Abstract
Gartanin, a 4-prenylated xanthone, has been identified from the purple mangosteen fruit as a potent growth inhibitor of various cancer cell lines, including prostate cancer. However, much of Gartanin’s anti-cancer mechanism remains unknown. We have discovered that Gartanin docked onto the regulatory subunit of the precursor cell-expressed developmentally down-regulated 8 (NEDD8)-activating enzyme (NAE) complex and next to the NEDD8 binding complex, which leads to inhibit NEDD8 conjugations to both Cullin1 and Ubc12 in prostate cancer cell lines and Ubc12 NEDDylation in an in vitro assay. S phase kinase-associated protein (Skp2) and F-box and WD-repeat domain-containing 2 (FBXW2), the NEDD8 family members of E3 ubiqutin ligases, were also down-regulated and up-regulated by Gartainin, respectively. Knock-down of NEDD8 expression by short harpin (Sh) RNAs blocked or attenuated these effects of Gartainin. Finally, Gartanin demonstrated its ability to inhibit growth of prostate cancer lines via autophagy initiation. Our data support that Gartanin is a naturally occurring NEDDylation inhibitor and deserves further investigation for prostate cancer prevention and treatment.
Keywords: Gartanin, Skp2, neddylation, chemoprevention, prostate cancer
INTRODUCTION
Prostate cancer is the most frequently diagnosed cancer in men. There will be an estimated 31,620 deaths from prostate cancer in the United States in 2019, a 7% increase from 2018 [1]. Prostate cancer is a highly heterogeneous disease. The course of prostate cancer is difficult to predict. Prostate cancer slowly progresses over several decades and in a significant proportion of elderly men with prostate cancer, the disease never becomes symptomatic [2]. This group of patients is easily over-treated and suffered from unnecessary adverse effects of treatments and loss of quality of life. In addition, between 15% and 50% of men who are thought to have clinically localized disease at diagnosis have failure in conventional therapy leading to a lethal disease. Based on these characteristics, prostate cancer has been considered to an ideal target for prevention by chemo/dietary approaches.
Fruits and vegetables contain a wide variety of new anti-cancer agents. Garcinia mangostana L(mangosteen), also called “The Queen of Fruits”, is one of the most popular tropical fruits widespread in Southeast Asian countries [3]. Mangosteen has been used as food and folk medicine by Southeast Asians for centuries [3] and sold as a health drink or dietary supplements in the United States. Mangosteen is rich in oxygenated and prenylated xanthones. Studies from cell cultures and animals have demonstrated that these xanthones have antioxidant, antitumor, antiallergic and anti-microbial activities [3–7]. Among these mangosteen xenthones, Gartanin has been identified to the most potent agent against the growth of bladder, breast, prostate and colon cancer, sarcoma, glioma, melanoma and leukemia cell lines via cell cycle arrest and apoptosis induction [8–15]. Molecular mechanisms of Gartanin’s anti-cancer effects were involved in inhibition of JNK, AKT, MAPK, and NF-κB pathways and CDK4 [9, 12, 14, 15]. In addition, our previous study has demonstrated that Gartanin induces autophagy through the inhibition of the mTOR pathway [10]. In prostate cancer, Gartanin has been shown to interact with androgen receptor and induce its degradation through the unfolded protein response and endoplasmic reticulum stress pathway [13]. However, the mechanism of Gartanin-induced protein degradation is still largely unclear.
NEDD8, an ubiquitin-like protein, plays an important role in the modification of Cullin-1 to turn on the Skp1-Cullin-F box protein (SCF) complex for regulation of the stability of its target proteins. The NEDDylation of Cullin1 occurs via a conjugation cascade-the NEDDylation pathway, which is initiated by an E1 (i.e. NAE) enzyme. Activated E1 then transfers NEDD8 to its E2 enzyme NEDD8-conjugating enzyme 2M (UBE2M), also called Ubc12, which causes covalent modulation of Cullin proteins with NEDD8 for activation of Cullin-RING ubiquitin ligases. Many components of the NEDDylation pathway, such as NEDD8 and NAE1, have been reported to be over-expressed in several cancers [16–18]. Currently, the knowledge about a role of NEDDylation in prostate cancer remains largely unclear. Limited studies have shown that the expression of the CUL1-interacting protein cullin-associated and neddylation-dissociated 1 (CAND1) and defective in cullin neddylation 1 (DCN1) that regulate the conjugation of NEDD8 to Cullins were aberrantly altered in prostate cancer [19, 20]. In addition, it has been thought that NEDDylation play an important role in prostate cancer by regulation of the equilibrium of cellular E3 ubiquitin ligase repertoire, such as Skp2, SPOP, etc., leading to enhanced survival of cancer cells and tumorigenesis [21, 22]. Therefore, the NEDDylation pathway could be targeted for development of novel cancer therapies. Indeed, a small molecule inhibitor of NAE, MLN4924 (a first-in-class inhibitor of NAE also named as pevonedistat), has been developed and currently in multiple phase I/II clinical trials for patients with advanced solid tumors or hematological tumors [23–27].
In this study, we discovered that Gartanin inhibits protein NEDDylation both in vitro and in vivo in prostate cancer cell lines (i.e. 22Rv1 and PC3) by interacting with the regulatory subunit of NAE1 and NEDD8, which results in down-regulation of Skp2 and Cdh1 and up-regulation of FBXW2, as well as induction of autophagy and growth inhibition.
MATERIALS AND METHODS
Cell lines and reagents
PC3 and 22Rv1 were obtained from American Type Culture Collection (ATCC, Manassas, VA). These cells were characterized and authenticated by ATCC. In addition, all cell lines were tested for known species of mycoplasma contamination using a kit from LONZA Inc. (Walkersville, MD). These prostate cancer cells were cultured in RPMI-1640 media (Fisher Scientific) supplemented with 10% fetal bovine serum (FBS), 1% L-Glutamine, and 1% penicillin-streptomycin as described previously in our publication. The NEDD8 knockdown and Skp2 transfected cell lines were obtained according to our reported methods [28, 29].
Compounds, antibodies and reagents
Gartanin was obtained from Chromadex with 99% purity (Irvine, CA). MG-132 and MLN4924 were obtained from Cayman Chemical Inc. (Ann Arbor, MI). Antibodies against Ubc12, ubiquitin, and β-tubulin were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-Skp2 antibodies were purchased from Invitrogen (Grand Island, NY). Anti-Myc-tag, anti-LC3B-II, anti-FBXW2, anti-Cdh1, and anti-β-TrPC antibodies were from Cell Signaling Inc. (Boston, MA). Anti-Cullin-1, anti-FBW7 and anti-NEDD8 antibodies were from Abcam (Cambridge, MA). Anti-p27/Kip1 antibody was from BD Biosciences (Billerica, MA). 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) was purchased from Sigma. Reverse Transcription System kit and was from Promega (Mandison, WI). A quantitative reverse transcription polymerase chain reaction (RT-PCR) kit was from Bio-Rad (Hercules, CA).
MTT assay
Cells (2 × 104 cells/well) were grown in 24-well culture dishes for 24 hours, and then treated as indicated in the figures. Cells were then incubated for another 72 hours before adding 1 mg/mL MTT in 20% PBS and 80% culture medium (v/v) for 2 hours. The absorbance was read at 570 nm, and the dose-response curves for reduction of cell viability were generated as percentage ratios of vehicle-treated controls.
Western blotting analysis
The treated cells were collected and lysed in RIPA buffer contained a protease inhibitor cocktail for 30 minutes followed by centrifugation at 15000 rpm for 30 minutes at 4 °C. After the collection of supernatants, the protein concentrations were detected. Clarified protein lysates (60–100 μg) were denatured in 2X loading buffer at 100 °C prior to adding into 8–16% SDS-PAGE. For non-denatured Western blotting analysis, non-reducing loading buffer were mixed with protein lysates prior to resolving it in non-denaturing gel (Biorad, CA). Proteins were transferred to nitrocellulose membranes, probed with indicated antibodies, and visualized by an enhanced chemiluminescence detection system. The western blotting bands were semi-quantified using Image J and adjusted for loading controls, β-actin or tubulin.
In vitro NEDD8 initiation conjugation assay
The NEDD8 initiation conjugation assay kit was purchased from Boston Biochem Inc. (Cambridge, MA). A master mix of 0.4 μM APP-BP1/UBA3 (NEDD8 ligase E1), 12.5 μM UbcH12 (NEDD8 ligase E2) and 62.5 μM NEDD8 were prepared in the reaction buffer (pH 8.0, 50 mM HEPES and 50 mM NaCl in final reaction) and distributed to individual tubes with a volume of 15 μl. A series of dilutions of Gartanin were made in DMSO. One microliter of Gartanin or DMSO was added to the indicated tubes and mixed well. The reactions were started by adding 2.5 mM Mg2+ and 1 mM ATP (4 μl in mixture), except the negative control tube was added by equal volume of ddH2O. The reaction tubes were incubated in 37 °C for 30 minutes and stopped by adding 5 μl 25 mM EDTA. Non-reducing western blot was performed with anti-Ubc12 antibody to detect both Ubc12 bands and NEDD8 conjugated Ubc12 bands.
Cell autophagy imaging
PC3 cell lines were stably transfected with pcDNA3-LC3-eGFP tagged vector. They were then treated for 24 hours with indicated concentration of Gartanin in a 24-well plate before imaging under a fluorescence microscope. Image J cell counter program was used to determine the number of fluorescence cells.
Molecular modeling studies
Molecular modeling was performed using the autodock 4 software from the Scripps Research Institute. Gartanin as first generated using Pymol software. Following generation, the files were converted to the. pdbqt format using Open Babel. Gartanin were then docked using AutoDockTools with NAE1 protein (PDB code: 3dbl) from MGLTools their resulting conformations were visualized using Pymol.
Statistical evaluation
Data were presented as means ± SD. Statistical analyses were performed by the analysis of variance (ANOVA). All statistical analyses were performed by SPSS 17.0 and Excel.
RESULTS
Gartanin inhibits in vitro neddylation.
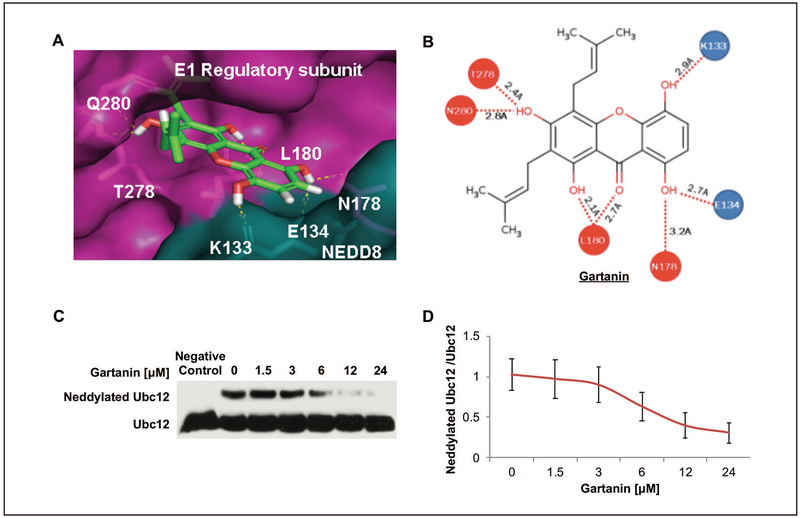
We have screened natural compounds for their inhibitory activity against NEDDylation and Gartanin was identified as a potent NEDDylation inhibitor (Data not shown). Autodock program was used to dock Gartanin with NAE1 protein (PDB code: 3dbl), which has a predicted IC50 value of 735 nM, and Pymol program was used to observe the superimposed binding of Gartanin to the regulatory subunit of NAE1 and next to NEDD8 (Fig. 1A). Gartanin was predicted to form multiple hydrogen bonds with the NAE1 regulatory subunit and with NEDD8 within the docking site (Fig. 1B).
Fig. 1. Gartanin inhibits NEDDylation in vitro.
A. Autodocktools program was used to dock Gartanin with NAE1 protein, which has a predicted IC50 of 735 nM, and Pymol program was used to observe the superimposed binding of Gartanin to NAE1. Gartanin was predicted to bind within the E1 regulatory subunit (Pink) of NAE1 and interacts with NEDD8 (Blue). B. 2D molecular structure of Gartanin is shown to form hydrogen bonds with the NAE1 regulatory subunit (Red) and NEDD8 (Blue) within the predicted binding site. C. In vitro Ubc12 NEDDylation initiation assay was performed. Negative control contains no addition of ATP. D. The ratio of neddylated Ubc12 compared with Ubc12 from above Western blotting analysis results.
Furthermore, in vitro Ubc12 NEDDylation initiation assay was performed by using Ubc12 antibody to detect the changes of NEDD8-conjugated Ubc12 bands by the indicated concentrations of Gartanin and negative control (with no addition of ATP) as shown in the Fig. 1.C. Densitometry analysis of the Western blotting results revealed a dose dependent decrease of Ubc12 NEDDylation by Gartanin treatment with an estimated IC50 value of ~10.33μM (Fig. 1D).
Gartanin inhibits in vivo NEDDylation in prostate cancer PC3 and 22Rv1 cells.
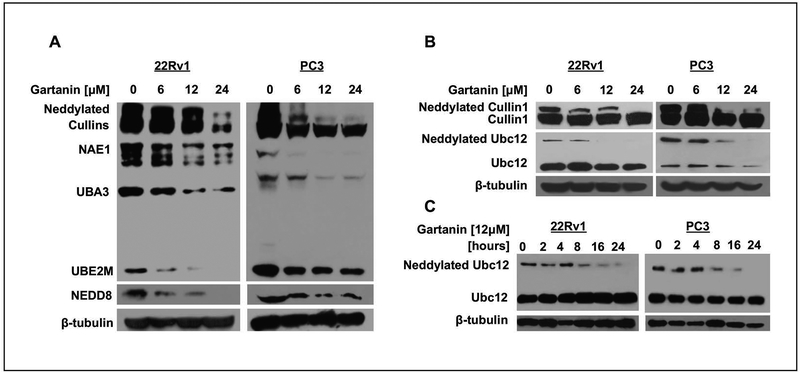
Western blotting analysis by anti-NEDD8 antibody shows that Gartanin inhibited the NEDDylation pathway by reducing the expression levels of NEDDylated Cullin1, NAE1, UBA3, and UBE2M, as well as protein levels of NEDD8 in a concentration-dependent manner in both 22Rv1 cells and PC3 cells (Fig. 2A). Using anti-Cullin1 and Ubc12 antibodies, we further confirmed that the NEDDylation of Cullin1 and Ubc12 was decreased by Gartanin treatment in a dose and time dependent manner (Fig. 2B and C), respectively. These results support that Gartainin is a cell-active NEDDylation inhibitor.
Fig. 2. Gartanin inhibits NEDDylation of Cullin1 and Ubc12 in prostate cancer PC3 and 22Rv1 cells.
A. The expression of neddylated proteins (Cullin1, Ubc12, NAE1, UBA3, UBE2M and NEDD8) in PC3 and 22Rv1 cells treated with vehicle control (0.1% DMSO) or indicated concentrations of Gartanin for 24 hours. B. Western blotting analysis of neddylated Cullin1 and Ubc12 protein levels in PC3 and 22 Rv1 cells treated with indicated concentrations of Gartanin for 24 hours. C. Western blotting analysis of neddylated Ubc12 protein levels after 22Rv1 cells were treated with 12 μM Gartanin for different times.
Gartanin induces proteasome dependent Skp2 degradation
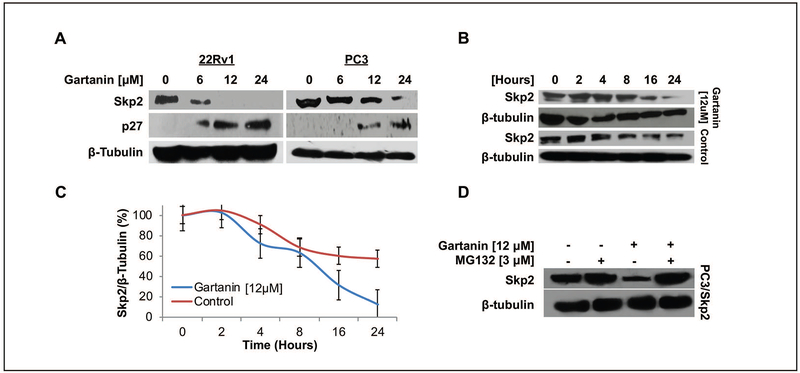
Skp2 is a critical oncogene and overexpressed in human prostate cancer [30]. Skp2 acts as a down-stream E3 ligase of the NEDDylation pathway for ubiquitination of tumor suppressors, such as CDK inhibitor p27/Kip1, for targeted degradation by proteasome [31]. We found that Gartanin treatment of 22Rv1 and PC3 cells for 24 hours dose-dependently down-regulated the protein expression of Skp2 and up-regulated the expression of p27/Kip1 in both 22Rv1 and PC3 cells (Fig. 3A).
Fig. 3. Gartanin induces Skp2 proteasome-dependent degradation.
A. The expression of Skp2 and p27 in PC3 and 22Rv1 cells after treatment with vehicle control (0.1% DMSO) or indicated concentrations of Gartanin for 24 hours. B. PC3 cells were treated with 10 μg/mL cyclohexamine for 8 hours to inhibit de novo protein synthesis, followed by 0.1% DMSO or 12 μM Gartanin treatment for 0, 2, 4, 8, 16, and 24 hours in order to follow up changes of Skp2 protein stability. C. Skp2 protein levels over time were semi-quantified by densitometry measurement of band densities using image J software. D. PC3/Skp2 cells were treated with 3 μM MG132 (proteasome inhibitor), 12 μM Gartanin, or a combination treatment of MG132 and Gartanin for 24 hours. Myc-Skp2 protein expression was observed under Western blotting analysis with anti Myc-tag antibody.
When PC3 cells were treated with 10 μg/mL cyclohexamine to inhibit de novo protein synthesis, Gartanin treatment at a concentration of 12μM accelerated the decrease in protein levels of Skp2 over time compared to control treatment, suggesting that Gartanin reduces the protein stability of Skp2 (Fig. 3B&C). MG132, which acts as a blocker in ubiquitin- proteasome pathway that is involved in >80% of intracellular protein degradation [32]. As shown in the Fig. 3D, 12μM Gartanin alone obviously downregulated the protein expression of Skp2 in PC3/Skp2 cells. However, the combination of 3μM MG132 and 12μM Gartanin reversed the effect of Gartanin on Skp2 down-regulation. This result suggests that Skp2 degradation by Gartanin was due to a proteasome-dependent manner.
The inhibitory effects of Gartanin on Skp2 protein expression at least in part require the expression of NEDD8 but not FBXW2, Cdh1 and β-TrCP1
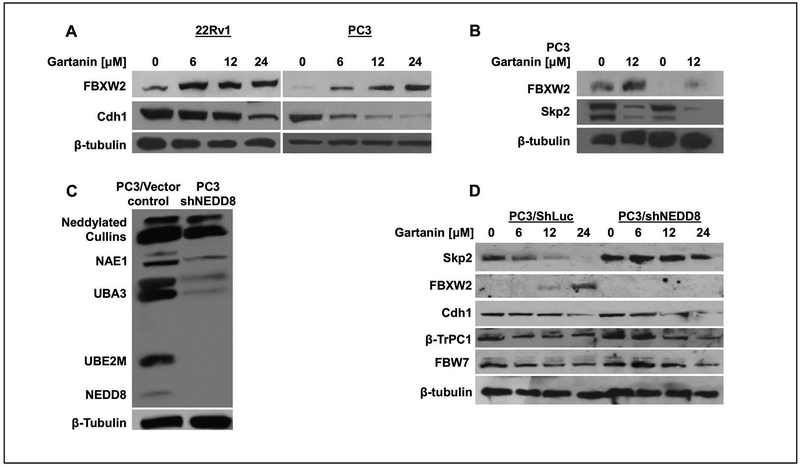
Several E3 ligases, such as FBXW2 and Cdh1, were reported to recognize Skp2 and cause Skp2 ubiquitination for targeted degradation by proteasome [33, 34].Fig.4A shows that Gartanin dose-dependently increased the protein expression of FBXW2 and decreased the expression of Cdh1 in both 22Rv1 and PC3 cells. However, siRNA knock-down of FBXW2 did not result in an increase in the expression Skp2 under Gartanin treatment (Fig.4B). These results suggest that Cdh1 and FBXW2 expression is not required for Gartanin induced Skp2 degradation.
Fig. 4. Gartanin induced Skp2 degradation at least in part requires the expression of NEDD8 protein.
A. The expression of E3 ligases FBXW2 and Cdh1 were analyzed by Western blotting after treatments with 0.1% DMSO or indicated concentrations of Gartanin for 24 hours in 22Rv1 and PC3 cells. B. PC3 cells were transfected with siRNA controls or FBXW2 siRNAs for 24 hours and then treated with 0.1% DMSO or 12 μM gartanin for additional 24 hours. Western blotting analysis of protein levels of FBXW2 and Skp2 is shown. C. Expression of NEDD8 and neddylated proteins was analyzed in PC3/ShLuc and PC3/shNEDD8 knockdown cells. D. The expression of E3 ligases, Skp2, FBXW2, Cdh1, FBW7, and β-TRCP, in PC3/ShLuc and PC3/shNEDD8 knockdown cells treated with 0.1%DMSO or indicated concentrations of Gartanin.
However, knocking-down the expression of NEDD8 by shRNAs decreased protein NEDDylation levels as detected by Western blotting analysis using anti-NEDD8 antibody (Fig. 4C), which completely blocked the Gartanin induced Skp2 protein degradation at concentrations of up to 12 μM (Fig. 4D). Knock-down of NEDD8 also blocked the effect of Gartanin on induction of FBXW2 expression, whereas other E3 ubiquitin ligases, Cdh1, FBW7 and β-TrCP were less or not affected by NEDD8 knock-down.
Taken together, our results indicate that Skp2 protein down-regulation is dependent on NEDD8 expression and that inhibition of neddylation byNEDD8 knock-down may regulate the protein expression or stability of specific groups of E3 ubiquitin ligases.
Growth inhibitory effects of Gartanin are associated with expression of NEDD8 and Skp2 expression but not with expression of FBXW2.
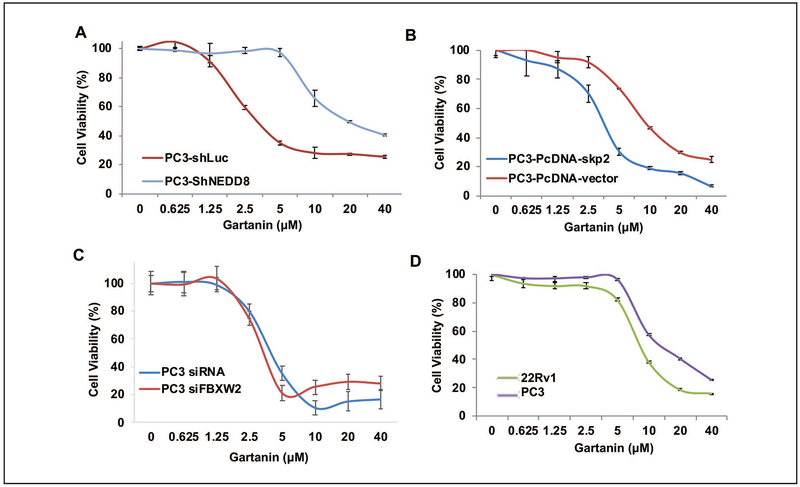
Fig. 5A demonstrates that NEDD8 knock-down also attenuated the growth inhibitory effect of Gartanin in PC3 cells. The IC50 values of Gartanin on the cell growth of PC3/vector control (ShLuc) and PC3/shNEDD8 cells were estimated to 4.91 ± 0.13 μM and 14.11 ± 0.16 μM (P<0.05), respectively. In addition, PC3 cells with stable overexpression of Skp2 are more sensitive to Gartanin treatment than those PC3 cells transfected with vector (PcDNA3) control with estimated IC50s of 3.55 ± 0.31 μM and 9.22 ± 0.10μM (P<0.05), respectively (Fig. 5B).
Fig. 5. The growth inhibitory effects of Gartanin is, at least in part dependent, on the expression of Skp2 and NEDD8.
Growth inhibitory effects of Gartanin in different cell lines as described below were examined by MTT assay after treatments with 0.1% DMSO or indicated concentrations of Gartanin for 72 hours. A. PC3/ShLuc and PC3/shNEDD8 knockdown cell lines, B. PC3/PcDNA3 vector control and PC3/Skp2 cell lines. C. PC3 cells after transfected with siRNA control and FBXW2 siRNAs for 24 hours. D. PC3 and 22Rv1 cell lines.
However, siRNA knock down of FBXW2 expression did not significantly change the growth inhibitory effects of Gartanin on PC3 cells (P>0.05) (Fig. 5C). The IC50 values of Gartanin against the growth of parental 22Rv1 and PC3 cells were estimated to be about 8.32 ± 0.18 μM and 13.56 ± 0.20 μM, respectively (Fig. 5D).
These results suggest that Gartanin target NEDDylation and Skp2 for its growth inhibitory effects on prostate cancer cell lines.
Gartanin induces autophagy in prostate cancer cells.
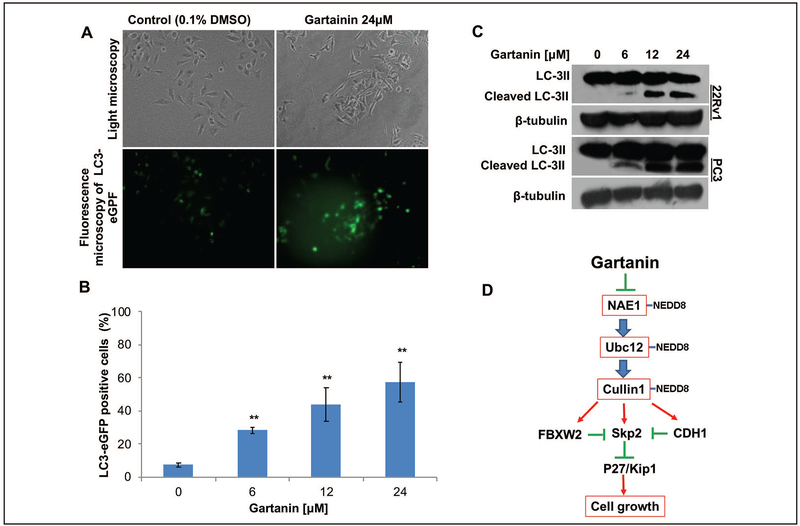
The autophagic effect of Gartanin on prostate cancer cells have not been reported yet. To determine whether Gartanin induces autophagy in prostate cancer cells, we established PC3 cell line with stable expression of pEGFP-LC3 to observe fluorescent LC3B protein. As shown in the Figs. 6A and B, the punctate staining of LC3B significantly increased after 24 hours of treatment with Gartanin (6μM, 12μM and 24μM) compared with vehicle control (0.1% DMSO) treatment. In addition, we observed accumulation of cleaved LC3 induced by Gartanin using Western Blotting analysis in 22Rv1 and PC3 cells (Fig. 6C), further confirming the initiation of autophagy by Gartanin.
Fig. 6. Gartanin induces autophagy.
A. PC3-LC3-eGFP cells were treated with 0.1% DMSO or indicated concentrations of Gartanin for 24 hours, and then observed under a fluorescence microscope. B. Quantitative analysis of cells with the punctate staining of LC3B after 24 hours of treatment with indicated concentrations of Gartanin compared with 0.1% DMSO treatment. “**” represents P < 0.01. C. 22Rv1 cells and PC3 cells were treated with indicated concentrations of Gartanin for 24 hours and LC3B antibody were used to detect LC3 cleavage. D. Proposed mechanisms of Gartanin’s action as a novel NEDDylation inhibitor.
In summary, the mechanism of Gartanin’s action can be simply depicted as in Fig. 6D. Gartanin act as a novel neddylation inhibitor for removing NEDD8 from Ubc12 and Cullin-1, which changes the cellular repertoire of specific SCF ubiquitin ligases, including Skp2, FBXW2, and others and eventually leads to cell growth inhibition and induction of autophagy.
DISCUSSION
Defects in the NEDD8 cycle leading to increased protein NEDDylation have been reported in several cancers, including lung adenocarcinomas, squamous-cell carcinoma, and hepatocellularcarcinoma, and thus the NEDDylation pathway has been considered as a major target for cancer intervention [19]. For the first time, we have provided evidence that Gartanin is a cell active NEDDylation inhibitor by inhibiting Ubc 12 and Cullin1 NEDDylation in vivo in prostate cancer cell lines and Ubc12 NEDDylation in vitro. Our results suggest that Gartanin is distinct from MLN-4294, a known NEDDylation inhibitor currently in clinical trials, which has been shown to increase the protein expression of the NEDD8 family of E3 ubiquitin ligases, such as Skp2 and FBXW2 [33]. In addition, MLN-4294 forms NEDD8-MLN-4294 adduct for inhibition of the NAE enzyme activity, whereas Gartanin is predicted to bind to the E1 regulatory subunit of the NAE.
The NEDD8 family of E3 ubiquitin ligases, such as Skp2, FBXW2, Cdh1, FBW7, and β-TrCP1, that act as down-stream events of the NEDDylation pathway, play a critical role in cell cycle regulation, cellular senescence, autophagy, apoptosis, metabolism, metastasis, and cancer stem cells by ubiquitinating a wide range of substrates for targeted degradation by the proteasome [33–37]. The gain or loss of function of these E3 ubiquitin ligases has been shown to contribute to tumorigenesis [33–37]. Therefore, these E3 ligases also represent promising targets for therapeutic manipulation [38]. The results from our experiments support the hypothesis that Gartanin is a novel NEDDylation inhibitor that can affect the equilibrium of cellular E3 ubiquitin ligase repertoire leading to its anti-proliferative effects. In particular, we have shown that both down-regulation of Skp2 expression and up-regulation of FBXW2 by Gartanin requires the expression of NEDD8 in prostate cancer cells (Fig. 4D).
Skp2 is a critical oncogene in human prostate cancer [39]. Skp2 was found to be overexpressed in high-grade prostatic intra-epithelial neoplasia lesions, primary, and metastatic prostate cancer [39]. In addition, Skp2 expression has been shown to be required for spontaneous tumor development that occurs in the pRb, the p19Arf and Pten deficient mouse models [40, 41]. More recently, multiple components of the SCFSKP Cullin F box containing complex including Skp2 has been identified to be candidates of highly penetrant, synthetic lethal interactions in RB defective triple negative breast cancer [42]. It is thus of import that new agents that attenuate Skp2 expression are discovered. We have demonstrated Gartanin, while acting primarily as a NEDDylation inhibitor, can selectively degrade Skp2 in prostate cancer cells.
FBXW2, a newly reported tumor suppressor, inhibits the growth, migration and invasion of lung cancer cell lines [33, 37]. FBXW2 functions as an E3 ligase to recognize Skp2 and β-catenin for targeted degradation [33, 37]. However, the function role and expression of FBXW2 in prostate cancer remains unknown and no reports discovered that natural products could affect FBXW2 expression in prostate cancer cells. In this work, Gartanin as a dietary xanthone from Garcinia mangostana L. increases the protein levels of FBXW2 in a concentration dependent manner against 22Rv1 and PC3 cells. However, the knock-down of FBXW2 did not attenuate the inhibitory effects of Gartanin on Skp2 expression and cell growth (Fig. 4B and Fig. 5C). Therefore, the contribution of FBXW2 expression in the anti-prostate cancer effects of Gartanin remains unclear and requires further investigation, which is beyond the scope of this manuscript.
In conclusion, many patients with prostate cancer are currently being over-treated and suffered from unnecessary adverse effects of treatments and loss of quality of life [2]. Dietary Gartanin, a polyphenolic xanthone that was isolated from the pericarp and whole fruit of mangosteen, has the potential of low toxicity. Dietary Gartanin may have a novel use as a NEDDylation inhibitor for selective degradation of oncogene Skp2 and up-regulation of tumor suppressor FBXW2 leading to induction of autophagy and growth inhibition of prostate cancer cells. Future studies should attempt to determine the in vivo efficacy and pharmacokinetics characteristics of dietary Gartanin for preventive intervention of prostate carcinogenesis in animal models.
Acknowledgement:
This work was supported in part by NIH award 1R01CA193967-01A1 and 1R21CA152804-01A1 (to X. Zi.). Victor Pham is currently supported by NSF graduate research fellowship program DGE-1321846.
Abbreviations:
- NEDD8
the precursor cell-expressed developmentally down-regulated 8
- NAE
NEDD8-activating enzyme
- Skp2
S phase kinase-associated protein
- ShRNAs
short harpin RNAs
- UBE2M or Ubc12
NEDD8-conjugating enzyme 2M
- FBXW2
F-box and WD-repeat domain-containing 2
Footnotes
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of Interest Disclosures: The authors declare no potential conflicts of interest.
References
- 1.https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html, accessed on September 30, 2019.
- 2.Sfanos KS, De Marzo AM: Prostate cancer and inflammation: the evidence. Histopathology 2012; 60:199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedraza-Chaverri J, Cárdenas-Rodríguez N, Orozco-Ibarra M, Pérez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana).Food Chem Toxicol. 2008;46:3227–39.3. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim SRM, Mohamed GA, Khayat MTA, Ahmed S, Abo-Haded H. α-Amylase inhibition of xanthones from Garcinia mangostana pericarps and their possible use for the treatment of diabetes with molecular docking studies. Food Biochem. 2019;43:e12844. [DOI] [PubMed] [Google Scholar]
- 5.Jung HA, Su BN, Keller WJ, Mehta RG, Kinghorn AD. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen).J Agric Food Chem. 2006;54:2077–82. [DOI] [PubMed] [Google Scholar]
- 6.Wang SN, Li Q, Jing MH, et al. Natural Xanthones from Garcinia mangostana with Multifunctional Activities for the Therapy of Alzheimer’s Disease. Neurochem Res. 2016;41:1806–17. [DOI] [PubMed] [Google Scholar]
- 7.Chin YW, Shin E, Hwang BY, Lee MK. Antifibrotic constituents from Garcinia mangostana. Nat Prod Commun. 2011;6:1267–8. [PubMed] [Google Scholar]
- 8.Suksamrarn S, Komutiban O, Ratananukul P, Chimnoi N, Lartpornmatulee N, Suksamrarn A. Cytotoxic prenylated xanthones from the young fruit of Garcinia mangostana.Chem Pharm Bull (Tokyo). 2006;54:301–5. [DOI] [PubMed] [Google Scholar]
- 9.Han AR, Kim JA, Lantvit DD, et al. Cytotoxic xanthone constituents of the stem bark of Garcinia mangostana (mangosteen).J Nat Prod. 2009;72:2028–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z, Antalek M, Nguyen L, et a. The effect of gartanin, a naturally occurring xanthone in mangosteen juice, on the mTOR pathway, autophagy, apoptosis, and the growth of human urinary bladder cancer cell lines. Nutrition and cancer 2013, 65 Suppl 1:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Z, Huang L, Chen XH, et al. Cytotoxic prenylated xanthones from the pericarps of Garcinia mangostana. Molecules. 2014;19:1820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MO, Lee HS, Chin YW, Moon DO, Ahn JS. Gartanin induces autophagy through JNK activation which extenuates caspase-dependent apoptosis. Oncol Rep. 2015;34:139–46. [DOI] [PubMed] [Google Scholar]
- 13.Li G, Petiwala SM, Yan M, Won JH, Petukhov PA, Johnson JJ.Gartanin, an isoprenylated xanthone from the mangosteen fruit (Garcinia mangostana), is an androgen receptor degradation enhancer. Mol Nutr Food Res. 2016; 60:1458–69. [DOI] [PubMed] [Google Scholar]
- 14.Luo M, Liu Q, He M, et al. Gartanin induces cell cycle arrest and autophagy and suppresses migration involving PI3K/Akt/mTOR and MAPK signalling pathway in human glioma cells. J Cell Mol Med. 2017; 21: 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vemu B, Nauman MC, Veenstra JP, Johnson JJ.Structure activity relationship of xanthones for inhibition of Cyclin Dependent Kinase 4 from mangosteen (Garcinia mangostana L.). Int J Nutr. 2019;4:38–45. [PMC free article] [PubMed] [Google Scholar]
- 16.Sarkaria I, O-charoenrat P, Talbot SG, Reddy PG, Ngai I, Maghami E, et al. Squamous cell carcinoma related oncogene/DCUN1D1 is highly conserved and activated by amplification in squamous cell carcinomas. Cancer Res. 2006; 66:9437–44. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Wang M, Yu G, et al. Overactivated neddylation pathway as a therapeutic target in lung cancer. J Natl Cancer Inst. 2014;106:dju083. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Huang WL, Xu QG, et al. Overactivated neddylation pathway in human hepatocellular carcinoma. Cancer Med. 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang ZH, Li J, Luo F, Wang YS. Clinical significance of SCCRO (DCUN1D1) in prostate cancer and its proliferation-inhibiting effect on Lncap cells. Eur Rev Med Pharmacol Sci. 2017; 21: 4283–4291. [PubMed] [Google Scholar]
- 20.Korzeniewski N, Hohenfellner M, Duensing S. CAND1 promotes PLK4-mediated centriole overduplication and is frequently disrupted in prostate cancer. Neoplasia. 2012;14:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choo YY, Boh BK, Lou JJ, et al. Characterization of the role of COP9 signalosome in regulating cullin E3 ubiquitin ligase activity. Mol Biol Cell. 2011; 22:4706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bornstein G, Ganoth D, Hershko A. Regulation of neddylation and deneddylation of cullin1 in SCFSkp2 ubiquitin ligase by F-box protein and substrate. Proc Natl Acad Sci U S A. 2006;103:11515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abidi N and Xirodimas DP. Regulation of cancer-related pathways by protein NEDDylation and strategies for the use of NEDD8 inhibitors in the clinic Endocr. Relat. Cancer 2015; 22: T55–T70. [DOI] [PubMed] [Google Scholar]
- 24.Shah JJ, Jakubowiak AJ, O’Connor OA, et al. Phase I study of the novel investigational NEDD8-activating enzyme inhibitor pevonedistat (MLN4924) in patients with relapsed/refractory multiple myeloma or lymphoma. Clin Cancer Res. 2016;22:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarantopoulos J, Shapiro GI, Cohen RB, et al. Phase I Study of the Investigational NEDD8-Activating Enzyme Inhibitor Pevonedistat (TAK-924/MLN4924) in Patients with Advanced Solid Tumors.Clin Cancer Res. 2016; 22: 847–57. [DOI] [PubMed] [Google Scholar]
- 26.Bhatia S, Pavlick AC, Boasberg P, et al. A phase I study of the investigational NEDD8-activating enzyme inhibitor pevonedistat (TAK-924/MLN4924) in patients with metastatic melanoma. Investig. New Drugs 2016; 34:439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swords RT, Coutre S, Maris MB, et al. Pevonedistat, a first-in-class NEDD8-activatingenzyme inhibitor, combined with azacitidine in patients with AML. Blood. 2018; 131: 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Yokoyama NN, Zhang S, et al. Flavokawain A induces deNEDDylation and Skp2 degradation leading to inhibition of tumorigenesis and cancer progression in the TRAMP transgenic mouse model. Oncotarget 2015;6:41809–41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Pham V, Tippin M, et al. Flavokawain B targets protein neddylation for enhancing the anti-prostate cancer effect of Bortezomib via Skp2 degradation. Cell Commun Signal. 2019;17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao H, Bauzon F, Fu H, et al. Skp2 deletion unmasks a p27 safeguard that blocks tumorigenesis in the absence of pRb and p53 tumor suppressors. Cancer Cell. 2013; 24: 645–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nature Cell Biology 1999, 1:193. [DOI] [PubMed] [Google Scholar]
- 32.Dang L, Wen F, Yang Y, et al. Proteasome inhibitor MG132 inhibits the proliferation and promotes the cisplatin-induced apoptosis of human esophageal squamous cell carcinoma cells. International journal of molecular medicine 2014, 33:1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu J, Zhou W, Yang F, et al. The β-TrCP-FBXW2-SKP2 axis regulates lung cancer cell growth with FBXW2 acting as a tumour suppressor. Nat Commun. 2017; 8:14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004; 428: 190–3. [DOI] [PubMed] [Google Scholar]
- 35.Kitagawa K, Kitagawa M. The SCF-type E3 Ubiquitin Ligases as Cancer Targets. Curr Cancer Drug Targets. 2016;16:119–29. [DOI] [PubMed] [Google Scholar]
- 36.Zheng N, Zhou Q, Wang Z, Wei W.Recent advances in SCF ubiquitin ligase complex: Clinical implications. Biochim Biophys Acta. 2016; 1866: 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang F, Xu J, Li H, Tan M, Xiong X, Sun Y. FBXW2 suppresses migration and invasion of lung cancer cells via promoting β-catenin ubiquitylation and degradation. Nat Commun. 2019; 10:1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chamberlain PP, Hamann LG.Development of targeted protein degradation therapeutics. Nat Chem Biol. 2019;15: 937–944. [DOI] [PubMed] [Google Scholar]
- 39.Drobnjak M, Melamed J, Taneja S, et al. Altered expression of p27 and Skp2 proteins in prostate cancer of African-American patients. Clin Cancer Res. 2003; 9: 2613–9. [PubMed] [Google Scholar]
- 40.Lin HK, Chen Z, Wang G, et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010; 464: 374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao H, Bauzon F, Fu H, et al. Skp2 deletion unmasks a p27 safeguard that blocks tumorigenesis in the absence of pRb and p53 tumor suppressors. Cancer Cell. 2013; 24: 645–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brough R, Gulati A, Haider S, et al. Identification of highly penetrant Rb-related synthetic lethal interactions in triple negative breast cancer. Oncogene. 2018; 37: 5701–5718. [DOI] [PMC free article] [PubMed] [Google Scholar]