato D, Rostam MA, Piva TJ, Babaahmadi Rezaei H, Getachew R, Thach L, Bernard R, Zheng W, Little PJ, Osman N. Transforming growth factor beta-mediated site-specific Smad linker region phosphorylation in vascular endothelial cells. J Pharm Pharmacol. 2014;66(12):1722–1733. doi: 10.1111/jphp.12298. [DOI] [PubMed] [Google Scholar]
- Kamato D, Rostam MA, Bernard R, Piva TJ, Mantri N, Guidone D, Zheng W, Osman N, Little PJ. The expansion of GPCR transactivation-dependent signalling to include serine/threonine kinase receptors represents a new cell signalling frontier. Cell Mol Life Sci. 2015;72(4):799–808. doi: 10.1007/s00018-014-1775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamato D, Thach L, Getachew R, Burch M, Hollenberg MD, Zheng W, Little PJ, Osman N. Protease activated receptor-1 mediated dual kinase receptor transactivation stimulates the expression of glycosaminoglycan synthesizing genes. Cell Signal. 2016;28(1):110–119. doi: 10.1016/j.cellsig.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Kamato D, Bhaskarala VV, Mantri N, Oh TG, Ling D, Janke R, Zheng W, Little PJ, Osman N. RNA sequencing to determine the contribution of kinase receptor transactivation to G protein coupled receptor signalling in vascular smooth muscle cells. PLoS One. 2017;12(7):e0180842. doi: 10.1371/journal.pone.0180842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamato D, Mitra P, Davis F, Osman N, Chaplin R, Cabot PJ, Afroz R, Thomas W, Zheng W, Kaur H, Brimble M, Little PJ. Gaq proteins: molecular pharmacology and therapeutic potential. Cell Mol Life Sci. 2017;74(8):1379–1390. doi: 10.1007/s00018-016-2405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamato Danielle, Burch Micah, Zhou Ying, Mohamed Raafat, Stow Jennifer L., Osman Narin, Zheng Wenhua, Little Peter J. Individual Smad2 linker region phosphorylation sites determine the expression of proteoglycan and glycosaminoglycan synthesizing genes. Cellular Signalling. 2019;53:365–373. doi: 10.1016/j.cellsig.2018.11.005. [DOI] [PubMed] [Google Scholar]
- Little PJ, Tannock L, Olin KL, Chait A, Wight TN. Proteoglycans synthesized by arterial smooth muscle cells in the presence of transforming growth factor-beta1 exhibit increased binding to LDLs. Arterioscler Thromb Vasc Biol. 2002;22(1):55–60. doi: 10.1161/hq0102.101100. [DOI] [PubMed] [Google Scholar]
- Little PJ, Rostam MA, Piva TJ, Getachew R, Kamato D, Guidone D, Ballinger ML, Zheng W, Osman N. Suramin inhibits PDGF-stimulated receptor phosphorylation, proteoglycan synthesis and glycosaminoglycan hyperelongation in human vascular smooth muscle cells. J Pharm Pharmacol. 2013;65(7):1055–1063. doi: 10.1111/jphp.12064. [DOI] [PubMed] [Google Scholar]
- Little PJ, Hollenberg MD, Kamato D, Thomas W, Chen J, Wang T, Zheng W, Osman N. Integrating the GPCR transactivation-dependent and biased signalling paradigms in the context of PAR1 signalling. British Journal of Pharmacology. 2016;173(20):2992–3000. doi: 10.1111/bph.13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki K. Smad phosphoisoform signaling specificity: the right place at the right time. Carcinogenesis. 2011;32(11):1578–1588. doi: 10.1093/carcin/bgr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki K, Kitano C, Murata M, Sekimoto G, Yoshida K, Uemura Y, Seki T, Taketani S, Fujisawa JI, Okazaki K. Smad2 and Smad3 phosphorylated at both linker and COOH-terminal regions transmit malignant TGF-beta signal in later stages of human colorectal cancer. Cancer Res. 2009;69(13):5321–5330. doi: 10.1158/0008-5472.CAN-08-4203. [DOI] [PubMed] [Google Scholar]
- Mohamed Raafat, Dayati Parisa, Mehr Reyhaneh Niayesh, Kamato Danielle, Seif Faezeh, Babaahmadi-Rezaei Hossein, Little Peter J. Transforming growth factor–β1 mediated CHST11 and CHSY1 mRNA expression is ROS dependent in vascular smooth muscle cells. Journal of Cell Communication and Signaling. 2018;13(2):225–233. doi: 10.1007/s12079-018-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJD, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Ohmichi M, Sawada T, Kanda Y, Koike K, Hirota K, Miyake A, Saltiel AR (1994) Thyrotropin-releasing hormone stimulates MAP kinase activity in GH3 cells by divergent pathways. Evidence of a role for early tyrosine phosphorylation. J Biol Chem 269(5):3783–3788 [PubMed]
- Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402(6764):884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- Rostam MA, Kamato D, Piva TJ, Zheng W, Little PJ, Osman N. The role of specific Smad linker region phosphorylation in TGF-beta mediated expression of glycosaminoglycan synthesizing enzymes in vascular smooth muscle. Cell Signal. 2016;28(8):956–966. doi: 10.1016/j.cellsig.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Rostam MA, Shajimoon A, Kamato D, Mitra P, Piva TJ, Getachew R, Cao Y, Zheng W, Osman N, Little PJ. Flavopiridol inhibits TGF-beta-stimulated biglycan synthesis by blocking linker region phosphorylation and nuclear translocation of Smad2. J Pharmacol Exp Ther. 2018;365:156–164. doi: 10.1124/jpet.117.244483. [DOI] [PubMed] [Google Scholar]
- Sharifat N, Mohammad Zadeh G, Ghaffari MA, Dayati P, Kamato D, Little PJ, Babaahmadi-Rezaei H. Endothelin-1 (ET-1) stimulates carboxy terminal Smad2 phosphorylation in vascular endothelial cells by a mechanism dependent on ET receptors and de novo protein synthesis. J Pharm Pharmacol. 2017;69(1):66–72. doi: 10.1111/jphp.12654. [DOI] [PubMed] [Google Scholar]
- Smiljanic K, Obradovic M, Jovanovic A, Djordjevic J, Dobutovic B, Jevremovic D, Marche P, Isenovic ER. Thrombin stimulates VSMC proliferation through an EGFR-dependent pathway: involvement of MMP-2. Mol Cell Biochem. 2014;396(1–2):147–160. doi: 10.1007/s11010-014-2151-y. [DOI] [PubMed] [Google Scholar]
- Sriram K, Insel PA. G protein-coupled receptors as targets for approved drugs: how many targets and how many drugs? Mol Pharmacol. 2018;93(4):251–258. doi: 10.1124/mol.117.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Fukui T, Kishimoto M, Miyamoto S, Takahashi Y, Takeo M, Mitsuyama T, Sakaguchi Y, Uchida K, Nishio A, Okazaki K. Smad2/3 linker phosphorylation is a possible marker of cancer stem cells and correlates with carcinogenesis in a mouse model of colitis-associated colorectal cancer. J Crohns Colitis. 2015;9(7):565–574. doi: 10.1093/ecco-jcc/jjv073. [DOI] [PubMed] [Google Scholar]
- Talati N, Kamato D, Piva TJ, Little PJ, Osman N. Thrombin promotes PAI-1 expression and migration in keratinocytes via ERK dependent Smad linker region phosphorylation. Cell Signal. 2018;47:37–43. doi: 10.1016/j.cellsig.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Tatler AL, John AE, Jolly L, Habgood A, Porte J, Brightling C, Knox AJ, Pang L, Sheppard D, Huang X, Jenkins G. Integrin alphavbeta5-mediated TGF-beta activation by airway smooth muscle cells in asthma. J Immunol. 2011;187(11):6094–6107. doi: 10.4049/jimmunol.1003507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueberham U, Ueberham E, Gruschka H, Arendt T. Altered subcellular location of phosphorylated Smads in Alzheimer's disease. Eur J Neurosci. 2006;24(8):2327–2334. doi: 10.1111/j.1460-9568.2006.05109.x. [DOI] [PubMed] [Google Scholar]
- Wang Zhixiang. Transactivation of Epidermal Growth Factor Receptor by G Protein-Coupled Receptors: Recent Progress, Challenges and Future Research. International Journal of Molecular Sciences. 2016;17(1):95. doi: 10.3390/ijms17010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu MY, Porte J, Knox AJ, Weinreb PH, Maher TM, Violette SM, McAnulty RJ, Sheppard D, Jenkins G. Lysophosphatidic acid induces alphavbeta6 integrin-mediated TGF-beta activation via the LPA2 receptor and the small G protein G alpha(q) Am J Pathol. 2009;174(4):1264–1279. doi: 10.2353/ajpath.2009.080160. [DOI] [PMC free article] [PubMed] [Google Scholar]