Abstract
Schizophrenia has been studied from the perspective of cognitive- or reward-related impairments, yet it cannot be wholly related to one or the other process and corresponding neural circuits. We posit a comprehensive circuit-based model proposing that dysfunctional interactions between the brain’s cognitive and reward circuits underlie schizophrenia. The model is underpinned by how the relationship between glutamatergic and dopaminergic dysfunction in schizophrenia drives interactions between cognition and reward circuits. We argue that this interaction is synergistic: that is, deficits of cognition and reward processing interact, and this interaction is a core feature of schizophrenia. In adopting this position, we undertake a focused review of animal physiology and human clinical data, and in proposing this synergistic model, we highlight dopaminergic afferents from the ventral tegmental area to nucleus accumbens (mesolimbic circuit) and frontal cortex (mesocortical circuit). We then expand on the role of glutamatergic inputs to these dopamine circuits, and dopaminergic modulation of critical excitatory pathways, with attention given to the role of glutamatergic hippocampal outputs onto nucleus accumbens. Finally, we present evidence for how in schizophrenia, dysfunction in the mesolimbic and mesocortical circuits, and their corresponding glutamatergic inputs gives rise to clinical and cognitive phenotypes, and is associated with positive and negative symptom dimensions. The synthesis attempted here provides an impetus for a conceptual shift that links cognitive and motivational aspects of schizophrenia and which can lead to treatment approaches that seek to harmonize network interactions between the brain’s cognition and reward circuits with ameliorative effects in each behavioral domain.
Keywords: Schizophrenia, circuits, reward, cognition, translation, genetics
Overview
The definitions and boundaries of what clinicians generally recognize as “schizophrenia” have evolved since its origins. In anticipating this (1), Bleuler accepted schizophrenia not as a unitary disease but an “umbrella” term representing a constellation of behavioral symptoms (2). Yet, positive (hallucinations, delusions and thought disorder), and negative symptoms (poverty of speech and thought (3, 4)) remain the most widely accepted and salient clinical and observational characteristics of schizophrenia. Bleuler’s work pre-dated modern neuroscience, and he remained agnostic about whether the core pathophysiology of schizophrenia was tractable or valuable to understand (5). He advocated for a focus on behavior over biology. However, semantic distinctions between behavior and biology have dissipated (6), and the search for biological mechanisms in terms of impaired functional brain circuits is central to understanding core characteristics of schizophrenia (7, 8).
Higher order neurocognitive domains (and corresponding brain circuits) are of traditional interest in schizophrenia; dysfunction is evident across a range of domains, with no aspect of function left intact. Studies have centered on: a) working memory, attention, and executive functioning, and their relationship to frontal-striatal circuits (9–12); and b) episodic and associative memory and learning and the relationship to frontal-hippocampal interactions, and synaptic dys-plasticity (13–19). Dys-functional dopamine (DA) and N-methyl-D-aspartate (NMDA) interactions in the prefrontal cortex, and altered glutamatergic neurotransmission in the hippocampus may underpin many of the neurocognitive deficits that characterize the illness (20), and animal models (e.g., 22q11.2 deletion, see below) show evidence of deficits in hippocampal neuronal function during memory-related processing (21). However, schizophrenia is also laced with motivational and salience deficits, assumed to stem in part from aberrant reward processing (22, 23). The relevance of reward-related deficits is most clearly shown in studies of reinforcement learning. Patients (particularly with pervasive negative symptoms), fail to represent the expected value of rewards. This failure results in impaired learning in the context of gains (but intact learning in the context of loss-avoidance) (24, 25), as patients appear not to make high-effort response choices in the service of maximizing reward (26). As response choice is closely associated with frontal and cingulate regions (27), impaired executive function in schizophrenia impacts reward sensitivity. A separate literature (reviewed below) links positive symptoms and abnormal salience with distorted representation of stimulus value, resulting in detrimental effects on cognitive integrity (28).
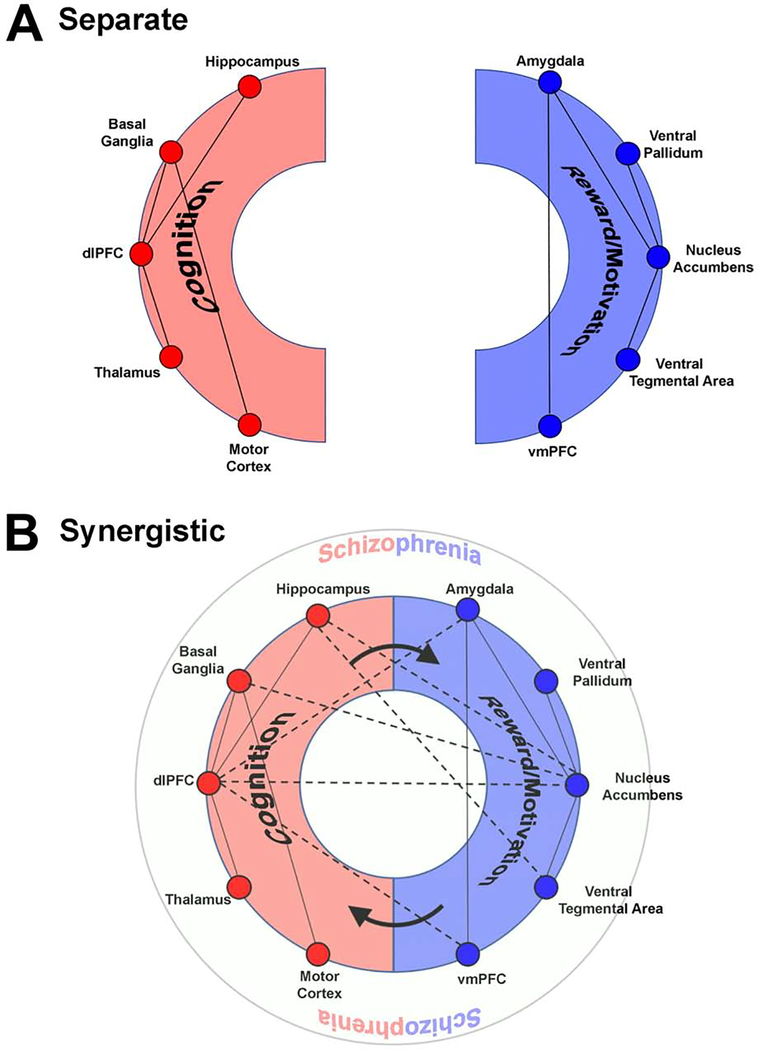
The corpus of empirical data is vast, but the field can benefit from an integrative framework which can distill how dys-functional interactions between distinct sets of sub-networks for cognition and reward interact to amplify the broad impairments, and the associated “infinite regress” of dysfunctional neuronal interactions characterizing schizophrenia (29). We argue that such amplification makes schizophrenia particularly insidious, resulting in well-known challenges to remediation. Our translational, circuit-based framework highlights the synergistic relationship between the brain’s cognitive and reward circuits, and their dopaminergic and glutamatergic innervations (30, 31). Figure 1 provides an initial schematic overview.
Figure 1: A synergistic interaction between cognition-related and reward/motivation related sub-networks underlies schizophrenia.
(a) Traditional approaches focus on dysfunction within circuits sub-serving cognitive processing including working memory, learning, executive and motor function. These include regions (not restricted to) the hippocampus, basal ganglia, the dorsolateral prefrontal cortex, the thalamus and regions of the motor cortex. Dys-functional interactions between these components explain many of the cognitive deficits in schizophrenia. Complementary deficits in reward/motivation circuits are presumed to underlie aberrant salience, anhedonia, impaired reward sensitivity and amotivation. These are driven by dysfunctional interactions between regions including the amygdala, ventral pallidum, nucleus accumbens, ventral tegmental area and the ventro-medial prefrontal cortex. (b) However, synergistic deficits between cognitive and reward/motivation circuits may be key to schizophrenia. In this view, dysfunction between cognitive circuits and reward/motivation circuits amplify deficits in cognition, which in parallel drive impaired reward/motivation. This interaction leads to the emergence of the complex phenotype that is schizophrenia (represented as the outer circle). The figure is a schematic depiction wherein the “connections” represent functional interactions between regions that may (or may not) exist in a one-to-one relationship with anatomical connections. Indeed, in presenting it in this way we acknowledge that the precise dialectic between brain structure (anatomy) and emergent function remains unresolved (171).
From the framework in Figure 1, we address the following: a) Is the interaction between cognitive deficits and motivational/hedonic dysfunction necessary and sufficient to explain core characteristics of schizophrenia?; b) by corollary, how do dys-functional interactions between the brain’s cognitive and reward circuits amplify impairment in the illness?; c) Is the complex relationship between negative and positive symptoms (32) related to dys-functional interactions between cognition and reward, or does this framework simply account for negative syndrome schizophrenia (33)?
Negative symptoms, reward processing and cognition
Altered reward processing in schizophrenia is associated with enduring and pervasive negative symptoms afflicting a significant portion of patients (34), and they are associated with bleaker outcomes and greater treatment resistance (35). Aberrant reward processing is assumed to be at the core of the major negative symptoms of avolition and amotivation (36). Although hedonic responses are frequently intact (37, 38), individuals with negative symptoms show alterations in reinforcement learning (39–41), reward anticipation (39), representing values (42), exploratory behavior (43), and effort allocation (44). Negative symptoms are also associated with cognitive deficits (45–47), with both bearing on real-world functioning, onset, and illness course.
Cross-sectional correlations between negative symptoms and cognitive deficits suggest that they are the same construct, or at least share a common etiology. However, a likelier explanation given extant data and neurobiology, is that they are independent constructs but with related etiologies (48). We suggest that these related etiologies are in fact dysfunctions between cognitive and reward circuits. By implication, impaired cognition in the illness undermines reward sensitivity, because cognitive success is inherently yoked to the internal motivational drive. Concurrently, a loss of internal motivational drive undermines cognition. There is no seriality to this model (49). Rather the loss of synergy drives schizophrenia, in the way that working memory deficits impair the generation and maintenance of value representations (50–52) and impaired reward processing reduces effort allocation in cognitively demanding situations (53).
Positive symptoms, salience and value sensitive for reward
Understanding relationships between positive and negative symptoms in schizophrenia is a foundational challenge (54). Though frequently co-observed (55), they represent distinct dimensions in illness etiology (32), with neither particularly predictive of the emergence of the other over time (56). Although the link between impaired cognition and reward systems is more naturally associated with negative symptom dimensions, recent evidence suggests that aberrant salience is related to distorted reward attribution/processing. We outline the evidence and the logic below.
Aberrant salience in schizophrenia is hypothesized to result from dysregulated dopaminergic signaling in the mesolimbic system (57, 58). Specifically, dysregulated dopaminergic signaling increases attribution of salience to external stimuli leading to the emergence of the classic positive symptoms of delusions and hallucinations (59). Stimulus novelty drives salience because novelty is intrinsically rewarding (60), and motivational salience which is linked with the search for novelty, is an adaptive feature of the primate brain linked to basic hedonic drives (61). This inextricable link between salience and reward has been discussed in classic reviews, highlighting how dopamine neurons in the ventral striatum signal anticipatory reward (62). Furthermore, in direct support of our central thesis, mounting evidence suggests that both prefrontal neurons (63) and neurons in the dorsal NAc are modulated during working memory processing (64), evidence that units in the ventral striatum are tuned to cognitive tasks.
The primate reward system attributes value to exogenous stimuli, the consequences of overt behaviors, and/or the experience of endogenous states (65, 66). Thus, incentive-based learning is associated with accurate representation of the value associated with achieving goals intrinsic to a task. Accurate representation of value that drives successful goal-directed behavior is impaired in schizophrenia (67), particularly associated with positive symptoms in a recent study (68), which replicated findings of low value sensitivity in schizophrenia, and its association with increases in self-reported aberrant salience (69). Moreover, low value sensitivity (emerging from aberrant salience) was strongly associated with impaired cognitive performance. Finally (28), patients have increased preference for novel images, a preference both correlated with hallucination severity and which interfered with task performance. From a mechanistic perspective, aberrant salience affects the reward system by distorting value sensitivity. This distortion is associated with the genesis of positive symptoms, suggesting a compelling link between positive symptoms and dysfunctional reward sensitivity.
We next present a sampling of animal studies outlining the reward circuitry and interactions with cognitive circuits (providing a physiological basis for our framework).
Overview of Reward Circuitry
The reward circuitry is centered upon the release of dopamine (DA) from ventral tegmental area (VTA) neurons into limbic brain regions that control processing of rewarding stimuli, with additional regions/connections (70). Reward processing includes perception and prediction, and association with context and cues (71). VTA DA neurons have two major projections: the mesocortical pathway to the prefrontal cortex (PFC) and the mesolimbic pathway to the nucleus accumbens (NAc). However, they also project to the hippocampus, amygdala, and several other forebrain regions (72). VTA DA release into cortex is important for emotional responses and cognition (73), while DA release in NAc is traditionally linked to reward and motivated behaviors (74). The primary DA responsive cells of the NAc are medium spiny neurons (MSNs), and GABAergic cells that comprise two largely separate populations predominantly expressing either D1 or D2 DA receptors (DRs) (75, 76). Although these populations in the dorsal striatum are largely distinct in their projections, NAc MSN projections are more heterogeneous. The NAc MSNs expressing the D1 DR (D1 MSNs) are generally equated with the “direct” pathway, projecting to several regions critical for processing motivation including VTA, ventral pallidum (VP), and lateral hypothalamus. By comparison, the D2 MSNs are more strongly represented in the “indirect” pathway and appear to project exclusively to VP (77).
Although outputs of NAc D1 and D2 MSNs are not as categorically distinct as those of the dorsal striatum, they retain differential and largely opposing roles in reward processing. D1 neuron activation is associated with increased motivation while D2 neuron activity generally reduces motivation (78–82). Because DA signaling at D1 DRs increases the excitability of neurons but D2 DR activity decreases excitability, DA release in NAc tilts the balance toward the direct pathway, with the combined effect of increased motivation and reward.
Dopaminergic inputs moderate the responsiveness of NAc MSNs to multiple glutamatergic inputs that drive behavioral reinforcement. Thus, the NAc acts as a central processor for glutamatergic activity generated by multiple structures including the PFC, amygdala, hippocampus, and thalamus (83, 84). Glutamatergic inputs from ventral hippocampus (vHPC) may relay information regarding previous experiences as well as emotional states linked to context, including both aversion-based learning like context-dependent fear conditioning, and motivated behaviors like feeding and responses to drugs (85–87). PFC inputs provide executive information for planning motivated behaviors, such as seeking and acquiring food, drugs, sex, or social reward (88, 89). While the amygdala generally regulates fear-related learning and behavior, glutamatergic inputs from the amygdala onto NAc MSNs drive reward seeking and positive reinforcement (90–92).
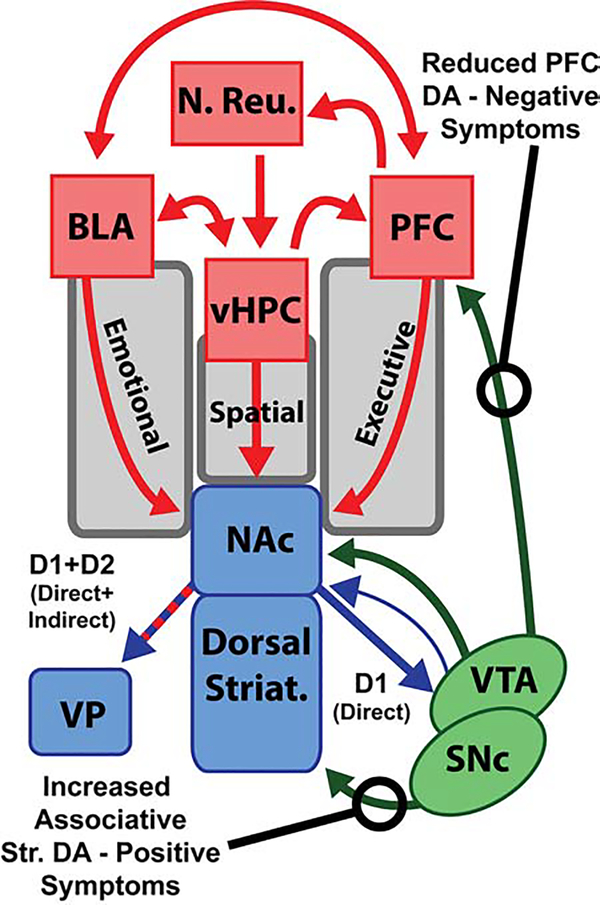
NAc MSNs send and receive GABAergic projections to/from the VTA, and many of the regions that send glutamatergic projections to NAc also share mutual projections. Figure 2 provides a mechanistic instantiation of the general framework (Figure 1). As seen, this reward network is complex with multiple internal circuits capable of positive and negative feedback (83, 84). Dysfunction of the basal ganglia reward network is ubiquitous in neurological and psychiatric conditions (93). In schizophrenia, dysfunction of dopaminergic signaling in the striatum, glutamatergic circuits in cortex and hippocampus, and integration of the entire reward network have been implicated by human imaging studies, pharmacology, and preclinical models. Next, we review how these disparate sources paint a synergistic picture of network dysfunction in the illness.
Figure 2: Schematic of brain reward circuitry and dysfunction in schizophrenia.
Dopaminergic (green) and glutamatergic (red) inputs converge on Y-aminobutyric acid (GABA)ergic (blue) medium spiny neurons in the nucleus accumbens (NAc). These inputs coordinate and regulate direct and indirect outputs that differentially contribute to reward-related behaviors. In schizophrenia, DA signalling is reduced in the cortex, in part driving negative symptoms. However, DA is increased in striatum, particularly in the dorsal associative striatum, contributing to the positive symptoms of schizophrenia. BLA = basolateral amygdala; D1 = dopamine type 1 receptor; D2 = dopamine type 2 receptor; PFC = prefrontal cortex; vHPC = ventral hippocampus; VP = ventral pallidum; VTA = ventral tegmental area.
Preclinical Studies relating to Schizophrenia Etiology
The symptoms of schizophrenia involve complex behaviors, therefore modeling the disease (as opposed to intermediate phenotypes) is difficult. Moreover, schizophrenia has a complex genetic basis; a simple genetic knockout or single animal model cannot encompass its symptoms. Nevertheless, the mouse model is a well-vetted compromise between sufficient complexity for behavioral analogy to schizophrenia, genetic tractability, and feasibility for high-throughput molecular studies (94, 95). Dozens of genetically altered mouse lines model one or more intermediate phenotypes (96, 97); here we restrict evidence to the most well-studied models demonstrating glutamatergic and dopaminergic synergistic molecular and behavioral phenotypes.
The most well-studied genetic mechanism of schizophrenia is DISC1. DISC1, dysfunction of which was originally linked families with high rates of schizophrenia (98), is a scaffolding protein with multiple protein-protein interaction domains allowing it to play structural and signaling roles in the nucleus, at mitochondria, and at synapses (99). DISC1 interacts with multiple proteins downstream of dopamine receptor signaling, thus controlling dopamine effects on neuronal function (100–102). Moreover, DISCI mutant mice have increased D2 dopamine receptor expression in the striatum (103), mimicking increased D2 signaling seen in the striatum of patients. Critically, DISCI also regulates the formation and function of glutamate synapses (104, 105). This evidence suggests that DISC1 disruption may synergistically drive dysfunction of both glutamate and dopamine signaling. Because mice with DISC1 mutations display deficits in pre-pulse inhibition that are reversed by antipsychotic treatment (106), uncoupled DISC1 regulation of dopamine and glutamate signaling could play a key role in schizophrenia.
Recurrent 22q 11.2 deletion is another promising neurogenic model of schizophrenia. 22q11.2 deletion syndrome (22q11DS) is caused by an autosomal dominant microdeletion within the 22nd chromosome, and ~25% of 22q11DS patients have schizophrenia (107). The deleted region contains genes including catechol-O-methyltransferase (COMT), a critical enzyme for dopamine catabolism (108) and proline dehydrogenase (PRODH), which degrades the amino acid proline (109), both of which have been independently linked to schizophrenia. Proline can bi-directionally regulate glutamate transmission (110, 111), and PRODH knockout mice have decreased glutamate biosynthesis and reduced sensorimotor gating, measured by pre pulse inhibition (112). A multi-gene deletion mouse model for 22q11DS mimics many of the anatomical and behavioral abnormalities of humans with this syndrome (113), indicating the causal nature of this mutation in schizophrenia symptoms and a role for both dopaminergic and glutamatergic dysfunction.
Schizophrenia has been directly associated with reduced hippocampal volume and hippocampal asymmetry in patients (114). Consistent with this, many mouse models display an immature dentate gyrus (iDG), where a preponderance of neurons are arrested in a pseudo-immature state at the gene expression, morphological, and functional levels (115). Critically, many of the mutations leading to this state in mice are associated with schizophrenia in humans. Multiple mutant mice with the iDG phenotype also display behaviors associated with schizophrenia, including hyperactivity and deficits in working memory. For instance, mice with reduced expression of calcium/calmodulin-dependent protein kinase II alpha (CaMKIIα) display iDG, with reduced hippocampal cell proliferation, and have multiple deficits in spatial learning, working memory, and hippocampal synaptic plasticity (116, 117). Functionally, CaMKIIα is critical for glutamatergic synaptic structure and function, primarily through regulating the insertion and function of AMPA receptors (118, 119), being implicated in a variety of neuropsychiatric disorders (120). Other genes are associated with iDG, including SNAP-25, a critical mediator of glutamate and dopamine synaptic vesicle fusion that is itself linked to schizophrenia (121), and calciuneurin, a protein phosphatase important for postsynaptic signaling at both glutamate and dopamine synapses also tied to the illness (122, 123). Although studies of iDG mouse models have predominantly focused on dorsal hippocampus and attendant memory phenotypes, ventral hippocampus (vHPC) is also dysfunctional in iDG models (115), and is likely to drive dysfunction of glutamatergic vHPC outputs to NAc (vHPC-NAc) critical for reward processing.
Dopamine, Glutamate and Schizophrenia
The dopamine and glutamate hypotheses are the leading pathophysiological accounts of schizophrenia. Both were formulated based on the psycho-mimetic effects of dopamine agonists (124) and N-methyl-D-aspartate (NMDA) receptor antagonists (125). The dopamine hypothesis garnered further clinical support because affinity for dopamine receptors was related to the effectiveness of antipsychotic drugs (126, 127), later bolstered by post-mortem studies (128, 129), human molecular imaging (reviewed in 130), and preclinical evidence (98, 131) of increased striatal D2/3 activation and baseline striatal dopamine. Furthermore, dopamine dysregulation is already present in the prodromal phase (reviewed in 132), meaning that it is not secondary to antipsychotic use or the psychosocial effects of illness chronicity.
The glutamate hypothesis of schizophrenia has been refined to posit a primary role of NMDA receptor hypofunction (133–135). In preclinical models, NMDAR blockade leads to a (somewhat counterintuitive) increase in glutamate release (136) and pyramidal neuron spiking (137), potentially via NMDAR hypofunction on fast- spiking gamma-aminobutyric acidergic (GABAergic) interneurons that regulate pyramidal neuron activity (138). NMDAR antagonists preferentially reduce the firing rate of these interneurons, leading to disinhibition of pyramidal neurons (139, 140). The clinical effects of NMDA receptor antagonists in healthy individuals mirror the full clinical picture of schizophrenia (including both negative and cognitive symptoms), more accurately than dopamine agonists (141). Furthermore, there is (equivocal) evidence for the clinical effectiveness of glutamatergic drugs; however, effects are modest, and it is unclear if effectiveness is directly related to a glutamatergic mechanism (reviewed in 142).
Post-mortem (143–145) and genetic studies (reviewed in 146) provide further support for the glutamate hypothesis, and proton magnetic resonance spectroscopy studies (1H-MRS) indicate altered levels of cortical and subcortical glutamate in high-risk and patient populations across brain regions relevant to reward processing (145). The glutamate hypothesis would predict reductions in GABA concentrations, but recent meta-analyses do not provide support for a significant reduction in schizophrenia (147, 148). Methodological issues may partially explain the mixed results (149, 150). In addition, limitations to MRS are relevant: Extracellular and intracellular metabolite concentrations cannot be teased apart; nor can MRS distinguish between GABA neuron subtypes (of which only the parvalbumin basket cell class is thought to be disrupted in schizophrenia).
The dopamine and glutamate hypotheses of schizophrenia are not mutually exclusive. Loss of glutamatergic projections from prefrontal cortex to midbrain dopaminergic neurons due to NMDAR hypofunction will produce decreased mesocortical DA but increased mesolimbic DA release, particularly under conditions of stress (151). Furthermore, enhanced hippocampal projections onto the NAc, likely secondary to decreased functioning of GABAergic interneurons (152), lead to enhanced inhibitory signaling onto the ventral pallidum. This in turn leads to reduced inhibition of the VTA, leading to increased dopamine release (153).
Neural circuit abnormalities underlying cognitive deficits, reward processing abnormalities and clinical symptoms
Negative symptoms, cognitive deficits, and reward processing abnormalities have traditionally been associated with impaired function of and between prefrontal areas and the basal ganglia (154). Emerging in vivo rsfMRI studies suggest that negative symptoms predict reduced spontaneous synchrony between the midbrain and prefrontal cortex (155), a Reward - Cognition pathway also sensitive to antipsychotic treatment (156).
Evidence from clinical and preclinical work additionally suggests that alterations in dopaminergic signaling due to vHPC abnormalities may explain both the psychotic and motivational symptoms of the illness. More specifically, abnormalities within the vHipp-NAc-VTA loop contribute to positive psychotic symptoms by leading to the abnormal generation and processing of novelty signals (157). This abnormality is central to several psychological accounts of psychotic symptoms that characterize acute stages of the illness. More specifically, psychosis may emerge due to reduced influence of statistical regularities stored in long-term memory on perception (158, 159). This results in undue salience and meaning being ascribed to irrelevant aspects of the environment (160). As noted earlier, aberrant salience subsequently engenders abnormal perceptions (hallucinations) and beliefs (delusions). Mechanistically, vHPC acts as a novelty detector, driving GABAergic neurons in the NAc that inhibit ventral pallidum, thus disinhibiting midbrain dopaminergic neurons (161, 162). In psychosis, resting vHPC activity may cause increased dopaminergic activity, resulting in inappropriate salience attributed to meaningless environmental stimuli (153).
Abnormal hippocampal afferents to the NAc also contribute to affective and motivational symptoms of schizophrenia. More specifically, acute stress leads to vHPC hyper-activity, which in turn leads to increased dopaminergic gain by way of NAc and ventral pallidum (163). Hyper-dopaminergia in response to acute stress leads to a relatively long-lasting decrease in dopaminergic gain, via increased activity of the infralimbic PFC (164, 165) homologue of the human sub-genual cingulate (166). This hypo-dopaminergic state has been associated with depressive phenotypes in rodent models. In support of this claim, glutamatergic transmission in the NAc is increased in a mouse model of chronic stress-induced depression (167–169), with a specific role for the vHPC-NAc projections in depressive behavior (170). Human neuroimaging work has revealed abnormal responses to rewarding stimuli in the NAc in clinical depression (171), and deep brain stimulation to the NAc has therapeutic effects in individuals with treatment-resistant depression (172), via inhibition of paralimbic cortical areas (173). Although negative syndrome schizophrenia and clinical depression are clinically similar with both characterized by abnormal reward processing and blunted NAc responses during reward anticipation (reviewed in 174), it unclear whether fully share pathophysiological mechanisms.
Toward a synergistic re-conception
The breath of the literature is impossible to review, but interactions between cognitive and reward pathways are unquestionably synergistic, and, as with all processing in the brain (175), contextually modulated. Schizophrenia is characterized by a loss of integrity of both cognition and reward circuits (176), resulting in a loss of a natural synergy that is necessary for healthy behavioral function. This loss, stems from both glutamatergic and dopaminergic dysregulation, fundamentally distorting behavioral processing in the illness, and resulting in global cognitive impairment (177) associated with foundational dys-connection (178) and aberrant cortical reorganization (132, 179).
Human behavioral function can be experimentally cleaved into cognitive or reward related processing. For example, human studies of reward processing typically study reward contingencies (e.g., delayed discounting) or reward choices (180). Such tasks are important probes for inducing experimentally controlled effects in the brain’s reward circuits or regions. In naturalistic settings, however, the balance between reward (or hedonic drives) or salience, and cognitive/control mechanisms is bi-directional and incessantly changing (181). Thus, even in the absence of explicit reward contingencies, judgments of implicit reward relate to a balance between task difficulty and individual ability, predicting increases in functional connectivity between reward structures and cognitive networks (182). These effects are associated with intrinsic motivational states and traits for autonomy and competence, attributes that underpin self-determination theory (183). In principle, intrinsic motivation is itself an inherently rewarding process that modulates, and is itself reinforced by, cognitive activity (184). This principle provides a measure of understanding how a loss of intrinsic motivation coupled with a loss of integrity of cognitive circuits forms a concurrent “hit” in schizophrenia. The subsequent loss of a fundamental synergy underlying typical human behavior exerts highly negative impacts on the course of the illness. Conversely, as previously discussed, in schizophrenia abnormal salience alters value sensitivity and the perception of novelty, both of which is related to core aspects of reward processing. Abnormal salience is associated with deficits in core cognitive domains (185), as well as phenomenological alterations in self processing (186), that ultimately undermine cognition (187).
Schizophrenia is a constellation of aberrant behaviors, and modern neuroscience is built around integrative and synergistic models of normative behavior. These models address how brain circuits and regions, with relative degrees of specialization, integrate outputs in the service of complex functions (188). A complex illness like schizophrenia arises from a synergistic dysregulation of reward and cognition circuits, not because there is a simple causative relationship between the two, but rather because there are sustained bi-directional effects by which each sub-network class continually undermines the other. To re-emphasize this point, we revert to Figure 1, where rather than characterizing schizophrenia as related to deficits in cognitive or reward processing circuits (Figure 1a), we suggest it more meaningful to characterize it as an emergent property of their interaction (Figure 1b).
We imagine that this framework can redirect the search for novel molecular, cellular, and behavioral targets for therapeutic or prophylactic intervention. For example, the centrality of cognition-reward interactions is implicit in a prominent cognitive model of negative symptoms used to guide behavioral interventions (189, 190). It is proposed that cognitive impairments result in underperformance and discouraging experiences, which in turn result in the development of defeatist beliefs (overgeneralized negative expectations about one’s abilities). Defeatist beliefs lead to decreased deployment of cognitive effort in the face of potential rewards (191) and a reduction in the pursuance of goal-directed behavior, which manifests clinically as negative symptoms. Defeatist beliefs, of which interactions between cognitive and reward processes are arguably key, are promising treatment targets of psychosocial interventions aimed at reducing historically intractable negative symptoms and functional deficits (192, 193). In addition, these defeatist beliefs emerge prior to formal illness onset (194), further intimating their relevance for negative symptom development, and possible importance in prevention efforts. In this way, re-conceptualizing negative symptoms as arising from abnormal cognition-reward interactions may have real and immediate implications for treatment development.
This framework also motivates obvious shifts in mechanistic and theoretical models of schizophrenia (as we attempt to provide in Figure 2), and therefore experimental approaches toward understanding its biology. For instance, evidence that a core memory region (the hippocampus) evinces spontaneous intrinsic connectivity with regions such as the NAc and the VTA (195), implies nascent task-free connectivity between cognitive and reward systems in the human brain. It will be imperative to understand how such intrinsic connectivity is contextually modulated during tasks with explicit and/or implicit reward processing, and whether schizophrenia is characterized by dys-modulation of connectivity between reward and cognition networks.
Acknowledgements
AJR acknowledges support from the National Institutes of Mental Health (MH111604), the National Institutes of Neurological Disease and Stroke (NS085171), the National Institutes of Drug Abuse (DA040621, and DA040621-03S1) and the Avielle Foundation. KT acknowledges support from the National Institutes of Mental Health (MH112644, MH115297). VAD acknowledges support from the National Institutes of Mental Health (MH111177, MH059299), the Mark Cohen Neuroscience Endowment, the Jack Dorsey Neuroscience Endowment, the Children’s Hospital of Michigan Foundation, the Prechter World Bipolar Foundation, the Ethel and James Flinn Foundation, the DMC Foundation, and the Lycaki-Young Funds from the State of Michigan.
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bleuler E (1908): Dementia Praecox or the Group of Schizophrenias. New York: International Press. [Google Scholar]
- 2.Fusar-Poli P, Politi P (2008): Paul Eugen Bleuler and the birth of schizophrenia (1908). The American journal of psychiatry. 165:1407. [DOI] [PubMed] [Google Scholar]
- 3.Tandon R, Nasrallah HA, Keshavan MS (2009): Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophrenia research. 110:1–23. [DOI] [PubMed] [Google Scholar]
- 4.Pienkos E, Giersch A, Hansen M, Humpston C, McCarthy-Jones S, Mishara A, et al. (2019): Hallucinations Beyond Voices: A Conceptual Review of the Phenomenology of Altered Perception in Psychosis. Schizophrenia bulletin. 45:S67–S77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heckers S (2011): Bleuler and the neurobiology of schizophrenia. Schizophrenia bulletin. 37:1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Churchland PS (1996): Neurophilosophy: Toward a Unified Science of the Mind-Brain. Cambridge, MA: MIT Press. [Google Scholar]
- 7.Silverstein B, Bressler S, Diwadkar VA (2016): Inferring the dysconnection syndrome in schizophrenia: Interpretational considerations on methods for the network analyses of fMRI data. Frontiers in psychiatry. 7:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friston KJ, Stephan KE, Montague R, Dolan RJ (2014): Computational psychiatry: the brain as a phantastic organ. The lancet Psychiatry. 1: 148–158. [DOI] [PubMed] [Google Scholar]
- 9.Goldman-Rakic PS (1999): The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biological psychiatry. 46:650–661. [DOI] [PubMed] [Google Scholar]
- 10.Lewis DA (2012): Cortical circuit dysfunction and cognitive deficits in schizophrenia-implications for preemptive interventions. Eur J Neurosci. 35:1871–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diwadkar VA, Bakshi N, Gupta G, Pruitt P, White R, Eickhoff SB (2014): Dysfunction and Dysconnection in Cortical-Striatal Networks during Sustained Attention: Genetic Risk for Schizophrenia or Bipolar Disorder and its Impact on Brain Network Function. Frontiers in psychiatry. 5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A (2005): Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Archives of general psychiatry. 62:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brambilla P, Riva MA, Melcangi R, Diwadkar VA (2007): The role of glutamatergic pathways in schizophrenia: From animal models to human imaging studies. Clinical Neuropsychiatry. 4:199–207. [Google Scholar]
- 14.Diwadkar VA, Flaugher B, Jones T, Zalanyi L, Ujfalussy B, Keshavan MS, et al. (2008): Impaired associative learning in schizophrenia: behavioral and computational studies. Cogn Neurodyn. 2:207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadehra S, Pruitt P, Murphy ER, Diwadkar VA (2013): Network dysfunction during associative learning in schizophrenia: Increased activation, but decreased connectivity: an fMRI study. Schizophrenia research. 148:38–49. [DOI] [PubMed] [Google Scholar]
- 16.Ragland JD, Layher E, Hannula DE, Niendam TA, Lesh TA, Solomon M, et al. (2017): Impact of schizophrenia on anterior and posterior hippocampus during memory for complex scenes. NeuroImage Clinical. 13:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, et al. (1998): Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1:318–323. [DOI] [PubMed] [Google Scholar]
- 18.Heckers S, Konradi C (2002): Hippocampal neurons in schizophrenia. J Neural Transm. 109:891–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stephan KE, Baldeweg T, Friston KJ (2006): Synaptic plasticity and dysconnection in schizophrenia. Biological psychiatry. 59:929–939. [DOI] [PubMed] [Google Scholar]
- 20.Castner SA, Williams GV (2007): Tuning the engine of cognition: a focus on NMDA/D1 receptor interactions in prefrontal cortex. Brain Cogn. 63:94–122. [DOI] [PubMed] [Google Scholar]
- 21.Zaremba JD, Diamantopoulou A, Danielson NB, Grosmark AD, Kaifosh PW, Bowler JC, et al. (2017): Impaired hippocampal place cell dynamics in a mouse model of the 22q11.2 deletion. Nature neuroscience. 20:1612–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barch DM, Pagliaccio D, Luking K (2016): Mechanisms Underlying Motivational Deficits in Psychopathology: Similarities and Differences in Depression and Schizophrenia. Current topics in behavioral neurosciences. 27:411–449. [DOI] [PubMed] [Google Scholar]
- 23.Strauss GP, Waltz JA, Gold JM (2014): A review of reward processing and motivational impairment in schizophrenia. SchizophrBull. 40 Suppl 2:S107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gold JM, Waltz JA, Matveeva TM, Kasanova Z, Strauss GP, Herbener ES, et al. (2012): Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Archives of general psychiatry. 69:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartmann-Riemer MN, Aschenbrenner S, Bossert M, Westermann C, Seifritz E, Tobler PN, et al. (2017): Deficits in reinforcement learning but no link to apathy in patients with schizophrenia. Scientific reports. 7:40352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gold JM, Waltz JA, Frank MJ (2015): Effort cost computation in schizophrenia: a commentary on the recent literature. Biological psychiatry. 78:747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shenhav A, Straccia MA, Botvinick MM, Cohen JD (2016): Dorsal anterior cingulate and ventromedial prefrontal cortex have inverse roles in both foraging and economic choice. Cognitive, affective & behavioral neuroscience. 16:1127–1139. [DOI] [PubMed] [Google Scholar]
- 28.Martinelli C, Rigoli F, Averbeck B, Shergill SS (2018): The value of novelty in schizophrenia. Schizophrenia research. 192:287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranganath C, Minzenberg MJ, Ragland JD (2008): The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biological psychiatry. 64:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mesulam MM (1998): From sensation to cognition. Brain. 121:1013–1052. [DOI] [PubMed] [Google Scholar]
- 31.Phelps EA (2006): Emotion and cognition: insights from studies of the human amygdala. Annual review of psychology. 57:27–53. [DOI] [PubMed] [Google Scholar]
- 32.Tandon R, Gaebel W, Barch DM, Bustillo J, Gur RE, Heckers S, et al. (2013): Definition and description of schizophrenia in the DSM-5. Schizophrenia research. 150:3–10. [DOI] [PubMed] [Google Scholar]
- 33.Carpenter WT Jr., Arango C, Buchanan RW, Kirkpatrick B (1999): Deficit psychopathology and a paradigm shift in schizophrenia research. Biological psychiatry. 46:352–360. [DOI] [PubMed] [Google Scholar]
- 34.Amador XF, Kirkpatrick B, Buchanan RW, Carpenter WT, Marcinko L, Yale SA (1999): Stability of the diagnosis of deficit syndrome in schizophrenia. The American journal of psychiatry. 156:637–639. [DOI] [PubMed] [Google Scholar]
- 35.Kirkpatrick B, Kopelowicz A, Buchanan RW, Carpenter WT, (2000): Assessing the efficacy of treatments for the deficit syndrome of schizophrenia. Neuropsychopharmacology. 22:303–310. [DOI] [PubMed] [Google Scholar]
- 36.Blanchard JJ, Cohen AS (2006): The structure of negative symptoms within schizophrenia: implications for assessment. SchizophrBull. 32:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen AS, Minor KS (2010): Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull. 36:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kring AM, Moran EK (2008): Emotional response deficits in schizophrenia: insights from affective science. Schizophr Bull. 34:819–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frank MJ (2008): Schizophrenia: a computational reinforcement learning perspective. Schizophr Bull. 34:1008–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waltz JA, Frank MJ, Robinson BM, Gold JM (2007): Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 62:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waltz JA, Gold JM (2007): Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 93:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA (2008): Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 34:835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strauss GP, Frank MJ, Waltz JA, Kasanova Z, Herbener ES, Gold JM (2011): Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biol Psychiatry. 69:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barch DM, Treadway MT, Schoen N (2014): Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. J Abnorm Psychol. 123:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heydebrand G, Weiser M, Rabinowitz J, Hoff AL, DeLisi LE, Csernansky JG (2004): Correlates of cognitive deficits in first episode schizophrenia. Schizophr Res. 68:1–9. [DOI] [PubMed] [Google Scholar]
- 46.Good KP, Rabinowitz J, Whitehorn D, Harvey PD, DeSmedt G, Kopala LC (2004): The relationship of neuropsychological test performance with the PANSS in antipsychotic naive, first-episode psychosis patients. Schizophr Res. 68:11–19. [DOI] [PubMed] [Google Scholar]
- 47.Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, et al. (2000): Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. AJ Psychiatry. 157:549–559. [DOI] [PubMed] [Google Scholar]
- 48.Harvey PD, Koren D, Reichenberg A, Bowie CR (2006): Negative symptoms and cognitive deficits: what is the nature of their relationship? Schizophr Bull. 32:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen MA, Dennett DC (2011): Consciousness cannot be separated from function. Trends in cognitive sciences. 15:358–364. [DOI] [PubMed] [Google Scholar]
- 50.Heerey EA, Bell-Warren KR, Gold JM (2008): Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biol Psychiatry. 64:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heerey EA, Robinson BM, McMahon RP, Gold JM (2007): Delay discounting in schizophrenia. Cogn Neuropsychiatry. 12:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weickert TW, Goldberg TE, Callicott JH, Chen Q, Apud JA, Das S, et al. (2009): Neural correlates of probabilistic category learning in patients with schizophrenia. J Neurosci. 29:1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Granholm E, Ruiz I, Gallegos-Rodriguez Y, Holden J, Link PC (2016): Pupillary Responses as a Biomarker of Diminished Effort Associated With Defeatist Attitudes and Negative Symptoms in Schizophrenia. Biol Psychiatry. 80:581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berrios GE (1985): Positive and negative symptoms and Jackson. A conceptual history. Archives of general psychiatry. 42:95–97. [DOI] [PubMed] [Google Scholar]
- 55.Pogue-Geile MF, Harrow M (1984): Negative and positive symptoms in schizophrenia and depression: a followup. Schizophrenia bulletin. 10:371–387. [DOI] [PubMed] [Google Scholar]
- 56.Carra G, Crocamo C, Angermeyer M, Brugha T, Toumi M, Bebbington P (2019): Positive and negative symptoms in schizophrenia: A longitudinal analysis using latent variable structural equation modelling. Schizophrenia research. 204:58–64. [DOI] [PubMed] [Google Scholar]
- 57.McCutcheon RA, Abi-Dargham A, Howes OD (2019): Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms. Trends Neurosci. 42:205–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kapur S (2003): Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. The American journal of psychiatry. 160:13–23. [DOI] [PubMed] [Google Scholar]
- 59.Howes OD, Murray RM (2014): Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 383:1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winton-Brown TT, Fusar-Poli P, Ungless MA, Howes OD (2014): Dopaminergic basis of salience dysregulation in psychosis. Trends Neurosci. 37:85–94. [DOI] [PubMed] [Google Scholar]
- 61.Muzik O, Diwadkar VA (2019): Hierarchical control systems for the regulation of physiological homeostasis and affect: Can their interactions modulate mood and anhedonia? Neuroscience and biobehavioral reviews. [DOI] [PubMed] [Google Scholar]
- 62.Schultz W (1998): Predictive reward signal of dopamine neurons. Journal of neurophysiology. 80:1–27. [DOI] [PubMed] [Google Scholar]
- 63.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF (2007): Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nature neuroscience. 10:376–384. [DOI] [PubMed] [Google Scholar]
- 64.Matsumoto M, Takada M (2013): Distinct representations of cognitive and motivational signals in midbrain dopamine neurons. Neuron. 79:1011–1024. [DOI] [PubMed] [Google Scholar]
- 65.Haber SN (2003): The primate basal ganglia: parallel and integrative networks. Journal of chemical neuroanatomy. 26:317–330. [DOI] [PubMed] [Google Scholar]
- 66.Schultz W (2015): Neuronal Reward and Decision Signals: From Theories to Data. Physiological reviews. 95:853–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haber SN, Behrens TE (2014): The neural network underlying incentive-based learning: implications for interpreting circuit disruptions in psychiatric disorders. Neuron. 83:1019–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinelli C, Rigoli F, Dolan RJ, Shergill SS (2018): Decreased value-sensitivity in schizophrenia. Psychiatry research. 259:295–301. [DOI] [PubMed] [Google Scholar]
- 69.Cicero DC, Kerns JG, McCarthy DM (2010): The Aberrant Salience Inventory: a new measure of psychosis proneness. Psychological assessment. 22:688–701. [DOI] [PubMed] [Google Scholar]
- 70.Ikemoto S (2010): Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci Biobehav Rev. 35:129–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cooper S, Robison AJ, Mazei-Robison MS (2017): Reward Circuitry in Addiction. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 14:687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duszkiewicz AJ, McNamara CG, Takeuchi T, Genzel L (2019): Novelty and Dopaminergic Modulation of Memory Persistence: A Tale of Two Systems. Trends Neurosci. 42:102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nestler EJ, Hyman SE, Holtzman DM, Malenka RC (2015): Molecular neuropharmacology : a foundation for clinical neuroscience, Third Edition 2015 ed Columbus, OH McGraw-Hill Education; pp xiv, 528 pages. [Google Scholar]
- 74.Salamone JD, Correa M, Mingote SM, Weber SM (2005): Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 5:34–41. [DOI] [PubMed] [Google Scholar]
- 75.Lobo MK (2009): Molecular profiling of striatonigral and striatopallidal medium spiny neurons past, present, and future. Int Rev Neurobiol. 89:1–35. [DOI] [PubMed] [Google Scholar]
- 76.Surmeier DJ, Ding J, Day M, Wang Z, Shen W (2007): D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 30:228–235. [DOI] [PubMed] [Google Scholar]
- 77.Francis TC, Lobo MK (2017): Emerging Role for Nucleus Accumbens Medium Spiny Neuron Subtypes in Depression. Biol Psychiatry. 81:645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kravitz AV, Tye LD, Kreitzer AC (2012): Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 15:816–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lobo MK, Covington HE 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. (2010): Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 330:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, et al. (2013): Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 16:632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chandra R, Francis TC, Konkalmatt P, Amgalan A, Gancarz AM, Dietz DM, et al. (2015): Opposing role for Egr3 in nucleus accumbens cell subtypes in cocaine action. J Neurosci. 35:7927–7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chandra R, Lenz JD, Gancarz AM, Chaudhury D, Schroeder GL, Han MH, et al. (2013): Optogenetic inhibition of D1R containing nucleus accumbens neurons alters cocaine-mediated regulation of Tiam1. Front Mol Neurosci. 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sesack SR, Grace AA (2010): Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 35:27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Floresco SB (2015): The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol. 66:25–52. [DOI] [PubMed] [Google Scholar]
- 85.Kanoski SE, Grill HJ (2015): Hippocampus Contributions to Food Intake Control: Mnemonic, Neuroanatomical, and Endocrine Mechanisms. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fanselow MS (2000): Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 110:73–81. [DOI] [PubMed] [Google Scholar]
- 87.Vezina P, Giovino AA, Wise RA, Stewart J (1989): Environment-specific cross-sensitization between the locomotor activating effects of morphine and amphetamine. Pharmacol Biochem Behav. 32:581–584. [DOI] [PubMed] [Google Scholar]
- 88.Kalivas PW, Volkow N, Seamans J (2005): Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 45:647–650. [DOI] [PubMed] [Google Scholar]
- 89.Gruber AJ, Hussain RJ, O’Donnell P (2009): The nucleus accumbens: a switchboard for goal-directed behaviors. PLoS One. 4:e5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ambroggi F, Ishikawa A, Fields HL, Nicola SM (2008): Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 59:648–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. (2011): Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 475:377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Janak PH, Tye KM (2015): From circuits to behaviour in the amygdala. Nature. 517:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Husain M, Roiser JP (2018): Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat Rev Neurosci. 19:470–484. [DOI] [PubMed] [Google Scholar]
- 94.Monteggia LM, Heimer H, Nestler EJ (2018): Meeting Report: Can We Make Animal Models of Human Mental Illness? Biol Psychiatry. 84:542–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wong AH, Josselyn SA (2016): Caution When Diagnosing Your Mouse With Schizophrenia: The Use and Misuse of Model Animals for Understanding Psychiatric Disorders. Biol Psychiatry. 79:32–38. [DOI] [PubMed] [Google Scholar]
- 96.Coyle JT (2017): Schizophrenia: Basic and Clinical. Advances in neurobiology. 15:255–280. [DOI] [PubMed] [Google Scholar]
- 97.Barnes SA, Der-Avakian A, Young JW (2017): Preclinical Models to Investigate Mechanisms of Negative Symptoms in Schizophrenia. Schizophr Bull. 43:706–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dahoun T, Trossbach SV, Brandon NJ, Korth C, Howes OD (2017): The impact of Disrupted-in-Schizophrenia 1 (DISC1) on the dopaminergic system: a systematic review. Translational psychiatry. 7:e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brandon NJ, Sawa A (2011): Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci. 12:707–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim JY, Duan X, Liu CY, Jang MH, Guo JU, Pow-anpongkul N, et al. (2009): DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron. 63:761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rodriguez-Seoane C, Ramos A, Korth C, Requena JR (2015): DISC1 regulates expression of the neurotrophin VGF through the PI3K/AKT/CREB pathway. J Neurochem. 135:598–605. [DOI] [PubMed] [Google Scholar]
- 102.Soda T, Frank C, Ishizuka K, Baccarella A, Park YU, Flood Z, et al. (2013): DISC1-ATF4 transcriptional repression complex: dual regulation of the cAMP-PDE4 cascade by DISC1. Mol Psychiatry. 18:898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jaaro-Peled H, Niwa M, Foss CA, Murai R, de Los Reyes S, Kamiya A, et al. (2013): Subcortical dopaminergic deficits in a DISC1 mutant model: a study in direct reference to human molecular brain imaging. Human molecular genetics. 22:1574–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, et al. (2010): Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 13:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Q, Charych EI, Pulito VL, Lee JB, Graziane NM, Crozier RA, et al. (2011): The psychiatric disease risk factors DISC1 and TNIK interact to regulate synapse composition and function. Mol Psychiatry. 16:1006–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, et al. (2007): Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 54:387–402. [DOI] [PubMed] [Google Scholar]
- 107.Murphy KC, Jones LA, Owen MJ (1999): High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 56:940–945. [DOI] [PubMed] [Google Scholar]
- 108.Gonzalez-Castro TB, Hernandez-Diaz Y, Juarez-Rojop IE, Lopez-Narvaez ML, Tovilla-Zarate CA, Fresan A (2016): The Role of a Catechol-O-Methyltransferase (COMT) Val158Met Genetic Polymorphism in Schizophrenia: A Systematic Review and Updated Meta-analysis on 32,816 Subjects. Neuromolecular Med. 18:216–231. [DOI] [PubMed] [Google Scholar]
- 109.Lang UE, Puls I, Muller DJ, Strutz-Seebohm N, Gallinat J (2007): Molecular mechanisms of schizophrenia. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 20:687–702. [DOI] [PubMed] [Google Scholar]
- 110.Cohen SM, Nadler JV (1997): Proline-induced potentiation of glutamate transmission. Brain Res. 761:271–282. [DOI] [PubMed] [Google Scholar]
- 111.Cohen SM, Nadler JV (1997): Proline-induced inhibition of glutamate release in hippocampal area CA1. Brain Res. 769:333–339. [DOI] [PubMed] [Google Scholar]
- 112.Gogos JA, Santha M, Takacs Z, Beck KD, Luine V, Lucas LR, et al. (1999): The gene encoding proline dehydrogenase modulates sensorimotor gating in mice. Nat Genet. 21:434–439. [DOI] [PubMed] [Google Scholar]
- 113.Lindsay EA, Botta A, Jurecic V, Carattini-Rivera S, Cheah YC, Rosenblatt HM, et al. (1999): Congenital heart disease in mice deficient for the DiGeorge syndrome region. Nature. 401:379–383. [DOI] [PubMed] [Google Scholar]
- 114.Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA (2006): Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 188:510–518. [DOI] [PubMed] [Google Scholar]
- 115.Hagihara H, Takao K, Walton NM, Matsumoto M, Miyakawa T (2013): Immature dentate gyrus: an endophenotype of neuropsychiatric disorders. Neural Plast. 2013:318596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Silva AJ, Wang Y, Paylor R, Wehner JM, Stevens CF, Tonegawa S (1992): Alpha calcium/calmodulin kinase II mutant mice: deficient long-term potentiation and impaired spatial learning. Cold Spring Harb Symp Quant Biol. 57:527–539. [DOI] [PubMed] [Google Scholar]
- 117.Matsuo N, Yamasaki N, Ohira K, Takao K, Toyama K, Eguchi M, et al. (2009): Neural activity changes underlying the working memory deficit in alpha-CaMKII heterozygous knockout mice. Front Behav Neurosci. 3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hell JW (2014): CaMKII: Claiming Center Stage in Postsynaptic Function and Organization. Neuron. 81:249–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Herring BE, Nicoll RA (2016): Long-Term Potentiation: From CaMKII to AMPA Receptor Trafficking. Annu Rev Physiol. 78:351–365. [DOI] [PubMed] [Google Scholar]
- 120.Robison AJ (2014): Emerging role of CaMKII in neuropsychiatric disease. Trends Neurosci. 37:653–662. [DOI] [PubMed] [Google Scholar]
- 121.Young CE, Arima K, Xie J, Hu L, Beach TG, Falkai P, et al. (1998): SNAP-25 deficit and hippocampal connectivity in schizophrenia. Cereb Cortex. 8:261–268. [DOI] [PubMed] [Google Scholar]
- 122.Gerber DJ, Hall D, Miyakawa T, Demars S, Gogos JA, Karayiorgou M, et al. (2003): Evidence for association of schizophrenia with genetic variation in the 8p21.3 gene, PPP3CC, encoding the calcineurin gamma subunit. Proc Natl Acad Sci U S A. 100:8993–8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miyakawa T, Leiter LM, Gerber DJ, Gainetdinov RR, Sotnikova TD, Zeng H, et al. (2003): Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 100:8987–8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Angrist BM, Gershon S (1970): The phenomenology of experimentally induced amphetamine psychosis--preliminary observations. Biol Psychiatry. 2:95–107. [PubMed] [Google Scholar]
- 125.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. (1994): Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 51:199–214. [DOI] [PubMed] [Google Scholar]
- 126.Seeman P, Lee T, Chau-Wong M, Wong K (1976): Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 261:717–719. [DOI] [PubMed] [Google Scholar]
- 127.Seeman P, Lee T (1975): Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 188:1217–1219. [DOI] [PubMed] [Google Scholar]
- 128.Seeman P, Kapur S (2000): Schizophrenia: more dopamine, more D2 receptors. Proc Natl Acad Sci U S A. 97:7673–7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee T, Seeman P (1980): Elevation of brain neuroleptic/dopamine receptors in schizophrenia. AJ Psychiatry. 137:191–197. [DOI] [PubMed] [Google Scholar]
- 130.Weinstein JJ, Chohan MO, Slifstein M, Kegeles LS, Moore H, Abi-Dargham A (2017): Pathway-Specific Dopamine Abnormalities in Schizophrenia. Biol Psychiatry. 81:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nielsen J, Fejgin K, Sotty F, Nielsen V, Mork A, Christoffersen CT, et al. (2017): A mouse model of the schizophrenia-associated 1q21.1 microdeletion syndrome exhibits altered mesolimbic dopamine transmission. Translational psychiatry. 7:1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Howes OD, McCutcheon R, Owen MJ, Murray RM (2017): The Role of Genes, Stress, and Dopamine in the Development of Schizophrenia. Biol Psychiatry. 81:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kantrowitz JT, Javitt DC (2010): N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 83:108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Olney JW, Newcomer JW, Farber NB (1999): NMDA receptor hypofunction model of schizophrenia. J PsychiatrRes. 33:523–533. [DOI] [PubMed] [Google Scholar]
- 135.Coyle JT (2012): NMDA receptor and schizophrenia: a brief history. Schizophr Bull. 38:920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moghaddam B, Adams B, Verma A, Daly D (1997): Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 17:2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jackson ME, Homayoun H, Moghaddam B (2004): NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci U S A. 101:8467–8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C (2004): Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 5:793–807. [DOI] [PubMed] [Google Scholar]
- 139.Farber NB, Wozniak DF, Price MT, Labruyere J, Huss J, St Peter H, et al. (1995): Age-specific neurotoxicity in the rat associated with NMDA receptor blockade: potential relevance to schizophrenia? Biol Psychiatry. 38:788–796. [DOI] [PubMed] [Google Scholar]
- 140.Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, et al. (1996): NMDA-dependent modulation of CA1 local circuit inhibition. The Journal of neuroscience : the official journal of the Society for Neuroscience. 16:2034–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Javitt DC (2010): Glutamatergic theories of schizophrenia. Isr J Psychiatry Relat Sci. 47:4–16. [PubMed] [Google Scholar]
- 142.Howes O, McCutcheon R, Stone J (2015): Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol. 29:97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE Jr., et al. (1995): Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 52:258–266. [DOI] [PubMed] [Google Scholar]
- 144.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA (2000): Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 57:237–245. [DOI] [PubMed] [Google Scholar]
- 145.Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK (2016): Nature of Glutamate Alterations in Schizophrenia: A Meta-analysis of Proton Magnetic Resonance Spectroscopy Studies. JAMA Psychiatry. 73:665–674. [DOI] [PubMed] [Google Scholar]
- 146.Harrison PJ, Owen MJ (2003): Genes for schizophrenia? Recent findings and their pathophysiological implications. Lancet. 361:417–419. [DOI] [PubMed] [Google Scholar]
- 147.Schur RR, Draisma LW, Wijnen JP, Boks MP, Koevoets MG, Joels M, et al. (2016): Brain GABA levels across psychiatric disorders: A systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp. 37:3337–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Egerton A, Modinos G, Ferrera D, McGuire P (2017): Neuroimaging studies of GABA in schizophrenia: a systematic review with meta-analysis. Transl Psychiatry. 7:e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rowland LM, Krause BW, Wijtenburg SA, McMahon RP, Chiappelli J, Nugent KL, et al. (2016): Medial frontal GABA is lower in older schizophrenia: a MEGA-PRESS with macromolecule suppression study. Mol Psychiatry. 21:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thakkar KN, Rosler L, Wijnen JP, Boer VO, Klomp DW, Cahn W, et al. (2017): 7T Proton Magnetic Resonance Spectroscopy of Gamma-Aminobutyric Acid, Glutamate, and Glutamine Reveals Altered Concentrations in Patients With Schizophrenia and Healthy Siblings. Biol Psychiatry. 81:525–535. [DOI] [PubMed] [Google Scholar]
- 151.Laruelle M, Kegeles LS, Abi-Dargham A (2003): Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci. 1003:138–158. [DOI] [PubMed] [Google Scholar]
- 152.Heckers S, Konradi C (2015): GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res. 167:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lodge DJ, Grace AA (2011): Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 32:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ziauddeen H, Murray GK (2010): The relevance of reward pathways for schizophrenia. Current opinion in psychiatry. 23:91–96. [DOI] [PubMed] [Google Scholar]
- 155.Xu P, Klaasen NG, Opmeer EM, Pijnenborg GHM, van Tol MJ, Liemburg EJ, et al. (2019): Intrinsic mesocorticolimbic connectivity is negatively associated with social amotivation in people with schizophrenia. Schizophrenia research. [DOI] [PubMed] [Google Scholar]
- 156.Hadley JA, Nenert R, Kraguljac NV, Bolding MS, White DM, Skidmore FM, et al. (2014): Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology. 39:1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lisman JE, Grace AA (2005): The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 46:703–713. [DOI] [PubMed] [Google Scholar]
- 158.Gray JA, Feldon J, Rawlings JNP, Hemsley DR, Smith AD (1991): The neuropsychology of schizophrenia. Behav Brain Sci. 14:1–81. [Google Scholar]
- 159.Hemsley DR (1987): An experimental psychological model for schizophrenia In: Hafner H, Gattaz WF, Janzavik W, editors. Search for the causes of schizophrenia. New York: Springer, pp 179–188. [Google Scholar]
- 160.Kapur S (2003): Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. The American journal of psychiatry. 160:13–23. [DOI] [PubMed] [Google Scholar]
- 161.Floresco SB, Todd CL, Grace AA (2001): Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 21:4915–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Floresco SB, West AR, Ash B, Moore H, Grace AA (2003): Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 6:968–973. [DOI] [PubMed] [Google Scholar]
- 163.Valenti O, Lodge DJ, Grace AA (2011): Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci. 31:4280–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Belujon P, Jakobowski NL, Dollish HK, Grace AA (2016): Withdrawal from Acute Amphetamine Induces an Amygdala-Driven Attenuation of Dopamine Neuron Activity: Reversal by Ketamine. Neuropsychopharmacology. 41:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chang CH, Grace AA (2013): Amygdala beta-noradrenergic receptors modulate delayed downregulation of dopamine activity following restraint. J Neurosci. 33:1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Reep R (1984): Relationship between prefrontal and limbic cortex: a comparative anatomical review. Brain Behav Evol. 25:5–80. [DOI] [PubMed] [Google Scholar]
- 167.Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC (2012): Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 487:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, et al. (2011): IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 31:314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vialou V, Robison AJ, Laplant QC, Covington HE, Dietz DM, Ohnishi YN, et al. (2010): DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 13:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bagot RC, Parise EM, Pena CJ, Zhang HX, Maze I, Chaudhury D, et al. (2015): Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun. 6:7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, et al. (2006): Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. AJ Psychiatry. 163:1784–1790. [DOI] [PubMed] [Google Scholar]
- 172.Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, et al. (2010): Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 67:110–116. [DOI] [PubMed] [Google Scholar]
- 173.McCracken CB, Grace AA (2007): High-frequency deep brain stimulation of the nucleus accumbens region suppresses neuronal activity and selectively modulates afferent drive in rat orbitofrontal cortex in vivo. J Neurosci. 27:12601–12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Whitton AE, Treadway MT, Pizzagalli DA (2015): Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 28:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Park HJ, Friston K (2013): Structural and functional brain networks: from connections to cognition. Science (New York, NY. 342:1238411. [DOI] [PubMed] [Google Scholar]
- 176.Millan MJ, Fone K, Steckler T, Horan WP (2014): Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol. 24:645–692. [DOI] [PubMed] [Google Scholar]
- 177.Aas M, Dazzan P, Mondelli V, Melle I, Murray RM, Pariante CM (2014): A Systematic Review of Cognitive Function in First-Episode Psychosis, Including a Discussion on Childhood Trauma, Stress, and Inflammation. Frontiers in psychiatry. 4:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Friston K, Brown HR, Siemerkus J, Stephan KE (2016): The dysconnection hypothesis (2016). Schizophrenia research. 176:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Palaniyappan L (2017): Progressive cortical reorganisation: A framework for investigating structural changes in schizophrenia. Neuroscience and biobehavioral reviews. 79:1–13. [DOI] [PubMed] [Google Scholar]
- 180.Frost R, McNaughton N (2017): The neural basis of delay discounting: A review and preliminary model. Neuroscience and biobehavioral reviews. 79:48–65. [DOI] [PubMed] [Google Scholar]
- 181.Diekhof EK, Gruber O (2010): When desire collides with reason: functional interactions between anteroventral prefrontal cortex and nucleus accumbens underlie the human ability to resist impulsive desires. J Neurosci. 30:1488–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Huskey R, Craighead B, Miller MB, Weber R (2018): Does intrinsic reward motivate cognitive control? a naturalistic-fMRI study based on the synchronization theory of flow. Cognitive, affective & behavioral neuroscience. 18:902–924. [DOI] [PubMed] [Google Scholar]
- 183.Reeve J, Lee W (2019): A neuroscientific perspective on basic psychological needs. J Pers. 87:102–114. [DOI] [PubMed] [Google Scholar]
- 184.Lee W, Reeve J (2017): Identifying the neural substrates of intrinsic motivation during task performance. Cognitive, affective & behavioral neuroscience. 17:939–953. [DOI] [PubMed] [Google Scholar]
- 185.Ceaser AE, Barch DM (2015): Striatal Activity is Associated with Deficits of Cognitive Control and Aberrant Salience for Patients with Schizophrenia. Front Hum Neurosci. 9:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pankow A, Katthagen T, Diner S, Deserno L, Boehme R, Kathmann N, et al. (2016): Aberrant Salience Is Related to Dysfunctional Self-Referential Processing in Psychosis. Schizophrenia bulletin. 42:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nelson B, Whitford TJ, Lavoie S, Sass LA (2014): What are the neurocognitive correlates of basic self-disturbance in schizophrenia? Integrating phenomenology and neurocognition: Part 2 (aberrant salience). Schizophrenia research. 152:20–27. [DOI] [PubMed] [Google Scholar]
- 188.Friston KJ (2005): Models of brain function in neuroimaging. Annual review of psychology. 56:57–87. [DOI] [PubMed] [Google Scholar]
- 189.Beck AT, Rector NA (2005): Cognitive approaches to schizophrenia: theory and therapy. Annu Rev Clin Psychol. 1:577–606. [DOI] [PubMed] [Google Scholar]
- 190.Grant PM, Beck AT (2009): Defeatist beliefs as a mediator of cognitive impairment, negative symptoms, and functioning in schizophrenia. Schizophr Bull. 35:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Reddy LF, Horan WP, Barch DM, Buchanan RW, Gold JM, Marder SR, et al. (2018): Understanding the Association Between Negative Symptoms and Performance on Effort-Based Decision-Making Tasks: The Importance of Defeatist Performance Beliefs. Schizophr Bull. 44:1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Granholm E, Holden J, Link PC, McQuaid JR (2014): Randomized clinical trial of cognitive behavioral social skills training for schizophrenia: improvement in functioning and experiential negative symptoms. J Consult Clin Psychol. 82:1173–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Granholm E, Holden J, Link PC, McQuaid JR, Jeste DV (2013): Randomized controlled trial of cognitive behavioral social skills training for older consumers with schizophrenia: defeatist performance attitudes and functional outcome. Am J Geriatr Psychiatry. 21:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Perivoliotis D, Morrison AP, Grant PM, French P, Beck AT (2009): Negative performance beliefs and negative symptoms in individuals at ultra-high risk of psychosis: a preliminary study. Psychopathology. 42:375–379. [DOI] [PubMed] [Google Scholar]
- 195.Kahn I, Shohamy D (2013): Intrinsic connectivity between the hippocampus, nucleus accumbens, and ventral tegmental area in humans. Hippocampus. 23:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]