Abstract
There is an increasing need to develop conducting hydrogels for bioelectronic applications. In particular, PEDOT:PSS hydrogels have become a research hotspot due to their excellent biocompatibility and stability. However, injectable PEDOT:PSS hydrogels have been rarely reported. Such syringe-injectable hydrogels are highly desirable for minimally invasive biomedical therapeutics. In this communication, we demonstrate an approach to develop injectable PEDOT:PSS hydrogels by taking advantage of the room-temperature gelation property of PEDOT:PSS. These PEDOT:PSS hydrogels form spontaneously after syringe-injection into the desired location, without the need of any additional treatments. We also present a facile strategy for large-scale production of injectable PEDOT:PSS hydrogel fibers at room temperature. Finally, we demonstrate that these room-temperature formed PEDOT:PSS hydrogels (RT-PEDOT:PSS hydrogel) and hydrogel fibers can be used for the development of soft and self-healable hydrogel bioelectronic devices.
Introduction
Conducting polymer poly(3,4-ethylenedioxythiophene) doped with poly(styrenesulfonate) (PEDOT:PSS) has gained great success in the past years due to its biocompatibility, high conductivity, and water stability[1]. Advances in research on PEDOT:PSS have unveiled its extensive applications for various functional devices, such as solar cells[2], light-emitting diodes[3], transparent electrodes[4], electrochemical transistors[5], neuromorphic computing[6], and supercapacitors[7]. Recently, we have witnessed an emerging trend of flexible electronics where PEDOT:PSS plays a key role in developing soft bioelectronic devices that can directly interface with the human body thanks to its inherently superior flexibility compared to its inorganic counterparts[8]. Despite these successes, the majority of bioelectronic devices still rely on PEDOT:PSS in the form of thin films, which are physically and mechanically dissimilar to biological tissues. Such physical and mechanical mismatches may pose potential difficulties in forming natural integration with biological tissues via long-term stable and conformal interfaces[9]. Thus, establishing a PEDOT:PSS-based bioelectronic interface with improved tissue-like properties will greatly promote its application in the field of soft bioelectronics.
Compared to thin films, PEDOT:PSS hydrogels are considered as more ideal interfacing alternatives to biological tissues because of their water-rich nature and tissue-like mechanical properties. They not only provide a suitable microenvironment for cell growth and differentiation, but also an electrically-conductive network that allows for the in situ investigation of cellular behaviors under electrical stimulation[10]. Correspondingly, tremendous attention has been paid to investigate the fundamental properties of PEDOT:PSS hydrogels. In this content, Shi and colleagues synthesized conductive PEDOT:PSS hydrogels by mixing a PEDOT:PSS suspension with concentrated H2SO4 at an elevated temperature of 90 °C[11]. High performance supercapacitors were constructed by using these hydrogels as electrodes. Bao and colleagues demonstrated a method to make interpenetrating networks in PEDOT:PSS hydrogels to tune their mechanical properties for better integration with biological tissues[9b]. Zhao and colleagues reported pure PEDOT:PSS hydrogels by controlled dry-annealing and rehydration of PEDOT:PSS thin films. High swelling ratio was obtained in the thickness direction without mechanical constraints[9a]. In addition, Xu and colleagues proposed a multi-functional hydrogel based on PEDOT:PSS which shows excellent photothermal conversion and stretchability via one-step polymerization[12]. Finally, Zhao and colleagues reported highly stretchable PEDOT:PSS hydrogel microfibers fabricated with multiflow microfluidics[13]. Despite these results, most of these hydrogels are fabricated at elevated temperatures which are beyond the tolerance limit of biological tissues. PEDOT:PSS hydrogels that can be formed spontaneously at room temperature (RT-PEDOT:PSS hydrogels) are highly favorable for direct processing and patterning under temperature-sensitive environments[9b, 14]. We hypothesize that a proper manipulation of an RT-PEDOT:PSS hydrogel would enable the development of injectable hydrogels where crosslinking and autonomous gelation can be controlled occurring only after the injection of PEDOT:PSS liquid into the desired location such as human body. Such injectable PEDOT:PSS hydrogels will be of great medical demand, especially for nerve regeneration and brain stimulation or recording. Further investigation on the fundamental properties of these hydrogels may promote their applications for next-generation organic and hydrogel bioelectronics.
In this communication, we present a strategy to develop injectable conductive hydrogels by taking advantage of the room-temperature gelation ability of PEDOT:PSS. Gelation of the PEDOT:PSS can be achieved spontaneously, at room temperature, after syringe-injecting surfactant-mixed PEDOT:PSS liquid into the desired location, without any additional treatments. A facile method for large scale production of injectable PEDOT:PSS hydrogel fibers is presented by injecting and crosslinking the PEDOT:PSS liquid within a confined cylinder tube. We demonstrate that these hydrogel fibers can be used as electroactive materials to develop organic bioelectronic devices such as organic electrochemical transistors (OECTs). Moreover, we reveal that these RT-PEDOT:PSS hydrogels own high volumetric swelling ratios which can be further used for water-healable hydrogel bioelectronics.
Results and discussions
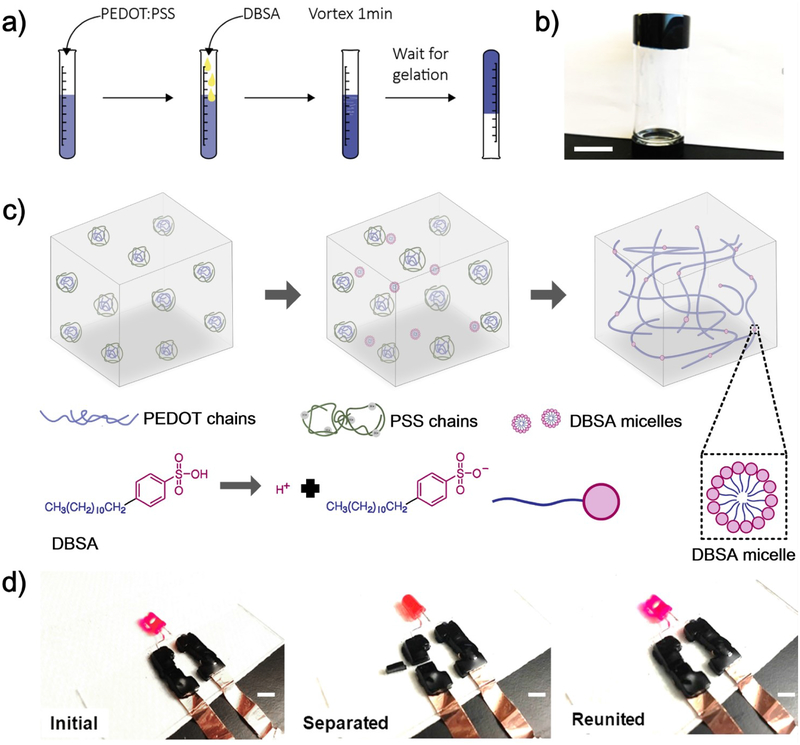
RT-PEDOT:PSS hydrogels were obtained by simply mixing PEDOT:PSS liquid with 4-dodecylbenzenesulfonic acid (DBSA) which is a widely used secondary dopant (surfactant) in processing PEDOT:PSS. Spontaneous gelation occurred when the concentration of DBSA reached a threshold value of ca. 3 v/v.%, without further treatment (Figure 1a,b). The time required for the gelation was controllable between 2 and 200 min, depending on the concentration of DBSA (Figure S1). The formation of PEDOT:PSS hydrogels at room temperature is tentatively attributed to the physical crosslinking between PEDOT+ polymer chains[11, 14], enabled by the addition of DBSA (Figure 1c). It is known that PEDOT:PSS has a core-shell structure. The coiled and hydrophilic PSS− shell surrounds the hydrophobic PEDOT+, and the complex forms as suspension in water[15]. PEDOT+ and PSS− chains interact via electrostatic interactions[14]. DBSA is an acidic surfactant which, once the concentration value is more than the critical micelle concentration (Figure 1c)[16], is capable of forming well-defined micellar structures in water, headed with negatively charged sulfuric acid groups (SO3−). The presence of sufficient DBSA molecules, as indicated by Fourier-transform infrared spectroscopy (FTIR) (Figure S2), increases the ionic strength in the solution and provides sufficient H+ to protonate the PSS−. The increased ionic strength is supposed to weaken the electrostatic attraction between PEDOT+ and PSS− chains[14], which subsequently exposes PEDOT+ chains to each other and promotes their interchain interactions to form a connected 3D network (Figure 1c) due to physical crosslinking induced by π–π stacking and hydrophobic attractions. Complex viscosity in physically crosslinked hydrogels normally decreases upon increasing shear frequency. This is confirmed by the result where we observed a decrease in complex viscosity of more than 2 orders of magnitude when increasing the shear frequency from 0.1 to 10 rad/s (Figure S3). Despite a relatively weak physical crosslinking in these hydrogels, they can be manipulated into three-dimensional (3D), self-standing structures with various shapes (Figure S4, Video S1 and S2).
Figure 1.
a) Schematic illustration of gelation processes of RT-PEDOT:PSS hydrogel; b) RT-PEDOT:PSS hydrogel formed after 10 min (4 v/v.% DBSA): the gel adhered at the bottom when flipping the vial; c) Crosslinking mechanism of our RT-PEDOT:PSS hydrogel. The addition of the DBSA into the suspension weakens electrostatic attraction between PEDOT+ and PSS−, exposing the PEDOT+ chains to water. The exposed PEDOT+ chains undergo a conformational change from a confined-coiled to an expanded-linear structure and subsequently physically crosslinked due to π–π stacking and hydrophobic attractions; d) RT-PEDOT:PSS hydrogels acting as conductive interconnects to drive a LED via a “cut and stick” approach. Scale bar: 5 mm.
The RT-PEDOT:PSS hydrogel showed a moderate conductivity of ~ 10−1 S/cm (Figure S5). Although it is lower than a PEDOT:PSS thin film (> 1 S/cm)[17], it has already surpassed those values of most tissues in the brain or spinal cord where these hydrogels are expected to functionalize[18]. For example, the cerebrospinal fluid (CSF) holds the highest conductivity among human tissues. However, its value (15.38*10−3 S/cm) is still much lower than the RT-PEDOT:PSS hydrogels[18], suggesting the conductivity should not be a limiting factor for future in vivo applications. Moreover, in practical application, these hydrogels are expected to be used in the form of thick gel, which will further lower their resistance as compared to thin films. For example, as shown in Figure 1d and Video S3, RT-PEDOT:PSS hydrogels with a thickness of 5 mm has a total resistance of less than 100 ohm (Figure S6) and is able to serve as interconnects to switch on the light-emitting diode (LED) under a low voltage of 1 V. Interestingly, the brightness of the LED was insensitive to external cuts in the hydrogel interconnects (Video S3), attributable to their favorable viscoelastic property. The current flowing across the RT-PEDOT:PSS hydrogel could only be interrupted by removing a bulky piece of the hydrogel from the circuit (Figure 1d, Video S3). Importantly, the current was able to recover to its initial value when we pushed the hydrogel piece back to the circuit, demonstrating these RT-PEDOT:PSS hydrogels can be manipulated in a customized “cut and stick” approach.
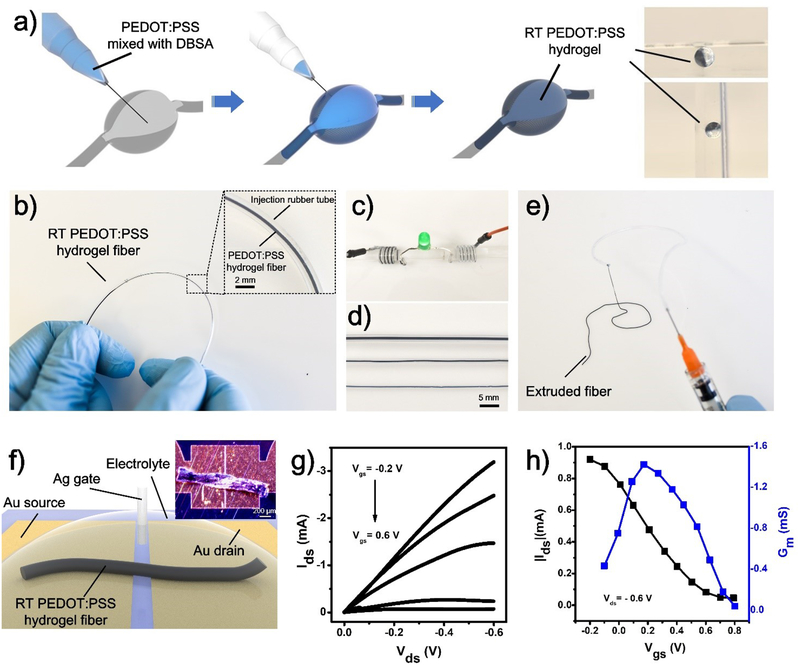
The unique room-temperature gelation ability of PEDOT:PSS enables us to demonstrate injectable PEDOT:PSS hydrogels[19], which can find potential application in minimally invasive therapeutics such as nerve regeneration and brain stimulation, without causing any trauma[20]. As a demonstration, we first injected the PEDOT:PSS liquid (premixed with DBSA) into a void polydimethylsiloxane (PDMS) mold with a syringe (Figure 2a and Video S4). RT-PEDOT:PSS hydrogels formed spontaneously in the mold after completing the injection process. These PEDOT:PSS hydrogels stayed stably in the mold even after inversion (Figure 2a). The RT-PEDOT:PSS hydrogel also enables direct fabrication of conductive interconnects on temperature-sensitive substrates such as gelatin, a skin-derived hydrogel that cannot tolerate heating because of its low melting temperature around 37 °C. To make conductive interconnects in gelatin with the injectable PEDOT:PSS hydrogel, serpentine channels were first created in the gelatin (Figure S7). Next, the PEDOT:PSS liquid was injected into these channels and hydrogel interconnects were subsequently obtained. These serpentine RT-PEDOT:PSS hydrogels in gelatin could tolerate mechanical deformations such as bending and twisting without any breakages. The current flow through the RT-PEDOT:PSS hydrogel maintained 80% of its initial value even after being strained up to 50% (Figure S7).
Figure 2.
a) Schematic of injectable RT-PEDOT:PSS hydrogels: Puncturing the soft tissue with syringe; Syringe-injecting PEDOT:PSS suspension (4 v/v.% DBSA) into the cavity; and RT-PEDOT:PSS hydrogel formed spontaneously after 10 min; The optical images show the RT-PEDOT:PSS hydrogel attached to the wall of the cavity even we tilted the mold; b) Formation of the RT-PEDOT:PSS hydrogel in a plastic tube via syringe injection. Inset: enlarged view of the RT-PEDOT:PSS hydrogel in tube; c) Application of RT-PEDOT:PSS hydrogel fibers for driving an LED; d) Injectable RT-PEDOT:PSS hydrogel fibers with different diameters of 875, 480, and 400 μm; e) Extruded RT-PEDOT:PSS hydrogel fibers with a syringe; f) Schematic of the fabricated OECTs with injected RT-PEDOT:PSS hydrogel fiber. The hydrogel fiber was freeze-dried after printing on the Au electrodes which have a gap of 10 μm; the inset shows the real optical image of the freeze-dried fiber on the source-drain electrodes. g-h) Output and transfer curves of the OECTs with RT-PEDOT:PSS hydrogel fibers as the channel.
The Young’s modulus of the obtained RT-PEDOT:PSS hydrogel is ~ 1 KPa (Figure S8a and Figure S8d), whereas the modulus of biological tissues in human body ranging from 1 to 100 KPa[21]. Therefore, an improved mechanical property could potentially minimize the potential mechanical mismatch between injectable hydrogel and various human tissues. We increased mechanical strength of the injectable RT-PEDOT:PSS hydrogel by infiltrating into it with precursors of a secondary hydrogel (polyacrylamide, PAAm) (Figure S9)[9b, 22]. The robust, covalent-bonded network in the PAAm hydrogel increases the modulus of the whole hydrogel by forming interpenetrated networks with the PEDOT:PSS hydrogel. Precursor of PAAm hydrogel is also manipulated to be injectable and crosslinkable at room temperature by using a unique initiator, the ammonium persulfate (APS) which can trigger the gelation of PAAm at 25 °C (Figure S9)[23]. With this method, we are able to control the Young’s modulus of the RT-PEDOT:PSS hydrogel between 1 and 100 KPa (Figure S8d), which covers the modulus range of most relevant tissues. For example, the brain has a Young’s modulus of 1~4 KPa and the heart has a Young’s modulus of 10~18 KPa[21]. Significantly, a comparable resistance was observed in the infiltrated hydrogel (Figure S6), suggesting the secondary network has a minor effect in the conductivity due to pre-formed conductive paths in the PEDOT networks before infiltrating. These results outline the feasibility to develop mechanically robust and injectable PEDOT:PSS hydrogel at room temperature, endowing PEDOT:PSS with fresh features for potential biomedical applications.
To further demonstrate the diverse application of these injectable RT-PEDOT:PSS hydrogels, we present a method to produce injectable hydrogel fibers by syringe-injecting and crosslinking PEDOT:PSS liquid into a plastic hollow tube (Figure 2b). The formed RT-PEDOT:PSS hydrogel fibers could be extruded by pressurizing the tube with controlled speed while maintaining their structural integrity because of their good mechanical properties and the lubricating effect of the DBSA. Hydrogel fibers of adjustable diameters can be obtained by using plastic tubes with different sizes (Figure 2c–e). These hydrogel fibers could be manipulated into various shapes through the syringe by simply hand-writing (Figure S10). It is worth mentioning that these hydrogel fibers can be injected into an aqueous solution in a way similar to wet spinning while maintaining their shapes even after entangling together (Video S5 and Video S6).
As a potential application, we exploited the possibility of using RT-PEDOT:PSS hydrogel fibers as channel material for the fabrication of organic bioelectronic device, such as OECT, which has been widely used for in vivo bioelectronics studies[5]. The OECT was fabricated by simply syringe-injecting a RT-PEDOT:PSS hydrogel fiber between two metallic electrodes (Figure 2f). The output and transfer curves of the OECT are shown in Figure 2g and 2h. The OECT shows typical transistor behavior working in the depletion mode. The resultant OECT has a maximum transconductance of ~1.4 mS (Vds= −0.6 V, Vgs= −0.2 V) (Figure 2h), which is comparable to conventional OECTs based on PEDOT:PSS thin films[24]. These results demonstrate the good electrochemical properties of these PEDOT:PSS hydrogel fibers, indicating their potential applications for organic and hydrogel bioelectronics.
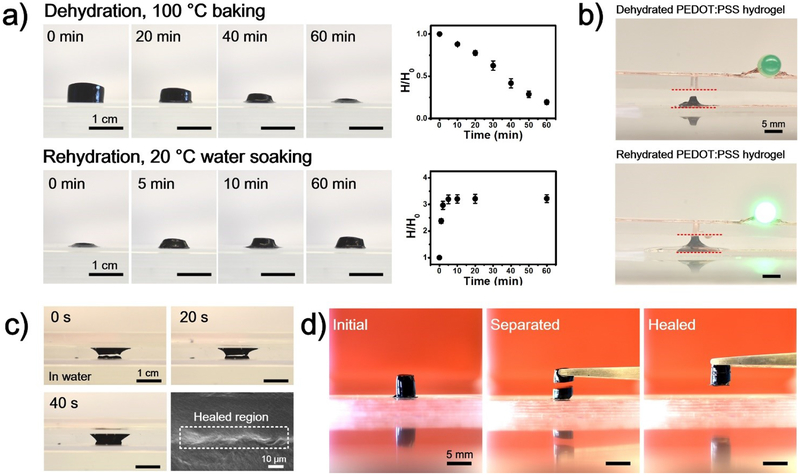
Recently we have discovered that PEDOT:PSS in the form of thin films demonstrates self-healing properties due to its excellent volumetric swelling ability upon exposing to water[25]. Accordingly, we investigated the volumetric swelling property in these RT-PEDOT:PSS hydrogels. Strikingly, we observed that these hydrogels showed greater volumetric swelling abilities compared to thin films. As shown in Figure 3a, the height of a dehydrated RT-PEDOT:PSS hydrogel (100 °C, 1 hour), swelled remarkably to ~ 300% of its initial value within ~5 min. Hydrogels dehydrated at room-temperature also showed high volumetric swelling ratio, regardless of their initial shapes (Figure S11). As a demonstration for self-healing hydrogel electronics, we placed dehydrated PEDOT:PSS hydrogel between two conductive copper tapes that adhered to glass slides (Figure 3b). No current flow was observed in the circuit due the gap between the hydrogel and the top electrode. Subsequently, we hydrated the device with water, which induced swelling of the PEDOT:PSS hydrogel and led to its physical connection to the top electrode, thereby switching on the LED. The healing process was further demonstrated by cutting the RT-PEDOT:PSS hydrogel with a razor blade, which created a gap of about 1 mm (Figure 3c). The presence of water healed the gap within only 40 seconds due to the rapid swelling of the RT-PEDOT:PSS hydrogel (Video S7). Additionally, the mechanical healing of RT-PEDOT:PSS hydrogels was achieved by connecting two separated hydrogels together. After 5 min, these two separated PEDOT:PSS hydrogels healed together and could be lifted up with tweezers (Figure 3d). Overall, these results demonstrate these RT-PEDOT:PSS hydrogels are promising candidates for self-healing electronics.
Figure 3.
a) Volumetric shrinking and swelling properties of the RT-PEDOT:PSS hydrogels under mild dehydration (N=3); (b) Application of the swellable RT-PEDOT:PSS hydrogel as a water-controllable switch for LED; (c) Optical images of the healing processes of a damaged RT-PEDOT:PSS hydrogel, and SEM images of the healed region; (d) Mechanical healing of the RT-PEDOT:PSS hydrogel by placing two separated RT-PEDOT:PSS hydrogels (mildly dehydrated) together for 5 min.
Finally, we evaluated biocompatibility of RT-PEDOT:PSS hydrogel by culturing C2C12 muscle cells on their surface to assess the cytotoxicity. Before the cell culture, the gel-coated polyethylene terephthalate (PET) substrate was rinsed thoroughly in dulbecco’s phosphate-buffered saline (DPBS) media to remove any access acidic species. Cell viability and proliferation analyses were carried out at a density of 100.000 cells/well. The cells proliferated on the RT-PEDOT:PSS hydrogel surfaces after 3 days, and only a slight difference was observed between cells cultured on reference surfaces and cells cultured on these hydrogel surfaces (Figure S5). These results indicate that RT-PEDOT:PSS hydrogel may provide a suitable environment for cell growth, thus ensuring their use towards biological applications[26].
Conclusion
In conclusion, we have presented a strategy to obtain injectable PEDOT:PSS hydrogels by using room-temperature crosslinkable PEDOT:PSS. We demonstrated that these injectable PEDOT:PSS hydrogels can be potentially used to obtain injectable conductors into human tissue, stretchable interconnects on temperature-sensitive substrates, and water-healable hydrogel conductors by taking advantage of their high volumetric swelling ratio. We also demonstrated these injectable hydrogels could enable the development of PEDOT:PSS hydrogel fibers which can be used to develop future hydrogel bioelectronic devices such as hydrogel-based OECTs. Future investigations and optimizations on the electrical and mechanical properties of these injectable PEDOT:PSS hydrogels and hydrogel fibers will promote their practical applications towards biomedical applications.
Experimental methods
Materials
PEDOT:PSS (Clevios™ PH1000) was purchased from Haraeus Electronic Materials, Germany. DBSA, Gelatin from porcine skin, and Gallium–Indium eutectic (EGaIn) were purchased from Sigma Aldrich, USA. DPBS was purchased from Gibco, USA.
Synthesis of RT-PEDOT:PSS hydrogels
The PEDOT:PSS hydrogels were obtained by adding 4 v/v% of DBSA solution into the PEDOT:PSS liquid (suspension), followed by a 3-min agitation. Then, the mixture was poured into pre-patterned molds and then placed in a desiccator or centrifuged to remove bubbles induced by vortex. PEDOT:PSS hydrogels formed spontaneously within about 10 min after mixing DBSA (4 v/v.%) with PEDOT:PSS liquid. Gelation time was defined by tube inversion method.
Characterization of RT-PEDOT:PSS hydrogel
The tensile test of the RT-PEDOT:PSS hydrogel was implemented with the INSTRON tensile tester (INSTRON 5943, USA) in extension mode (extension rate of 1 mm/min).
Rheology tests were performed with the Anton-Paar rheometer (MCR 302, Austria). All samples had a diameter of 8 mm and height of 3 mm. The samples were cut by a circular blade and had a diameter of 8 mm. The storage and loss modulus as well as complex viscosity were measured via small amplitude shear oscillation with constant shear rates (0.1–10 rad/s) in the linear region. The infiltration process for increasing the mechanical property of the RT-PEDOT:PSS hydrogel is detailed in Figure S9.
The conductivity of RT-PEDOT:PSS hydrogel was characterized by biasing the hydrogel at a constant direct current (DC) voltage of 1 V with the source measure unit Agilent B2901A (Keysight Technologies, USA). Conductive copper tape and EGaIn were used to connect the PEDOT:PSS hydrogel (length × width × thickness: 20 mm × 20 mm × 5 mm) to the source measure unit. The current was collected after biasing the PEDOT:PSS hydrogel for 100 seconds at 1 V, which allowed us to extract a stable DC conductance. Conductivity of the dehydrated PEDOT:PSS hydrogel was measured after oven-baking the gel at 100 °C for 1 hour. The length, width and thickness of RT-PEDOT:PSS hydrogels for conductivity characterization are 20 mm, 20 mm and 5 mm, respectively.
The electromechanical properties of patterned PEDOT:PSS hydrogel on gelatin substrates were tested with a tensile tester. Liquid metal (EGaIn) and conductive tape (3M 9703) were used to facilitate the electrical connection to the samples.
Biocompatibility evaluation of RT-PEDOT:PSS hydrogel
Cell culture was performed with C2C12 cells (mouse muscle cells). Cells were seeded on RT-PEDOT:PSS hydrogel coated and uncoated PET surfaces as control. A Live/Dead calcein AM/ethidium homodimer assay (Thermo Scientific) was used to quantify the viability of the cells according to the manufacturer instruction until day-3. Cell proliferation was quantified using Presto Blue (Thermo Scientific) by measuring the metabolic activity of cells until day-7. 100 μL of staining solution for each condition was transferred into ELISA (enzyme-linked immunosorbent assay) microplates (96-wells plates, Corning Life Sciences, Lowell, MA, USA) for spectrophotometric measurement. The absorbance of the solutions was measured spectrophotometrically at 570 nm.
Patterning of RT-PEDOT:PSS hydrogel
Patterned RT-PEDOT:PSS hydrogels were obtained with laser ablated PMMA molds (VLS 2.30, Universal Laser, USA). For pattering on gelatin, patterned and stretchable gelatin substrate was first obtained with a PMMA mold. Next, PEDOT:PSS solution (with 4 v/v.% DBSA) was quickly syringe-injected into the microchannels in gelatin substrate. PEDOT:PSS hydrogels formed spontaneously in gelatin microchannels within 10 min after injection.
Fabrication of OECTs with RT-PEDOT:PSS hydrogel fibers
Silicon substrates were pre-cleaned with acetone, IPA and DI water. Then photoresist (SPR 700 1.2) was spin-coated on top of the substrates, followed by UV exposure under an optical shadow mask to define the desired patterns. After photoresist developing (MA-26A, 30s), Ti/Au (10 nm / 100 nm) was deposited. Patterned source and drain electrodes were obtained after stripping the photoresist with acetone in a sonication bath. Next, parylene was selectively deposited on top of the electrodes as insulating layer. The RT-PEDOT:PSS hydrogel fiber was subsequently syringe-injected in between the source and drain electrode as the transistor channel, followed by a freeze-drying process. The channel length is 10 μm, the channel width is 1000 μm, and the diameter of the RT-PEDOT:PSS hydrogel is about 200 μm which shrinks to 20 μm after baking (100 °C, 1 hour). Before transistor measurements, chitosan solution was coated on top of the RT-PEDOT:PSS hydrogel to prevent delamination during the experiments. Finally, a silver wire was inserted into CTAB solution as gate electrode. The characterization of the OECTs was performed with Agilent B2902A, controlled with LabVIEW software for output and transfer curves measurements.
Supplementary Material
Acknowledgement
S. Z, Y. C and H. L equally contributed to this work. This work was supported by the National Institutes of Health (Grant Nos. 1R01HL140951–01A1 and 1R01HL140618–01). The authors would like to thank Dr. Halima Alem for discussions on the gelation of the hydrogel.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
References:
- [1].Groenendaal L, Jonas F, Freitag D, Pielartzik H, Reynolds JR, Advanced Materials 2000, 12, 481. [Google Scholar]
- [2].(a) Kim YH, Sachse C, Machala ML, May C, Müller-Meskamp L, Leo K, Advanced Functional Materials 2011, 21, 1076; [Google Scholar]; (b) Lipomi DJ, Tee BC-K, Vosgueritchian M, Bao Z, Advanced Materials 2011, 23, 1771. [DOI] [PubMed] [Google Scholar]
- [3].Liu Y.-s., Feng J, Ou X-L, Cui H.-f., Xu M, Sun H-B, Organic Electronics 2016, 31, 247. [Google Scholar]
- [4].Crispin X, Jakobsson FLE, Crispin A, Grim PCM, Andersson P, Volodin A, van Haesendonck C, Van der Auweraer M, Salaneck WR, Berggren M, Chemistry of Materials 2006, 18, 4354. [Google Scholar]
- [5].Rivnay J, Inal S, Salleo A, Owens RM, Berggren M, Malliaras GG, Nature Reviews Materials 2018, 3, 17086. [Google Scholar]
- [6].(a) van de Burgt Y, Lubberman E, Fuller EJ, Keene ST, Faria GC, Agarwal S, Marinella MJ, Alec Talin A, Salleo A, Nature Materials 2017, 16, 414; [DOI] [PubMed] [Google Scholar]; (b) Fuller EJ, Keene ST, Melianas A, Wang Z, Agarwal S, Li Y, Tuchman Y, James CD, Marinella MJ, Yang JJ, Salleo A, Talin AA, Science 2019, 364, 570. [DOI] [PubMed] [Google Scholar]
- [7].Gund GS, Park JH, Harpalsinh R, Kota M, Shin JH, Kim T.-i., Gogotsi Y, Park HS, Joule 2019, 3, 164. [Google Scholar]
- [8].Kayser LV, Lipomi DJ, Advanced Materials 2019, 31, 1806133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].(a) Lu B, Yuk H, Lin S, Jian N, Qu K, Xu J, Zhao X, Nature Communications 2019, 10, 1043; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Feig VR, Tran H, Lee M, Bao Z, Nature communications 2018, 9, 2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].(a) Spencer AR, Primbetova A, Koppes AN, Koppes RA, Fenniri H, Annabi N, ACS Biomaterials Science & Engineering 2018, 4, 1558; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tondera C, Akbar TF, Thomas AK, Lin W, Werner C, Busskamp V, Zhang Y, Minev IR, Small 2019, 0, 1901406. [DOI] [PubMed] [Google Scholar]
- [11].Yao B, Wang H, Zhou Q, Wu M, Zhang M, Li C, Shi G, Advanced Materials 2017, 29, 1700974. [DOI] [PubMed] [Google Scholar]
- [12].Cao S, Tong X, Dai K, Xu Q, Journal of Materials Chemistry A 2019, 7, 8204. [Google Scholar]
- [13].Guo J, Yu Y, Wang H, Zhang H, Zhang X, Zhao Y, Small 2019, 15, 1805162. [DOI] [PubMed] [Google Scholar]
- [14].Leaf MA, Muthukumar M, Macromolecules 2016, 49, 4286. [Google Scholar]
- [15].Zhou J, Anjum DH, Chen L, Xu X, Ventura IA, Jiang L, Lubineau G, Journal of Materials Chemistry C 2014, 2, 9903. [Google Scholar]
- [16].Khan MS, Ali Z, Chinese Journal of Polymer Science 2005, 23, 29. [Google Scholar]
- [17].Zhang S, Kumar P, Nouas AS, Fontaine L, Tang H, Cicoira F, APL materials 2015, 3, 014911. [Google Scholar]
- [18].Ramon C, Schimpf PH, Haueisen J, BioMedical Engineering Online 2006, 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu J, Fu T-M, Cheng Z, Hong G, Zhou T, Jin L, Duvvuri M, Jiang Z, Kruskal P, Xie C, Suo Z, Fang Y, Lieber CM, Nature Nanotechnology 2015, 10, 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].(a) Ashammakhi N, Ahadian S, Darabi MA, El Tahchi M, Lee J, Suthiwanich K, Sheikhi A, Dokmeci MR, Oklu R, Khademhosseini A, Advanced Materials 2019, 31, 1804041; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sun W, Lee J, Zhang S, Benyshek C, Dokmeci MR, Khademhosseini A, Advanced Science 2019, 6, 1801039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Handorf AM, Zhou Y, Halanski MA, Li W-J, Organogenesis 2015, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].(a) Goding J, Gilmour A, Martens P, Poole‐Warren L, Green R, Advanced healthcare materials 2017, 6, 1601177; [DOI] [PubMed] [Google Scholar]; (b) Yuk H, Lu B, Zhao X, Chemical Society Reviews 2019, 48, 1642; [DOI] [PubMed] [Google Scholar]; (c) Warren H, MRS Online Proceedings Library Archive 2013, 1569, 219; [Google Scholar]; (d) Sun J-Y, Zhao X, Illeperuma WR, Chaudhuri O, Oh KH, Mooney DJ, Vlassak JJ, Suo Z, Nature 2012, 489, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].(a) Huang SH, Sheth S, Jain E, Jiang X, Zustiak SP, Yang L, Optics express 2018, 26, 51; [DOI] [PubMed] [Google Scholar]; (b) Zhang E, Bai R, Morelle XP, Suo Z, Soft matter 2018, 14, 3563. [DOI] [PubMed] [Google Scholar]
- [24].Zhang S, Hubis E, Girard C, Kumar P, DeFranco J, Cicoira F, Journal of Materials Chemistry C 2016, 4, 1382. [Google Scholar]
- [25].Zhang S, Cicoira F, Advanced Materials 2017, 29, 1703098. [DOI] [PubMed] [Google Scholar]
- [26].Zhang YS, Khademhosseini A, Science 2017, 356, eaaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.