SUMMARY
Background
Tumor grade, size, resection potential, and extent of disease influence outcome in pediatric non-rhabdomyosarcoma soft tissue sarcoma (NRSTS) but no risk stratification systems exist and the standard of care is poorly defined. This trial evaluated a risk stratification system developed from known prognostic factors in the context of risk-adapted therapy for young NRSTS patients. Treatment goals were to limit radiotherapy use in low-risk disease, decrease radiotherapy doses in those requiring it, and evaluate the feasibility/efficacy of a neoadjuvant chemoradiotherapy approach for higher-risk disease.
Methods
The primary objective of ClinicalTrials.gov was to assess event-free survival (EFS), overall survival (OS), and the pattern of treatment failure in patients with NRSTS treated with a risk-based strategy. Age < 30 years, performance status ≥ 50 [Lansky (≤16 years),Karnofsky (>16 years)] and a new diagnosis of a World Health Organization (2002 criteria) intermediate (rarely metastasizing) or malignant soft tissue tumor (except tumor types eligible for other Children’s Oncology Group studies and those for whom the therapy in this trial was deemed inappropriate), malignant peripheral nerve sheath tumor, non-metastatic and grossly resected dermatofibrosarcoma protuberans, undifferentiated embryonal sarcoma of the liver, or unclassified malignant soft tissue sarcoma were eligible. Patients were assigned to 3 risk groups: low [non-metastatic R0 (negative-margin resection)/R1 (microscopic positive-margin resection) low-grade or ≤5 cm high-grade tumor], intermediate (nonmetastatic R0/R1 >5 cm high-grade or non-metastatic unresected tumor of any size or grade) or high (metastatic tumor) and 4 treatment arms: A (surgery only): Nonmetastatic R0/R1 low-grade or ≤5 cm R0 high-grade tumor; B (55·8 Gy radiotherapy): Non-metastatic ≤5 cm R1 high-grade tumor; C (ifosfamide/doxorubicin, 55·8 Gy radiotherapy): Non-metastatic R0/R1 >5 cm highgrade or metastatic R0/R1 tumor; D (ifosfamide/doxorubicin and 45 Gy radiotherapy, then surgery and radiotherapy boost based on margins: R0 none, R1 10·8 Gy, R2/no surgery 19·8 Gy): unresected tumor +/− metastases. Chemotherapy included 6 cycles of ifosfamide 3 g/m2/dose intravenously on days 1–3 and 5 cycles of doxorubicin 75 mg/m2/dose intravenously on days 1–2 every 3 weeks with sequence adjusted based on timing of surgery/radiotherapy. This analysis of the completed trial excluded 21 patients treated on the incorrect arm.
Findings
Five-hundred twenty-nine eligible/evaluable patients enrolled between 2/5/07 and 2/20/12: low-risk (n=222), intermediate-risk (n=227), high-risk (n=80); Arm A (n=205), B (n=17), C (n=111), D (n=196). Tumors included 33 histologies; 72% highgrade, 63% >5 cm, 15% metastatic. At a median follow-up of 6·5 years (IQR 2·9 years), 5-year EFS and OS were: low-risk [88·9% (95% CI, 84·0–93·8%) and 96·2% (95% CI, 93·2–99·2%)], intermediate-risk [65·0% (95% CI, 58·2–71·8%) and 79·2% (95% CI, 73·4–85·0%)], high-risk [21·2% (95% CI, 11·4–31·1%) and 35·5% (95% CI, 23·6–47·4%)]. No toxic deaths were reported. Ten patients experienced unexpected grade 4 adverse events (2 Arm C, 8 Arm D), including 4 wound complications that required surgery (all Arm D). Eleven additional wound complications precluded initiation of postoperative therapy within 5 weeks of tumor resection in Arm D patients. Neoadjuvant chemoradiotherapy in intermediate-/highrisk Arm D patients proved feasible: isolated local tumor progression occurred in 5% during this therapy, delayed R0/R1 resection was achieved in 91% who completed neoadjuvant chemoradiotherapy, and 11% who underwent delayed surgery experienced significant wound complications. Risk group predicted EFS and OS (p<0·0001).
Interpretation
Pre-treatment clinical features can be used to effectively define treatment failure risk and to stratify young NRSTS patients for risk-adapted therapy. Most low-risk patients can be cured without adjuvant therapy, thereby avoiding known long-term treatment complications. The radiotherapy dose may be safely lowered to 55·8 Gy for patients in all risk groups requiring adjuvant treatment. A neoadjuvant chemoradiotherapy and delayed surgery strategy permits less radiotherapy exposure while still achieving a high rate of local disease control with few complications, so is the preferred approach for unresected intermediate-/high-risk tumors. Survival remains suboptimal for intermediate-/high-risk patients and novel therapies are needed. Funded by grants from the National Institutes of Health to the Children’s Oncology Group, the Imaging and Radiation Oncology Core, and St. Jude Children’s Research Hospital, and by the St. Baldrick’s Foundation, the Seattle Children’s Foundation from Kat’s Crew Guild through the Sarcoma Research Fund, and the American Lebanese Syrian Associated Charities.
Keywords: soft tissue sarcoma, chemoradiotherapy, neoadjuvant therapy, adjuvant chemoradiotherapy, prognostic factors, child, adolescent, young adult
INTRODUCTION
Long-term survival in childhood rhabdomyosarcoma has improved from about 35% in the 1960s to nearly 70% today,1 due partly to the participation of thousands of patients in North American and European cooperative group therapeutic trials since the inception of the first Intergroup Rhabdomyosarcoma Study (IRS-I) in 1972. In contrast, fewer than 200 pediatric patients with non-rhabdomyosarcoma soft tissue sarcomas (NRSTS) were enrolled in prospective therapeutic trials in North America in the 35 years following the successful launch of IRS-I.2–4 In Europe, certain rhabdomyosarcoma studies included NRSTS patients, yielding modest information about outcomes for these patients with therapy designed for rhabdomyosarcoma.5,6 Data from patients over 16 years of age has been used in the development of most published nomograms for predicting outcomes in soft tissue sarcoma patients. An attempt to apply one of these nomograms to a pediatric population showed that although the variables included were relevant, actual mortality in pediatric patients was higher than in adults and tumor size and depth were stronger predictors of outcome in the pediatric population. The limited pediatric data suggest that the primary drivers of outcome in pediatric NRSTS are tumor grade, maximal tumor diameter, presence/absence of metastases, and extent of surgical resection.7–10 These prognostic factors formed the basis for a novel risk stratification system developed for pediatric NRSTS. Children’s Oncology Group (COG) study ARST0332 prospectively evaluated this risk stratification system in the context of risk-adapted therapy designed to limit treatment exposures for lower risk patients and to intensify therapy for higher risk patients (Figure 1).
Figure 1.
Risk Group and Treatment Assignment Schema
Patients with low-grade or ≤5 cm high-grade tumors who have undergone negative (R0) or microscopically positive (R1) tumor resection are known to have favorable outcomes without systemic therapy and constituted the low-risk subgroup.4,7,10 ARST0332 sought to limit radiotherapy-related toxicity in these patients by eliminating its use or lowering the dose administered. Although previous studies had documented that low-grade NRSTS is readily cured with R0 resection alone, several retrospective adult studies in the 1990s suggested that radiotherapy might also be safely omitted following R0 resection of high-grade NRSTS.11,12 Although there was no clear evidence that tumor size correlated with the risk of local recurrence in these studies, we elected a conservative approach of omitting radiotherapy only for ≤ 5 cm high-grade tumors following R0 resection.
Limited retrospective data documented that omission of adjuvant radiotherapy for R1 low-grade tumors produced a local recurrence rate of only 25% without any effect on disease-specific survival.13 Therefore, we also adopted a surgery-only strategy for patients with low-grade tumors with R1 margins following maximal surgery, aiming to prevent the majority of these patients from receiving radiotherapy while recognizing that a small subset would require further treatment for local recurrence. Low-risk patients with ≤ 5 cm high-grade R1 tumors received adjuvant radiotherapy, although the dose was restricted to 55·8 Gy with the goal of decreasing the risk of secondary neoplasia, which had been shown to be radiation dose-dependent with doses ≥ 60 Gy carrying the highest risk in young Ewing sarcoma patients.14
Patients with non-metastatic >5 cm, high-grade or unresectable tumor of any grade or size have an approximately 50% survival rate and constituted the intermediaterisk group,2,7,9 whereas patients with metastatic disease have a less than 20% survival rate and comprised the high-risk group.2,3,8 A dose-intensive regimen of ifosfamide and doxorubicin was used for these patients because these agents are the most active overall in soft tissue sarcomas,15 a prior pilot study had confirmed the safety and efficacy of this combination in pediatric patients,2 and the dose intensity of both agents might be important.16,17 Although some histologic subtypes such as alveolar soft part sarcoma were thought to be chemoresistant, patients with all histologic subtypes were included on the chemotherapy treatment arms due to the lack of prospective data on chemoresponsiveness in pediatric patients and evidence that some tumor types differ biologically and clinically in young patients. Patients with unresected tumors received neoadjuvant chemotherapy and radiotherapy because a retrospective pediatric analysis suggested that combined modality therapy might facilitate more complete surgical resection,9 this approach was proven safe and effective in adults,18 and the potentially lower radiotherapy doses and smaller field sizes might reduce long-term toxicity in this young patient population.
This article evaluates the predictive value of the risk-stratification system used in ARST0332, the outcomes observed with the risk-based treatment approach utilized, and the analysis to identify other prognostic features in the context of this therapeutic approach. Other study objectives of ARST0332 will be reported in future publications.
METHODS
Study Design and Participants
Patients under 30 years of age with NRSTS of any stage diagnosed within the prior 12 weeks who met the inclusion and exclusion criteria described below were eligible for this therapeutic study after the protocol (Appendix, page 1) was approved by the institutional review board at the treating institution. Written informed consent was required of patients and/or their parents or legal guardians as appropriate. With the exception of tumor types eligible for other COG studies (rhabdomyosarcoma, Ewing sarcoma, desmoplastic small round cell tumor, malignant rhabdoid tumor) and those for whom the therapy in this trial was deemed inappropriate (infantile fibrosarcoma, unresectable/metastatic dermatofibrosarcoma protuberans), all malignant and intermediate (rarely metastasizing) soft tissue tumors included in the 2002 World Health Organization (WHO) criteria19 were included. Additionally, malignant peripheral nerve sheath tumor, embryonal sarcoma of the liver, non-metastatic grossly resected dermatofibrosarcoma protuberans, and undifferentiated and unclassified soft tissue sarcomas were eligible. Submission of representative tumor tissue via enrollment on the COG soft tissue sarcoma biology study, D9902, was compulsory and submission of additional tissue for banking for research studies was encouraged. The diagnosis by the 2002 WHO criteria19 and histologic grade by POG (Pediatric Oncology Group)20 and FNCLCC (Federation Nationale des Centres de Lutte Contre le Cancer)21 criteria were confirmed in all cases by central review of submitted tumor tissue by two pediatric soft tissue pathologists; discrepancies were resolved by consensus review. Intermediate (rarely metastasizing) tumors were considered POG grade 1 for the purpose of treatment assignment. After the study was completed, all diagnoses were updated according to the 2013 WHO soft tissue tumor classification22 by two study pathologists who re-reviewed submitted tumor tissue when required. Tumors that were thoroughly evaluated and found to be undifferentiated but contained multiple morphologic patterns or otherwise precluded categorization within a single subtype according to the WHO guidelines were coded as undifferentiated sarcoma NOS. Tumors that were confirmed to be eligible soft tissue sarcomas but were inadequately evaluated to define a specific diagnosis, usually due to insufficient specimen, were coded as unclassified soft tissue sarcoma. The extent of tumor resection was defined as R0 (no malignant cells microscopically evident at the resection margin), R1 (malignant cells evident microscopically at the resection margin), or R2 (malignant cells grossly evident at the resection margin).23 R0/R1 resection of the primary tumor was required within 6 weeks prior to study entry except for those with metastatic disease, unresectable tumor (defined as inability to achieve an R0/R1 resection without unacceptable morbidity), or nonmetastatic high-grade tumor > 5 cm in maximal diameter with gross or microscopic residual tumor anticipated following resection. Primary re-excision was recommended in all R1 tumors where R0 margins were thought to be achievable. Sentinel lymph node biopsy or lymph node sampling was required for epithelioid sarcoma and clear cell sarcoma and for those with clinical or radiographic evidence of regional lymph node enlargement; lymph node dissection was required for those with involved lymph nodes. Biopsy confirmation of metastases was required if all metastases were ≤ 1 cm in maximal diameter.
A performance status score ≥ 50 (Lansky age ≤16 years, Karnofsky age >16 years) and a ≥3 month life expectancy with appropriate therapy were required. Organ function requirements included absolute neutrophil count ≥ 1000/mm3, platelet count ≥ 100,000/mm3, creatinine clearance or radioisotope glomerular filtration rate (GFR) ≥ 70 ml/min/1·73 m2 or normal serum creatinine for age, unimpeded urinary flow via decompression of obstructed portions of the urinary tract, total bilirubin ≤ 1·5x the upper limit of normal for age, shortening fraction ≥ 24% by echocardiogram or ejection fraction ≥ 50% by radionuclide angiogram, no dyspnea at rest, no exercise intolerance, and for those with respiratory symptoms, a resting pulse oximetry reading of >94% on room air. Patients eligible for Arm C or D were required to meet all of the organ function requirements; those eligible for Arm B were required to meet the bone marrow function requirements as well as the requirements pertaining to the organs within the radiotherapy field. A negative pregnancy test was required for female patients of childbearing age eligible for Arms B, C, or D. Sexually active patients of childbearing potential were required to agree to use effective contraception during and for at least 1 month after therapy completion, and lactating women eligible for Arm C or D were required to agree not to breastfeed during treatment and for at least 1 month after therapy completion. Exclusion criteria included prior anthracycline or ifosfamide chemotherapy (only for patients eligible for Arms C and D) and prior radiotherapy to tumor-involved sites.
Primary tumor features including anatomic site, maximal diameter, depth, and bone or neurovascular invasiveness were defined by two pediatric radiologists who reviewed baseline imaging studies; discrepancies were resolved by consensus review. For patients who had undergone tumor resection before baseline imaging, primary tumor features were assigned by central review of operative notes and pathology reports by orthopedic and pediatric surgeons. Sites of metastatic disease and the extent of resection of the primary tumor and metastases were confirmed by central review of imaging studies, operative notes, and pathology reports; discrepancies between radiology and surgery reviewers were resolved by the study chair.
Procedures
Based on tumor features (maximal diameter, POG grade, presence/absence of metastatic disease) and the extent of surgery prior to enrollment, each patient was assigned to one of three risk groups (low, intermediate or high) and one of four treatment arms (A, B, C or D) (Figure 1). The low-risk group included patients who had undergone R0 or R1 resection of a non-metastatic low-grade or ≤ 5 cm highgrade tumor. The intermediate-risk group comprised patients who had undergone R0 or R1 resection of a non-metastatic > 5 cm high-grade tumor or had an unresected, non-metastatic tumor of any grade or size. The high-risk group included patients with nodal or distant metastases.
Patients on Arm A had undergone R0/R1 resection of a non-metastatic low-grade tumor or R0 resection of a non-metastatic, ≤5 cm high-grade tumor before study entry and received no adjuvant therapy. Patients on Arm B had undergone an R1 resection of a ≤ 5 cm high-grade tumor and received 55·8 Gy (31 fractions of 1·8 Gy) adjuvant radiotherapy starting within 6 weeks of surgery.
Table 1 shows the therapy schema for patients on Arms C and D. Although the sequence of every 3 week drug administration differed for the two treatment arms and depended on the type and timing of radiotherapy, the dose per cycle [ifosfamide: 3 g/m2/dose (1·5 g/m2/dose for age < 1 year) intravenously over 3 hours on days 1, 2, and 3; doxorubicin: 37·5 mg/m2/dose (18·75 mg/m2/dose for age < 1 year) intravenously over 24 hours on days 1 and 2] and cumulative dose [ifosfamide 54 g/m2 (27 g/m2 for age < 1 year); doxorubicin 375 mg/m2 (187·5 mg/m2 for age < 1 year)] were identical for all patients assigned to receive chemotherapy. Those assigned to Arm C had undergone R0/R1 resection of a non-metastatic >5 cm highgrade or metastatic tumor. They received ifosfamide/doxorubicin chemotherapy and 55·8 Gy (31 fractions of 1·8 Gy) primary site radiotherapy starting at week 4 after the second cycle of chemotherapy.
Table 1.
Treatment Arm C and D Therapy
| Week 1 | Week 4 | Week 7 | Week 10 | Week 13 | Week 16 | Week 19 | Week 22 |
|---|---|---|---|---|---|---|---|
| Treatment Arm C: Chemotherapy and Radiotherapy | |||||||
| I | I | I | I | I | I | ||
| D# | D | D | D* | D#* | |||
| Radiotherapy | |||||||
| Treatment Arm D: Neoadjuvant Chemoradiotherapy | |||||||
| I | I | I | I | Surgery | I | I | |
| D | D | D† | D‡ | D | |||
| Radiotherapy | Radiotherapy | ||||||
Ifosfamide: Age < 1 year: 1·5 g/m2/dose IV over 3 hours on days 1, 2, 3 with Mesna 300 mg/m2/dose IV over 15 minutes immediately before and 3, 6, and 9 hours after ifosfamide; age ≥ 1 year: 3 g/m2/dose IV over 3 hours on days 1, 2, 3 with Mesna 600 mg/m2/dose IV over 15 minutes immediately before and 3, 6, and 9 hours after ifosfamide
Doxorubicin: Age < 1 year: 18·75 mg/m2/dose IV over 24 hours on days 1, 2; age ≥ 1 year: 37·5 mg/m2/dose IV over 24 hours on days 1, 2 (maximum 75 mg/dose)
Growth Factor: Required except when Doxorubicin administered alone; Filgrastim 5 mcg/kg SQ or Sargramostim 250 mcg/m2 SQ daily starting on day 4 until ANC > 2,000/μl after the nadir or Pegfilgrastim 100 mcg/kg SQ once on day 4
Weeks 1 and 19 Doxorubicin moved to weeks 7 and 10 in patients receiving brachytherapy
Weeks 16 and 19 Doxorubicin moved to weeks 7 and 10 in patients not receiving RT
Week 16 Doxorubicin moved to week 25 in patients receiving intraoperative RT or brachytherapy following week 13 surgery
Week 19 Doxorubicin moved to week 25 in patients receiving external beam RT after week 13 surgery
ANC, absolute neutrophil count; D, doxorubicin; I, ifosfamide; RT, radiotherapy; SQ, subcutaneous
Patients assigned to Arm D had undergone R2 or no resection at the primary site at study entry, with or without metastases, and received neoadjuvant chemotherapy (2 cycles ifosfamide/doxorubicin, 2 cycles ifosfamide alone) and 45 Gy (25 fractions of 1·8 Gy) primary site radiotherapy starting at week 4 after the second cycle of chemotherapy. Definitive primary tumor resection was performed at week 13, if feasible, with the goal of achieving R0 margins; re-excision to achieve R0 margins was encouraged. Regardless of the extent of surgery, chemotherapy was continued postoperatively starting 2–5 weeks after surgery depending on wound healing. A primary site radiotherapy boost was given immediately after the first cycle of postoperative chemotherapy for those with residual tumor after surgery [10·8 Gy (6 fractions of 1·8 Gy) for R1 (cumulative dose 55. 8 Gy) and 19·8 Gy (11 fractions of 1·8 Gy) for R2 or no surgery (cumulative dose 64·8 Gy)].
Primary site cumulative radiotherapy doses of 45 Gy (R0 margin at delayed surgery), 55·8 Gy (R0 margin at upfront surgery or R1 margin at delayed surgery), and 64·8 Gy (R2 margin or no surgery) were selected based on high local control rates achieved in adult soft tissue sarcoma trials with similar doses18 and evidence that higher radiotherapy doses in pediatric sarcoma patients increase secondary neoplasia risk.14 Radiotherapy target volumes and dosimetry was centrally reviewed at the Imaging and Radiation Oncology Core (IROC) within 3 days of the start and end of all radiotherapy for compliance with protocol guidelines. Pathologically involved lymph nodes were excised at the time of primary tumor resection. Clinically or pathologically involved lymph nodes were included with the primary tumor site in the radiotherapy clinical target volume and treated at the same time. Distant metastases were excised at the end of therapy when feasible. Radiotherapy to all residual metastases was recommended at the end of therapy (total dose 50 Gy in 2 Gy fractions). Whole lung or whole abdomen/pelvis radiotherapy was not recommended. Radiotherapy was optional for children ≤24 months of age and for those with hepatic primary tumors, although postoperative radiation for R1 margins after liver tumor resection was encouraged. Protocol-specified radiotherapy was recommended for intraabdominal and retroperitoneal tumors arising outside of the liver.
Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4·0. Adverse event reporting was required within 5 calendar days of learning of the event for grade 5 and unexpected grade 4 adverse events. Patients with intolerable toxicity and those who developed a second malignant neoplasm were removed from protocol therapy. Required laboratory monitoring included a complete blood count with differential, electrolytes, blood urea nitrogen, creatinine, aspartate aminotransferase, alanine aminotransferase, total bilirubin, albumin, and urinalysis before each chemotherapy cycle. Initiation of each chemotherapy cycle required an absolute neutrophil count ≥ 750/microliter, platelet count ≥ 75,000/microliter, total bilirubin ≤ 1·5x the upper limit of normal, and for doxorubicin-containing cycles except at week 4, a shortening fraction 24% or ejection fraction 50% on echocardiogram or multigated acquisition scan. For delays due to either neutropenia or thrombocytopenia at the time of scheduled chemotherapy, dose modifications were made only if both ifosfamide and doxorubicin were being given and included ifosfamide dose reduction first to 7·5 g/m2/cycle (2·5 g/m2/dose × 3 days) and then to 6 g/m2/cycle (2 g/m2/dose × 3 days) if neutropenia recurred. For grade 3 or 4 mucositis following doxorubicin, future cycles utilized the same total dose but shortened the infusion duration to 24 hours. If grade 3 or 4 mucositis recurred, the doxorubicin dose was decreased to 60 mg/m2/cycle (30 mg/m2/dose over 24 hours on days 1 and 2) and the infusion was subsequently shortened to 24 hours for recurrent grade 3/4 mucositis. A ≥33% drop from baseline or abnormal estimated or measured creatinine clearance or GFR required a 1 week delay; persistence for >1 week resulted in replacement of all future ifosfamide doses with cyclophosphamide 700 mg/m2/dose IV over 1 hour on days 1, 2, and 3. Cyclophosphamide was substituted for ifosfamide in an identical manner for development of Fanconi syndrome. Doxorubicin-containing chemotherapy was delayed for 1 week for shortening fraction <24% or ejection fraction <50%, with permanent discontinuation of doxorubicin for persistence of left ventricular systolic dysfunction despite correction of malnutrition. The doxorubicin dose was decreased by 50% for the next cycle after liver irradiation. Direct hyperbilirubinemia prompted omission of doxorubicin for total bilirubin > 6 mg/dL and a dose reduction of 50% for total bilirubin 4·1–6 mg/dL or 25% for total bilirubin 2·1–4 mg/dL. Acute ifosfamide-related neurotoxicity was treated with methylene blue every 6 hours until resolution; methylene blue prophylaxis was recommended daily prior to each subsequent ifosfamide dose. Transient gross and microscopic hematuria was managed with increasing IV hydration and mesna dosing. Omission of all further ifosfamide doses was recommended for recurrent gross hematuria or persistent microscopic hematuria. In Arm D patients, failure to initiate postoperative chemotherapy within 5 weeks of surgery due to wound complications required removal from protocol therapy.
Required baseline imaging included magnetic resonance (MR) or computed tomography (CT) imaging of the primary tumor and draining lymph node bed, CT chest, and either 18FDG-PET scan or Tc99 bone scan. On Arms C and D, MR or CT imaging of the primary tumor, CT chest, and imaging of other metastatic sites identified at study entry were also performed at week 13 and at the end of protocol therapy. Overall imaging response in patients with gross disease at study entry was coded as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) using centrally reviewed volumetric measurements of the primary tumor and the Response Evaluation Criteria in Solid Tumors (RECIST) for metastases (Appendix page 136).24 Patients with tumor progression or recurrence were removed from protocol therapy but remained on study until death, loss to follow-up, withdrawal of consent for data submission, enrollment onto another COG study with tumor therapeutic intent, or the tenth anniversary of study entry. Submission of imaging studies and reports, operative notes, and pathology reports for central review was mandatory for patients with tumor progression/recurrence and secondary malignant neoplasms. Post-treatment imaging surveillance was performed at lengthening intervals over a 5-year period. (Appendix page 137).
Outcomes
The primary objective of ARST0332 was to assess event-free survival (EFS), overall survival (OS), and the pattern of treatment failure in patients under 30 years of age with NRSTS treated with a risk-based strategy. Secondary objectives reported in this manuscript include evaluation of the feasibility of a neoadjuvant chemoradiotherapy approach in patients with intermediate- and high-risk disease, prospective identification of clinical prognostic factors associated with EFS and OS, and assessment of the predictive value of imaging response following neoadjuvant chemoradiotherapy. Due to the large amount of data to be reported, secondary objectives that will be addressed in future manuscripts include: 1) prospective identification of clinical prognostic factors associated with local and distant recurrence, 2) correlation of findings of biologic studies performed on tumor tissue from enrolled patients with their outcomes, 3) assessment of the concordance between institutional and central pathology review of diagnosis and histologic grade, 4) comparison of the POG and FNCLCC pathologic grading systems for predicting outcomes, and 5) determine whether imaging or pathologic response correlates best with clinical outcomes.
Statistical Analysis
The primary aim of this trial was to assess if a risk stratification system based on clinical prognostic factors that had been identified in retrospective studies would separate young NRSTS patients into significantly different prognostic subgroups in the context of risk-based therapy and, in this setting, if each of the factors utilized for treatment allocation would be a strong predictor of outcome. Because this was the first COG protocol to study NRSTS and the anticipated accrual rate was uncertain, a fixed 5-year accrual period was planned with the goal of evaluating outcomes in each unique risk subgroup. Due to lower than anticipated accrual to the low-risk arm, plans to separately evaluate outcomes in each of the 4 low-risk subsets were removed and replaced with a plan to evaluate outcome in the low-risk subset overall, and a fixed 5-year accrual period was maintained.
Three protocol amendments were approved that affected trial recruitment or conduct: 1) eligibility criteria modified to permit enrollment prior to central pathology review and statistical plan updated to reflect lower than anticipated accrual to the low-risk arm (6/1/09), 2) clarified that bone primary tumors were not eligible (3/23/11), 3) set a maximum accrual number (600 patients) anticipated to be necessary to adequately address the study questions (5/6/11).
To monitor for an unacceptably high death rate in low-risk patients, a formal power calculation was performed: a total sample size of 100 would provide 90% power (testing at the 10% level of significance, one-sided) to detect an increased risk of death of 1·97 (long-term survival of 81%). Interim monitoring of survival of low-risk patients was planned at 30% and 70% of the expected information. No formal interim monitoring of EFS and OS was planned for intermediate- and high-risk patients because the planned systemic therapy was considered to be standard-ofcare. Interim monitoring of the cumulative incidence of isolated local recurrence for patients treated on arm D was planned after 15, 30, and 45 isolated local failures were observed.
Since the goal was to evaluate outcomes among patients treated according to the risk-based treatment plan, the final data analysis was performed per protocol guidelines (i.e., the “as treated” principle”). The only patients excluded from the toxicity and EFS/OS analyses were those deemed ineligible for the study and those treated on the incorrect arm (Figure 2). EFS was defined as the time from study enrollment to disease progression or recurrence, second malignant neoplasm, or death from any cause, whichever occurred first. OS was defined as the time from study enrollment to death from any cause. EFS and OS were censored at the patient’s last contact date. Patient follow-up was current through 6/30/18. EFS and OS rates were estimated using the Kaplan-Meier method25 with confidence intervals estimated by the Peto-Peto method26 and were compared between groups using the log-rank test.27
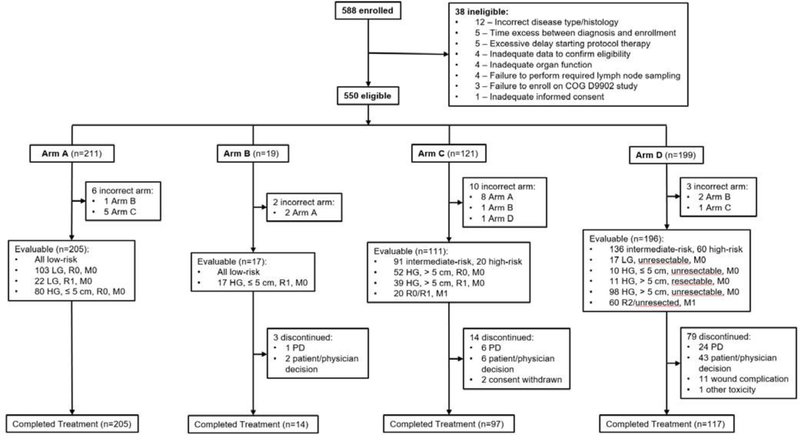
Figure 2.
Study Subject Flow Diagram
HG: high-grade, LG: low-grade, M0: non-metastatic, M1: metastatic, PD: progressive disease, R0: completely excised with negative microscopic margins, R1: grossly excised but with positive microscopic margins, R2: less than complete gross excision
The study protocol specified that the following factors would be evaluated for their influence on EFS and OS: primary site, tumor size, tumor depth, tumor invasiveness, histologic grade (POG and FNCLCC), metastatic status, extent of primary tumor resection, extent of resection of metastases, microscopic surgical margins, imaging response to therapy, pathologic response to therapy, risk group, and treatment arm. Post-hoc, demographic features including age, sex, race, and ethnicity as well as histologic diagnosis (synovial sarcoma vs. other diagnosis) were analyzed. Since intermediate-risk patients could undergo tumor resection prior to study entry or in a delayed fashion, we also evaluated whether the timing of surgery and the extent of resection (R0/R1 vs. R2/no resection, R0 vs. R1) influenced EFS/OS. This analysis was not performed in high-risk patients because their EFS/OS were poor and most treatment failures were due to metastases. Clinical prognostic factors were evaluated independently with the log-rank test. Associations between prognostic factors were evaluated with the Chi-square test. Statistical significance was determined at the 0·05 level. No correction for multiple comparisons was considered. SAS version 9·4 software was used for the statistical analyses. This study is registered with ClinicalTrials.gov, number .
Role of the Funding Source
The National Institutes of Health (NIH), through the Cancer Therapy Evaluation Program, reviewed and approved the study design and original and amended versions of the protocol. COG approved the study design and all versions of the protocol, oversaw the study conduct including data collection, cleaning, analysis, and interpretation, and approved the final manuscript for publication. IROC (Imaging and Radiation Oncology Core Cooperative) participated in radiotherapy case review. Other funding sources provided salary support to protect investigator time for study conduct but had no role in study design, conduct, or reporting. SLS, YYC, JA, JT, and EH had access to the raw data. None of the sponsors participated in writing the manuscript. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
RESULTS
Figure 2 shows the disposition of the 588 patients enrolled between 2/5/07 and 2/20/12 (see Appendix page 138 for a list of participating institutions and number of patients enrolled at each site). Thirty-eight ineligible patients and 21 patients treated on an arm that differed from the treatment arm assigned by the protocol were excluded from all analyses, leaving 529 patients in the analytic cohort who were treated on Arm A (n=205, 39%), B (n=17, 3%), C (111, 21%), or D (196, 37%). Table 2 shows the patient characteristics at study entry.
Table 2.
Patient Characteristics at Study Entry
| Characteristic | Number (%) |
|---|---|
| Age | |
| Median | 13·6 years |
| Range | 0·1–29·8 years |
| Sex | |
| Female | 284 (54%) |
| Male | 245 (46%) |
| Race | |
| White | 375 (71%) |
| Black | 80 (15%) |
| Other/Unknown | 74 (14%) |
| Ethnicity | |
| Non-Spanish, non-Hispanic | 430 (81%) |
| Spanish/Hispanic | 80 (15%) |
| Unknown | 19 (4%) |
| Histologic Diagnosis | |
| Synovial sarcoma | 138 (26%) |
| Malignant peripheral nerve sheath tumor | 58 (11%) |
| Undifferentiated sarcoma | |
| Epithelioid Cell Type | 10 (2%) |
| Pleomorphic Cell Type | 16 (3%) |
| Round Cell Type | 18 (3%) |
| Spindle Cell Type | 15 (3%) 4 |
| Not Otherwise Specified | (1%) |
| Undifferentiated embryonal sarcoma of the liver | 39 (7%) |
| Epithelioid sarcoma | 28 (5%) |
| Liposarcoma | 25 (5%) |
| Alveolar soft part sarcoma | 24 (5%) |
| Dermatofibrosarcoma protuberans | 21 (4%) |
| Low-grade fibromyxoid sarcoma | 16 (3%) |
| Leiomyosarcoma | 10 (2%) |
| Other specific entity | 63 (12%) |
| Unclassified soft tissue sarcoma | 44 (8%) |
| World Health Organization Soft Tissue Tumor Classification | |
| Malignant | 473 (89%) |
| Intermediate, rarely metastasizing | 56 (11%) |
| Primary Tumor Site | |
| Extremity | 289 (55%) |
| Visceral | 111 (21%) |
| Body wall | 75 (14%) |
| Head/neck | 54 (10%) |
| Extent of Disease | |
| Non-metastatic | 449 (85%) 80 |
| Metastatic | (15%) |
| Sites of Metastases (80 patients with metastases) | |
| Lung | 57 (71%) |
| Lymph nodes | 20 (25%) |
| Visceral surface (pleura, peritoneum, etc.) | 11 (14%) |
| Liver | 7 (9%) |
| Bone | 6 (8%) |
| Brain | 2 (3%) |
| Leptomeninges | 1 (<1%) |
| POG Grade | |
| 1 | 60 (11%) |
| 2 | 86 (16%) |
| 3 | 383 (73%) |
| FNCLCC Grade | |
| 1 | 70(13%) |
| 2 | 222 (42%) |
| 3 | 236 (45%) |
| Indeterminate | 1 (0%) |
| Maximal Tumor Diameter | |
| <= 5 cm | 195 (37%) |
| 5.1–10 cm | 168 (32%) |
| 10.1–15 cm | 106 (20%) |
| 15.1–20 cm | 46 (9%) |
| 20.1–25 cm | 13 (2%) |
| 25.1–30 cm | 1 (<1%) |
| Tumor Depth | |
| Superficial | 92 (17%) |
| Deep | 437 (83%) |
| Bone or Neurovascular Invasiveness | |
| Non-invasive | 229 (43%) |
| Invasive | 300 (57%) |
| Extent of Primary Tumor Resection | |
| Completely excised with negative microscopic margins (R0) | 252 (48%) 81 |
| Grossly excised but with positive microscopic margins (R1) | (15%) |
| Less than complete gross excision (R2) or unresected | 196 (37%) |
| Extent of Resection of Metastases (80 patients with metastases) | |
| Complete | 5 (6%) |
| Microscopic residual | 6 (8%) |
| Gross residual | 69 (86%) |
FNCLCC: Federation Nationale des Centres de Lutte Contre le Cancer, POG: Pediatric Oncology Group
The 205 low-risk Arm A patients with non-metastatic R0/R1 low-grade and <= 5 cm R0 high-grade tumors were observed without adjuvant therapy. Among the 17 lowrisk Arm B patients (including 5 patients with <5 mm margins after R0 resection), 15 (88%) received 55·8 Gy of adjuvant radiotherapy and 2 (12%) went off protocol therapy before radiotherapy. The 111 Arm C patients (91 intermediate-risk, 20 highrisk) received chemotherapy and 85 (77%) received primary site radiotherapy (median dose 55·8 Gy, range 28·5–57·6 Gy).
The 196 Arm D patients (136 intermediate-risk, 60 high-risk) received neoadjuvant chemotherapy and 155 (79%) received radiotherapy to the primary tumor (median dose 45·0 Gy, range 14·4–68·8 Gy). Thirty-one patients were removed from protocol therapy prior to week 13 for reasons other than PD (22 physician decision, 8 patient/guardian refusal, 1 toxicity). Excluding these 31 patients and the 13 patients with inevaluable imaging, imaging response was evaluable in 152 patients: CR (n=2, 1%), PR (n=47, 31%), SD (n=82, 54%), PD (n=21, 14% including 15 removed from protocol therapy for PD before week 13). Eight of the 21 PD patients (38%) had isolated local tumor progression. Five of these 8 patients (63%) survived > 3 years from study entry, including 2 patients with PD by imaging criteria but who remained on protocol therapy (pseudoprogression); two patients died of metastatic disease progression (1 with stable lung metastases at the time of primary tumor PD) and one died in a car accident. Excluding the 52 patients removed from protocol therapy (22 physician decision, 8 patient/guardian refusal, 1 toxicity, 21 PD), 144 patients were eligible for surgery at week 13. Extent of resection in these 144 patients was R0 (n=111, 77%), R1 (n=21, 15%), R2 (n=3, 2%), and no surgery (n=9, 6%). Among 222 low-risk patients, 219 (99%) completed protocol therapy. Reasons for non-completion were PD (n=1) and physician/patient decision (n=2). Among 227 intermediate-risk patients, 175 (77%) completed protocol therapy. Reasons for non-completion in the remaining 52 patients were consent withdrawn (n=2, 4%), PD (n=11, 21%), patient/physician decision (n=31, 60%), and wound complications (n=8, 15%). Among 80 high-risk patients, 39 (49%) completed protocol therapy. Reasons for non-completion in the 41 remaining patients were PD (n=19, 46%), patient/physician decision (n=18, 44%), wound complications (n=3, 7%), or other intolerable toxicity (n=1, 3%). Nineteen patients (24% of high-risk patients) underwent gross resection of all metastases, 11 prior to study entry and 8 during protocol therapy. Ten patients received radiotherapy to one or more metastatic sites (13% of high-risk patients), most commonly to lungs (n=3), soft tissues (n = 3), and bone (n=2).
Overall compliance with protocol treatment guidelines was good. Eighteen of 307 patients treated with chemotherapy (6%) had unplanned chemotherapy dose modifications, 12 intermediate-risk and 6 high-risk. Of the 324 patients assigned to a radiotherapy-containing treatment arm, 66 (20%) did not receive radiotherapy due to: amputation (3, 4%), age ≤ 24 months (5, 8%), hepatic primary tumor (29, 44%), removal from protocol therapy before radiotherapy due (27, 41%), or protocol violation (2, 3%). Among 34 patients eligible for a radiotherapy boost after delayed surgery, 5 did not receive it (15%) secondary to removal from protocol therapy due to patient/physician decision (n=4) or spinal cord tolerance (n=1), and 2 patients received a higher boost dose than prescribed by the protocol. Among the 7 patients with radiotherapy deviations, 5 were intermediate-risk and 2 were high-risk. Four-hundred twelve of the 529 evaluable patients (78%) were alive at the time of this analysis, with a median follow-up of 6·5 years (IQR 2·9 years); 92% of surviving patients (379 of 412) had more than 2 years of follow-up. At 5 years, estimated EFS and OS were 68% (95% CI: 63·4–72·5%) and 79·4% (95% CI: 75·5–83·4%) for the entire cohort, and both EFS and OS differed significantly by risk group and treatment arm (all p<0·0001) (Figures 3–5). Table 3 shows EFS and OS by clinical features, risk group, treatment administered, and response to therapy. The 214 first events included local recurrence/progression (n=42, 20%; 13 Arm A, 2 Arm B, 9 Arm C, 18 Arm D), metastatic recurrence/progression +/− local recurrence/progression (n=118, 74%; 7 Arm A, 2 Arm B, 32 Arm C, 77 Arm D), second malignant neoplasm (n=12, 6%; 1 Arm A, 1 Arm B, 5 Arm C, 5 Arm D), and death (n=1, <1%; Arm C).
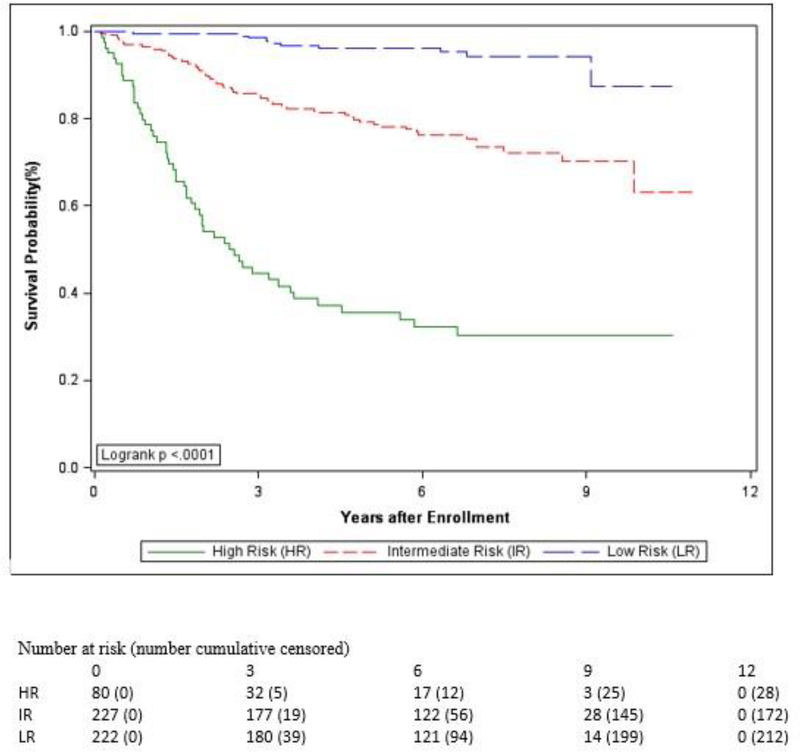
Figure 3.
Estimated Event-Free Survival by Risk Group
Figure 5.
Estimated Event-Free Survival by Treatment Arm
Table 3.
Univariate Analysis of Outcomes by Clinical Features, Risk Group, Treatment, and Response to Therapy
| Subgroup | # (%) | 5-year EFS (95% CI) | p-Value | 5-year OS (95% CI) | p-Value |
|---|---|---|---|---|---|
| Clinical Features at Study Entry | |||||
| Age | |||||
| < 2 years | 17 (3%) | 69·7 (43·1, 96·3) | 0·0005 | 93·8 (79·2, 100·0) | 0·0087 |
| 2–9.99 years | 129 (24%) | 81·5 (73·9, 89·1) | 86·6 (80·0, 93·2) | ||
| > 10 years | 383 (72%) | 63·3 (57·7, 68·8) | 76·4 (71·5, 81·2) | ||
| Sex | |||||
| Female | 284 (54%) | 70·9 (64·9, 76·9) | 0·13 | 80·8 (75·6, 86·0) | 0·21 |
| Male | 245 (46%) | 64·6 (57·7, 71·4) | 77·9 (71·9, 83·8) | ||
| Race | |||||
| White | 375 (71%) | 70·7 (65·5, 75·9) | 0·09 | 81·1 (76·7, 85·6) | 0·11 |
| Non-White/Unknown | 154 (29%) | 61·2 (52·1, 70·2) | 75·1 (67·0, 83·2) | ||
| Ethnicity | |||||
| Hispanic | 80 (15%) | 61·1 (48·4, 73·7) | 0·25 | 74·0 (62·7, 85·3) | 0·29 |
| Non-Hispanic/Unknown | 449 (85%) | 69·2 (64·4, 74·1) | 80·4 (76·2, 84·6) | ||
| Primary Site | |||||
| Head/neck | 54 (10%) | 63·8 (49·6, 78·0) | 80·5 (68·8, 92·3) | ||
| Body wall | 75 (14%) | 64·4 (51·5, 77·4) | 0·27 | 84·1 (74·0, 94·1) | 0·53 |
| Visceral | 111 (21%) | 63·2 (53·6, 72·7) | 74·0 (65·3, 82·7) | ||
| Extremity | 289 (55%) | 71·8 (65·7, 77·8) | 80·3 (74·9, 85·6) | ||
| Histologic Diagnosis | |||||
| Synovial sarcoma | 138 (26%) | 64·9 (55·7, 74·1) | 0·70 | 80·8 (73·3, 88·3) | 0·93 |
| Other diagnosis | 391 (74%) | 69·1 (63·8, 74·3) | 78·9 (74·3, 83·5) | ||
| Maximal Tumor Diameter | |||||
| ≤ 5 cm | 195 (37%) | 85·4 (79·7, 91·1) | <0·0001 | 93·2 (89·1, 97·2) | <0·0001 |
| > 5 cm | 334 (63%) | 57·8 (51·8, 63·8) | 71·4 (65·9, 76·9) | ||
| Tumor Depth | |||||
| Superficial | 92 (17%) | 93·2 (87·3, 99·2) | <0·0001 | 95·2 (90·1, 100·0) | <0·0001 |
| Deep | 437 (83%) | 62·7 (57·6, 67·9) | 76·1 (71·6, 80·7) | ||
| Tumor invasiveness | |||||
| Non-invasive | 229 (43%) | 82·1 (76·2, 87·9) | <0·0001 | 89·3 (84·6, 93·9) | <0·0001 |
| Invasive | 300 (57%) | 57·4 (51·2, 63·7) | 72·1 (66·4, 77·7) | ||
| POG grade | |||||
| Low (1/2) | 146 (28%) | 86·9 (80·2, 93·6) | <0·0001 | 95·0 (90·7, 99·4) | <0·0001 |
| High (3) | 383 (72%) | 61·0 (55·6, 66·3) | 73·8 (69·0, 78·7) | ||
| FNCLCC grade* | |||||
| Low (1) | 70 (13%) | 98·4 (95·0, 100·0) | <0·0001 | 100·0 | <0·0001 |
| High (2/3) | 458 (87%) | 63·8 (58·8, 68·7) | 76·7 (72·3, 81·1) | ||
| Metastases | |||||
| No | 449 (85%) | 76·5 (72·0, 81·0) | <0·0001 | 87·4 (83·9, 90·9) | <0·0001 |
| Yes | 80 (15%) | 21·2 (11·4, 31·1) | 35·5 (23·6, 47·4) | ||
| Extent of resection | |||||
| R0/R1 | 333 (63%) | 79·3 (74·3, 84·3) | <0·0001 | 89·5 (85·7, 93·3) | <0·0001 |
| R2/Unresected | 196 (37%) | 49·2 (41·4, 57·0) | 62·7 (55·2, 70·3) | ||
| Microscopic surgical margin | 333 | ||||
| R0 | 252 (76%) | 83·6 (78·3, 89·0) | 0·0006 | 92·8 (89·1, 96·5) | 0·0043 |
| R1 | 81 (24%) | 66·2 (54·8, 77·5) | 79·7 (70·0, 89·5) | ||
| Risk Group and Treatment | |||||
| Risk Group | 529 | 68·0 (63·4, 72·5) | 79·4 (75·5, 83·4) | ||
| Low | 222 (42%) | 88·9 (84·0, 93·8) | 96·2 (93·2, 99·2) | ||
| Intermediate | 227 (43%) | 65·0 (58·2, 71·8) | <0·0001 | 79·2 (73·4, 85·0) | <0·0001 |
| High | 80 (35%) | 21·2(11·4, 31·1) | 35·5 (23·6, 47·4) | ||
| Arm A: Surgery alone | 205 (39%) | 89·9 (85·0, 94·8) | 97·0 (94·2, 99·8) | ||
| LG, R0 | 103 | 96·5 (92·1, 100·0) | 100·0 | ||
| LG, R1 | 22 80 | 80·6 (59·6, 100·0) | 0·011 | 100·0 | 0·15 |
| HG, ≤ 5 cm, R0 | 84·2 (75·0, 93·3) | 92·9 (86·6, 99·3) | |||
| Arm B: Adjuvant radiotherapy | 17 (3%) | 76·5 (54·6, 98·4) | 86·7 (68·8, 100·0) | ||
| All HG, ≤ 5 cm, R1 | |||||
| Arm C: Chemoradiotherapy | 111 (21%) | 61·2 (51·5, 70·9) | 76·9 (68·4, 85·4) | ||
| Non-metastatic, HG, > 5 cm, R0 | 52 | 71·4 (58·2, 84·6) | <0·0001 | 89·7 (80·7, 98·7) | <0·0001 |
| Non-metastatic, HG, > 5 cm, R1 | 39 20 | 67·2 (51·4, 82·9) | 78·0 (64·2, 91·8) | ||
| Metastatic R0/R1 | 24·0 (3·5, 44·5) | 42·4 (18·6, 66·3) | |||
| Arm D: Neoadjuvant chemoradiotherapy | 196 (37%) 17 | 49·2 (41·4, 57·0) | <0·0001 | 62·7 (55·2, 70·3) | <0·0001 |
| Non-metastatic, LG, unresectable at study entry | 54·5 (27·3, 81·8) | 79·6 (57·2, 100·0) | |||
| Non-metastatic, HG, > 5 cm, resectable at study entry | 11 10 | 70·7 (40·1, 100·0) | 75·0 (45·0 100·0) | ||
| 98 60 | 68·6 (37·8, 99·3) | 80·0 (53·5, 100·0) | |||
| Non-metastatic, HG, ≤ 5 cm, unresectable at study entry | 61·4 (50·7, 72·1) | 74·5 (65·0, 83·9) | |||
| Non-metastatic, HG, > 5 cm, unresectable at study entry | 20·3 (9·1, 31·5) | 33·1 (19·4, 46·8) | |||
| Metastatic, R2/unresected | |||||
| Intermediate risk patients: Extent of resection including delayed surgery | <0·0001 | <0·0001 | |||
| R0/R1 | 196 31 | 70·5 (63·5, 77·4) | 82·5 (76·7, 88·2) | ||
| R2/no resection | 29·6 (9·7, 49·5) | 58·0 (37·6, 78·5) | |||
| Intermediate risk patients: Timing of R0/R1 resection | 0·67 | 0·50 | |||
| At study entry | 91 | 69·7 (59·5, 79·8) 71·2 | 84·6 (76·6, 92·6) | ||
| At delayed surgery | 105 | (61·8, 80·8) | 80·6 (72·3, 88·8) | ||
| Intermediate risk patients: Surgical margins including delayed surgery | 0·36 | 0·79 | |||
| R0 | 138 58 | 71·8 (63·6, 80·0) 67·3 | 83·5 (76·8, 90·3) | ||
| R1 | (54·4, 80·2) | 79·9 (69·0, 90·9) | |||
| Response to Therapy | |||||
| Arm D patients evaluable for response at week 13 | 152 | 51·5 (42·7, 60·3)60·9 | 65·6 (57·2, 73·9) | ||
| Complete response/partial response | 49 (32%) 82 | (46·0, 75·8) | <0·0001 | 75·2 (62·2, 88·1) | <0·0001 |
| Stable disease | (54%) 21 | 56·7 (44·8, 68·5) | 68·1 (57·0, 79·3) | ||
| Progressive disease^ | (14%) | 9·5 (0·0, 27·3) | 33·3 (6·7, 60·0) | ||
EFS: event-free survival, FNCLCC: Federation Nationale des Centres de Lutte Contre le Cancer, HG: high-grade, LG: low-grade, OS: overall survival, POG: Pediatric Oncology Group, R0: completely excised with negative microscopic margins, R1: grossly excised but with positive microscopic margins, R2: less than complete gross excision
1 case missing FNCLCC grade
Includes patients with progressive disease prior to week 13
In univariate analysis (Table 3), the 5 factors used for treatment stratification [metastases (present vs. absent), POG grade (low [1/2] vs. high [3]), tumor size (≤ 5 cm vs. > 5 cm), extent of resection (R0/R1 vs. R2/unresectable), and margin status (R0 vs. R1)] were each strongly associated with EFS and OS. Other factors with strong association with EFS and OS were tumor depth (superficial vs. deep), invasiveness (non-invasive vs. invasive), and FNCLCC grade (1 vs. 2/3). These 3 factors were each strongly associated with all of the 5 treatment stratification factors (Appendix page 142). Patients 2–10 years of age had a better EFS than either younger or older patients (p=0·0005), but OS progressively declined with increasing age group (p=0·0087). Sex, race, ethnicity, primary site, and histologic diagnosis were not significant predictors of outcomes.
Overall outcomes in the low-risk group were excellent: 5-year EFS 88·9% (95% CI, 84·0–93·8%) and OS 96·2% (95% CI, 93·2–99·2%). Events (n=26) included isolated local recurrence/progression (n=15, 58%), metastatic recurrence/progression +/− local recurrence/progression (n=9, 34%), and second malignant neoplasm (n=2, 8%). Isolated local recurrence/progression occurred in 4 of 103 low-grade R0 tumors (4%), 4 of 22 low-grade R1 tumors (18%), 5 of 80 ≤5 cm high-grade R0 tumors (9%), and 2 of 17 ≤5 cm high-grade R1 tumors (18%). The 9 patients who developed metastases had 5 different high-grade histology tumors that ranged from 2·3 to 4·5 cm in size (7 R0, 2 R1). Ten deaths were reported among the 222 low-risk patients (5%): 9 due to PD (7 Arm A, 2 Arm B) and 1 due to a second malignant neoplasm (Arm B).
Intermediate-risk patients had a 5-year EFS of 65% (95% CI, 58·2–71·8%) and OS of 79.2% (95% CI, 73·4–85%). Events (n=84) included isolated local recurrence/progression (n=22, 26%), metastatic recurrence/progression +/− local recurrence/progression (n=52, 62%), second malignant neoplasm (n=9, 11%), and death (n=1, 1%). Fifty-five deaths were reported among the 227 intermediate-risk patients (24%): 48 due to PD (13 Arm C, 35 Arm D), 3 due to a second malignant neoplasm (2 Arm C, 1 Arm D), 2 due to toxicity after protocol therapy completion (1 infection during chemotherapy for tumor recurrence, 1 surgical complication during neurofibroma treatment); 1 Arm C, 1 Arm D), and 2 due to other reasons (car accident, cardiac failure due to tricuspid regurgitation; 1 Arm C, 1 Arm D). As shown in Table 3, unresectable disease in intermediate-risk patients predicted poor EFS [29·6% (95% CI 9·7–49·5%) for R2/no resection vs. 70·5% (95% CI 63·5–77·4%) for R0/R1 resection, p<0·0001] and OS [58·0% (95% CI 37·6–78·5%) for R2/no resection vs. 82·5% (95% CI 76·7–88·2%) for R0/R1 resection, p<0·0001].
Outcomes for upfront R0/R1 resection [EFS 69·7% (95% CI 59·5–79·8%) and OS 84·6% (95% CI 76·6–92·6%)] were similar to those for delayed R0/R1 resection [EFS 71·2% (95% CI 61·8–80·8%) and OS 80·6% (95% CI 72·3–88·8%)] (p=0·67 for EFS, p=0·50 for OS). The likelihood of R0 resection was similar whether it was performed upfront (52 of 91, 57%) or in a delayed fashion (85 of 136, 63%). However, there was not a significant difference in outcome for those who had an R0 resection compared to those who had an R1 resection: EFS [71·8% (95% CI 63·680·0%) for R0 vs. 67·3% (95% CI 54·4–80·2%) for R1, p=0·36] and OS [83·5% (95% CI 76·8–90·3%) for R0 vs. 79·9% (95% CI 69·0–90·9%) for R1, p=0·79]. Outcomes for high-risk patients were poor, with estimated 5-year EFS of 21·2% (95% CI 11·4–31·1%) and OS of 35·5% (95% CI 23·6–47·4%), respectively. Events (n=63) included isolated local recurrence/progression (n=5), metastatic recurrence/progression +/− local recurrence/progression (n=57), and second malignant neoplasm (n=1). Fifty-two deaths (12 Arm C, 40 Arm D) were reported among the 80 high-risk patients (65%), 4 due to isolated local recurrence/progression and 48 due to metastatic recurrence/progression +/− local recurrence/progression. Since only 19 patients underwent gross resection of the primary tumor and metastases, it was not possible to determine whether complete resection of all disease predicted a better outcome.
There were no toxic deaths during protocol therapy, and only one patient discontinued therapy early (grade 4 encephalopathy due to ifosfamide, Arm D). Ten patients (2%) experienced unexpected grade 4 adverse events (2 Arm C, 8 Arm D; 7 intermediate-risk, 3 high-risk). Four of these were Arm D wound complications: one developed an open wound over the primary tumor during neoadjuvant chemoradiotherapy and underwent above-knee amputation; the other 3 had wound complications following definitive surgery that required operative intervention. Eleven additional Arm D patients were removed from protocol therapy due to wound complications that precluded initiation of postoperative therapy within 5 weeks of surgery. These 15 wound complications that occurred in 135 Arm D patients who underwent delayed surgery represents a wound complication rate of 11%. Thirteen second malignant neoplasms have been reported among the 529 eligible/evaluable patients (2%; 1 Arm A, 1 Arm B, 5 Arm C, 6 Arm D; 2 low-risk, 10 intermediate-risk, 1 high-risk), including 6 in patients with neurofibromatosis type I (5 MPNST and 1 brainstem glioma, all outside of the prior radiotherapy field) and 1 in a patient with Li-Fraumeni syndrome (spinal cord astrocytoma at the margin of the prior radiotherapy field). Other cancers not associated with a documented genetic cancer predisposition syndrome included acute myelogenous leukemia (n=3), papillary thyroid cancer (n=2, both outside the prior radiotherapy field), and chondroblastic osteosarcoma (n=1, within the prior radiotherapy field).
DISCUSSION
This trial met its primary aim of confirming that the risk stratification system developed for young patients with NRSTS separated patients into low-, intermediate, and high-risk prognostic subgroups with statistically different EFS and OS in the context of risk-adapted therapy. The 5 factors utilized in the risk stratification system (POG grade, tumor size, metastatic status, extent of resection, and margin status) were each strongly predictive of both EFS and OS. Adequate surgery, including primary re-excision whenever possible to achieve negative margins, is the only one of these risk factors that is potentially modifiable and continues to be important for achieving a cure. Further testing of the predictive value of the risk model used in this study will be required to confirm its validity with alternate therapeutic approaches. Additional analyses will also be needed to determine whether certain patient subsets, such as those with particular histologic subtypes, had outcomes consistent with their risk assignment and benefitted equally from the risk-based therapeutic approach utilized. Like other childhood cancer risk stratification systems that have been developed over the last several decades, the risk model used in this clinical trial will help clinicians plan risk-adapted therapy for young NRSTS patients that optimizes the likelihood of cure while minimizing treatment exposures and also provides an important foundation for future studies of pediatric NRSTS.
In this study, low-risk patients had excellent outcomes (5-year EFS 88·9% and OS 96·2%) despite the fact that only 8% received adjuvant radiotherapy and none received chemotherapy. Although high rates of local tumor control and universal survival were anticipated and confirmed in low-grade tumors observed after R0 resection,4,7,10 92% of patients with ≤5 cm high-grade tumors observed after R0 resection also remained free of local recurrence. This suggests that radiotherapy can be safely avoided in this patient subset, thereby avoiding long-term radiotherapy risks. Unfortunately 9% of low-risk patients with high-grade tumors developed metastases. We were unable to identify unique features of these patients that would permit them to be identified prospectively and treated with systemic therapy.
Although retrospective case series in adults have reported similar outcomes for lowrisk patients,11,12 to our knowledge this is the first prospective clinical trial of patients of any age treated with wide resection alone for small high-grade soft tissue sarcomas. Based on our findings, we recommend omission of RT following R0 resection of both low-grade and ≤5 cm high-grade soft tissue sarcomas in young patients although further research is needed to identify the few patients with highgrade tumors who deserve systemic therapy. Whether radiotherapy can be safely omitted in patients with >5 cm high-grade tumors or in older patients following R0 resection is uncertain, although in a multivariate analysis of 684 soft tissue sarcoma patients over 16 years of age treated with surgery alone, tumor size > 5 cm (p=0·05) and age > 50 years (p=0·02) both predicted a higher risk of local recurrence.28 Of interest is that despite receiving no adjuvant therapy, only 4 of 22 patients (18%) with low-grade R1 NRSTS experienced events, all of which were local recurrence/progression. Three of these 4 patients were effectively salvaged; the remaining patient died of metastatic disease 5·6 years from local recurrence. This single death likely reflects aggressive tumor biology, as local recurrence seems to be an indicator of biologic aggressiveness rather than a cause of subsequent metastatic recurrence.13 Although the small number of enrolled patients did not permit a precise estimate of the likelihood of local failure in this particular patient subgroup, the observed local recurrence rate was lower than expected.13 This, in combination with the high rate of effective salvage for local recurrence of low-grade tumors documented in this study and elsewhere,10,29 suggests that omission of radiotherapy should be considered in patients with microscopic residual low-grade NRSTS after maximal resection. The only potential exception is where local recurrence would create substantial risks. This conservative approach to the use of radiotherapy could spare many children from the burdens, cost, and toxicity of adjuvant radiotherapy, although it does produce a higher therapy burden for those who experience local tumor recurrence.
Comparing outcomes for intermediate-risk patients in this study to historical controls is difficult due to challenges in documenting past outcomes for this exact subgroup. Nevertheless, it appears that our EFS and OS were similar to or better than those reported in the literature.2,3,7–10 Use of a standardized therapeutic approach with dose-intensive ifosfamide/doxorubicin chemotherapy, image-guided conformal radiotherapy, improvements in surgical techniques, and better supportive care may all have contributed to more favorable outcomes.
Among patients with unresected tumor at study entry, the combination of ifosfamide/doxorubicin chemotherapy and 45 Gy of radiotherapy proved feasible. Wound complications that significantly delayed postoperative therapy or required operative intervention occurred in 11% of patients who underwent surgery after neoadjuvant chemoradiotherapy. This rate is similar to or perhaps slightly lower than the 11–29% wound complication rate reported in adults treated with neoadjuvant chemoradiotherapy.18,30 In our study, neoadjuvant chemoradiotherapy produced CR/PR rates and PD rates very similar to the 22–39% CR/PR rate reported in pediatric and adult patients treated with either chemotherapy or chemoradiotherapy7,9,18 and the 13–14% PD rate reported in adults treated with chemoradiotherapy.18 Our PD rate was substantially lower than the 26% rate reported previously in pediatric patients.9 Although this may reflect better disease control due to combining dose-intensive ifosfamide/doxorubicin with radiotherapy in a standardized fashion, it is difficult to prove that low PD rates could not be achieved with either modality alone.
Importantly, despite only a 32% CR/PR rate following neoadjuvant chemoradiotherapy, 92% of patients who reached week 13 underwent an R0/R1 resection. Whether the combination of chemotherapy and radiotherapy enhanced the resection potential is uncertain, but chemoradiotherapy studies in adult soft tissue sarcoma have documented similarly high rates of resection.18 Considering that tumors assigned to delayed resection likely were larger and more invasive than those that underwent upfront resection, it is notable that the proportion of R0 resections was similar whether the operation was done prior to study entry or at week 13. Since the timing of resection (upfront or delayed) did not impact EFS or OS and those who underwent delayed R0 resection received a lower total dose of radiotherapy (45 Gy, versus 55·8 Gy for those undergoing upfront R0 resection), a delayed surgery approach should be considered for patients who will require chemotherapy and radiotherapy. Other theoretical considerations supporting radiotherapy administration prior to surgery include smaller radiotherapy field sizes, greater efficacy of radiotherapy in tissues that are not hypoxic following surgery, and a reduction in risk of secondary neoplasia following resection of the majority of irradiated tissues. One potential downside of administering neoadjuvant therapy prior to surgery is the risk of local tumor progression that renders the primary tumor unresectable. However, we found that only 5% of patients evaluable for response after neoadjuvant chemoradiotherapy experienced isolated local tumor progression and several of these patients were salvaged, suggesting that delaying surgery until after neoadjuvant therapy does not create significant risks. Based on all of these considerations, there is a strong argument that pediatric NRSTS patients requiring both chemotherapy and radiotherapy should receive combined modality therapy prior to tumor resection rather than undergoing upfront surgery followed by chemotherapy and radiotherapy. However, careful monitoring of the primary site by physical examination and/or imaging during neoadjuvant therapy is important to identify the small percentage of patients with disease progression warranting earlier surgical intervention.
Despite intensive multimodality therapy, patients with high-risk metastatic disease fared poorly with 5-year EFS 21·2% and OS 35·5%. These outcomes compare favorably to those of past pediatric NRSTS trials,2,3 suggesting that the treatment strategy used in this study (dose-intensive ifosfamide/doxorubicin chemotherapy, radiotherapy often administered in a neoadjuvant setting, and surgical resection of all sites of disease) was modestly efficacious. Our observation that only 49% of patients with metastatic disease completed protocol therapy is concerning, though, and nearly half who discontinued treatment early did so due to disease progression, usually metastatic. Since further intensification of cytotoxic chemotherapy is not feasible, improvements in outcome for high-risk patients await identification of novel therapies.
Major limitations of this study are the heterogeneity of the patient population in terms of age, primary tumor sites, and histologic subtypes, which may have masked important therapy considerations for selected patient subsets, and the absence of randomized comparisons that could add confidence to our conclusions. Using historical controls to inform our interpretation of this study is fraught with challenges including difficulty identifying comparable patients, variability in therapy, and confounding factors including unmeasurable advances in medical care over time that may have influenced outcomes. Unfortunately, given the rarity of pediatric NRSTS, it is unlikely that randomized, controlled clinical trials will be feasible even in the most common histologic subtypes without trans-Atlantic collaboration or inclusion of adults. Therefore, the findings of this study and those of the similar European paediatric Soft Tissue Sarcoma Group’s NRSTS 2005 study conducted during the same timeframe will likely determine the standard of care for most pediatric NRSTS patients for the foreseeable future.
In this era of precision medicine and targeted cancer therapy, our study demonstrates the continued value of careful risk stratification and optimization of standard therapy. Many questions about standard therapy remain. Is adjuvant radiotherapy needed for > 5 cm, high-grade tumors that are adequately excised? What is the minimum dose of radiotherapy needed to achieve adequate local tumor control following R1 resection? In which settings does chemotherapy measurably improve outcome, and what magnitude of outcome improvement is worth exposing young patients to chemotherapy that carries significant long-term risks? Is doseintensive ifosfamide and doxorubicin the most effective and least toxic chemotherapy regimen? There is clearly much more progress to be made, considering the suboptimal cure rates for intermediate- and high-risk patients and the substantial long-term complications of therapy. Studies to further refine the approach to local tumor control, optimize systemic therapy, and integrate targeted agents are needed.
Supplementary Material
Figure 4.
Estimated Overall Survival by Risk Group
Panel: Research in Context.
Evidence before this study
In designing this clinical trial, the authors considered both retrospective and prospective published data from pediatric and adult soft tissue sarcoma studies excluding those focusing on rhabdomysoasrcoma that were reported in English in the PubMed database between 1/1/80 and 12/31/2005. MeSH (Medical Subject Heading) terms searched included sarcoma; soft tissue neoplasms; neoplasms, connective and soft tissue; clinical trial; risk factors; prognosis; infant; child, preschool; child; adolescent; chemotherapy, adjuvant; antineoplastic combined chemotherapy protocols; antineoplastic protocols; ifosfamide; cyclophosphamide; dacarbazine; doxorubicin; epirubicin; neoadjuvant therapy; radiotherapy; radiotherapy, adjuvant; surgery; second-look surgery. Non-MeSH terms searched included histologic grading, grading, prognostic factor, primary re-excision, excision margin, resection margin, surgical margin, chemoradiotherapy, neoadjuvant chemotherapy, neoadjuvant radiotherapy. Further, separate searches were conducted for each tumor type eligible for this trial, using the MeSH term if one was available or a non-MeSH term if not. Prognostic factor analyses and clinical trials reporting survival and disease-specific outcomes were evaluated to identify the most important clinical features to be included in the risk stratification system tested in this clinical trial. Retrospective case series and prospective clinical trials were reviewed to develop the treatment guidelines to be used in this study, which were dependent on risk classification and clinical features.
Added value of this study
This study confirms that the risk stratification system developed for young patients with NRSTS separated patients effectively into low-, intermediate-, and high-risk prognostic subgroups with statistically different EFS and OS in the context of riskadapted therapy. Although many low-risk patients previously received adjuvant radiotherapy following R0 resection of a ≤ 5 cm high-grade soft tissue sarcoma, the high rate of local tumor control (92%) in our study with surgery alone suggests that radiotherapy can be safely omitted in this clinical setting. A surgery only strategy in low-risk patients with low-grade soft tissue sarcomas who underwent an R1 resection yielded a relatively low local failure rate and a high salvage rate, indicating that omission of radiotherapy should also be considered in this setting. For patients in all risk groups who require adjuvant radiotherapy, this study showed that a slightly lower radiotherapy dose than is typically used (55.8 Gy) produced high rates of local tumor control. Neoadjuvant dose-intensive ifosfamide/doxorubicin chemotherapy combined with radiotherapy was feasible and safe for unresected intermediate- and high-risk soft tissue sarcoma patients and produced a high rate of delayed R0/R1 resection with a low likelihood of isolated local tumor progression or significant postoperative wound complications.
Implications of all the available evidence
The risk stratification system utilized in this study will help clinicians plan riskadapted therapy for young NRSTS patients that optimizes the likelihood of cure while minimizing treatment exposures. Importantly, our study demonstrates that radiotherapy either can be omitted or the dose reduced in most patients without compromising survival, thereby potentially reducing the known long-term risks of radiotherapy in survivors. Since the small number of available pediatric NRSTS patients precludes a randomized clinical trial to confirm our results, our findings will inform the standard of care while providing benchmark outcome data against which outcomes in future clinical trials will be compared.
Data Sharing
Deidentified individual patient data from this clinical trial and a data dictionary defining each field in the dataset will be made available on the NCI NCTN/NCORP Data Archive (https://nctn-data-archive.nci.nih.gov/) within one year of print publication of this manuscript according to the NIH Data Sharing Policy (grants.nih.gov/grants/policy/data_sharing/). Data access requires a user account with an official institutional email address and completion of an online Data Request Form that includes a brief research plan and a Data Use Agreement with legallybinding signatures by the requestor and an Authorized Representative from his/her institution.
Acknowledgments
This research was supported in part by grants from the National Institutes of Health (NIH) to the Children’s Oncology Group, the Imaging and Radiation Oncology Core, and St. Jude Children’s Research Hospital, and by funding from the St. Baldrick’s Foundation, the Seattle Children’s Foundation from Kat’s Crew Guild through the Sarcoma Research Fund, and the American Lebanese Syrian Associated Charities. None of the authors are employed by NIH but SLS, YYC, JA, JT, EH, CC, MBM, DMP, SCK, SXS, WM, and DSH are recipients of NIH grants to support this research. We gratefully acknowledge all of the patients and their families, care providers, and research personnel who participated in this study.
Funding Sources
This research was supported in part by Children’s Oncology Group grants U10CA180886, U10CA180899, U10CA098543, U10CA098413; Quality Assurance Review Center (QARC) grant U10CA29511; Imaging and Radiation Oncology Core (IROC) grant U10CA180803; St. Baldrick’s Foundation; Seattle Children’s Foundation from Kat’s Crew Guild through the Sarcoma Research Fund; Solid Tumor Program Project Grant CA23099 to SLS and ASP; Cancer Center Support CORE Grant P30 CA 21765 from the National Cancer Institute to SLS and ASP; and by funding from the American Lebanese Syrian Associated Charities (ALSAC) to SLS, RS, and ASP. No author received an honorarium or other form of financial support related to the development of this manuscript.
Declaration of Interests
SLS - National Cancer Institute/Children’s Oncology Group (grant to support salary of study chair to permit study conduct), F. Hoffman-LaRoche Ltd, Novartis, Bayer Healthcare Pharmaceuticals Inc, Sanofi US Services, Inc, Bristol Myers Squibb, Loxo Oncology, Incyte Corporation, Pfizer, St. Baldrick’s Foundation, Cookies for Kids’ Cancer, and University of California, Santa Cruz (clinical trial fees paid to institution to offset cost of study conduct unrelated to this publication), Alex’s Lemonade Stand Foundation (infrastructure grant to institution to support early phase clinical trial development unrelated to this publication)
LM - None
YYC - None
JA – Merck and Co. (salary)
JT - None
EH - None CC
- None
MBM - None
RLR – Zimmer Biomet (consultation fees and travel unrelated to this publication), Daiichi Sankyo (consultation fees and travel unrelated to this publication), Lilly USA LLC (consultation fees unrelated to this publication)
DMP – National Cancer Institute (grant)
JB - None
SCK - None
AHJ - None SW
- None
FL - None
RS - None
EK - None
SXS - None
WM - None
ASP – Bayer (personal fees outside the submitted work), Abbvie (personal fees outside the submitted work)
DSH – Loxo Oncology, Bayer, Bristol Myers Squibb, Lilly (clinical trial fees paid to institution to offset costs of study conduct; reimbursed for or provided travel, housing, and food to attend medical advisory board meetings unrelated to this publication), Celgene (reimbursed for or provided travel, housing, and food to attend medical advisory board meetings unrelated to this publication), Merck Sharpe Dohme, Eisai, Glaxo Smith Kline, Novartis, Sanofi, Amgen, Seattle Genetics, Jazz Pharmaceuticals, Incyte (clinical trial fees paid to institution to offset cost of study conduct unrelated to this publication)
Footnotes
Clinicaltrials.gov Registry No.:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sheri L. Spunt, Stanford University School of Medicine, Palo Alto, CA, USA
Lynn Million, Stanford University School of Medicine, Palo Alto, CA, USA.
Yueh-Yun Chi, University of Florida, Gainesville, FL, USA.
James Anderson, Merck and Co., North Wales, PA, USA.
Jing Tian, University of Florida, Gainesville, FL, USA.
Emily Hibbitts, University of Florida, Gainesville, FL, USA.
Cheryl Coffin, Vanderbilt University Ingram Cancer Center, Nashville, TN, USA.
M. Beth McCarville, St. Jude Children’s Research Hospital, Memphis, TN, USA.
R. Lor Randall, University of California Davis, Sacramento, CA, USA.
David M. Parham, Children’s Hospital of Los Angeles and USC Keck School of Medicine, Los Angeles, CA, USA
Jennifer O. Black, Children’s Hospital Colorado, Aurora, CO, USA
Simon C. Kao, University of Iowa Carver College of Medicine, Iowa City, IA, USA
Andrea Hayes-Jordan, University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, NC, USA.
Suzanne Wolden, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Fran Laurie, Imaging and Radiation Oncology Core Rhode Island, Lincoln, RI, USA.
Roseanne Speights, St. Jude Children’s Research Hospital, Memphis, TN, USA.
Ellen Kawashima, Children’s Oncology Group, Monrovia, CA, USA.
Stephen X. Skapek, University of Texas Southwestern Medical Center, Dallas, TX, USA
William Meyer, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA.
Alberto S. Pappo, St. Jude Children’s Research Hospital, Memphis, TN, USA
Douglas S. Hawkins, Seattle Children’s Hospital, Seattle, WA, USA
References
- 1.Punyko JA, Mertens AC, Baker KS, Ness KK, Robison LL, Gurney JG. Longterm survival probabilities for childhood rhabdomyosarcoma. A population-based evaluation. Cancer 2005; 103: 1475–83. [DOI] [PubMed] [Google Scholar]
- 2.Pappo AS, Devidas M, Jenkins J, et al. Phase II trial of neoadjuvant vincristine, ifosfamide, and doxorubicin with granulocyte colony-stimulating factor support in children and adolescents with advanced-stage nonrhabdomyosarcomatous soft tissue sarcomas: a Pediatric Oncology Group Study. J Clin Oncol 2005; 23: 4031–8. [DOI] [PubMed] [Google Scholar]
- 3.Pratt CB, Maurer HM, Gieser P, et al. Treatment of unresectable or metastatic pediatric soft tissue sarcomas with surgery, irradiation, and chemotherapy: a Pediatric Oncology Group study. Med Pediatr Oncol 1998; 30: 201–9. [DOI] [PubMed] [Google Scholar]
- 4.Pratt CB, Pappo AS, Gieser P, et al. Role of adjuvant chemotherapy in the treatment of surgically resected pediatric nonrhabdomyosarcomatous soft tissue sarcomas: A Pediatric Oncology Group Study. J Clin Oncol 1999; 17: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 5.Carli M, Ferrari A, Mattke A, et al. Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol 2005; 23: 8422–30. [DOI] [PubMed] [Google Scholar]
- 6.Orbach D, Mc Dowell H, Rey A, Bouvet N, Kelsey A, Stevens MC. Sparing strategy does not compromise prognosis in pediatric localized synovial sarcoma: experience of the International Society of Pediatric Oncology, Malignant Mesenchymal Tumors (SIOP-MMT) Working Group. Pediatr Blood Cancer 2011; 57: 1130–6. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari A, Casanova M, Collini P, et al. Adult-type soft tissue sarcomas in pediatric-age patients: experience at the Istituto Nazionale Tumori in Milan. J Clin Oncol 2005; 23: 4021–30. [DOI] [PubMed] [Google Scholar]
- 8.Pappo AS, Rao BN, Jenkins JJ, et al. Metastatic nonrhabdomyosarcomatous soft-tissue sarcomas in children and adolescents: the St. Jude Children’s Research Hospital experience. Med Pediatr Oncol 1999; 33: 76–82. [DOI] [PubMed] [Google Scholar]
- 9.Spunt SL, Hill DA, Motosue AM, et al. Clinical features and outcome of initially unresected nonmetastatic pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Clin Oncol 2002; 20: 3225–35. [DOI] [PubMed] [Google Scholar]
- 10.Spunt SL, Poquette CA, Hurt YS, et al. Prognostic factors for children and adolescents with surgically resected nonrhabdomyosarcoma soft tissue sarcoma: an analysis of 121 patients treated at St Jude Children’s Research Hospital. J Clin Oncol 1999; 17: 3697–705. [DOI] [PubMed] [Google Scholar]
- 11.Baldini EH, Goldberg J, Jenner C, et al. Long-term outcomes after functionsparing surgery without radiotherapy for soft tissue sarcoma of the extremities and trunk. J Clin Oncol 1999; 17: 3252–9. [DOI] [PubMed] [Google Scholar]
- 12.Rydholm A, Gustafson P, Rooser B, et al. Limb-sparing surgery without radiotherapy based on anatomic location of soft tissue sarcoma. J Clin Oncol 1991; 9: 1757–65. [DOI] [PubMed] [Google Scholar]
- 13.Brennan MF. The enigma of local recurrence. The Society of Surgical Oncology. Ann Surg Oncol 1997; 4: 1–12. [DOI] [PubMed] [Google Scholar]
- 14.Kuttesch JF Jr., Wexler LH, Marcus RB, et al. Second malignancies after Ewing’s sarcoma: radiation dose-dependency of secondary sarcomas. J Clin Oncol 1996; 14: 2818–25. [DOI] [PubMed] [Google Scholar]
- 15.Demetri GD, Elias AD. Results of single-agent and combination chemotherapy for advanced soft tissue sarcomas. Implications for decision making in the clinic. Hematol Oncol Clin North Am 1995; 9: 765–85. [PubMed] [Google Scholar]
- 16.Le Cesne A, Judson I, Crowther D, et al. Randomized phase III study comparing conventional-dose doxorubicin plus ifosfamide versus high-dose doxorubicin plus ifosfamide plus recombinant human granulocyte-macrophage colony-stimulating factor in advanced soft tissue sarcomas: A trial of the European Organization for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group. J Clin Oncol 2000; 18: 2676–84. [DOI] [PubMed] [Google Scholar]
- 17.Patel SR, Vadhan-Raj S, Papadopolous N, et al. High-dose ifosfamide in bone and soft tissue sarcomas: results of phase II and pilot studies--dose-response and schedule dependence. J Clin Oncol 1997; 15: 2378–84. [DOI] [PubMed] [Google Scholar]
- 18.Kraybill WG, Harris J, Spiro IJ, et al. Phase II study of neoadjuvant chemotherapy and radiation therapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. J Clin Oncol 2006; 24: 619–25. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher CDM, Unni KK, Mertens F (Eds.): World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone IARC Press: Lyon, 2002. [Google Scholar]
- 20.Parham DM, Webber BL, Jenkins JJ 3rd, Cantor AB, Maurer HM. Nonrhabdomyosarcomatous soft tissue sarcomas of childhood: formulation of a simplified system for grading. Mod Pathol 1995; 8: 705–10. [PubMed] [Google Scholar]
- 21.Coindre JM, Trojani M, Contesso G, et al. Reproducibility of a histopathologic grading system for adult soft tissue sarcoma. Cancer 1986; 58: 306–9. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F (Eds.): WHO Classification of Tumours of Soft Tissue and Bone. IARC: Lyon, 2013. [Google Scholar]
- 23.Manual for Staging of Cancer 1977. American Joint Committee for Cancer Staging and End Results Reporting: Chicago, 1977. [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81. [Google Scholar]
- 26.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Statist Soc A 1972; 135: 185–207. [Google Scholar]
- 27.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer 1977; 35: 1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cahlon O, Brennan MF, Jia X, Qin LX, Singer S, Alektiar KM. A postoperative nomogram for local recurrence risk in extremity soft tissue sarcomas after limbsparing surgery without adjuvant radiation. Ann Surg 2012; 255: 343–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis JJ, Leung D, Heslin M, Woodruff JM, Brennan MF. Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol 1997; 15: 646–52. [DOI] [PubMed] [Google Scholar]
- 30.Rivard JD, Puloski SS, Temple WJ, et al. Quality of life, functional outcomes, and wound complications in patients with soft tissue sarcomas treated with preoperative chemoradiation: a prospective study. Ann Surg Oncol 2015; 22: 2869–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.