Abstract
The second-generation anti-androgen, enzalutamide (ENZ), is approved for castrate-resistant (CR) PC and targets androgen receptor (AR) activity in CRPC. Despite initial clinical activity, acquired resistance to ENZ arises rapidly and most patients develop terminal disease. Previous work has established Stat5 as a potent inducer of PC growth. Here, we investigated the significance of Jak2-Stat5 signaling in resistance of PC to ENZ. The levels of Jak2 and Stat5 mRNA, proteins and activation were evaluated in PC cells, xenograft tumors and clinical PCs before and after ENZ therapy. Jak2 and Stat5 were suppressed by genetic knockdown using lentiviral shRNA or pharmacological inhibitors. Responsiveness of primary and ENZ resistant PC to pharmacological inhibitors of Jak2-Stat5 signaling was assessed in vivo in mice bearing PC xenograft tumors. Patient-derived PCs were tested for responsiveness to Stat5 blockade as second line treatment after ENZ ex vivo in tumor explant cultures. ENZ-liganded AR induces sustained Jak2-Stat5 phosphorylation in PC leading to a formation of a positive feed-forward loop, where activated Stat5, in turn, induces Jak2 mRNA and protein levels contributing to further Jak2 activation. Mechanistically, ENZ-liganded AR induced Jak2 phosphorylation through a process involving Jak2-specific phosphatases. Stat5 promoted PC growth during ENZ treatment. Jak2-Stat5 inhibition induced death of PC cells and patient-derived PCs surviving ENZ treatment and blocked ENZ-resistant tumor growth in mice. This work introduces a novel concept of a pivotal role of hyperactivated Jak2-Stat5 signaling in ENZ-resistant PC which is readily targetable by Jak2 inhibitors in clinical development.
Keywords: Jak2, enzalutamide, resistance, therapy development, prostate cancer
Introduction
The standard treatment of locally advanced or metastatic prostate cancer (PC) is androgen deprivation therapy (ADT) (1,2). Response to ADT is limited, and PC becomes castrate-resistant (CR), a stage for which there is no cure (1-4). ADT targets androgen receptor (AR) which translocates from cytoplasm to nucleus upon ligand binding followed by chromatin engagement (5). ADT is carried out by luteinizing hormone-releasing hormone (LHRH) agonists/antagonists or by anti-androgens which block the AR activity at the tissue level (1-5). The first-generation anti-androgen, bicalutamide, competitively inhibits androgen binding to AR (1-6) and recruitment of AR corepressors (6). The recently developed second-generation anti-androgen, enzalutamide (ENZ), has gained increasing dominance in the clinical space and is currently FDA approved as a monotherapy in both pre- and post-chemotherapy settings (7-10). ENZ is a more effective competitive inhibitor of steroid binding to the androgen binding pocket on the ligand binding domain of the AR and retains AR more effective in the cytoplasmic compartment of PC cells (11). However, despite its initial promise, ENZ provides an improvement in patient survival only by 4-6 months due to rapid development of resistance (8,10,12,13).
Mechanisms underlying PC resistance to ENZ are incompletely understood. The current proposed mechanisms include emergence of AR splice variants (14,15), glucocorticoid receptor expression (16), a ligand-binding domain mutation F876L in the AR that promotes an antagonist-to-agonist switch of ENZ (17,18), and neuroendocrine differentiation (NE) (19). Moreover, emergence of an AR-null, NE-null PC phenotype driven by MAPK signaling pathway was recently reported (20). However, no single mechanism has been reliably shown to completely account for progress to ENZ resistance in PC in experimental models or in patients, and it is likely that additional mechanisms are involved.
Stat5a/b (Stat5), which is both a signaling protein and a nuclear transcription factor (21,22), sustains viability of PC (23-29). Blockade of Stat5 signaling induces apoptotic death of hormone-naïve PC cells, suppresses growth of both xenografted and autochthonous PC tumors as well as clinical patient-derived PCs ex vivo in culture (23-28,30). Conversely, overexpression of active Stat5 has been shown to induce proliferation of PC cells in vitro and growth of PC tumors in mice (31). Stat5 induces metastatic progression of PC, as evidenced by Stat5 promotion of metastasis formation in vivo, and induction of hallmarks of EMT and stem-like cancer cell properties through induction of Twist1 and BMI1 expression in PC (30,32). In 30-40% of advanced CRPCs, the chromosome 17 locus encompassing STAT5A and STAT5B genes undergoes amplification resulting in increased Stat5 protein levels (31). Notably, high nuclear Stat5 protein expression at the time of the initial PC treatment predicted recurrence of the disease in three independent cohorts totaling 1,035 patients (33,34). The predictive role of active Stat5 for clinical PC progression to lethal CR state (33,34) corroborates involvement of Stat5 in PC progression in preclinical PC models.
Activation of Stat5 occurs in the cytoplasm through inducible phosphorylation of a conserved C-terminal tyrosine residue (21). Phosphorylated Stat5 (pY694/699) forms functional dimers that translocate to the nucleus and bind to specific DNA response elements to regulate transcription (21). In PC, Stat5 is activated predominantly by the Jak2 tyrosine kinase (35), a member of Jak family including Jak1, Jak3 and Tyk2 (36). The binding of cytokines, hormones and growth factors to the specific receptor-Jak2 complex leads to a conformational change in Jak2 which results in autophosphorylation and kinase activation. Phosphorylation of amino acid residues Tyr1007/1008 in the activation loop of the kinase domain are required for full catalytic activity of Jak2 (36). Besides canonical cytokine activated activation, Jak2 phosphorylation in non-hematopoietic tissues is regulated by a set of Jak2-specific phosphatases, which include SHP-2 (37), PTP1B (38) and PTBƐ (39,40). In addition, Jak2 phosphorylation state is affected by the levels of Jak2 protein in cells where overexpression of Jak2 leads to Jak2 autophosphorylation in the absence of cytokine stimulation (41).
The findings of Stat5 as a PC growth promoter and a predictor of PC recurrence implies involvement of Stat5 in the development of CRPC, which led us to hypothesize that Jak2-Stat5 signaling sustains viability of PC cells following disruption of AR signaling by ENZ. We demonstrate, for the first time, that ENZ induces a robust increase in Stat5 activation in PC cells in vitro, in xenograft tumors in vivo and in patient-derived PCs during ENZ treatment. Mechanistically, ENZ-liganded AR induces rapid and sustained Jak2 phosphorylation in PC cells via Jak2-specific phosphatases PTPƐ and SHP2. ENZ-induced Jak2 activation leads to Stat5 phosphorylation, and the formation of a feed-forward loop in PC cells where active Stat5 increases Jak2 mRNA and protein levels. We further demonstrate that inhibition of Stat5 as a second-line treatment induces extensive death of PC cells surviving ENZ treatment. Stat5 blockade inhibited CR growth of PC xenograft tumors after ENZ resistance developed and induced further death after ENZ treatment in patient-derived PCs ex vivo in tumor explant cultures. In summary, this work supports the new concept of a critical role of a hyperactive Jak2-Stat5 signaling loop in promoting resistance of PC to ENZ. Pharmacological Jak2-Stat5 inhibition may provide an effective therapy for Stat5-positive advanced PC in combination with ENZ or after ENZ fails.
Materials and Methods
Prostate cancers from ENZ-treated patients.
Paraffin-embedded tissues sections of PCs from ENZ treated patients were obtained from the pathology archives at Helsinki University Hospital (Helsinki, Finland) and Medical College of Wisconsin (MCW). In addition, tissue sections of hormone -naïve PCs of corresponding histological grades were from patients treated by radical prostatectomy (RP) without adjuvant hormone therapy (Helsinki University Hospital and MCW). Patient demographics and clinicopathological data are presented in Supplementary Table 1a. The study protocol for the samples obtained from archives in Finland was approved by the Ethical Committee of the University of Helsinki, and the National Data Protection Ombudsman was notified about the collection of the information. Tissue sections of PCs from patients before and after ENZ treatment were obtained from MCW and the patient demographics and clinicopathological data are presented in Supplementary Table 1b. These samples were de-identified archival tissues and were granted an exemption from the MCW Institutional Review Board and is in compliance with federal regulations governing research [45 CFR 46102(f)]. PC specimens for 3D explant cultures were obtained from patients with localized or locally advanced PC undergoing RP at MCW (Supplementary Table 2) and were de-identified excess tissue available for research purposes granted an exemption by the MCW Institutional Review Board. All the studies were conducted with the guidelines of Declaration of Helsinki and U.S. Common Rule.
Ex vivo tumor explant cultures of clinical prostate cancers.
Detailed information is provided in Supplementary Materials and Methods.
Cell lines and reagents.
Human PC cell lines LNCaP, PC-3, DU145 (from ATCC) and CWR22Pc (42) were cultured in RPMI 1640 growth media (Mediatech) containing 10% fetal bovine serum (FBS; Quality Biological) and penicillin/streptomycin (50 IU/ml and 50 μg/ml, respectively; Mediatech). LAPC-4 cells (from Dr. Charles Sawyers, Sloan-Kettering Memorial Cancer Center, NY) were cultured under the same conditions, with substitution of RPMI 1640 for IMDM (Mediatech). LNCaP, LAPC-4 and CWR22Pc cells were cultured in the presence of (DHT) (Sigma-Aldrich) (LNCap: 0.5 nM, LAPC-4: 1 nM, CWR22Pc: 0.8 nM). CWR22Pc subline expressing AR-F876L (from Dr. Charles Sawyers, Sloan-Kettering Memorial Cancer Center) was cultured in RPMI 1640 supplemented with ENZ (10 μM). All cell lines were regularly authenticated by observation of cell morphology, androgen-responsiveness and expression of cell line specific markers and tested for mycoplasma contamination (PCR Mycoplasma Detection Set; Takara Bio Inc.,) every 3 months. ENZ and AZD1480 were purchased from MedChem Express, MG132 and cyclohexamide (CHX) from Calbiochem, LFA102 (43) from Novartis, sodium orthovanadate from Sigma-Aldrich and IST5-002 was provided by Fox Chase Chemical Diversity Center. Recombinant human prolactin (Prl) was obtained from NIDDK Hormone and Peptide Program, Torrance, CA.
ShRNA and cDNA constructs and lentiviral production shRNA.
Detailed information is provided in Supplementary Materials and Methods.
Adenovirus generation.
Detailed information is provided in Supplementary Materials and Methods.
Protein solubilization, immunoprecipitation and immunoblotting.
Detailed information is provided in Supplementary Materials and Methods, and antibodies used are listed in Supplementary Table 3.
Quantitative real-time RT-PCR.
Detailed information is provided in Supplementary Materials and Methods.
Cell viability analysis.
Detailed information is provided in Supplementary Materials and Methods.
Immunohistochemistry.
Detailed information is provided in Supplementary Materials and Methods.
Scoring of cell viability and nuclear Stat5a/b.
Detailed information is provided in Supplementary Materials and Methods.
Immunofluorescence cytochemistry.
Detailed information is provided in Supplementary Materials and Methods.
Human prostate cancer xenograft tumor studies.
All the animal studies have been conducted in accordance with and approved Institutional Animal Care and Use Committee (IACUC). Castrated male athymic nude mice (Taconic) were cared for according to the institutional guidelines. Mice were implanted with sustained release DHT pellets (60-day release, 1 pellet/mouse, Innovative Research of America) 7 days prior to PC cell inoculation. Briefly, 1.5×107 CWR22Pc cells were mixed with 0.2 ml of Matrigel (BD Biosciences) and inoculated subcutaneously (s.c.) into flanks of nude mice (one tumor/mouse) as previously described (24,29). Bicalutamide (BIC) and ENZ were dissolved in 0.5% Tween 80 (Sigma-Aldrich)/PBS, AZD1480 in 0.1% Tween 80/0.5% hydroxypropyl methyl cellulose (HPMC, K4M prep, Dow Chemical)/H2O and IST5-002 in 0.3% hydroxypropyl cellulose (HPC, Sigma-Aldrich)/H2O.
For the monotherapy study (Fig. 6A), mice (5 mice/group) were treated daily for 32 days by oral gavage with vehicle (0.5% Tween 80/PBS), BIC (30 mg/kg) or ENZ (30 mg/kg), or by intraperitoneal (i.p.) injection with IST5-002 (50 mg/kg). DHT pellets were removed from the mice in the castration group concurrently with the start of the treatment period. Tumor dimensions were measured using vernier calipers three times per week and tumor volumes calculated using the following formula: (3.14 × length × width × depth)/6. Mice were sacrificed, and the tumor tissues were harvested at the end of the 32-day treatment period, or prior to this endpoint if tumor sizes reached 15-20 mm in diameter, and tumor tissues were harvested. Tumor growth rates were calculated from the beginning of drug treatment and are presented as fold changes in tumor volume of each group.
For the sequential therapy study (Fig. 6 B and C), mice (10 mice/ treatment group) were treated daily for 13 days (phase I) by oral gavage with vehicle (0.5% Tween 80/PBS) or ENZ (30 mg/kg) as the first-line therapy. On day 13, mice were randomly distributed into the indicated second-line therapy groups and treated daily for an additional 18 days (phase II) by oral gavage with vehicle, ENZ (30 mg/kg), AZD1480 (30 mg/kg), or by i.p. injection with IST5-002 (50 mg/kg), or by combination of ENZ (30 mg/kg) and IST5-002 (50 mg/kg). Tumor dimensions were measured twice per week and tumor volumes calculated as described for the monotherapy study. Mice were sacrificed when tumor sizes reached 15-20 mm in diameter in the vehicle-treated group (day 31), and the tumor tissues were harvested.
Statistical analyses.
Detailed information is provided in Supplementary Materials and Methods.
Results
Enzalutamide-liganded androgen receptor induces phosphorylation and activation of Stat5 in prostate cancer.
Immunohistochemical analyses of the activation status of Stat5 in PCs of patients treated with ENZ show that the levels of nuclear active Stat5 were significantly (p<0.0001) elevated in biopsies of ENZ-treated PCs when compared to hormone-naïve PCs of corresponding histological grades (Fig. 1A, Supplementary Table 1a). In paired PC samples from patients before and after ENZ treatment (Supplementary Table 1b), active Stat5 levels were robustly elevated in the biopsies after ENZ treatment compared to the biopsies taken prior to ENZ treatment (Fig. 1B). In CWR22Pc and LAPC4 cell lines, ENZ induced a rapid increase in Stat5 phosphorylation at 6 h which was sustained and further increased at day 7 (Fig. 1C). At the same time, phosphorylation of Stat3 was not induced by ENZ in the same cell lines (Fig. 1C). In a CWR22Pc cell subline expressing AR-F876L (CW22Pc-ENZ-R) (17), which emerges in CWR22Pc cells surviving extended treatment with ENZ (≥6 months) (18), active Stat5 levels were markedly higher compared to the parental CWR22Pc cells (Fig. 1C). ENZ induction of Stat5 phosphorylation resulted in nuclear translocation of Stat5 to an extent comparable to cytokine-induced Stat5 nuclear translocation (Supplementary Fig.1), indicating that ENZ induces formation of Stat5 dimers capable of nuclear localization in PC cells.
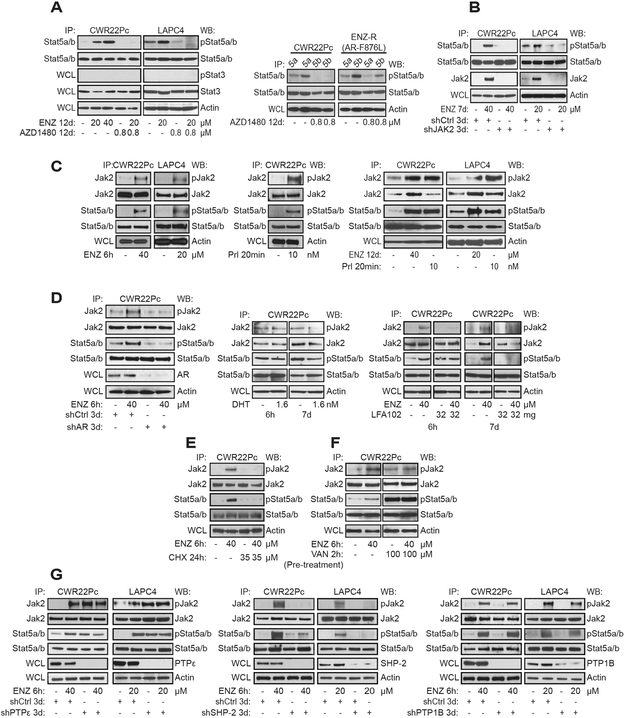
Figure 1. ENZ induces Jak2-Stat5a/b signaling in prostate cancer.
A, Active Stat5 levels were robustly elevated in PCs from patients treated by ENZ compared to hormone naïve PCs, demonstrated by immunostaining of paraffin-embedded tissue sections (image magnification 40X; scale bar: 50μm). B, Active Stat5 levels were elevated in paired biopsies after ENZ treatment compared to biopsies taken prior to ENZ treatment from the same patient (image magnification 40X; scale bar: 50μm). C, ENZ induces Stat5 phosphorylation in PC cells. PC cells were treated with ENZ or vehicle for the indicated periods of time. Expression levels of active Stat5 and Stat3 were determined by immunoprecipitation (IP) of Stat5 and Stat3 followed by immunoblotting (WB) for pStat5a/b, pStat3, total Stat5 and total Stat3. Whole cell lysates (WCL) were immunoblotted for Actin. Stat5 phosphorylation levels are elevated in ENZ-resistant (R) (CWR22Pc AR-F876L) cells compared to parental CWR22Pc cells (right panel). D, AR is required for ENZ induction of Stat5 phosphorylation. AR-negative DU145 PC cells were treated with ENZ or vehicle, and CWR22Pc and LAPC4 cells were transduced with lentiviral AR shRNA (shAR) or lentiviral shCtrl for 3 days followed by treatment with ENZ or vehicle for 7 days at the indicated concentrations. E, DHT, androgen deprivation, or genetic AR suppression do not increase Stat5 phosphorylation levels in PC cells. PC cells were androgen-deprived (charcoal-stripped FBS) or cultured with additional DHT (1.5 μM) for 7 days. Alternatively, AR was suppressed by lentiviral AR shRNA for 7 days. Expression levels of active Stat5, Stat5 and Jak2 were determined by immunoprecipitation (IP) followed by immunoblotting (WB) with Actin blotting as loading control.
To investigate whether AR is required for ENZ induction of Stat5 signaling, we first analyzed ENZ-activation of Stat5 in DU145 PC cell line which is AR-negative. In a parallel set of experiments, we inhibited AR expression in CWR22Pc and LAPC4 cells by genetic knockdown using lentiviral expression of AR shRNA. In the absence of AR, ENZ failed to induce Stat5 activation (Fig. 1D) demonstrating that ENZ-induction of Stat5 signaling is not an AR-independent off-target effect of the ENZ compound. We next evaluated if androgen deprivation, additional androgen treatment or genetic knockdown of AR would lead to an increase in Stat5 phosphorylation in PC cells. CWR22Pc and LAPC4 cell were androgen-deprived, cultured with additional DHT supplementation (1.5 μM) or AR was genetically depleted by lentiviral expression of AR shRNA (Fig. 1E). Androgen deprivation or AR knockdown did not stimulate Stat5 phosphorylation levels in PC cells. In addition, when the AR was liganded by DHT, Stat5 phosphorylation status remained unchanged. Taken together, these results indicate that ENZ-liganded AR induces sustained Stat5 activation in human PC.
Enzalutamide-induced Stat5 activation in prostate cancer cells occurs via Jak2.
To assess if ENZ-induced Stat5 activation in PC cells is dependent on Jak2 signaling, Jak2 activity was pharmacologically inhibited by Jak2-inhibitor AZD1480 (44) during ENZ-treatment. AZD1480 blocked ENZ-induced Stat5 phosphorylation in CWR22Pc, LAPC4 and CWR22Pc-ENZ-R cells (Fig. 2A). To further assess the involvement of Jak2 in ENZ-induced Stat5 activation, Jak2 was genetically knocked down by lentiviral expression of Jak2 shRNA in CWR22Pc and LAPC4 cells for 3 days followed by ENZ treatment for 7 days (Fig. 2B). In the absence of Jak2, ENZ failed to induce Stat5 phosphorylation in both cell lines. Unexpectedly, the protein levels of Jak2 on day 7 were robustly increased in ENZ-treated CWR22Pc and LAPC4 cells compared to non-treated cells (Fig. 2B). Collectively, these results indicate that ENZ induction of Stat5 activation in PC cells requires Jak2.
Figure 2. ENZ-liganded androgen receptor induces Jak2 phosphorylation in prostate cancer cells.
A, CWR22Pc, LAPC4 and ENZ-R cells were cultured with ENZ or Jak2 inhibitor AZD1480 alone or in combination for 12 days at indicated concentrations. Expression levels of active Stat5 were determined by immunoprecipitation (IP) of Stat5 followed by immunoblotting (WB) for pStat5a/b and total Stat5. Whole cell lysates (WCL) were immunoblotted for pStat3, Stat3 and Actin. B, Genetic knockdown of Jak2 blocks ENZ-induced Stat5 phosphorylation in PC cells. Jak2 was suppressed by lentiviral Jak2 shRNA vs. shCtrl in PC cells for 3 days followed by ENZ or vehicle for 7 days. Active Stat5, Stat5 and Jak2 levels were evaluated by IP and WB, as depicted. C, CWR22Pc and LAPC-4 cells were treated with ENZ or vehicle for 6 h at the indicated concentrations. Control cells were stimulated with prolactin (Prl) (10 nM) for 20 min as control for cytokine-induced Jak2 phosphorylation. Alternatively, PC cells were treated with ENZ or vehicle for 12 days or stimulated with Prl for 20 min at the indicated concentrations. Stat5 and Jak2 were IP:ed and immunoblotted (WB) for pStat5, pJak2, total Stat5, and total Jak2. WCLs were immunoblotted for Actin. D, AR is required for ENZ induction of Jak2 activation. AR was suppressed in CWR22Pc cells by lentiviral AR shRNA (shAR) vs. shCtrl for 3 days followed by ENZ for 6 h. In parallel experiments, CWR22Pc cell were cultured with 1.5 μM DHT for 6 h or 7 days. ENZ induction of Jak2-Stat5 activation requires a cytokine receptor shown by treatment of CWR22Pc cells with ENZ alone or in combination with a prolactin receptor antagonist LFA102 for 6 h or 7 days at the indicated concentrations. E, ENZ induction of Jak2-Stat5 activation requires ongoing protein synthesis in PC cells. CWR22Pc cells were treated with cyclohexamide (CHX) (35 μM) for 24 h followed by ENZ treatment (40 μM) for 6 h. F, Phosphatase inhibitor Vanadate (VAN) abolished ENZ induction of Jak2 phosphorylation. CWR22Pc cells were treated with ENZ (6 h) or VAN (2 h) or vehicle alone or pre-treated with VAN (2 h) followed by ENZ (6 h). G, ENZ-induced Jak2 phosphorylation is decreased by depletion of either PTPƐ or SHP-2 phosphatases. CWR22Pc and LAPC4 cells were infected with lentivirus expressing shRNA targeting PTPƐ, SHP-2, PTP1B or shCtrl for 3 days followed by ENZ or vehicle treatment for 6 h at the indicated concentrations. IPs and WBs in were conducted as described in A.
We next investigated if ENZ-liganded AR influences the phosphorylation status of Jak2 in PC cells. ENZ induced a rapid phosphorylation of Jak2 already at 6 h in both CWR22Pc and LAPC4 cells to almost the same extent as stimulation of the cells with the cytokine prolactin (Prl) (Fig. 2C). ENZ-induced Jak2 phosphorylation was sustained at 12 days and accompanied with a strong induction of Stat5 phosphorylation in both CWR22Pc and LAPC4 cells (Figs. 2C). To further examine if AR is required for ENZ induction of Jak2 activation, AR was suppressed by lentiviral AR shRNA in CWR22Pc cells, which blocked the ENZ-induced Jak2 activation (Fig. 2D). In addition, AR agonists were not able to increase Jak2 phosphorylation in PC cells, because supplementation of culture medium with additional DHT (1.5 nM) for 6 h and 7 days cell did not elevate Jak2 phosphorylation in CWR22Pc PC cells (Fig. 2D, middle panel). To further understand if ENZ-liganded AR induction of Jak2-Stat5 activation requires the presence of a cytokine receptor, we disrupted Prl receptor (PrlR) dimerization by a specific Prl antagonist LFA102 (43) for 6 h and 7 days. As shown in Figure 2D, ENZ failed to activate Jak2 and Stat5 in the presence of LFA102 suggesting that docking of Stat5 and Jak2 to the cytokine receptor complex is required for ENZ induction of Jak2-Stat5 signaling. In summary, the data shown here demonstrate that AR-liganded ENZ induces rapid and sustained Jak2 phosphorylation in PC cells which involves association of Jak2 with a cytokine receptor.
Enzalutamide-induction of Jak2 phosphorylation in prostate cancer cells involves Jak2 phosphatases.
To investigate the mechanisms underlying ENZ activation of Jak2 in PC cells, CWR22Pc cells were treated with cyclohexamide (CHX) (35 μM) for 24 h followed by ENZ (40 μM) for 6 h, which blocked ENZ-induced Jak2 phosphorylation (Fig. 2E). This suggests that ENZ induction of Jak2-Stat5 signaling is mediated by a protein that is regulated by ENZ and, therefore, requires ongoing protein synthesis. To evaluate if ENZ-induction of Jak2 phosphorylation is dependent on phosphatase activity in PC cells, we treated PC cells with a broad-spectrum phosphatase inhibitor vanadate (VAN). Pre-treatment of CWR22Pc with VAN led to a general increase in Jak2 phosphorylation, as expected, but also attenuated the induction of Jak2 phosphorylation by ENZ (Fig. 2F) suggesting involvement of Jak2 phosphatases in ENZ-induction of Jak2 phosphorylation. The key known Jak2 phosphatases expressed in solid tumors are SHP-2 (37), PTPƐ (38) and PTP1B (39,40), while SHP-1 is expressed in hematopoietic cells. Using lentiviral expression of shRNA, we depleted each of the three phosphatases for 3 days prior to ENZ treatment (6 h) of CWR22Pc and LAPC4 cells (Fig. 2G). In both PC cell lines, ENZ-induced Jak2 phosphorylation was significantly attenuated by depletion of either SHP-2 or PTPƐ. Genetic knockdown of PTPƐ resulted in generally elevated levels of Jak2 phosphorylation, which indicates that PTPƐ is a crucial negative regulator of Jak2 phosphorylation in PC cells. Importantly, when PTPƐ was genetically depleted in PC cells, there was no difference between ENZ treatment vs. non-treated cells suggesting that the mechanism of action of ENZ on Jak2 phosphorylation involves suppression of PTPƐ activity (Fig. 2G, left panel). At the same time, genetic depletion of SHP-2 decreased ENZ-induction of Jak2 phosphorylation while not increasing the general phosphorylation levels of Jak2 (Fig. 2G, middle panel). This, in turn, suggests that SHP-2 is a positive regulator of ENZ-induced Jak2-Stat5 signaling and is required for ENZ induction of Jak2 phosphorylation in PC cells. A positive regulatory role of SHP-2 for Jak2-Stat5 signaling has been previously reported for several different cell types (45,46). Genetic knockdown of PTP1B did not affect Jak2 phosphorylation levels nor ENZ-induction of Jak2 phosphorylation (Fig. 2G, right panel). In conclusion, these data indicate involvement of Jak2 phosphatases SHP-2 and PTPƐ in ENZ-induced Jak2 phosphorylation in PC cells.
Enzalutamide-induced Stat5 activation increases Jak2 mRNA and protein levels and a formation of a positive feed-forward loop in prostate cancer cells.
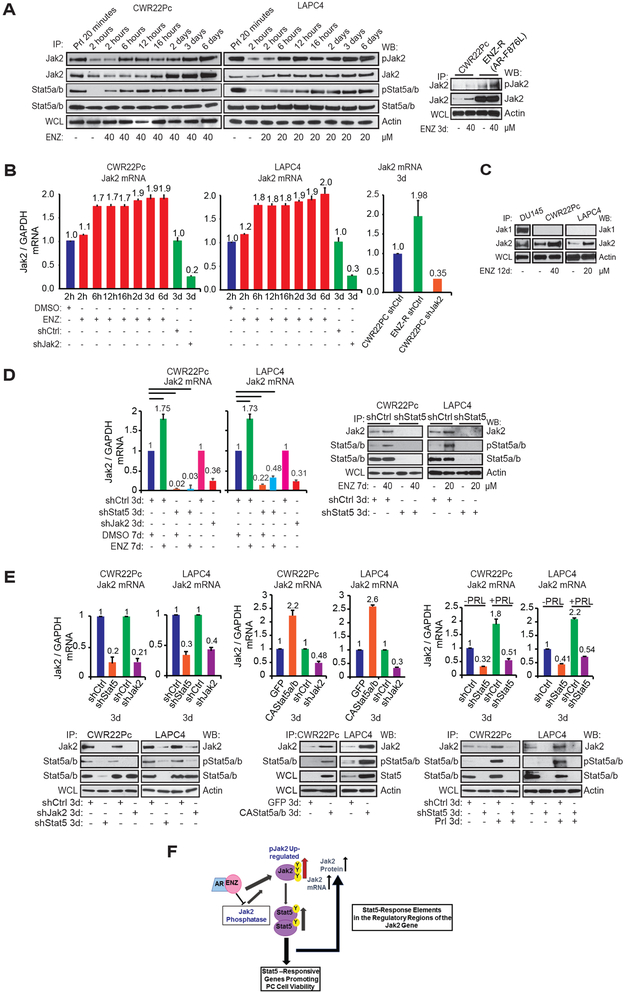
The finding demonstrating that ENZ may increase Jak2 protein levels in CWR22Pc and LAPC4 cells (Fig. 2B), led us to evaluate the effects of ENZ on phosphorylation, protein levels and mRNA levels of Jak2 and Stat5 during a time-course ranging from 2 hours to 6 days in CWR22Pc and LAPC4 cells (Fig. 3A and B). ENZ increased phosphorylation of Jak2 in both CWR22Pc and LAPC4 cells at 6 h, which was accompanied by an increase in Stat5 phosphorylation. Activation of Stat5 reached maximum at 12-16 h and was sustained at 6 days of exposure to ENZ (Fig. 3A). The levels of Jak2 protein, but not Stat5, increased starting at approximately 16 h (CWR22Pc) and 6 h (LAPC4) after initiation of the ENZ treatment. Concomitant with ENZ-induced activation of Jak2 and Stat5, Jak2 mRNA levels were increased by 70% at the 6 h time-point of ENZ treatment, which was sustained at the day 6 (Fig. 3B). In parallel experiments, PC cells resistant to ENZ (CWR22Pc-ENZ-R) displayed markedly higher levels of Jak2 protein (Fig. 3A) and mRNA (Fig. 3B) in comparison to the parental CWR22Pc cells. While inducing Jak2 levels, ENZ did not induce Jak1 levels in CWR22Pc or LAPC-4 cells indicating specificity of ENZ regulation of the phosphorylation state of Jak2 (Fig. 3C).
Figure 3. ENZ induces a formation of a positive Jak2-Stat5 feed-forward loop in prostate cancer.
A, ENZ induced Jak2 phosphorylation is accompanied by an increase in Stat5 phosphorylation and Jak2 protein levels in PC cells. CWR22Pc, LAPC-4 and ENZ-R cells were treated with ENZ or vehicle during a time-course at the indicated concentrations. PC cells were stimulated with Prl (10 nM) as control for Jak2 and Stat5 phosphorylation. The phosphorylation status of Stat5 and Jak2 were determined by IP of Stat5 and Jak2 followed by immunoblotting for pStat5, total Stat5, pJak2 and total Jak2. WCLs were immunoblotted for Actin. B, ENZ induces Jak2 mRNA levels in PC cells. CWR22Pc and LAPC-4 cells were treated with ENZ or vehicle for indicated periods of time or infected with lentiviral Jak2 shRNA (shJak2) vs. shCtrl (Jak2 mRNA control) (3 days) followed by qRT-PCR. C, Jak2, but not Jak1, levels are induced by ENZ. PC cells were treated with ENZ or vehicle for 12 days. Jak1 and Jak2 were immunoprecipitated and blotted for Jak1 and Jak2. WCLs were immunoblotted for Actin. D, ENZ increases Jak2 protein levels by Stat5-driven up-regulation of Jak2 mRNA expression in PC cells. When Stat5 was depleted in PC cells by lentiviral Stat5 shRNA (shStat5) or shCtrl for 3 days followed by ENZ or vehicle for 7 days, ENZ failed to increase Jak2 mRNA levels. For Jak2 mRNA control, Jak2 was suppressed by lentiviral Jak2 shRNA (shJak2) or shCtrl (left panel) for 3 days. Jak2 mRNA levels were determined by qPCR and protein levels by IP and WB as described in A. E, CWR22Pc and LAPC-4 cells were infected with lentiviral Stat5 shRNA (shStat5), shCtrl (upper and bottom panels), constitutively active (CA) Stat5 or GFP for 3 days (middle panel). After 3 days, PC cells were treated with Prl for 3 days (bottom panel). IP, WB and qPCR were conducted as described in A and B. F, Schematic representation of the proposed ENZ induction of hyperactivated Jak2-Stat5 feed-forward loop in PC.
To further understand the mechanisms underlying ENZ-induction of Jak2 mRNA levels, we analyzed the significance of Stat5 in this process by genetic knockdown of Stat5 by lentiviral shRNA in CWR22Pc and LAPC4 cells. In the absence of Stat5, ENZ failed to induce Jak2 mRNA and protein expression and Jak2 mRNA levels were generally decreased by more than 90% (Fig. 3D). This finding suggests that Stat5 is critical for ENZ-induced up-regulation of Jak2 mRNA expression in PC cells. Since Stat5 has not been shown to up-regulate Jak2 mRNA expression in other cell types, we investigated this in PC cells in more detail. First, we suppressed Stat5 by lentiviral Stat5 shRNA in CWR22Pc and LAPC4 cells for 3 days and assessed Jak2 mRNA levels by qRT-PCR. Depletion of Stat5 led to a 60-80% decrease in Jak2 mRNA levels in both CWR22PC and LAPC4 cells (Fig. 3E, left panel). In parallel experiments, lentiviral expression of constitutively active (CA) Stat5 (32) in CWR22Pc and LAPC4 cells increased Jak2 mRNA expression by 120-160%, which was also reflected at Jak2 protein levels (Fig. 3E, middle panel). In addition, Prl-induced Stat5 activation increased Jak2 mRNA levels by 80-120% in PC cells, which was blocked by genetic knockdown of Stat5 by shRNA (Fig. 3E, right panel). In summary, these results demonstrate that ENZ induces Jak2 mRNA and protein levels by Stat5-driven mechanisms in PC. Collectively, these findings indicate that ENZ induces a positive Jak2-Stat5 feed-forward loop in PC, depicted in Figure 3F. In this proposed scheme, ENZ-liganded AR induces Jak2 phosphorylation in PC cells through regulation of the activity and function of Jak2 phosphatases. Activated Jak2 leads to phosphorylation of Stat5 which, in turn, increases Jak2 mRNA and protein levels. It is known that elevated Jak2 protein levels result in Jak2 autophosphorylation (41). In addition, increased levels of Jak2 are likely to result in up-regulation of Jak2-Stat5 activation in PC cells induced by ENZ.
Stat5 promotes viability of prostate cancer cells surviving enzalutamide treatment.
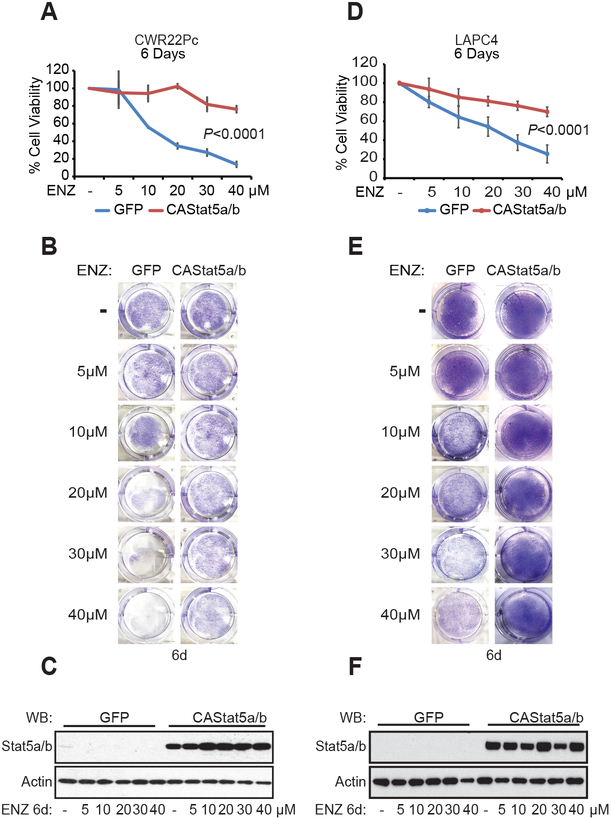
Having established that ENZ induces Jak2-Stat5 signaling in PC, we investigated if active Stat5 promotes CRPC growth during ENZ treatment. First, constitutively active (CA) Stat5 (32) was expressed in CWR22Pc and LAPC4 cells using lentivirus during treatment of the cells with increasing doses of ENZ ranging from 5 to 40 μM for 6 days. Expression of CAStat5 increased the fraction of viable CWR22Pc and LAPC4 cells at all concentrations of ENZ with the maximum increase of 60% (P<0.0001) and 45% (P<0.0001) in CWR22Pc and LAPC4 cells (Fig. 4A, B, D and E). The efficacy of lentiviral CAStat5 expression in PC cells was confirmed by immunoblotting (Fig. 4C and F).
Figure 4. Stat5 promotes viability of prostate cancer cells during ENZ treatment.
Constitutively active (CA) Stat5 or GFP were lentivirally expressed in CWR22Pc and LAPC-4 cells for 2 days followed by ENZ treatment (5, 10, 20, 30 and 40 μM) or vehicle for 6 days (A and D). The fractions of viable cells were determined by counting three separate fields from each of the three parallel wells, and the averages are indicated by a line graph with SDs. Crystal violet staining and (B and E) immunoblotting of whole cell lysates (WCL) for Stat5 and Actin on day 6 (C and F).
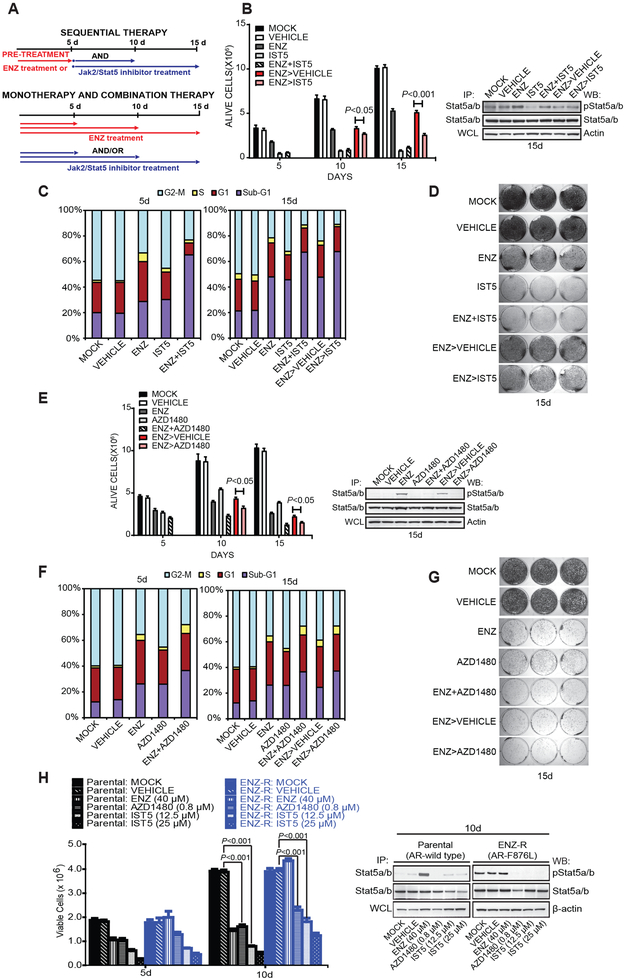
To investigate the efficacy of inhibition of Jak2-Stat5 signaling in eliminating PC cells that survive ENZ treatment, we used a specific Stat5 inhibitor IST5-002 (47) or Jak2 inhibitor AZD1480 (44). PC cells were exposed to ENZ for 5 days followed by treatment with IST5-002 or AZD1480 for 5 or 10 days (Fig. 5A). Control cells were grown in culture medium (MOCK), in the presence of vehicle (VEH), ENZ, IST5-002 (IST5), AZD148 or ENZ combined with IST5-002 (ENZ+IST5) or AZD1480 (ENZ+AZD1480) for 5, 10 or 15 days, as depicted in Figure 5A. In comparison to the control group (ENZ followed by vehicle: ENZ>vehicle), CWR22Pc cells treated at the start of the experiment with ENZ and later switched to IST5-002 after 5 days (ENZ>IST5-002) incurred additional loss of viability at both time points with the maximal reduction of 50% at 10 days of ENZ treatment (15 d timepoint) (P<0.001) (Fig. 5B). Notably, IST5-002 alone or in combination with ENZ was more efficacious (P<0.001) in reducing viability of CWR22Pc cells than ENZ applied as a single treatment (Fig. 5B). This may be due to ENZ induction of hyperactive Jak2-Stat5 signaling loop which leads to Stat5 promotion of PC cell viability and growth. Moreover, when the cells were pretreated with ENZ, inhibition of Stat5 was less efficient in reducing PC cell viability compared to Stat5 inhibition monotherapy or combination treatment of the cells with both IST5-002 and ENZ (Fig. 5B). Similarly, this may be explained by ENZ activation of positive Jak2-Stat5 feed-forward loop, which sets a barrier for a second-line therapy to overcome. Viability of CWR22Pc in each group was visualized and quantified by cell cycle analysis and crystal violet staining (Figs. 5C and D).
Figure 5. Pharmacological inhibition of Jak2-Stat5 signaling increases death of prostate cancer cells when used in combination with ENZ or alone after ENZ.
A, The experimental design for a sequential treatment of PC cells with AR inhibition followed by genetic or pharmacological knockdown of Stat5. B, Stat5 inhibitors alone or in combination with ENZ are more effective than ENZ alone in suppressing PC cell viability. Sequential therapy with ENZ followed by IST5-002 decreases viability of PC cells surviving ENZ treatment. CWR22Pc cells were treated at the start of the experiment (day 0) with vehicle, ENZ (40 μM) for 5 days, IST5-002 (12.5 μM) alone or in combination, as indicated or left untreated (MOCK). For sequential treatment, cells were treated at the start of the experiment (day 0) with ENZ (40 μM) followed by treatment with vehicle (ENZ>vehicle) or IST5-002 (12.5 μM) (ENZ>IST5-002) for 5 or 10 days. The fractions of viable cells are indicated by columns with SDs. Activation of Stat5 was determined by IP of followed by WB for pStat5 and total Stat5. WCLs were immunoblotted for Actin. C, Fluorescence-activated cell sorting (FACS) analysis of cell cycle distribution at the indicated timepoints. D, Pictures of PC cells stained with crystal violet after the treatments, as described in B. E, Combination of ENZ with AZD1480 is more effective than ENZ alone in decreasing viability of PC cells surviving ENZ treatment. Sequential treatment of CWR22Pc cells with ENZ (40 μM) for 5 days followed by AZD1480 (0.8 μM) for 5 or 10 days suppresses viability of PC cells surviving ENZ treatment. CWR22Pc cells were treated and analyzed for viable cells as described in A. F, FACS analysis of cell cycle distribution at the indicated timepoints. G, Crystal violet staining of the parallel wells. H, IST5-002 and AZD1480 decreased viability of both parental CWR22Pc cells and ENZ-R cells (AR-F876L) after 5 and 10 days of treatment. ENZ decreased viability of parental cells but did not affect viability of ENZ-R cells after 5 or 10 days of treatment. The fractions of viable cells are indicated by columns with SDs.
Given that ENZ induces a marked increase in Jak2 phosphorylation in PC cells, we next assessed the efficacy of a sequential application of ENZ followed by AZD1480 (44) as a representative of Jak2 inhibitors. Compared to control (ENZ>vehicle), CWR22Pc cells initially receiving ENZ at the start of the experiment for 5 days and switched to AZD1480 for 5 or 10 days (ENZ>AZD1480) exhibited additional loss of 15-20% of cell viability at both time points (P<0.05) (Fig. 5E, F and G). Stat5 inhibition was verified by immunoblotting in cells receiving AZD1480, combined ENZ and AZD1480, or ENZ followed by AZD1480 (Fig. 5E). In summary, Jak2 inhibition by AZD1480 induced extensive death of CWR22Pc cells that survived ENZ treatment.
To investigate if PC cells encompassing AR with the mutation conferring ENZ resistance are sensitive to inhibition of Jak2-Stat5 signaling with cell viability as the end-point, Jak2-Stat5a/b signaling was inhibited in both parental CWR22Pc and ENZ-R (AR-F876L) cells by AZD1480 or IST5-002. While parental CWR22Pc cells remained sensitive to ENZ, viability of ENZ-resistant (AR-F876L) CWR22Pc cells was unaffected by ENZ, as expected (Fig. 5H). Viability of both parental and ENZ-resistant CWR22Pc cells was decreased by both IST5-002 (12.5 μM) (P < 0.001) or AZD1480 (P < 0.001) (0.8 μM) at 10 days, indicating that ENZ-resistant CWR22Pc cells remained sensitive to Stat5a/b inhibition (Fig. 5H). ENZ-resistant cells displayed uniformly high levels of active Stat5 (Fig. 5H). Collectively, these findings support the concept of an emergence of sustained upregulation of Jak2-Stat5 activation during long-term ENZ treatment. To summarize, these data demonstrate that active Stat5 increases viability and growth of PC cells during ENZ treatment, and pharmacological inhibition of Jak2-Stat5 signaling induces death of PC cells surviving ENZ treatment.
Stat5 inhibition decreases growth of ENZ resistant prostate cancer xenograft tumors and patient-derived prostate cancers in tumor explant cultures ex vivo.
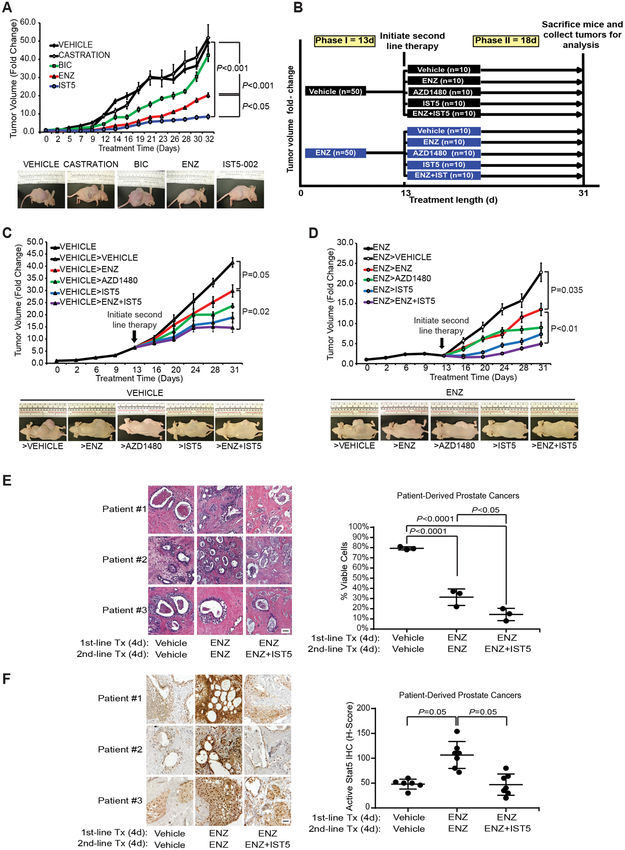
Given that Stat5a inhibition potently induced apoptotic death of PC cells depleted of AR signaling, we sought to extend our in vitro findings to PC xenograft tumor growth in vivo. Castrated athymic nude mice, implanted with sustained-release DHT pellets to normalize circulating androgen levels, were inoculated subcutaneously with CWR22Pc cells. CWR22Pc tumors are known to initially display androgen-sensitive growth and regress upon castration, but later recur as castrate-resistant tumors (42). The mice were treated with bicalutamide (BIC), ENZ or IST5-002 daily for 32 days with vehicle or surgical castration as control groups (Fig. 6A). Tumors in the castration group rapidly resumed the same growth rate as tumors in the vehicle-treated mice, suggesting the presence of adrenal and/or intracrine androgen synthesis. As expected, ENZ was more effective than BIC in blocking androgen-sensitive CWR22Pc tumor growth (P<0.001) (Fig. 6A). In line with the higher efficacy of IST5-002 than ENZ in inducing death of PC cells in vitro shown in Figure 5, IST5-002 was superior to ENZ (P<0.05) at suppressing in vivo androgen-sensitive growth of CWR22Pc xenograft tumors in the presence of circulating androgens (Fig. 6A). Immunohistochemical analyses showed decreased nuclear Stat5 content within tumor cells treated with IST5-002, while nuclear Stat5 levels were elevated in tumors treated with ENZ (Supplementary Fig. 2A). Collectively, these results indicate that Stat5 inhibition by IST5-002 blocked CWR22Pc xenograft tumor growth equally or more effectively than ENZ.
Figure 6. Second-line treatment by pharmacological inhibitors of Jak2-Stat5 signaling suppresses growth of both androgen-dependent and ENZ-resistant xenograft tumors in nude mice and in patient-derived prostate cancers ex vivo in tumor explant cultures.
A, Inhibition of Stat5 by IST5-002 suppressed androgen-sensitive CWR22Pc xenograft tumor growth more effectively than bicalutamide (BIC) or ENZ, as shown by tumor growth curves (upper panel) and representative tumor images (lower panel). CWR22Pc cells were inoculated subcutaneously (s.c.) into flanks of castrated athymic nude mice supplied with sustained-release DHT pellets. Mice were surgically castrated or treated daily with vehicle, BIC (30 mg/kg), ENZ (30 mg/kg) or IST5-002 (IST5) (50 mg/kg), and tumor growth rates were calculated for each treatment group and are presented as fold changes in tumor volume (volume at timepoint/volume at treatment start). B, Experimental design for the sequential therapy of the xenograft tumors in vivo. A two-phase in vivo experiment using vehicle or ENZ as first-line therapy (phase I, 13 days) and vehicle, ENZ, AZD1480, IST5-002 or ENZ+IST5-002 as second-line therapy (phase II, 18 days). On day 31, mice were sacrificed and CWR22Pc xenograft tumors collected for analyses. C-D, Sequential, second-line therapy with IST5-002 (IST5) or ENZ+IST5-002 (IST5) effectively suppressed the growth of androgen-sensitive (left panel) (C) and ENZ-resistant (right panel) (D). CWR22Pc xenograft tumors, as shown by tumor growth curves (upper panels) and representative tumor images (lower panels). CWR22Pc cells were inoculated s.c. into flanks of castrated athymic nude mice supplied with sustained release DHT pellets. Mice were treated with vehicle or ENZ (30 mg/kg) for 13 days. On day 13, mice were randomly distributed into subgroups and treated daily for an additional 18 days with vehicle, ENZ (30 mg/kg), AZD1480 (30 mg/kg), IST5-002 (IST5) (50 mg/kg) or ENZ (30 mg/kg)+IST5-002 (IST5) (50 mg/kg). Tumor growth rates are calculated and presented as described in (A). E, To test the efficacy of a second-line therapy with combined ENZ and IST5-002 (IST5) vs. ENZ monotherapy in clinical PCs, seven localized PCs obtained from radical prostatectomies were cultured ex vivo in tumor explant cultures in the presence of vehicle (8 days), ENZ (40 μM) alone (8 days) or ENZ (40 μM) as first-line therapy (4 days) followed by ENZ (40 μM) and IST5-002 (IST5) (25 μM) as a second-line therapy (4 days). Second-line therapy with combined ENZ and IST5-002 (IST5) induced cell death in clinical PCs more effectively than ENZ alone, as demonstrated by extensive loss of viable acinar epithelium. The number of viable epithelial cells in the explants at the end of the cultures were counted and presented as percentage per epithelial cells prior to culture. Representative histologies are shown (image magnification 40X; scale bar: 50μm). F, Levels of nuclear Stat5 in the PC explants were determined by immunostaining using biotin-streptavidin-amplified peroxidase-antiperoxidase immunodetection at the end of the cultures and expressed as percentages of Stat5 positive cells per 100 epithelial cells in explants for each treatment group (12-20 explants) (image magnification 40X; scale bar: 50μm).
To assess therapeutic efficacy of sequential targeting of AR and Stat5 in PC tumors in vivo, we designed a two-phase in vivo experiment, depicted in Figure 6 B. Mice received first-line therapy of vehicle or ENZ daily until emergence of resistance to ENZ occurred (day 13), followed by randomization and switch to a second-line therapy (vehicle, ENZ, AZD1480, IST5-002 or ENZ+IST5-002) daily for additional 18 days. Tumors in mice receiving vehicle during the first phase represent androgen-sensitive PC growth (Fig. 6C), and final fold-changes in tumor volume (volume at day 31/day 0±SEM) at the end of the second phase (day 31) were as follows: Vehicle>vehicle, 41.6±1.9; vehicle>ENZ, 29.8±2.5; vehicle>AZD1480, 23.7±0.9; vehicle>IST5-002, 18.9±2.0; vehicle>ENZ+IST5-002, 14.6±2.0 (Fig. 6C). As expected, ENZ reduced androgen-sensitive CWR22Pc xenograft tumor growth significantly compared to vehicle-treated tumors (P=0.05). At the same time, Stat5 inhibition by IST5-002 alone suppressed androgen-sensitive PC xenograft tumor growth equally effectively as ENZ when compared to the control group (vehicle) (P<0.01). However, the growth of androgen-sensitive PC tumors was suppressed more effectively by combination of IST5-002 with ENZ when compared to ENZ alone (P=0.02) (Fig. 6C). In conclusion, while ENZ alone suppressed growth in the presence of androgens as expected, combination of ENZ with IST5-002 was remarkably more effective than ENZ alone.
PC tumors in mice receiving ENZ during the first phase developed resistance as evidenced by re-growth of tumors in ENZ-treated mice starting on day 13. At the end of the second-line treatment on day 31, final fold-changes in ENZ-resistant tumor volumes were as follows: ENZ>vehicle, 22.8±2.3; ENZ>ENZ, 13.5±1.5; ENZ>AZD1480, 8.9±1.3; ENZ>IST5-002, 7.3±0.9; ENZ>ENZ+IST5-002, 4.9±0.6 (Fig. 6D). Importantly, IST5-002 alone suppressed ENZ-resistant PC tumor growth. At the same time, combined ENZ with IST5-002 was most effective in suppressing ENZ-resistant CRPC tumor growth when compared to continued ENZ treatment (P<0.01). Immunohistochemical analysis of Stat5 demonstrated that ENZ increased nuclear Stat5 in both androgen-induced and castrate-resistant tumors (Supplementary Fig. 2). In conclusion, growth of ENZ-resistant CRPC xenograft tumors was most effectively suppressed by a second-line therapy of combined ENZ and IST5-002.
To evaluate the efficacy of combined ENZ and IST5-002 as a second-line therapy in clinical patient-derived PCs, we utilized an ex vivo 3D tumor explant culture system of patient-derived PCs which we have established and characterized previously (25,32,47-49). Crucial interactions between prostate epithelium and stroma are retained in tumor explant cultures and, therefore, it is considered a more physiological model of PC growth than cell lines or primary cell cultures. PCs from seven individual patients (Supplementary Table 2) were cultured ex vivo in tumor explant cultures with ENZ alone for 4 days followed by combined ENZ and IST5-002 for 4 days with explants cultured with ENZ or vehicle alone for 8 days as control groups. PCs receiving combined ENZ and IST5-002 as the second-line therapy demonstrated extensive loss of viable epithelium at the end of the culture period, with only 15% viable cells at the end of the treatment period compared to ENZ monotherapy (30%) (P<0.05) or vehicle (80%) (P<0.0001) (Fig. 6E). Nuclear levels of active Stat5 evaluated by immunohistochemistry, were increased in the explants treated with ENZ compared to vehicle-treated explants (P=0.05). At the same time, treatment of the explants with the combination of ENZ and IST5-002 as a second-line therapy reduced the nuclear active Stat5 levels compared to ENZ (P=0.05) (Fig. 6F). Overall, these data suggest that second-line therapy with combined ENZ and IST5-002 was more effective than ENZ alone in inducing epithelial cell death in patient-derived clinical PCs ex vivo.
Discussion
Prostate cancer (PC) cells require androgen receptor (AR) signaling for growth and survival, a dependency exploited by androgen deprivation therapy (ADT) for locally advanced, recurrent or metastatic PC. A more potent second-generation AR antagonist, ENZ, was developed to re-target the persistent AR activity in CRPC tumors and has become standard-of-care in this setting (7-9). ENZ is approved as front-line therapy for metastatic and non-metastatic CRPC (8,10). However, despite these advances, CRPC remains a uniformly lethal disease due to rapid development of resistance. Identification of molecular mechanisms and pathways that drive growth of ENZ resistant PC cells holds potential for development of effective first-line combination therapies that suppress development of ENZ resistance or second-line therapies once ENZ fails. In this study, we show, for the first time, that ENZ induces a hyperactive Jak2-Stat5 signaling loop in PC through mechanisms that involve Jak2 phosphatases PTPƐ and SHP-2. Active Stat5 increased PC cell survival during ENZ treatment and, conversely, Stat5 inhibition blocked growth of PC cells surviving ENZ treatment. Pharmacological inhibition of Stat5 suppressed ENZ-resistant growth of xenograft tumors that emerged during ENZ treatment and reduced cell viability in patient-derived clinical PCs cultured ex vivo in tumor explant cultures. Pharmacological inhibitors of Jak2-Stat5 signaling may be effective agents to overcome ENZ-resistant CRPCs driven by Jak2-Stat5.
One of the key results of the work presented here is that ENZ induced a hyperactive Jak2-Stat5 feed-forward signaling loop in PC, as demonstrated in PC cell lines, PC xenograft tumors and clinical PCs from patients treated with ENZ. Eight out of eight ENZ-treated clinical PC specimens examined showed high Stat5 signaling. This finding has high translational significance because Stat5 is a potent inducer of PC growth and a therapeutically targetable survival factor in PC (23-28,32). We show here that the initial step in the hyperactive Jak2-Stat5 signaling in ENZ-treated PC cells was induction of Jak2 phosphorylation by ENZ-liganded AR. ENZ-induced Jak2 activation was accompanied by simultaneous and robust up-regulation of Stat5 phosphorylation in PC. In addition, the levels of both Jak2 mRNA and protein were increased by exposure of PC cells to ENZ, as demonstrated by parallel evaluation of Stat5 and Jak2 phosphorylation, protein levels and Jak2 mRNA expression in two PC cell lines during a time-course ranging from 2 h to 6 days. ENZ-induction of Jak2 mRNA levels preceded ENZ induction of Jak2 protein expression, which suggests that Jak2 mRNA levels are predominantly induced by ENZ and lead to increased Jak2 protein levels in PC. When Stat5 was genetically suppressed, ENZ failed to induce Jak2 mRNA and protein levels. This demonstrates that ENZ-induced Stat5 activation is crucial for ENZ-induced up-regulation of Jak2 in PC. Separate evaluation of Stat5-regulation of Jak2 mRNA and protein expression by genetic knockdown or over-expression approaches revealed that Jak2 mRNA levels are tightly regulated by Stat5 in PC. Future work will need to determine whether Stat5 induces Jak2 mRNA stability and/or the transcription of the Jak2 gene in PC and the associated enhancer elements and their genomic locations.
Jak2 phosphorylation triggered by ENZ in PC cells was rapid occurring already at 6 h and was further increased over the 12-day period tested here. The fact that ongoing protein synthesis was required for ENZ-induction of Jak2 phosphorylation and that a broad-spectrum phosphatase-inhibitor blocked ENZ-induced Jak2 phosphorylation both suggest mechanistic involvement of Jak2 phosphatases in this process. Genetic AR knockdown or androgen-depletion failed to increase Jak2-Stat5 signaling, which indicates that phosphorylation of Jak2 is not triggered by cellular stress of androgen pathway suppression in general. We further showed that genetic depletion of PTPƐ, but not SHP-2 or PTP1B, resulted in elevated levels of Jak2 phosphorylation, suggesting that PTPƐ is a key phosphatase and a negative regulator of Jak2 phosphorylation in PC. PTPƐ knockdown abolished the difference in Jak2 phosphorylation levels in ENZ -treated vs. non-treated cells, which implies that ENZ-liganded AR directly or indirectly suppresses the activity of PTPƐ in PC cells. At the same time, depletion of SHP-2 levels decreased ENZ-induction of Jak2 phosphorylation. This is consistent with a previously reported role of SHP-2 as a positive regulator of Jak2 activation (45,46) and is required for ENZ induction of Jak2 phosphorylation in PC cells. The finding that AR liganded by DHT did not increase phosphorylation of either Stat5 or Jak2 implies that ENZ-induction of Jak2 phosphorylation may be caused by cytoplasmic actions of the AR. Future studies will need to establish the mechanisms underlying ENZ regulation of enzymatic activity of PTPƐ as well as the mechanistic role of SHP-2 in ENZ-enhanced coupling of Jak2-Stat5 signaling in PC.
Active Stat5 increased the viability of PC cells during ENZ treatment and, conversely, Stat5 inhibition induced death of the residual PC cells surviving ENZ therapy. In addition, pharmacological inhibition of Stat5 in combination with ENZ displayed greater efficacy in suppressing growth of PC in vitro and in vivo than ENZ alone in both androgen-sensitive and castrate-resistant settings. Combined ENZ and IST5-002 as second-line therapy was more effective than ENZ monotherapy in suppressing in vivo growth of CRPC xenograft tumors and ex vivo growth of clinical patient-derived PCs in tumor explant cultures. Importantly, Stat3 has been implicated in ENZ-resistant CRPC growth in different preclinical models than those used in this study (50). Evaluation of the efficacy of Jak2 inhibitors that are currently in the clinical development for myeloproliferative disorders for blocking ENZ-resistant PC tumor growth will be essential for transition to phase I/II studies in PC and may provide efficacious second-line treatment for ENZ-resistant PC. Clinical evaluation of the efficacy of Jak2-Stat5 inhibitors in ENZ-resistant PCs should utilize positive Stat5 activation status as a biomarker for patient selection for accrual.
In summary, the present work introduces a concept of a hyperactive Jak2-Stat5 signaling loop as a mechanism mediating resistance of PC to ENZ. The Jak2-Stat5 pathway provides an attractive therapeutic target for ENZ-resistant PC as a second-line treatment or for first-line therapy in combination with ENZ. Future work will need to evaluate the efficacy of Jak2 inhibitors in the treatment of ENZ-resistant PC with positive status for active Stat5 as one of the inclusion criteria.
Supplementary Material
Acknowledgements
This work was financially supported by a National Cancer Institute (NCI)/National Institutes of Health (NIH) Research Project Grant (2RO1CA11358-06), an NCI/NIH Exploratory/Developmental Research Grant (1R21CA178755-01), Advancing a Healthier Wisconsin (#5520368) and Wisconsin Cancer Showhouse Grant (#15437) to M.T. Nevalainen. D.T. Hoang was supported by an NCI/NIH Predoctoral Individual National Research Service Award (NRSA) Fellowship (1F31CA180626-01) and A. Banerjee was supported by National Cancer Institute (NCI)/National Institutes of Health (NIH) Research Project Grant (R21CA231892).
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
References
- 1.Attard G, Parker C, Eeles RA, Schroder F, Tomlins SA, Tannock I, et al. Prostate cancer. Lancet 2016;387(10013):70–82 doi 10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- 2.Litwin MS, Tan HJ. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 2017;317(24):2532–42 doi 10.1001/jama.2017.7248. [DOI] [PubMed] [Google Scholar]
- 3.Yap TA, Zivi A, Omlin A, de Bono JS. The changing therapeutic landscape of castration-resistant prostate cancer. Nat Rev Clin Oncol 2011;8(10):597–610. [DOI] [PubMed] [Google Scholar]
- 4.Wong YN, Ferraldeschi R, Attard G, de Bono J. Evolution of androgen receptor targeted therapy for advanced prostate cancer. Nat Rev Clin Oncol 2014;11(6):365–76 doi 10.1038/nrclinonc.2014.72. [DOI] [PubMed] [Google Scholar]
- 5.Labrie F, Belanger A, Simard J, Labrie C, Dupont A. Combination therapy for prostate cancer. Endocrine and biologic basis of its choice as new standard first-line therapy. Cancer 1993;71:1059–67. [DOI] [PubMed] [Google Scholar]
- 6.Masiello D, Cheng S, Bubley GJ, Lu ML, Balk SP. Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor. J Biol Chem 2002;277:26321–6. [DOI] [PubMed] [Google Scholar]
- 7.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N Engl J Med 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ning YM, Pierce W, Maher VE, Karuri S, Tang SH, Chiu HJ, et al. Enzalutamide for treatment of patients with metastatic castration-resistant prostate cancer who have previously received docetaxel: U.S. Food and Drug Administration drug approval summary. Clin Cancer Res 2013;19(22):6067–73 doi 10.1158/1078-0432.CCR-13-1763. [DOI] [PubMed] [Google Scholar]
- 9.Beer TM, Armstrong AJ, Rathkopf D, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in Men with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur Urol 2017;71(2):151–4 doi 10.1016/j.eururo.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ning YM, Brave M, Maher VE, Zhang L, Tang S, Sridhara R, et al. U.S. Food and Drug Administration Approval Summary: Enzalutamide for the Treatment of Patients With Chemotherapy-Naive Metastatic Castration-Resistant Prostate Cancer. Oncologist 2015;20(8):960–6 doi 10.1634/theoncologist.2015-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009;324(5928):787–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claessens F, Helsen C, Prekovic S, Van den Broeck T, Spans L, Van Poppel H, et al. Emerging mechanisms of enzalutamide resistance in prostate cancer. Nat Rev Urol 2014;11(12):712–6 doi 10.1038/nrurol.2014.243. [DOI] [PubMed] [Google Scholar]
- 13.Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer 2015;15(12):701–11 doi 10.1038/nrc4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014;371(11):1028–38 doi 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res 2008;68(13):5469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 2013;155(6):1309–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph JD, Lu N, Qian J, Sensintaffar J, Shao G, Brigham D, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov 2013;3(9):1020–9. [DOI] [PubMed] [Google Scholar]
- 18.Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S, et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide). Cancer Discov 2013;3(9):1030–43. [DOI] [PubMed] [Google Scholar]
- 19.Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med 2016;22(3):298–305 doi 10.1038/nm.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bluemn EG, Coleman IM, Lucas JM, Coleman RT, Hernandez-Lopez S, Tharakan R, et al. Androgen Receptor Pathway-Independent Prostate Cancer Is Sustained through FGF Signaling. Cancer Cell 2017;32(4):474–89 e6 doi 10.1016/j.ccell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy DE, Darnell JE Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 2002;3(9):651–62. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Robinson GW, Gouilleux F, Groner B, Hennighausen L. Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci U S A 1995;92(19):8831–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahonen TJ, Xie J, LeBaron MJ, Zhu J, Nurmi M, Alanen K, et al. Inhibition of transcription factor Stat5 induces cell death of human prostate cancer cells. J Biol Chem 2003;278(29):27287–92. [DOI] [PubMed] [Google Scholar]
- 24.Dagvadorj A, Kirken RA, Leiby B, Karras J, Nevalainen MT. Transcription factor signal transducer and activator of transcription 5 promotes growth of human prostate cancer cells in vivo. Clin Cancer Res 2008;14(5):1317–24. [DOI] [PubMed] [Google Scholar]
- 25.Maranto C, Udhane V, Hoang DT, Gu L, Alexeev V, Malas K, et al. STAT5A/B Blockade Sensitizes Prostate Cancer to Radiation through Inhibition of RAD51 and DNA Repair. Clin Cancer Res 2018;24(8):1917–31 doi 10.1158/1078-0432.CCR-17-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazansky AV, Spencer DM, Greenberg NM. Activation of signal transducer and activator of transcription 5 is required for progression of autochthonous prostate cancer: evidence from the transgenic adenocarcinoma of the mouse prostate system. Cancer Res 2003;63(24):8757–62. [PubMed] [Google Scholar]
- 27.Thomas C, Zoubeidi A, Kuruma H, Fazli L, Lamoureux F, Beraldi E, et al. Transcription factor Stat5 knockdown enhances androgen receptor degradation and delays castration-resistant prostate cancer progression in vivo. Mol Cancer Ther 2010;10(2):347–59. [DOI] [PubMed] [Google Scholar]
- 28.Tan SH, Dagvadorj A, Shen F, Gu L, Liao Z, Abdulghani J, et al. Transcription factor Stat5 synergizes with androgen receptor in prostate cancer cells. Cancer Res 2008;68(1):236–48. [DOI] [PubMed] [Google Scholar]
- 29.Gu L, Dagvadorj A, Lutz J, Leiby B, Bonuccelli G, Lisanti MP, et al. Transcription factor Stat3 stimulates metastatic behavior of human prostate cancer cells in vivo, whereas Stat5b has a preferential role in the promotion of prostate cancer cell viability and tumor growth. Am J Pathol 2010;176(4):1959–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu L, Vogiatzi P, Puhr M, Dagvadorj A, Lutz J, Ryder A, et al. Stat5 promotes metastatic behavior of human prostate cancer cells in vitro and in vivo. Endocr Relat Cancer 2010;17(2):481–93 doi 10.1677/ERC-09-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haddad BR, Gu L, Mirtti T, Dagvadorj A, Vogiatzi P, Hoang DT, et al. STAT5A/B gene locus undergoes amplification during human prostate cancer progression. Am J Pathol 2013;182(6):2264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talati PG, Gu L, Ellsworth EM, Girondo MA, Trerotola M, Hoang DT, et al. Jak2-Stat5a/b Signaling Induces Epithelial-to-Mesenchymal Transition and Stem-Like Cell Properties in Prostate Cancer. Am J Pathol 2015;185(9):2505–22 doi 10.1016/j.ajpath.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Zhang Y, Glass A, Zellweger T, Gehan E, Bubendorf L, et al. Activation of signal transducer and activator of transcription-5 in prostate cancer predicts early recurrence. Clin Cancer Res 2005;11(16):5863–8. [DOI] [PubMed] [Google Scholar]
- 34.Mirtti T, Leiby BE, Abdulghani J, Aaltonen E, Pavela M, Mamtani A, et al. Nuclear Stat5a/b predicts early recurrence and prostate cancer-specific death in patients treated by radical prostatectomy. Hum Pathol 2013;44(3):310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dagvadorj A, Collins S, Jomain JB, Abdulghani J, Karras J, Zellweger T, et al. Autocrine prolactin promotes prostate cancer cell growth via Janus kinase-2-signal transducer and activator of transcription-5a/b signaling pathway. Endocrinology 2007;148(7):3089–101. [DOI] [PubMed] [Google Scholar]
- 36.Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev 2009;228(1):273–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin T, Shen R, Feng GS, Yang YC. Molecular characterization of specific interactions between SHP-2 phosphatase and JAK tyrosine kinases. J Biol Chem 1997;272(2):1032–7. [DOI] [PubMed] [Google Scholar]
- 38.Myers MP, Andersen JN, Cheng A, Tremblay ML, Horvath CM, Parisien JP, et al. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J Biol Chem 2001;276(51):47771–4 doi 10.1074/jbc.C100583200. [DOI] [PubMed] [Google Scholar]
- 39.Tanuma N, Nakamura K, Shima H, Kikuchi K. Protein-tyrosine phosphatase PTPepsilon C inhibits Jak-STAT signaling and differentiation induced by interleukin-6 and leukemia inhibitory factor in M1 leukemia cells. J Biol Chem 2000;275(36):28216–21. [DOI] [PubMed] [Google Scholar]
- 40.Lee JH, Kim C, Baek SH, Ko JH, Lee SG, Yang WM, et al. Capsazepine inhibits JAK/STAT3 signaling, tumor growth, and cell survival in prostate cancer. Oncotarget 2017;8(11):17700–11 doi 10.18632/oncotarget.10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duhe RJ, Farrar WL. Characterization of active and inactive forms of the JAK2 protein-tyrosine kinase produced via the baculovirus expression vector system. J Biol Chem 1995;270(39):23084–9. [DOI] [PubMed] [Google Scholar]
- 42.Dagvadorj A, Tan SH, Liao Z, Cavalli LR, Haddad BR, Nevalainen MT. Androgen-regulated and highly tumorigenic human prostate cancer cell line established from a transplantable primary CWR22 tumor. Clin Cancer Res 2008;14(19):6062–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damiano JS, Rendahl KG, Karim C, Embry MG, Ghoddusi M, Holash J, et al. Neutralization of prolactin receptor function by monoclonal antibody LFA102, a novel potential therapeutic for the treatment of breast cancer. Mol Cancer Ther 2013;12(3):295–305. [DOI] [PubMed] [Google Scholar]
- 44.Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell 2009;16(6):487–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Futami M, Zhu QS, Whichard ZL, Xia L, Ke Y, Neel BG, et al. G-CSF receptor activation of the Src kinase Lyn is mediated by Gab2 recruitment of the Shp2 phosphatase. Blood 2011;118(4):1077–86 doi 10.1182/blood-2009-12-261636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pazdrak K, Adachi T, Alam R. Src homology 2 protein tyrosine phosphatase (SHPTP2)/Src homology 2 phosphatase 2 (SHP2) tyrosine phosphatase is a positive regulator of the interleukin 5 receptor signal transduction pathways leading to the prolongation of eosinophil survival. J Exp Med 1997;186(4):561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao Z, Gu L, Vergalli J, Mariani SA, De Dominici M, Lokareddy RK, et al. Structure-Based Screen Identifies a Potent Small Molecule Inhibitor of Stat5a/b with Therapeutic Potential for Prostate Cancer and Chronic Myeloid Leukemia. Mol Cancer Ther 2015;14(8):1777–93 doi 10.1158/1535-7163.MCT-14-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nevalainen MT, Harkonen PL, Valve EM, Ping W, Nurmi M, Martikainen PM. Hormone regulation of human prostate in organ culture. Cancer Res 1993;53(21):5199–207. [PubMed] [Google Scholar]
- 49.Nevalainen MT, Valve EM, Ingleton PM, Nurmi M, Martikainen PM, Harkonen PL. Prolactin and prolactin receptors are expressed and functioning in human prostate. J Clin Invest 1997;99(4):618–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu C, Zhu Y, Lou W, Cui Y, Evans CP, Gao AC. Inhibition of constitutively active Stat3 reverses enzalutamide resistance in LNCaP derivative prostate cancer cells. Prostate 2014;74(2):201–9 doi 10.1002/pros.22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.