Abstract
Pancreatic ductal adenocarcinoma (PDAC) continues to have a dismal prognosis in part due to ineffective treatment strategies. The efficacy of some chemotherapies and especially radiotherapy are mediated partially by the immune system. Therefore, we hypothesized that profiling the immune response following chemotherapy and/or irradiation can be used as a readout for treatment efficacy, but also to help identify optimal therapeutic schedules for PDAC. Using murine models of PDAC, we demonstrated that concurrent administration of stereotactic body radiotherapy (SBRT) and a modified dose of FOLFIRINOX (mFX) resulted in superior tumor control when compared to single or sequential treatment groups. Importantly, this combined treatment schedule enhanced the magnitude of immunogenic cell death, which in turn amplified tumor antigen presentation by dendritic cells and intratumoral CD8+ T cell infiltration. Concurrent therapy also resulted in systemic immunity contributing to the control of established metastases. These findings provide rationale for pursuing concurrent treatment schedules of SBRT with mFX in PDAC.
Keywords: Pancreatic cancer, Stereotactic body radiation therapy (SBRT), Modified dose FOLFIRINOX (mFX), Immunogenic cell death (ICD), Immune response
Introduction
Pancreatic ductal adenocarcinoma (PDAC) cancer is the fourth leading cause of cancer related deaths in the United States with a five-year survival rate of only 8% (1). 80–85% patients are diagnosed with advanced stage disease and are thus ineligible for curative intent surgery (2). New therapeutic approaches are urgently needed to improve outcome of patients with locally advanced/metastatic PDAC.
There is controversy regarding the most effective therapeutic approach for patients with locally-advanced PDAC. Randomized clinical trials have explored whether neoadjuvant chemoradiotherapy (CRT) is superior to chemotherapy alone, however, results have been conflicting (3). Most of these trials utilized conventional radiotherapy (RT) schedules (3). Stereotactic body radiotherapy (SBRT) has emerged as a strategy that incorporates precision tumor-targeting to deliver higher doses of radiation in fewer fractions for patients with PDAC (4). This schedule offers better local tumor control and limited toxicity when compared to conventional RT (3, 4). Only a few clinical trials incorporate the combination of SBRT and chemotherapy in locally-advanced pancreatic cancer (LAPC), some of which show promising local control rates (5). However, survival rates remain poor with most patients dying of metastatic disease (3). Due to the relatively new emergence of SBRT to treat PDAC, there is limited clinical and preclinical information examining the optimal scheduling of SBRT in combination with chemotherapy. Developing an optimal schedule of neoadjuvant chemotherapy in combination with SBRT is crucial to achieve improved outcome for patients with advanced PDAC.
Toxicity is a concern when considering the combination of chemotherapy with radiotherapy. In PDAC, the predominant chemotherapy regimens utilize FOLFIRINOX (FX) and gemcitabine/paclitaxel, with FX increasing survival (11.1 vs. 6.8 months) for patients with non-operable disease. However, FX is associated with increased toxicity such as leukopenia and/or diarrhea (6). To overcome these side effects, modified FX (mFX, defined as a reduction of dose), has demonstrated similar survival benefits with fewer adverse effects such as neutropenia and lymphopenia, when compared to the conventional dosage (7). In addition to modifying chemotherapy dose, toxicity can also be managed by modulating the schedule of chemoradiotherapy. For example, traditional schedules in an adjuvant setting often consist of an initial treatment of chemotherapy followed by sequential radiotherapy (8). However, current clinical evidence suggests that concurrent chemoradiotherapy is superior to sequential use of combination treatment (9, 10). In patients with PDAC, concurrent chemoradiotherapy is possible due to the shorter treatment schedule of SBRT, which allows for better integration of chemotherapy (4). Given the potential overlapping toxicities associated with chemotherapy and radiotherapy, it is imperative to identify the optimal combination and schedule that provides both efficacy and reduced toxicity.
Studies have demonstrated that the efficacy of some chemotherapies and radiotherapy are mediated partially by the immune system via immunogenic cell death (ICD) (11), resulting in activation of innate and adaptive antitumor immune responses (12, 13). Therefore, it may be possible to monitor treatment efficacy by measuring the magnitude of corresponding immune responses. ICD is characterized by the release or cell-surface expression of highly immunostimulatory damage-associated molecular patterns (DAMPs) by the dying tumor cells, such as calreticulin (CRT) and/or high mobility group box 1 (HMGB1) (12). These DAMPs enhance the stimulation and antigen presentation of dendritic cells (DCs) which in turn promote activation and expansion of antitumor T cells (14). Chemoradiation may serve as an endogenous vaccine, thus we propose that treatment efficacy can be assessed by monitoring the quantity of DAMPs and antitumor activity from various immune cells.
Here, we demonstrated in murine models of PDAC that concurrent administration of SBRT and mFX enhanced antitumor effects and ICD as measured by heightened DAMPs, elevated tumor antigen presentation by DCs, and increased tumor-reactive T cells. This schedule of chemoradiotherapy was well-tolerated. Concurrent SBRT + mFX also promoted systemic antitumor immunity that led to significant protection from the formation of liver metastases. These findings provide rationale for pursuing concurrent treatment schedules of SBRT with mFX in PDAC patients and elucidated a potential mechanism for the observed benefit of combining SBRT and mFX, even in patients with metastatic disease.
Material and methods
Cell lines and reagents
The murine PDAC cell line parental KCKO (15, 16) or luciferase expressing KCKO (KCKO-luc) were a gift from Dr. Pinku Mukherjee (2010). Panc 02 (17), luciferase expressing Panc 02 (Panc 02-luc) and OVA expressing KCKO (KCKO-OVA) cell lines were a gift from Dr. David DeNardo (2016). All cell lines were cultured in RPMI 1640 supplemented with 10% FBS and penicillin/streptomycin and tested to exclude mycoplasma contamination. All these cell lines were used for experiments within 3 passages of subsequent culture, but were not authenticated in the past year.
5-fluorouracil (5-FU, Teva), irinotecan (Areva) and oxaliplatin (Athenex) were obtained from the pharmacy at University of Rochester Medical Center (URMC, Rochester, NY). For mouse chemotherapy treatments, the maximal tolerated dose (MTD)(18) of FOLFIRINOX [5-FU (25 mg/kg), irinotecan (50 mg/kg) and oxaliplatin (5 mg/kg)] or modified FOLFIRINOX (mFX, 75%MTD) was administered with retroorbital injection of irrinotecan and oxaliplatin (diluted in PBS, 100μl per mouse) and intraperitoneal injection of 5-FU (diluted in PBS, 100μl per mouse) weekly for three cycles. Cultured tumor cells were treated with 5-FU (18.75 μg/ml), irinotecan (37.5 μg/ml) and oxaliplatin (3.75 μg/ml) for the indicated times. For depletion experiments, 200 μg of anti-CD8 (clone: 53–6.7) antibody or isotype rat IgG (diluted in PBS, 100μl per mouse) was administered intraperitoneally every 3 days.
Murine orthotopic model and hepatic metastases model of pancreatic cancer
6–8 week old female C57BL/6J mice were purchased from Jackson Laboratory and maintained in a pathogen-free facility under an approved animal studies protocol at URMC. Mice were anesthetized with vaporized isoflurane and injected orthotopically in the tail of the pancreas with 2×105 KCKO-luc cells or 1×105 Panc 02-luc cells in a 1:1 PBS to Matrigel (Corning) as previously described (19). For the hepatic metastases model, mice that had tumor clearance after mFX + SBRT were re-challenged with hemisplenic injection of 4×105 KCKO-luc cells to develop hepatic metastases as previously described with some modifications (20). Briefly, the spleen was divided by placing two medium size ligating clips in the center of the spleen, the upper pole of spleen was pushed back into peritoneum and 100 μl of KCKO-luc cells (4×105) were slowly injected into the exposed hemispleen. The injected hemispleen was resected after applying one medium size ligating clip on the most distal aspect of the pancreas and splenic vessels. For the murine model with both orthotopic pancreatic tumor (KCKO cells) and hepatic metastases (KCKO-luc cells), hepatic metastases were established similarly with sutures instead of metal ligating clips used above to avoid confusion with pancreatic tumor markers (two small size of metal ligation clips on two sides of pancreatic tumor) used to identify tumors for SBRT.
Pancreatic tumor or liver metastases were monitored over time by an In Vivo Imaging System (IVIS). Briefly, the mice were administered D-luciferin (75mg/kg) subcutaneously. The mice were anaesthetized with isoflurane and imaged with IVIS 12 minutes after D-luciferin injection. Regions of interest (ROI) of the same size and shape were applied to all acquired images to measure average radiance (photons/sec/cm2/sr).
SBRT treatment
As previously described (21), radiation was delivered using a small animal radiation research platform (SARRP, XStrahl) equipped with a computed tomography (CT) scanning devise and controlled by Muriplan software. Briefly, a CT image of anesthetized mice was obtained to identify the pancreatic tumor based on two small metal fiducial clips placed on both sides of the tumor at the time of injection. A radiation treatment plan positioned a dosing isocenter and delivered a dose of 6 Gy (X-ray) for 4 consecutive days using a 5mm collimator and beam angle that precisely targets the tumor while minimizing normal tissue radiation exposure. A dose volume histogram (DVH) was obtained for each experiment to certify dose deposition to the tumor.
Blood biochemistry and body weight
Blood samples from treated mice were collected from the submandibular facial vein on day −2 (as baseline), 4, 11, and 18. Blood biochemistry was analyzed by i-STAT handheld with i-STAT CHEM8+ cartridges (Abbott Point of Care Inc., Princeton, NJ) according to the manufacturer’s instructions. Body weight was measured every 3–4 days.
Flow cytometry
Mouse blood was retroorbitally collected in heparinized capillary tubes followed by RBC lysis (BioLegend). Mouse tumors were collected after sacrifice and dissociated using 30% collagenase digestion (30 mins, 37°C). Single cell suspensions in 5% FBS in PBS by passing through 40 μm cell strainer were assessed for viability with Trypan Blue and manually counted, then incubated with Fc receptor blocking solution followed by fluorophore-conjugated antibodies.
For cell surface staining, fluorescence-labeled mouse antibodies (including anti-CD45, anti-CD3, anti-CD4, anti-CD8, anti-CD11b, anti-Ly6C, anti-Ly6G, anti-F4/80, anti-CD11c, anti-CD80, anti-CD86, anti-MHCII purchased from BD Biosciences, BioLegend or eBioscience) were added to samples for 30 min at 4 °C in the dark. For further intracellular staining, cells were washed with PBS supplemented with 5% FBS, permeabilized with Permeabilization Buffer (BD Bioscience) and stained with fluorescence-labeled mouse antibodies (including anti-IFNγ, anti-GzmB, and anti-TNFa purchased from BD Biosciences or BioLegend) for 30 min at 4 °C in the dark. Cells were washed and resuspended in 5% FBS in PBS. Flow cytometry was performed on an LSRII (BD Biosciences), and data analyzed using FlowJo Version X (Tree Star).
Immunohistochemistry
The cleaved caspase 3, HMGB1 or calreticulin positive cells were determined using immunohistochemical staining, as described previously (22). Briefly, freshly dissected murine pancreatic tumor tissue was placed into a pre-labeled tissue base mold, covered with OCT and frozen immediately with dry ice. Frozen tissue blocks were stored at −80 °C until ready for sectioning. Tissue sections of 10 um thickness were prepared using a cryostat (Leica). Tissue sections were air-dried at room temperature for 30 min and fixed in pre-cooled acetone for 10 min at −20°C. Acetone was poured off and allowed to evaporate from tissue sections for 20 min at room temperature (RT). Tissue sections were rinsed twice in PBS for 5 min, incubated in 0.3% H2O2 solution in PBS for 10 min at RT to block the endogenous peroxidase activity and rinsed with PBS two times. Sections were incubated with blocking buffer (DAKO serum free protein block), followed by primary antibodies (including cleaved caspase-3 (Asp175) (5A1E), rabbit mAb (#9664S, Cell Signaling Technology), anti-HMGB1 antibody (ab18256, Abcam), or anti-calreticulin antibody (ab4109, Abcam)). The sections were stained with HRP-labeled 2nd antibodies for 20 min at RT and rinsed with PBS three times. DAB was applied as substrates. Hematoxylin was used for counterstaining. Positive cells were enumerated using a computerized Olympus DP80 image system. For cleaved caspase 3 or HMGB1 staining, ten randomly selected fields (×400, magnification) of each tumor tissue section were enumerated, and the means reported. The calreticulin expression in tumor tissue was examined and scored with help from a licensed pathologist (blinded): 0, no staining; 1, low intensity of staining, or <25% of tumor cells were positive; 2, medium level of intensity of staining, or >25% and <50% of tumor cells are positive; 3, high level intensity of staining, or >50% and <75% of tumor cells are positive; or 4, maximum high level intensity of staining, or >75% of tumor cells are positive.
Antigen presentation assay (B3Z T hybridoma activation assay)
Mice were injected with KCKO-OVA (2×105 cells/mouse) in the pancreas, and 5 days later treated with SBRT (6 Gy on day 7, 8, 9 and 10), mFX (day 5), or a combination of SBRT and mFX. Mice were sacrificed on day 11, and tumor draining lymph nodes (pancreaticoduodenal nodes), non-draining lymph nodes (axillary, inguinal and iliac nodes), and the spleen collected. Dissociated cells (passed through a 70 μm strainer) were washed three times with RPMI 1640 medium and then incubated with the B3Z T cell hybridoma to assess antigen presentation as previously described (23). Briefly, B3Z cells recognize the OVA257–264 peptide (SIINFEKL) presented by H-2b MHC and express lacZ upon activation. B3Z cells are OVA/Kb-specific cytotoxic T cell clone transfected with lacZ gene under IL-2 promoter elements. Recognition of OVA peptide 257–264 (SIINFEKL) by T cell receptor leads to transcriptional activation of IL-2 promoter elements resulting in production of the enzyme β-galactosidase. Activated B3Z cells will turn blue after addition of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside. A total of 5×105 lymph node cells or spleen cells was incubated with 5×105 B3Z cells in 96-well flat-bottom plates and incubated in MAT/P medium (US patent 4.816.401) supplemented with 100 u/ml penicillin, 100 ug/ml streptomycin and 5% fetal calf serum for 18 h at 37°C. The cultures were washed with PBS, and cells were fixed with cold 2% formaldehyde with 0.2% glutaraldehyde for 10 min at 4°C. They were washed again with PBS and overlaid with 0.5 mg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (Fisher Scientific, cat#15520034). The number of blue cells in each well was counted microscopically after 24 hours incubation at 37°C.
Antigen-specific T cell detection
Mice were injected with 2×105 OVA labeled KCKO cells (KCKO-OVA) in the pancreas, and treated with mFX on day 5 and/or SBRT (6 Gy) on day 7, 8, 9 and 10 after tumor implantation. Mice were sacrificed on day 11 and H-2Kb (SIINFEKL) dextramer binding CD8+ T cells in tumor were determined by flow cytometry as instructions provided by IMMUDEX with small adjustments. Briefly, single cells digested from tumor tissue were washed with PBS, stained with Aqua fluorescent reactive dye to identify the dead cells. Cells were then stained with H-2Kb (SIINFEKL) dextramer-PE (Immudex, Copenhagen, Denmark) at RT for 10 min, followed by staining of other surface markers (CD45-APC, CD3-FITC, CD8-PerCP-Cy5.5) for 20 min at 4°C. Stained cells were analyzed by flow cytometry within two hours. Aqua−Dextramer+ CD45+CD3+CD8+ cells were determined as tumor antigen specific CD8 T cells.
Immunization studies in vivo
KCKO-luc cells were treated with mFX (3 days), radiation (6 Gy on day 0, 1, 2 and 3 respectively) or mFX + radiation in vitro as described above. C57BL/6J mice were inoculated subcutaneously (s.c.) in the flank with 5 × 105 cells (suspended in 100μl PBS) per mouse of treated KCKO-luc cells. Frozen (dry ice) and thawed (37°C water bath) KCKO-luc cells (4 times) were injected as controls. After 7 days, 2 × 105 live KCKO-luc cells suspended in 50 μl of 1:1 PBS to Matrigel were injected in the pancreas tail. Tumor growth was monitored over time by IVIS.
Adoptive T cells transfer
CD8+ T cells were isolated from lymph nodes and spleen of naïve mice or mFX plus SBRT cured mice using EasySep™ Mouse CD8+ T cell negative isolation kit (Stem Cell Technology) according to the manufacturer’s instructions. Isolated CD8+ T cells (1×107) resuspended in 100 PBS were adoptively transferred to recipient mice by tail vein injection (1:1 donor:recipient transferal). 24hrs later, 2×105 cells of KCKO-luc cells suspended in 50 μl of 1:1 PBS to Matrigel were injected into the pancreas tail.
DCs phagocytosis
CD11c+DCs were generated from C57BL/6J bone marrow as previously described (24). Briefly, BM cells were harvested from the femurs of C57BL/6J mice and 1×107 cells were cultured in 10 ml complete RPMI containing mouse recombinant GMCSF (50 ng/ml) and IL-4 (25 ng/ml) (Peprotech) for 7 days to generate CD11c+ DCs (fresh media containing GMCSF and IL4 was added on day 4). KCKO-luc-mCherry cells were treated with mFX for 3 days, radiation (6 Gy on day 0, 1, 2 and 3), or a combination of the two. Treated tumor cells were then co-cultured with DCs at 2:1 ratio for 24 hours. Cell cultures were stained with anti-CD11c, anti-MHCII, anti-CD80, and anti-CD86 and analyzed by flow cytometry. Tumor cell phagocytosis was detected via analysis of mCherry (tumor)/CD11c double-positive signal.
Detection of apoptosis, surface calreticulin and ERp57, and release of HMGB1
mFX and/or radiation-induced tumor cell death was assessed using Annexin V-7AAD apoptosis kit (BD Biosciences, San Diego, CA) according to the manufacturer’s instructions. As previously described (25), cell surface calreticulin or ERp57 was detected by staining of anti-calreticulin antibody (1:1000, ab4109, Abcam) or anti-ERp57 antibody (1:1000, ab10287, Abcam) for 30 min at 4 °C in the dark. Cells were washed with PBS containing 5% FBS, followed by AF488-labeled 2nd antibody for 30 min at 4 °C in the dark. Cells were washed again with PBS containing 5% FBS, and analyzed by flow cytometry. Cell culture supernatant was assayed for extracellular HMGB1 by ELISA (Fisher Scientific) according to the manufacturer’s instructions.
Generation of syngeneic CD11c.DTR-eGFP chimeras and DC depletion
CD11c.DTR-eGFP syngeneic chimeras were generated as previous described (26). Briefly, 8–10 week-old wild type female C57BL/6J mice were irradiated with 9.5 Gy and immediately transplanted i.v. with 1×107 bone marrow cells from CD11c.DTR-eGFP B6 mice. Chimeras were used for experiments 8 weeks after bone marrow transplant. For long-term DC depletion, chimeras were injected intraperitoneally with diphtheria toxin (Sigma Cat#D0564, 100ng in PBS/mouse) every other day starting 1 day before treatment (mFX plus SBRT) for 5× total (26). For confirmation of DC depletion, mice were sacrificed 1 day after the second DT injection. Spleen, lymph node and tumors (digested with 30% collagenase) were isolated and passed through 70 μl cell strainer to get single cell suspension in PBS with 5% FBS. Cells were stained with fluorescence-labeled mouse antibodies (including anti-CD45 and anti-CD11c from BD Bioscience). Flow cytometry was performed and data analyzed using FlowJo Version X (Tree Star) by gating on CD45, CD11c and GFP.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 7 software. Unless indicated, data are expressed as mean ± SE. For multiple group–comparison in vivo studies, one-way ANOVA was used, followed by the Dunnett test for comparing experimental groups against a single control. For a single comparison between two groups, the paired Student t test was used. P < 0.05 or P < 0.01 was considered to be significant.
Results
Concurrent administration of mFX and SBRT enhanced anti-pancreatic tumor efficacy
To establish a treatment schedule of modified FX, KCKO-luc cells were injected into the tail of pancreas and treated weekly with the maximum tolerated dose (MTD)(18) or 75, 50 or 25% of the MTD FX starting on day 5. We determined that 75% maximal tolerated dose (MTD) was most effective [Supplemental Fig. S1] therefore, we chose 75% MTD of FX as modified FX (mFX) in all subsequent experiments.
Concurrent administration of conventional RT and chemotherapy was more efficacious than sequential treatment; however, prospective clinical studies examining concurrent treatment of SBRT and chemotherapy have not been performed (9, 10). To test this, mice with established PDAC of KCKO-luc cells were left untreated or treated with mFX, SBRT, or concurrent administration of mFX and SBRT [Fig. 1A]. We observed that mFX or SBRT alone could partially slow tumor growth whereas concurrent administration of both significantly enhanced tumor control [Fig. 1B and C]. 57% of the mice in the combination treatment group demonstrated tumor clearance, whereas 100% of mice died in the other groups [Fig. 1D].
Figure 1. SBRT synergistically enhanced the antitumor efficacy of modified FOLFIRINOX in a murine orthotopic model of pancreatic cancer.
(A) Schematic of the experimental design. (B-D) Tumor growth was analyzed by IVIS twice a week. (B) Representative IVIS images from day 19 after tumor implantation. (C) Tumor growth curve based on IVIS imaging. Data represent at least 2 independent experiments (n=5–7 mice/group). *p<0.05, mFX + SBRT compared with mFX treatment group by one-way ANOVA with Dunnett post-test. (D) Kaplan Meier survival plots for tumor-bearing mice in the various treatment groups. Shown is the percentage of tumor-free mice. **p<0.01, mFX + SBRT compared with mFX treatment group, by log-rank (Mantel-Cox) test. (E, F) C57BL/6J mice were injected with Panc 02-luc in tail of pancreas, and treated with mFX and/or SBRT as in (A). Tumor growth was monitored by IVIS and survival was observed. (E) Tumor growth curve based on IVIS imaging. Data shown are mean ± SEM (n=5 mice/group). *p<0.05, mFX + SBRT compared with mFX treatment group by one-way ANOVA with Dunnett post-test. (F) Kaplan Meier survival curve. *p<0.05, mFX + SBRT compared with mFX treatment group by log-rank (Mantel-Cox) test.
To generalize our findings across other PDAC models, mice were injected with Panc 02-luc followed by the same treatment illustrated in Fig. 1A. Similar to KCKO, concurrent treatment of mFX and SBRT significantly reduced tumor growth and improved survival of Panc 02-luc-bearing mice compared with either treatment alone [Fig. 1E and F]. Taken together, concurrent use of SBRT synergistically enhanced the antitumor effect of mFX in PDAC.
SBRT and mFX concurrent administration had better efficacy than sequential administration
We determined if the concurrent administration of SBRT and mFX (described in Fig. 1A) resulted in measurable toxicity in mice. No significant changes in body weight were observed [Supplemental Fig. S2]. Blood chemistry evaluations indicated slightly decreased hematocrit and hemoglobin on day 11 and 18 post tumor implantation in the concurrently treated group that was comparable to mFX alone [Supplemental Table S1 and S2]. Therefore, concurrent mFX and SBRT exhibited minimal side effects that were similar to mFX alone.
We compared the efficacy of concurrent treatment of mFX and SBRT (Fig. 1A) with sequential treatment of both therapies (3 cycles of mFX followed by 4 consecutive 6 Gy SBRT treatment-illustrated in [Supplemental Fig. S3A]) in a PDAC model. Concurrent administration of SBRT and mFX demonstrated better antitumor efficacy than sequential treatment [Supplemental Fig. S3B]. Concurrent delivery of therapy resulted in over half the mice surviving past 100 days whereas no mice displayed tumor clearance in the sequential treatment group [supplemental Fig. S3C]. Thus, our data demonstrated that concurrent use of mFX and SBRT was superior to sequential administration.
Treatment with mFX and SBRT increased immunogenic cell death
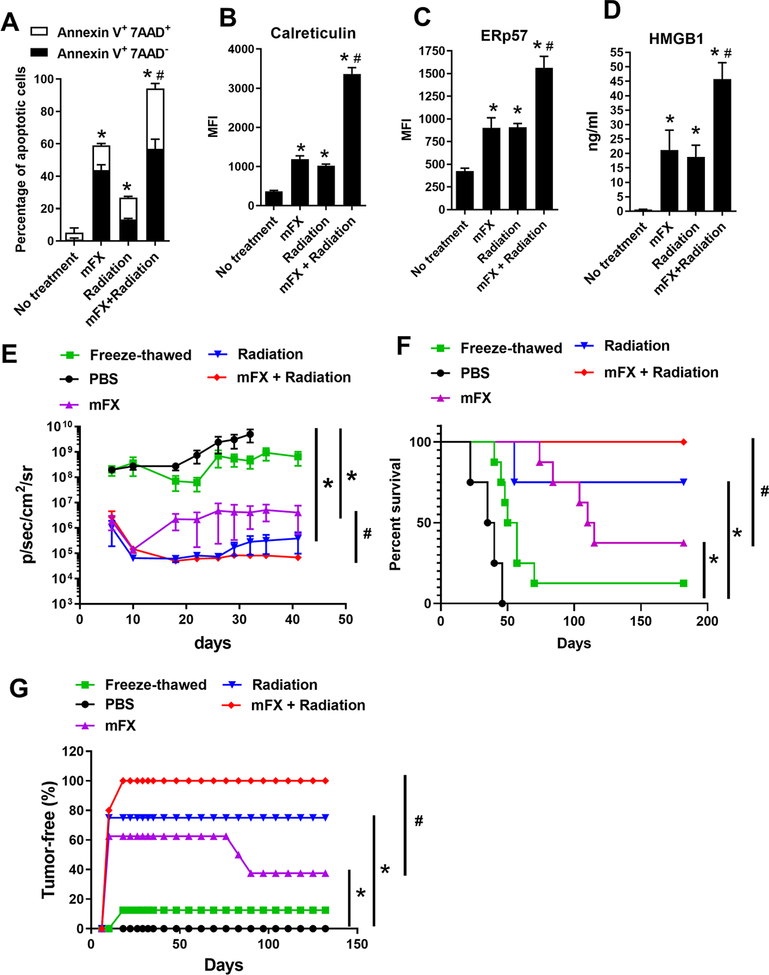
To determine whether concurrent treatment of radiation and mFX enhanced tumor cell ICD, KCKO-luc cells were treated in vitro with a similar schedule as in Fig. 1A and apoptosis was assessed by annexin V staining. Modified FX or radiation-alone induced apoptosis, whereas the combination of both therapies given concurrently resulted in a significantly higher percentage of apoptosis [Fig. 2A and supplemental Fig. S4]. To further assess the therapeutic induction of ICD, we focused on calreticulin and ER-associated protein disulfide isomerase ERp57, which provide an “eat-met” signal to DCs when translocated to the cell surface of dying cells (27, 28). The combination of mFX and radiation significantly increased calreticulin and ERp57 cell surface expression on in vitro treated tumor cells than either therapy alone (Fig. 2B–C). Additionally, we found significantly higher secretion of HMGB1 in the combination treatment when compared with either strategy alone [Fig. 2D].
Figure 2. Enhanced ICD induced by combination of radiation and mFX.
KCKO-luc cells were treated or not treated with mFX (24 hours), radiation (6 Gy on day 0, 1, 2 and 3) or both in combination (radiation + mFX). (A) Apoptosis was determined by annexin V and 7-aminoactinomycin D (7-AAD). Early apoptotic cells were stained as annexin V positive and 7-AAD negative; late apoptotic cells stained as both annexin V and 7-AAD positive. (B, C) Cell surface calreticulin (B) and ERp57 (C) were stained and determined by flow cytometry. (D) HMGB1 secretion in culture medium was determined by ELISA. Data shown in (A-D) are mean ± SEM from three independent experiments and analyzed by one-way ANOVA with Dunnett post-test. *, p<0.05, compared with no treatment group; #, p<0.05, the combination was compared to mFX treated group. (E-G) KCKO-luc cells treated with mFX, radiation, combination of radiation and mFX, or freeze-thawed were inoculated s.c. into C57BL/6J mice. After 7 days, mice were re-challenged with live KCKO-luc cells in pancreas tail. (E) Tumor growth curve based on IVIS imaging. Data shown are mean ± SEM (n=5–8 mice/group). *p<0.05, compared with mFX treatment group by one-way ANOVA with Dunnett post-test. (F) The Kaplan Meier survival curve. (G) Percentage of tumor-free mice determined by IVIS and analyzed by log-rank(Mantel-Cox) test. *, p<0.05, compared with PBS group; #, p<0.05, the combination was compared to mFX treated group.
To test if the heightened ICD from SRBT plus mFX treatment elevated DC function, we examined the in vitro phagocytosis and maturation status of DCs following coculture with KCKO cells treated with mFX, radiation, or both. Mouse bone marrow-derived DCs (BMDCs) cocultured with KCKO cells treated with concurrent treatment resulted in increased phagocytosis compared to coculture with either KCKO cells treated with mFX or radiation alone [Supplemental Fig. S5A and B]. An increased maturation phenotype, indicated by the surface expression of MHCII, CD80 and CD86, was observed in DCs in the concurrent treatment group [Supplemental Fig. S5C–F]. To complement these in vitro experiments, we vaccinated mice with KCKO cells treated with mFX, radiation, or the concurrent schedule of both therapies. Freeze/thawed KCKO cells were used as a negative control. Mice were challenged with live KCKO-luc cells in pancreas 7 days following vaccination. Mice vaccinated s.c. with radiation or mFX-treated KCKO cells displayed significant tumor growth inhibition [Fig. 2E], resulting in 75% or 37.5% survival respectively [Fig. 2F and G] after live KCKO pancreatic tumor challenge. Mice vaccinated with KCKO cells treated with the combination of radiation and mFX were 100% protected against tumor development [Fig. 2F and G]. Only 12.5% of control mice vaccinated with freeze/thawed KCKO cells demonstrated protection against tumor challenge, and non-vaccinated mice (PBS group) all developed tumors [Fig. 2E, F and G]. Thus, concurrent combination treatment displayed the greatest efficacy of ICD.
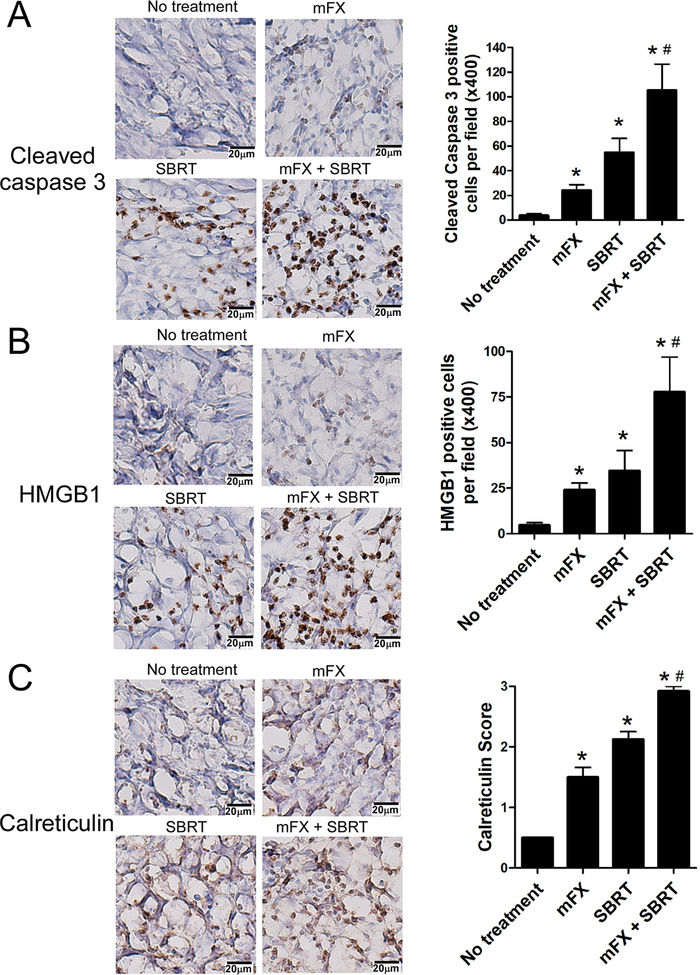
We next assessed ICD in tumor tissue in vivo by IHC staining for cleaved caspase 3 (as a measure of apoptotic cell death), HMGB1 and calreticulin. Both mFX and SBRT alone could induce apoptosis, however the concurrent combination strategy induced significantly higher percentage of cell death [Fig. 3A]. HMGB1 and calreticulin staining were significantly elevated in the concurrent therapy [Fig. 3B and C]. Collectively, these data indicate that combination of SBRT and mFX induced enhanced ICD of PDAC cells.
Figure 3. Enhanced tumor immunogenic cell death in mice treated with concurrent administration of mFX and SBRT.
Mice bearing KCKO-luc orthotopic pancreatic tumors and treated with mFx followed by SBRT were sacrificed on day 11 and immunogenic cell death was determined by immunohistochemistry staining of cleaved caspase 3, HMGB1 and calreticulin in tumor tissue. (A) Representative immunohistochemistry of cleaved caspase 3 (left) and quantification (right) in the pancreatic tumor treated with no treatment, mFX, SBRT or combination. (B) Representative immunohistochemistry of HMGB1 (left) and quantification (right). (C) Representative immunohistochemistry of calreticulin (left) and quantification (right). Results are expressed as mean ± SEM from 5 mice/group, and analyzed by one-way ANOVA with Dunnett post-test. *, p<0.05, compared with no treatment group; #, p<0.05, mFX + SBRT compared with mFX treated group.
Treatment with mFX and SBRT induced increased tumor antigen presentation
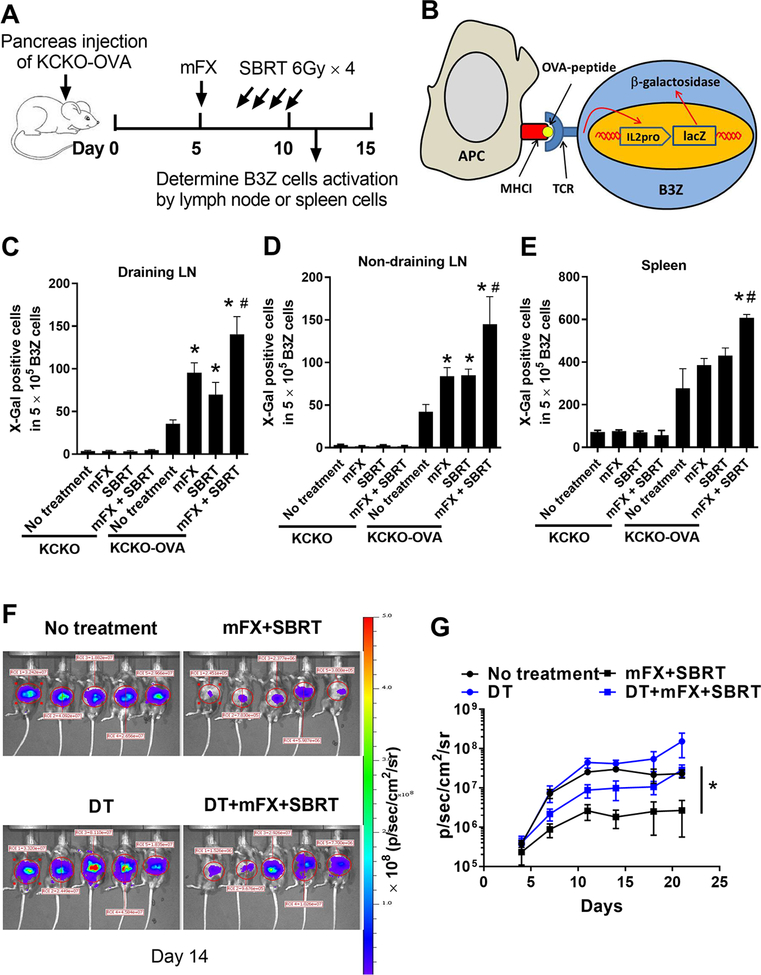
DAMP release upon ICD can efficiently recruit DCs to the tumor microenvironment (TME), facilitating the engulfment of dying cells and promoting DC maturation and their ability to cross-present antigen (13). To test whether concurrent combination treatment with SBRT and mFX enhanced tumor antigen presentation, OVA-expressing KCKO cells (KCKO-OVA) were injected into mouse pancreas and treated with mFX, SBRT, or the combination (Fig. 4A). Mice were sacrificed on day 11 and antigen presentation in the lymph node and spleen was detected by an established B3Z hybridoma assay [Fig. 4B] (23). Modified FX or SBRT alone treatment resulted in increased numbers of activated B3Z cells by tumor-draining lymph node cells, however the concurrent combination treatment induced significantly higher percentages of activated B3Z cells [Fig. 4C]. Concurrent combination treatment with mFX and SBRT also induced increased antigen presentation in non-draining LN and spleen [Fig. 4D and E], suggesting the generation of both local and systemic antitumor immunity. Mice with control non-OVA expressing KCKO tumors failed to stimulate B3Z cells in LN or spleen samples [Fig. 4C–E]. We observed increased tumor-infiltrating DCs along with a greater number of mature DCs, determined by enhanced CD80 and CD86 expression, in tumors from mice following concurrent combination treatment [Supplemental Fig. S6A–C]. To further assess the critical role of DCs in our model, we generated syngeneic CD11c.DTR-eGFP chimeric mice as described in the materials and methods, and implanted these mice with KCKO-luc cells followed by mFX plus SBRT treatment as described in Fig. 1. DCs depletion was confirmed by flow cytometry [Supplemental Fig. S6D]. Loss of DCs significantly abrogated the antitumor effect induced by concurrent treatment of mFX and SBRT [Fig. 4F and G]. These results suggest that concurrent treatment with mFX and SBRT induced increased DC tumor antigen presentation, which were crucial for treatment efficacy.
Figure 4. Enhanced tumor antigen presentation after concurrent mFX and SBRT treatment.
(A) Schematic of experimental design. Mice were sacrificed on day 11 and antigen presentation was detected with lymph node cells or splenocytes from tumor-bearing mice. (B) Schematic of B3Z cell activation by antigen presentation. (C-E) B3Z cell activation after coculture with draining lymph node cells (C), non-draining lymph node cells (D) or spleen cells (E) from tumor-bearing mice treated with the various treatments. KCKO was utilized as control to KCKO-OVA to exclude the possible non-specific antigen presentation to B3Z cells. Results are expressed as mean ± SEM (5 mice per group) and analyzed by one-way ANOVA with Dunnett post-test. *, p<0.05, treated group compared with no treatment group; #, p<0.05, mFX + SBRT compared with mFX treated group. (F, G) CD11c.DTR-eGFP chimeric mice were implanted with KCKO-luc cells in pancreas and treated with mFX plus SBRT as in Figure 1. DCs were depleted by diphtheria toxin (DT). Tumor growth was analyzed by IVIS imaging twice a week. (F) Representative image on day 14 after tumor implantation. (G) Tumor growth curve based on IVIS imaging. Results are expressed as mean ± SEM (5 mice per group) and analyzed by one-way ANOVA with Dunnett post-test. *, p<0.05, mFX + SBRT compared with DCs depleted treatment group (DT+mFX+SBRT).
CD8+ T cells were essential for the antitumor effect elicited by concurrent SBRT and mFX therapy
We hypothesized that enhanced DC antigen presentation and maturation would promote adaptive immunity, especially in CD8+ T cells. To address this, we utilized the same experimental setup as in Fig. 1A and assessed the role of CD8+ T cells from the various treatment groups. Concurrent combination therapy resulted in the highest intratumoral infiltration of CD8+ T cells [Fig. 5A] and IFNγ+CD8+ T cells [Fig. 5B]. We examined the expression of PD-1 and CTLA-4; two receptors often found on activated T cells, but also potential signatures of T cell exhaustion (29). The majority (~80%) of tumor-infiltrating CD8+ T cells expressed PD-1 regardless of treatment [Fig. 5C], whereas CTLA-4 expression was elevated in the SBRT or combination treatment group [Fig. 5D]. We also determined that peripheral blood IFNγ+CD8+ T cells increased in both SBRT alone and the concurrent combination treatment group [Fig. 5E] and expressed phenotypic markers for effector CD8+ T cells (e.g. CD62L–CD44+) in all treatment groups with the highest value in the concurrent therapy administration [Fig. 5F]. Thus, concurrent treatment resulted in an increase of effector CD8+ T cells intratumorally and systemically.
Figure 5. CD8+ T cells were essential for the antitumor effect by SBRT plus mFX (A-F).
Orthotopic pancreatic tumors were established and treated with mFX and SBRT as in figure. 1. On day 11 (1 day after SBRT), mice were sacrificed, peripheral blood and tumors collected, and tumor-infiltrating CD8+ T cells (A), IFNγ+CD8+ T cells (B), PD1+CD8+ T cells (C), CTLA4+CD8+ T cells (D), peripheral IFNγ+CD8+ T cells (E), and effector CD8+ T cells (F) were determined by flow cytometry. (G-H) Mice were injected with 2×105 KCKO-OVA cells in tail of pancreas, and treated with mFX on day 5 and SBRT on day 7, 8, 9 and 10 after tumor implantation. Mice were sacrificed on day 11 and SIINFEKL/H-2Kb dextramer binding CD8+ T cells in tumor were determined by flow cytometry. (G) Representative flow cytometry plots of SIINFEKL/H-2Kb dextramer positive CD8+ T cells. (H) Quantitative analysis of G. Data in (A-F) and (H) are expressed as mean ± SEM from 5 mice/group and analyzed by one-way ANOVA with Dunnett post-test. *, p<0.05, treated group compared with no treatment group; #, p<0.05, mFX+SBRT or SBRT treated group compared to mFX treated group. (I) Orthotopic pancreatic tumors were established and treated with mFX and SBRT as in figure 1. Left panel, tumor growth was determined by IVIS imaging. Data shown are mean ± SEM from 5 mice/group. *p<0.05, mFX+SBRT-IgG compared with mFX+SBRT-CD8 depletion, using one-way ANOVA with Dunnett post-test. Right panel, survival was observed. *p<0.05, mFX+SBRT-IgG compared with mFX+SBRT-CD8 depletion group by log-rank (Mantel-Cox) test. (J) Orthotopic pancreatic tumor were established in WT or Rag1 KO mice and treated with mFX and SBRT as in Fig. 1. Tumor growth was determined by IVIS imaging and the value of IVIS in wide type mice on day 5 set as 1. Data shown are mean of fold change of IVIS value ± SEM from 5 mice/group. *p<0.05, WT-mFX+SBRT compared with Rag1KO-mFX+SBRT using one-way ANOVA with Dunnett post-test.
To determine whether concurrent combination treatment generated an increase in antigen-specific CD8+ T cells, we examined intratumoral MHC dextramer+ CD8+ T cells that recognize the SIINFEKL peptide from KCKO-OVA tumors. Concurrent combination treatment induced a significantly higher percentage of tumor-infiltrating antigen-specific CD8+ T cells when compared with any other treatment group [Fig. 5G and H].
Depletion of CD8+ T cells attenuated the antitumor effect (both tumor burden and survival- Fig. 5I) observed in the concurrent combination treatment group. To complement these depletion experiments, we performed a similar experiment in Rag1 KO mice, which lack T and B cells, and determined that the antitumor efficacy observed in concurrent mFX and SBRT was completely abrogated [Fig. 5J]. These data demonstrated that CD8+ T cells were critical for the antitumor effect of concurrent combination treatment with mFX and SBRT.
Concurrent mFX and SBRT treatment resulted in long-term immunologic antitumor memory
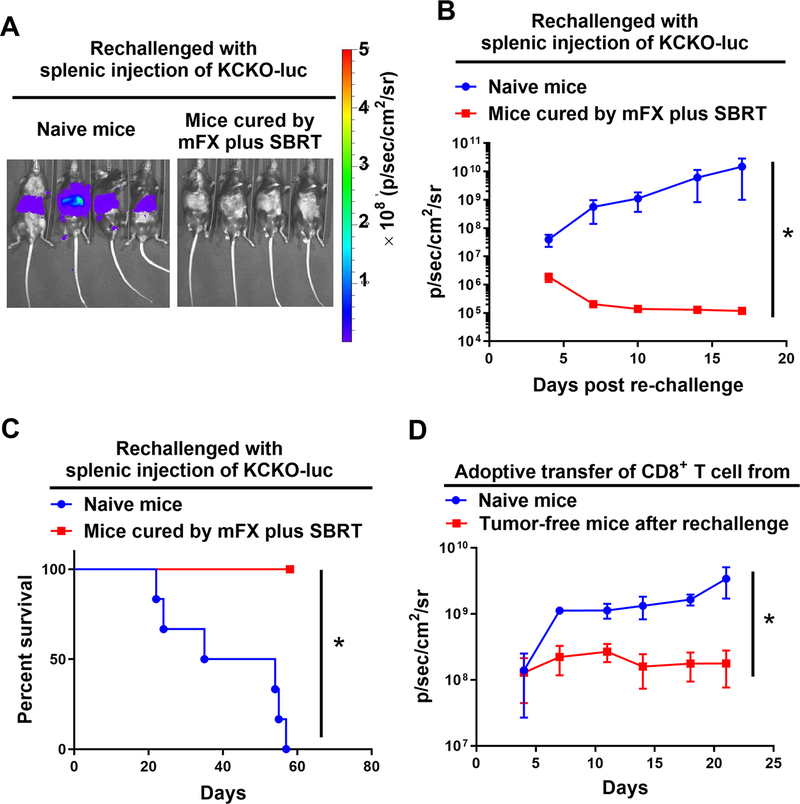
We investigated whether concurrent treatment of mFX and SBRT induced long-term antitumor immunity, which might provide protection from both recurrence and metastases. Mice that rejected primary challenge with KCKO-luc following concurrent mFX and SBRT (see Fig. 1) were maintained tumor free for 90 days and then re-challenged with KCKO-luc cells by hemisplenic injection. Naïve mice that received hemisplenic KCKO-luc developed liver metastasis and died; however, all mice that originally underwent tumor clearance after concurrent treatment with SBRT and mFX failed to develop hepatic metastases after hemisplenic re-challenge with KCKO-luc cells suggesting that concurrent treatment confers long-term systemic antitumor immunity (Fig. 6A–C).
Figure 6. Mice that cleared tumors after mFX plus SBRT treatment were refractory to tumor rechallenge. (A-C).
Naïve mice or mice that cleared tumors after mFX plus SBRT treatment were given hemisplenic KCKO-luc cell. (A) Representative IVIS imaging of hepatic tumor metastasis day 14 post tumor injection. (B) Tumor growth in mice. Data are expressed as mean ± SEM. *, p<0.05 by paired t-test. (C) Survival of mice. *, p<0.05 by log-rank (Mantel-Cox) test. (D) Conferred protection by adoptive transfer of CD8+ T cells isolated from mice that rejected hemisplenic tumor rechallenge. Tumor growth monitored by IVIS imaging. Data are expressed as mean ± SEM. *, p<0.05 by paired t-test.
To further assess whether CD8+ T cells were mediating this conferred protection, we performed an adoptive T cell transfer experiment from cured mice to naïve mice. Purified CD8+ T cells were isolated from lymph nodes and spleens of mice from Figure 6 that cleared tumors after concurrent SBRT and mFX. These cells were adoptively transferred into naïve mice followed by orthotopic pancreatic injection of KCKO-luc cells one day later. As a control, CD8+ T cells isolated from naïve mice were transferred to naïve recipients followed by injection of KCKO-luc cells one day later. Mice that received adoptive transfer of CD8+ T cells from animals that cleared tumors exhibited significantly reduced tumor growth when compared to naïve mice (Fig. 6D). These data indicated that CD8+ T cells were largely responsible for conferring antitumor immunity following concurrent therapy.
Local SBRT treatment enhanced efficacy of systemic chemotherapy on distal metastasis
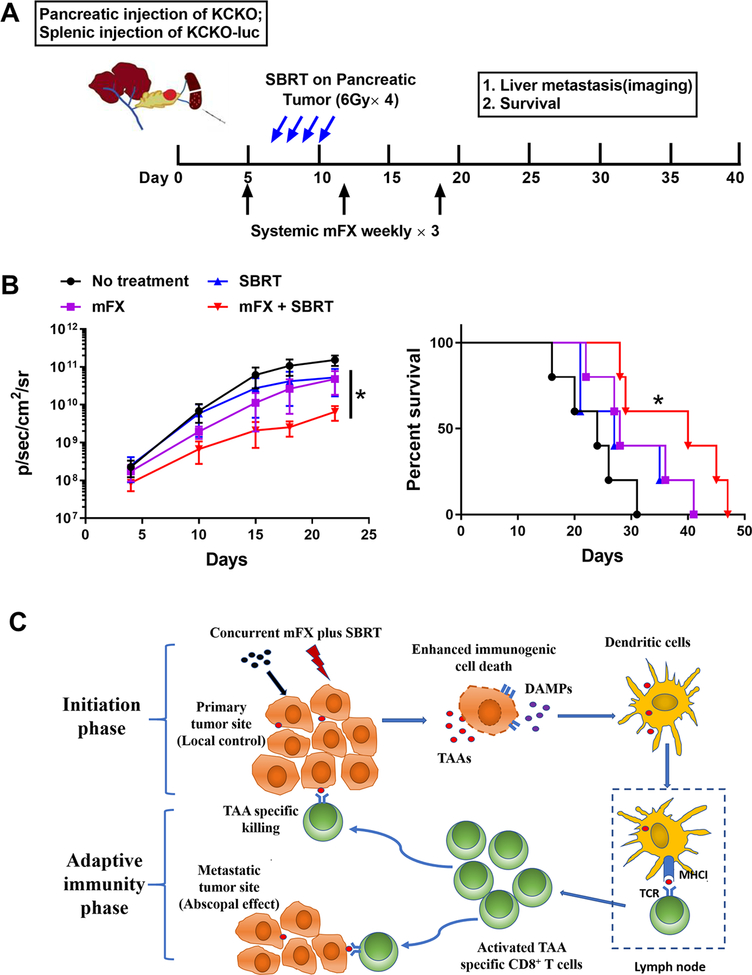
Our data suggested that systemic antitumor immunity was generated by concurrent SBRT and mFX treatment. This is of particular importance as many patients present both primary and metastatic lesions to which there is limited effective therapies. We developed a preclinical model that recapitulates metastatic PDAC where a primary pancreatic tumor was established with KCKO cells (these cells do not express luciferase) followed immediately by a hemisplenic injection of KCKO-luc cells to generate hepatic metastases (Fig. 7A): tracking tumors by bioluminescence only detected hepatic metastatic PDAC lesions. Tumor-bearing mice were left untreated, treated with mFX alone, SBRT alone, or concurrent therapy as described in Figure 1A. SBRT was localized to the primary tumor only; liver metastases were outside the field of treatment. Only the concurrent treatment group resulted in a significant decrease of hepatic metastases and enhanced survival when compared to other groups (Fig. 7B). These data demonstrated that concurrent use of systemic mFX and localized SBRT could achieve stronger systemic immune responses that was capable of controlling metastases in PDAC.
Figure 7. Local SBRT treatment of pancreatic cancer enhanced efficacy of systemic chemotherapy on liver metastasis.
(A) Schematic of mice orthotopic pancreatic cancer and hepatic metastasis establishment and SBRT and mFX treatment. (B) The growth of liver metastases (KCKO-luc) was analyzed by IVIS imaging and survival was observed. Left panel, tumor growth(IVIS imaging). Data are expressed as mean ± SEM from 5 mice/group. *p<0.05, mFX+SBRT compared with mFX treatment group by one-way ANOVA with Dunnett post-test. Right panel, mice survival. *p<0.05, mFX+SBRT compared with no treatment group by log-rank (Mantel-Cox) test. (C) Proposed model of SBRT plus mFX-induced antitumor immune response consisting of both initiating and adaptive immune elements. In the initiating phase, the concurrent treatment amplified ICD, which was evident by increased DAMPs expressed or released by dying tumor cells. These DAMPs facilitated the recruitment and activation of DCs, which uptake, process, and present tumor-associated antigens to CD8+ T cells, resulting in activated tumor-specific CD8+ T cells. In adaptive immune phase, activated tumor-specific CD8+ T cells migrated to the primary tumor site, leading to a local antitumor response. Concurrently, CD8+ T cells may have migrated to distal tumor metastatic sites, resulting in systemic or abscopal antitumor effect.
Discussion:
Immune cells play an integral role in mediating many of the antitumor effects of cancer treatment modalities such as radiation and chemotherapy, as loss of therapeutic efficacy occurs when components of the immune system are experimentally depleted (30–32). The TME consists of a multitude of immune cells that can ultimately influence, either positively or negatively, treatment outcome, thus these cells must be considered when designing cancer treatments (33). Here, we used ICD to determine the optimal treatment schedule of SBRT and modified chemotherapy to treat a recalcitrant malignancy such as PDAC. Changes in the dose and schedule of both SBRT and mFX greatly impacted the immune response, which subsequently dictated outcome. Our data not only provided mechanistic insight into how the concurrently administered combination of SBRT and mFX was eliciting a potent antitumor effect (both local and systemic), but also highlighted the importance of tumor immunology when designing treatment regimens. The proposed immunological mechanism is illustrated in Figure 7C and consists of both initiating and adaptive immune elements.
Initiation Phase (top of Figure 7C):
Tumors, including PDAC, can have immunosuppressive TMEs, making it difficult to initiate and/or sustain a productive antitumor response (34). Therapies that recondition the immunosuppressive TME to a immunostimulatory TME can drive antitumor immunity (35). To repolarize an established immunosuppressive microenvironment, factors that initiate immune responses, such as DAMPs can be activated to first trigger innate immune cells (36). Therefore, we proposed that measuring the magnitude of induction of intratumoral DAMPs, which are expressed or released by tumor cells undergoing ICD, could serve as readout for immune response initiation. Administration of either SBRT or chemotherapy alone induced DAMPs (13, 37), however changing the administration schedule of both treatment modalities resulted in a marked increase of these molecules and dictated efficacy and outcome. Both our in vitro and in vivo data indicated that concurrent, but not sequential, administration of SBRT + mFX stimulated the greatest release of DAMPs. In our model, the possible mechanism of action supporting the observation that DAMPs were increased following concurrent SBRT + mFX may be that mFX acts as a radiosensitizing agent, which allows for heightened tumor cell death (and ICD) by SBRT. Both 5-FU and Oxaliplatin (components of FOLFIRINOX) augment double-stranded DNA breaks by either inhibiting DNA repair pathways (38) or by facilitating interstrand crosslinks that promote nucleotide damage and cell cycle arrest/cell death (39). These factors may directly amplify the cytotoxic nature of SBRT or simply be an additive effect to the existing ICD-inducing properties of radiotherapy. Regardless, concurrent use of SBRT and mFX initiated a potent initiation phase, which in turn, generated stronger antitumor immunity.
DCs were critical during the initiation phase as depletion of this cell subset significantly decreased treatment efficacy, suggesting that DCs may have stimulated the antitumor immune response observed concurrent therapy. Only concurrent therapy resulted in a heightened mature DC phenotype (increases of co-stimulatory marker and MHCII), that was closely associated with the augmentation of DAMPs in the TME. Concurrent therapy resulted in greater tumor antigen-presenting DCs in the draining lymph node and spleen, suggestive of increased DC trafficking from the tumor and/or a greater propensity of DC to process and present tumor antigen ultimately resulting in an elevated density of antigen-carrying DCs in lymphoid tissue. Our data cannot dissociate whether this enhanced response was solely due to increased antigen availability, a qualitative difference in DCs on a per cell basis, or a combination of both factors. Collectively, our data demonstrated that the magnitude of the initial immune phase was important and suggests that targeting this stage, either by promoting ICD with radiosensitizing reagents, anthracyclines, photodynamic therapy, or oncolytic viruses (13, 40, 41), along with DC maturation (e.g. TLR agonists, etc.) (42), may help to optimize antitumor responses.
Adaptive Immunity Phase (Bottom of Figure 7C):
Concurrent SBRT + mFX therapy induced a potent effector phase. The link between the magnitude of the initial antitumor response and subsequent effector phase was not surprising; however, a number of mechanistic factors were likely contributing to the heightened CD8+ T cell responses. Concurrent therapy increased the density of intratumoral antigen-specific CD8+ T cells that were capable of secreting IFNγ. The typical PDAC TME is highly immunosuppressive and inhospitable to adaptive T cell responses (43). For example, PDAC is comprised of abundant immunosuppressive myeloid cells (44). Fortunately, these myeloid cells are very plastic suggesting that a therapy (such as concurrent therapy) that results in a profound change in the TME can reprogram these cells to be immunostimulatory, rather than suppressive (44). This is likely the first step that allows intratumoral T cells a legitimate chance to elicit effector function. Once this occurs, CD8+ T cells can produce IFNγ, which amplifies the antitumor response causing a self-sustaining type of mechanism (45). For example, dampening of the immunosuppressive TME allows CD8+ T cells to produce IFNγ, induce IFNγ-mediated events, such as establishment of chemokine gradients (mediated by CXCL10, CXCL9, etc.), and upregulate vascular adhesion molecules (e.g. ICAM-1, VCAM-1), which facilitate extravasation of antitumor cells capable of recognizing and lysing tumor cells (46). It is possible that intratumoral myeloid cells may repolarize back to an immunosuppressive phenotype or heightened concentrations of IFNγ may result in exhausted T effector cells. In this scenario, immunotherapy targeting myeloid cell elimination/reprogramming, or immune checkpoint inhibitors may be added to sustain the antitumor T cell response initiated by concurrent therapy (18, 47–49).
The abscopal effect, defined as a systemic antitumor response generated by local radiotherapy, has been reported preclinically and clinically, but is overall infrequent, suggesting that radiotherapy alone is insufficient to induce a strong enough systemic response (50, 51). We hypothesized that increased ICD elicited by concurrent use of SBRT and mFX may amplify the systemic immune response and result in the control/elimination of distal disease. Our data supports the generation of a systemic immune response following concurrent therapy as rechallenge of mice that cleared tumors failed to develop new tumors, immunity could be adoptively transferred to another mouse, and therapy targeting the primary pancreatic tumor reduced distal liver metastases. Tumor antigen-presenting DCs were present in draining lymph node, distal lymph nodes, and spleen, suggestive of systemic immunity at that particular time point. Overall, our data advocates for establishing optimal treatment combination/schedules that promote the abscopal effect.
Toxicity is a clinical concern when both SBRT and mFX are given concurrently (8). However, our data demonstrated that the minimal toxicity was similar between the concurrent treatment group and mFX alone. The observed tolerable levels of toxicity were likely a result of the treatment modality and dose. Due to the inherent toxic nature of chemotherapy, using a lesser dose of FX therapy reduces toxicity and, in our model, was more effective than full dose chemotherapy. Our preclinical toxicity observations are supported by clinical retrospective studies demonstrating that concurrent administration of SBRT and chemotherapy achieved effective local tumor control with minimal/well tolerated toxicity (52–54). The concurrent dosing regimen reported here is expected to promote an optimal immune response as SBRT utilizes fewer fractions than conventional radiotherapy meaning that radiosensitive antitumor lymphocytes are likely not destroyed by the repeated radiation fractions given in the conventional schedule. Along those same lines, the modified (lesser) dose of chemotherapy will likely spare these critical immune cells, which would have been eliminated or functionally inhibited to a greater extent by the full dose chemotherapy schedule (55).
Altogether, our preclinical data provide rationale for the clinical translation of concurrent SBRT and mFX to treat PDAC in a safe and efficacious manner. Concurrent therapy converted tumor cells into an endogenous vaccine by exposing tumor-associated antigen in an inflammatory context, which stimulated both local and systemic antitumor immunity. Moving forward, utilizing ICD as a therapeutic readout may allow for optimal treatment scheduling and improved cancer patient outcome.
Supplementary Material
Acknowledgements:
The authors would like to thank Eric Hernady for SBRT treatment of our mice. This work was partially supported by grants from the National Institutes of Health (R01CA168863 to DCL; 1P50CA196510-01A1 to SAG & DCL; R01CA230277 to SAG; R01CA28332 to EML/SAG) and the Pancreatic Cancer Action Network (PANCAN) Translational Research Grant (15-65-25 LINE to DCL).
Footnotes
Conflict-of-interest disclosure
The authors declare no competing financial interests.
References
- 1.Siegel RL, Miller KD, and Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, and Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer research. 2014;74(11):2913–21. [DOI] [PubMed] [Google Scholar]
- 3.De Bari B, Porta L, Mazzola R, Alongi F, Wagner AD, Schafer M, et al. Hypofractionated radiotherapy in pancreatic cancer: Lessons from the past in the era of stereotactic body radiation therapy. Critical reviews in oncology/hematology. 2016;103:49–61. [DOI] [PubMed] [Google Scholar]
- 4.Rosati LM, Kumar R, and Herman JM. Integration of Stereotactic Body Radiation Therapy into the Multidisciplinary Management of Pancreatic Cancer. Seminars in radiation oncology. 2017;27(3):256–67. [DOI] [PubMed] [Google Scholar]
- 5.Petrelli F, Comito T, Ghidini A, Torri V, Scorsetti M, and Barni S. Stereotactic Body Radiation Therapy for Locally Advanced Pancreatic Cancer: A Systematic Review and Pooled Analysis of 19 Trials. International journal of radiation oncology, biology, physics. 2017;97(2):313–22. [DOI] [PubMed] [Google Scholar]
- 6.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. The New England journal of medicine. 2011;364(19):1817–25. [DOI] [PubMed] [Google Scholar]
- 7.Tong H, Fan Z, Liu B, and Lu T. The benefits of modified FOLFIRINOX for advanced pancreatic cancer and its induced adverse events: a systematic review and meta-analysis. Scientific reports. 2018;8(1):8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaib WL, Hawk N, Cassidy RJ, Chen Z, Zhang C, Brutcher E, et al. A Phase 1 Study of Stereotactic Body Radiation Therapy Dose Escalation for Borderline Resectable Pancreatic Cancer After Modified FOLFIRINOX (). International journal of radiation oncology, biology, physics. 2016;96(2):296–303. [DOI] [PubMed] [Google Scholar]
- 9.O’Rourke N, Roque IFM, Farre Bernado N, and Macbeth F. Concurrent chemoradiotherapy in non-small cell lung cancer. The Cochrane database of systematic reviews. 2010(6):CD002140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golden EB, and Apetoh L. Radiotherapy and immunogenic cell death. Seminars in radiation oncology. 2015;25(1):11–7. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y, Kepp O, Ghiringhelli F, Apetoh L, Aymeric L, Locher C, et al. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Seminars in immunology. 2010;22(3):113–24. [DOI] [PubMed] [Google Scholar]
- 12.Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. 2014;3(9):e955691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garg AD, More S, Rufo N, Mece O, Sassano ML, Agostinis P, et al. Trial watch: Immunogenic cell death induction by anticancer chemotherapeutics. Oncoimmunology. 2017;6(12):e1386829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kroemer G, Galluzzi L, Kepp O, and Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. [DOI] [PubMed] [Google Scholar]
- 15.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4(6):437–50. [DOI] [PubMed] [Google Scholar]
- 16.Besmer DM, Curry JM, Roy LD, Tinder TL, Sahraei M, Schettini J, et al. Pancreatic ductal adenocarcinoma mice lacking mucin 1 have a profound defect in tumor growth and metastasis. Cancer research. 2011;71(13):4432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbett TH, Roberts BJ, Leopold WR, Peckham JC, Wilkoff LJ, Griswold DP Jr., et al. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer research. 1984;44(2):717–26. [PubMed] [Google Scholar]
- 18.Nywening TM, Belt BA, Cullinan DR, Panni RZ, Han BJ, Sanford DE, et al. Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut. 2018;67(6):1112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim MP, Evans DB, Wang H, Abbruzzese JL, Fleming JB, and Gallick GE. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc. 2009;4(11):1670–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soares KC, Foley K, Olino K, Leubner A, Mayo SC, Jain A, et al. A preclinical murine model of hepatic metastases. Journal of visualized experiments : JoVE. 2014(91):51677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mills BN, Connolly KA, Ye J, Murphy JD, Uccello TP, Han BJ, et al. Stereotactic Body Radiation and Interleukin-12 Combination Therapy Eradicates Pancreatic Tumors by Repolarizing the Immune Microenvironment. Cell Rep. 2019;29(2):406–21 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye J, Ma C, Wang F, Hsueh EC, Toth K, Huang Y, et al. Specific recruitment of gammadelta regulatory T cells in human breast cancer. Cancer research. 2013;73(20):6137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, and Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516–23. [DOI] [PubMed] [Google Scholar]
- 24.Hossain DMS, Javaid S, Cai M, Zhang C, Sawant A, Hinton M, et al. Dinaciclib induces immunogenic cell death and enhances anti-PD1-mediated tumor suppression. The Journal of clinical investigation. 2018;128(2):644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–91. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Divito SJ, Shufesky WJ, Sumpter T, Wang H, Tkacheva OA, et al. Dendritic cell therapies in transplantation revisited: deletion of recipient DCs deters the effect of therapeutic DCs. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(6):1398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, and Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(12):3100–4. [DOI] [PubMed] [Google Scholar]
- 28.Obeid M. ERP57 membrane translocation dictates the immunogenicity of tumor cell death by controlling the membrane translocation of calreticulin. Journal of immunology. 2008;181(4):2533–43. [DOI] [PubMed] [Google Scholar]
- 29.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kieler M, Unseld M, Bianconi D, and Prager G. Challenges and Perspectives for Immunotherapy in Adenocarcinoma of the Pancreas: The Cancer Immunity Cycle. Pancreas. 2018;47(2):142–57. [DOI] [PubMed] [Google Scholar]
- 31.Walle T, Martinez Monge R, Cerwenka A, Ajona D, Melero I, and Lecanda F. Radiation effects on antitumor immune responses: current perspectives and challenges. Therapeutic advances in medical oncology. 2018;10:1758834017742575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang YJ, Fletcher R, Yu J, and Zhang L. Immunogenic effects of chemotherapy-induced tumor cell death. Genes & diseases. 2018;5(3):194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wormann SM, Diakopoulos KN, Lesina M, and Algul H. The immune network in pancreatic cancer development and progression. Oncogene. 2014;33(23):2956–67. [DOI] [PubMed] [Google Scholar]
- 34.Foucher ED, Ghigo C, Chouaib S, Galon J, Iovanna J, and Olive D. Pancreatic Ductal Adenocarcinoma: A Strong Imbalance of Good and Bad Immunological Cops in the Tumor Microenvironment. Frontiers in immunology. 2018;9:1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson BA, 3rd, Yarchoan M, Lee V, Laheru DA, and Jaffee EM. Strategies for Increasing Pancreatic Tumor Immunogenicity. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(7):1656–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, and Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–75. [DOI] [PubMed] [Google Scholar]
- 37.Vacchelli E, Vitale I, Tartour E, Eggermont A, Sautes-Fridman C, Galon J, et al. Trial Watch: Anticancer radioimmunotherapy. Oncoimmunology. 2013;2(9):e25595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rich TA, Shepard RC, and Mosley ST. Four decades of continuing innovation with fluorouracil: current and future approaches to fluorouracil chemoradiation therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(11):2214–32. [DOI] [PubMed] [Google Scholar]
- 39.Hill EJ, Nicolay NH, Middleton MR, and Sharma RA. Oxaliplatin as a radiosensitiser for upper and lower gastrointestinal tract malignancies: what have we learned from a decade of translational research? Critical reviews in oncology/hematology. 2012;83(3):353–87. [DOI] [PubMed] [Google Scholar]
- 40.Garg AD, and Agostinis P. ER stress, autophagy and immunogenic cell death in photodynamic therapy-induced anti-cancer immune responses. Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology. 2014;13(3):474–87. [DOI] [PubMed] [Google Scholar]
- 41.Guo ZS, Lu B, Guo Z, Giehl E, Feist M, Dai E, et al. Vaccinia virus-mediated cancer immunotherapy: cancer vaccines and oncolytics. Journal for immunotherapy of cancer. 2019;7(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roses RE, Datta J, and Czerniecki BJ. Radiation as immunomodulator: implications for dendritic cell-based immunotherapy. Radiation research. 2014;182(2):211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez-Bosch N, Vinaixa J, and Navarro P. Immune Evasion in Pancreatic Cancer: From Mechanisms to Therapy. Cancers. 2018;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pergamo M, and Miller G. Myeloid-derived suppressor cells and their role in pancreatic cancer. Cancer gene therapy. 2017;24(3):100–5. [DOI] [PubMed] [Google Scholar]
- 45.Tsukumo SI, and Yasutomo K. Regulation of CD8(+) T Cells and Antitumor Immunity by Notch Signaling. Frontiers in immunology. 2018;9:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peske JD, Woods AB, and Engelhard VH. Control of CD8 T-Cell Infiltration into Tumors by Vasculature and Microenvironment. Advances in cancer research. 2015;128:263–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer research. 2013;73(3):1128–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zappasodi R, Wolchok JD, and Merghoub T. Strategies for Predicting Response to Checkpoint Inhibitors. Current hematologic malignancy reports. 2018;13(5):383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kabacaoglu D, Ciecielski KJ, Ruess DA, and Algul H. Immune Checkpoint Inhibition for Pancreatic Ductal Adenocarcinoma: Current Limitations and Future Options. Frontiers in immunology. 2018;9:1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siva S, MacManus MP, Martin RF, and Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer letters. 2015;356(1):82–90. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Dong Y, Kong L, Shi F, Zhu H, and Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. Journal of hematology & oncology. 2018;11(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lischalk JW, Burke A, Chew J, Elledge C, Gurka M, Marshall J, et al. Five-Fraction Stereotactic Body Radiation Therapy (SBRT) and Chemotherapy for the Local Management of Metastatic Pancreatic Cancer. Journal of gastrointestinal cancer. 2018;49(2):116–23. [DOI] [PubMed] [Google Scholar]
- 53.Lin JC, Jen YM, Li MH, Chao HL, and Tsai JT. Comparing outcomes of stereotactic body radiotherapy with intensity-modulated radiotherapy for patients with locally advanced unresectable pancreatic cancer. European journal of gastroenterology & hepatology. 2015;27(3):259–64. [DOI] [PubMed] [Google Scholar]
- 54.Gurka MK, Kim C, He AR, Charabaty A, Haddad N, Turocy J, et al. Stereotactic Body Radiation Therapy (SBRT) Combined With Chemotherapy for Unresected Pancreatic Adenocarcinoma. American journal of clinical oncology. 2017;40(2):152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu J, and Waxman DJ. Immunogenic chemotherapy: Dose and schedule dependence and combination with immunotherapy. Cancer letters. 2018;419:210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.