Abstract
Poly (ADP-ribose) Polymerase (PARP) inhibitors (PARPi) are approved to treat recurrent ovarian cancer with BRCA1 or BRCA2 mutations, and as maintenance therapy for recurrent platinum sensitive ovarian cancer (BRCA wild-type or mutated) after treatment with platinum. However, the acquired resistance against PARPi remains a clinical hurdle. Here, we demonstrated that PARP inhibitor (olaparib)-resistant epithelial ovarian cancer (EOC) cells exhibited an elevated aldehyde dehydrogenase (ALDH) activity, mainly contributed by increased expression of ALDH1A1 due to olaparib-induced expression of BRD4, a member of bromodomain and extraterminal (BET) family protein. We also revealed that ALDH1A1 enhanced microhomology-mediated end joining (MMEJ) activity in EOC cells with inactivated BRCA2, a key protein that promotes homologous recombination (HR) by using an intra-chromosomal MMEJ reporter. Moreover, NCT-501, an ALDH1A1 selective inhibitor, can synergize with olaparib in killing EOC cells carrying BRCA2 mutation in both in vitro cell culture and the in vivo xenograft animal model. Given MMEJ activity has been reported to be responsible for PARPi resistance in HR deficient cells, we conclude that ALDH1A1 contributes to the resistance to PARP inhibitors via enhancing MMEJ in BRCA2−/− ovarian cancer cells. Our findings provide a novel mechanism underlying PARPi resistance in BRCA2 mutated EOC cells, and suggest that inhibition of ALDH1A1 could be exploited for preventing and overcoming PARPi resistance in EOC patients carrying BRCA2 mutation.
Introduction
Ovarian cancer is the most lethal malignancy of the female reproductive tract with a five-year survival rate of only 29% in distant stages, at which approximately 60% of cases are diagnosed (1). It is estimated that in 2019, about 22,530 new cases of ovarian cancer will be diagnosed and 13,980 women will die of ovarian cancer in the United States (1). Over 90% of ovarian cancers are epithelial in origin, and epithelial ovarian cancer (EOC), especially the most aggressive subtype high-grade serous ovarian cancer (HGSOC), accounts for the majority of ovarian cancer deaths (2, 3). Despite the progress of cancer treatment, long-term survival in women with EOC has not increased significantly in the last 25 years (4).
Poly (ADP-ribose) polymerase (PARP) inhibitors are an exciting and promising new class of anticancer drugs. PARP inhibitors (PARPi) induce stalled replication forks by trapping the inactive PARP protein on DNA and/or inhibiting single strand breaks (SSBs) repair (5, 6). The stalled replication forks, if not rescued, can be converted to more deleterious double strand breaks (DSBs). DSBs are mainly repaired by error-free homologous recombination (HR), which is mediated by BRCA1 and BRCA2, as well as error-prone non-homologous end joining (NHEJ). The alternative NHEJ (alt-NHET), also called microhomology-mediated end joining (MMEJ), also plays a role in repairing DSBs, particularly in HR-deficient cells (7, 8). PARPi has been shown to be synthetically lethal with defective HR repair (9, 10) because the DSBs caused by PARP inhibition depends on HR to repair. In contrast, enhanced classical NHEJ (c-NHEJ) promotes the cytotoxicity of HR-deficient cells treated with PARPi (11). PARPi have been approved by FDA for recurrent ovarian cancer with BRCA1 or BRCA2 mutations, and as maintenance therapy after frontline therapy for BRCA mutated ovarian cancer, and as maintenance for recurrent platinum sensitive ovarian cancer after treatment with platinum regardless of BRCA mutation. Thus, the number of patients taking PARPi is increasing rapidly. However, resistance has been observed, and patients receiving PARPi eventually develop cancer progression. Given that the greatest benefit of PARPi is seen in patients with BRCA mutations (>3 yrs improvement in PFS) than those without BRCA mutations (3-15 months improvement in PFS) (12), understanding the mechanism underlying PARPi resistance in BRCA mutated EOCs is particularly important.
Aldehyde dehydrogenase (ALDH) is a superfamily of 19 known enzymes participated in metabolism of endogenous and exogenous aldehydes (13). High ALDH activity is observed in cancer stem cells (CSCs) of multiple cancer types, and is often used to isolate and functionally characterize CSCs (14). In addition, the high ALDH activity has also been correlated with chemotherapy resistance in various cancers (15-18). ALDH1A1 is a major member in the ALDH superfamily contributing to the ALDH activity. ALDH1A1 is upregulated more than 100-fold in ovarian cancer cells selected for taxane resistance in vitro, and ALDH1A1 knockdown reversed this chemotherapy resistance (19). Chemotherapy can also increase ALDH1A1 expression in patients and patient-derived ovarian tumor xenografts (20, 21). ALDH can mediate resistance to chemotherapy via direct drug metabolism and by regulation of reactive oxygen species (ROS), preventing ROS-mediated apoptosis in the drug-tolerant subpopulation (22). ALDH1A1-mediated platinum resistance also correlates to altered DNA repair networks in the A2780 ovarian cancer cell line (23). However, it is unknown whether ALDH activity affects the sensitivity of EOC cells to PARPi, and whether ALDH1A1 can be proposed as a therapeutic target to enhance PARPi efficacy in EOC.
In this study, we demonstrated that PARPi can enhance the ALDH activity in BRCA2 mutated EOC cells, mainly through Bromodomain-containing protein 4 (BRD4)-mediated enhancement of ALDH1A1 expression. ALDH1A1 reduces the sensitivity of BRCA2−/− EOC cells to PARPi, probably by augmenting MMEJ-mediated DSB repair. Selectively targeting ALDH1A1 by its inhibitor NCT-501 significantly sensitized BRCA2−/− EOC cells to PARPi and rescued the sensitivity of PARPi-resistant BRCA2−/− EOC cells to olaparib.
Materials and Methods
Cell lines and reagents
Epithelial ovarian cancer cell lines PEO1 (BRCA2 −/−) and PEO4 (BRCA2 wild-type) (24) were kindly provided by Dr. Thomas C. Hamilton (Fox Chase Cancer Center), and Kuramochi (BRCA2−/−) (25) were kindly provided by Dr. Adam Karpf (University of Nebraska Medical Center). All cell lines were authenticated by ATCC using the DNA (short tandem repeat) profiling and tested for mycoplasma contamination on 1/22/2019. PEO1-Olaparib-Resistant cell line (PEO1-R) and Kuramochi-Olaparib-Resistant cell line (Kura-R) were generated from the parental PEO1 and Kuramochi cells, respectively, by intermittent, incremental, in vitro treatment with PAPRi olaparib from 2 μM to 20 μM for 6 months. PEO1, PEO1-R, Kuramochi, Kura-R cells were maintained in RPMI-1640 medium supplemented with 10% FBS, 100 μg/ml streptomycin and 100 units/ml penicillin. The H1299-pCAM-1810-GFP cell line was established by stably transfecting a MMEJ reporter vector pCMV/I-SceI/GFP into H1299 cells (26). NHEJ reporter cells HEK293-pPHW1 were kindly provided by Dr. Kay Huebner (The Ohio State University). These cell lines were maintained in DMEM supplemented with 10% FBS, 100 μg/ml streptomycin and 100 units/ml penicillin. All cells were grown at 37° C in humidified atmosphere of 5% CO2, and used within 20 passages after recovered from liquid nitrogen. ALDH1A1 selective inhibitor NCT-501 was purchased from MedChemExpress (MCE, Monmouth Junction, NJ). PARPi olaparib, rucaparib, and niraparib were purchased from Selleckchem (Houston, TX). Olaparib and NCT-501 were dissolved in DMSO for in vitro cell treatment. For treating mice, olaparib was dissolved in DMSO and 10% 2-hydroxy-propyl-β-cyclodextrin (HPβCD)/saline to yield a solution of 10 mg/mL; NCT-501 was dissolved in 5% HPβCD/saline to a final concentration of 2 mg/mL.
Plasmid and siRNA transfection
pCDNA3.1-ALDH1A1 plasmids were generated in our laboratory. 1 μg ALDH1A1 expression vector or pCDNA3.1 empty vector was transfected into PEO1 cells using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA) according to the manufacture’s instruction or by electroporation with NEPA-21 Electroporator (Nepa Gene Co., Ltd). siRNA designed to target human ALDH1A1 or BRD4 (Supplementary Table S1) were purchased from Dharmacon Inc (Denver, CO). 100 nM of siRNA was transfected into cells by Lipofectamine 2000 transfection reagent.
Cell survival measurement
Cells were seeded in 96-well plates at an initial density of 1-2 × 103, incubated for 24 h, and treated with various doses of PARPi or the ALDH1A1 inhibitor for 7 days. Cells were then washed with PBS, fixed with 3.7% formaldehyde for 30 min, and stained with 1.0% methylene blue for 60 min. The plate was rinsed in running water and then left to dry. 100 μl of solvent (10% acetic acid, 50% methanol and 40% H2O) was added to each well to dissolve the cells. Optical density (OD) of the released color was read at 630 nm. The relative cell survival was calculated with the values of vehicle-treated cells set as 100%. Combination index (CI) was calculated by Chou’s median-effect method (27) using CompuSyn Software. CI < 0.9, CI= 0.9-1.1, and CI > 1.1 denote synergistic effect, additive effect, and antagonistic effect, respectively.
ALDH analysis and cell sorting
The ALDEFLUOR Assay kit (STEMCELL Technology) was used to analyze ALDH activity in cells, and sort ALDH-dim (ALDHdim) and ALDH-bright (ALDHbr) cells by using flow cytometry. Briefly, cells were incubated with ALDEFLUOR reagents at 37°C for 45 min according to the manufacture’s instruction. For each sample, one portion of cells was treated with 50 mM diethylaminobenzaldehyde (DEAB) to define the negative gate. After incubation, ALDEFLUOR reagents were removed; cells were re-suspended in assay buffer and subjected to a BD LSR II Flow cytometer for analysis, or a BD Aria III Flow Cytometer for sorting.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was purified from various cell samples using Trizol (ThermoFisher Scientific). The cDNA was synthesized by the reverse transcription system (Applied Biosystem) in a 20 μl reaction containing 1 μg of total RNA. An aliquot of 0.5 μl cDNA was used in each 20 μl PCR reaction, using Fast SYBR Green PCR Master Mix (Applied Biosystem) and reactions were run on an ABI 7500 Fast Real-Time PCR system. The primers used for PCR are listed in Supplemental Table S2.
BRCA2 gene mutation analysis
Total RNA was extracted from Kuramochi cells and cDNA was generated as described above. 40 ng cDNA was amplified by PCR in a 25 μL reaction containing 20 pmol of each primer, 200 μM of each dNTP, 1 unit of Taq DNA polymerase, and 2 mM MgSO4. PCR products were then purified by using QIAquick PCR purification Kit (QIAGEN, Cat #28106). 5 ng of final purified PCR product was added in a 12 μL system containing 6.4 pmol primer and subjected to Sanger Sequencing analysis (Genomics Shared Resource, OSUCCC). The primers used for PCR amplification and sequencing of fragment covering c.6952 are listed in Supplemental Table S2.
Immunoblotting
Whole-cell lysates were prepared by boiling cell pellets for 10 min in SDS lysis buffer [2% SDS, 10% glycerol, 62 mmol/L Tris-HCl, pH 6.8, and a complete mini-protease inhibitor mixture (Roche Applied Science)]. After protein quantification, equal amounts of proteins were loaded, separated on a polyacrylamide gel, and transferred to a nitrocellulose membrane. Protein bands were immuno-detected with appropriate antibodies: anti-ALDH1A1 (Cell Signaling, #54135), anti-BRD4 (Cell Signaling, #13440), anti-β-Tubulin (Cell Signaling, #2148), and anti-GAPDH (Santa Cruz, Sc-47724).
Immunofluorescence
PEO1 cells sorted by flow cytometry after staining with ALDEFLUOR reagent or transfected with ALDH1A1 expression plasmid were grown on the coverslips, and then treated with 10 μM olaparib for 1h. Cells were further cultured for 1, 8 or 24 h in the drug-free medium. Cells were fixed and permeabilized with 2% paraformaldehyde and 0.5% Triton X-100. After blocking with 20% normal goat serum, cells were stained with mouse anti-γH2AX or rabbit anti-Rad51 antibody for 1 h at room temperature, washed with TBST 4 times, and then incubated with anti-mouse IgG conjugated with FITC or Texas Red, or anti-rabbit IgG conjugated with Texas Red. Fluorescence images were obtained with a Nikon fluorescence microscope E80i (Nikon, Tokyo, Japan). The digital images were then captured with a Nikon camera and processed with the help of its software.
MMEJ activity detection
H1299 cells stably transfected with a single-copy of a MMEJ reporter vector pCMV/I-SceI/GFP (28) were generated in Dr. Junran Zhang’s lab (26). These cells were first transfected with empty vector (EV) or ALDH1A1 expression vector by electroporation for 2 days. Cells were then cotransfected with EV or ALDH1A1 plasmids, along with I-SceI expression vector (pCBASce). Cells were harvested after 2 days, and the GFP-positive cells were analyzed using flow cytometry.
NHEJ activity detection
The effect of ALDH1A1 on the NHEJ activity was analyzed as described in (29). HEK293 cells containing the NHEJ reporter plasmid pPHW1 were transfected with ALDH1A1 and I-SceI expression plasmids. After 2 days, the genomic DNA was isolated, the NHEJ product (Probe C, 5’-TGC GCC CAT TAC CCT GTT ATC CCT AGA TCT-3’) was quantitated using TaqMan real-time PCR. The primer sequences for the religation substrate were as follows: forward, 5’-GAG GCC TAG GCT TTT GCA AA-3’; and reverse, 5’-TGT ATT TTT CGC TCA TGT GAA GTG T-3’. RNase P probe (ThermoFisher Scientific) was used as an internal control for quantitating ΔΔCt.
Xenograft tumor study
Athymic nude mice (6–8 weeks, female, 20–25 g body weight) were obtained from The Jackson laboratory. Animals’ care was in accordance with institutional guidelines, and all studies were performed with approval of the Institutional Animal Care and Use Committee (IACUC) at the Ohio State University. 2 × 106 PEO1 cells stably expressing Luciferase (PEO1-Luc) were injected into mice intraperitoneally, or 2 ×106 PEO1-R cells were injected into mice subcutaneously, to generate ovarian xenografts. After two weeks, mice were divided into 4 groups, administrated with olaparib (50 mg/kg, once a day) or/and NCT-501 (10 mg/kg, once a day) intraperitoneally for 10 days. Mice in the control group were injected with vehicle reagents (10% HPβCD in saline). Bioluminescence imaging (BLI) was carried out to show the intraperitoneal xenografts. Tumor size was measured using caliper every two days for subcutaneous xenografts.
Statistical analysis
Sample sizes were determined using Power analysis. Descriptive statistics, i.e., means ± SD, are shown on the figures. Two-sample t-tests or ANOVA were performed for data analysis for experiments with two groups or more than two groups’ comparisons. Linear mixed effects models including an interaction term between cell line and dose or time were used to analyze trends across changing doses or times. For all statistical methods, P < 0.05 was considered statistically significant. All tests were two-sided.
Results
PARPi treatment induces ALDH activity in BRCA2 mutated EOC cells
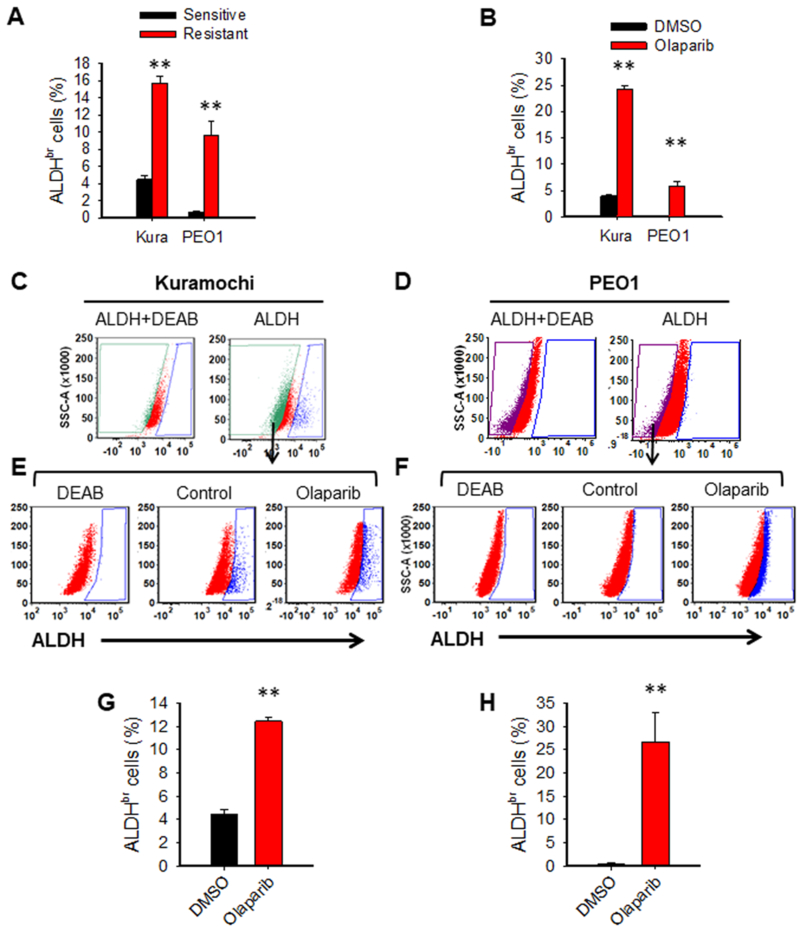
To investigate the mechanisms underlying PARPi resistance, we established two olaparib-resistant cell lines PEO1-R and Kura-R cells by treating two BRCA2 mutated EOC cell lines PEO1 and Kuramochi with low dose of olaparib for 6 months. Both cell lines are not only resistant to olaparib treatment, but also exhibit resistance to another two PARPi, niraparib and rucaparib (Supplementary Fig. S1A, B). Given that ALDH activity is associated to chemotherapy resistance in various cancers, we sought to determine whether ALDH activity is enhanced in PARPi-resistant EOC cells. ALDH activity was measured in PARPi-resistant EOC cells along with their sensitive parental cells using the flow cytometry-based assay, and ALDH-bright (ALDHbr) cells were analyzed with DEAB serving as a negative control. Both PEO1-R and Kura-R cell lines possess increased fraction of ALDHbr cells compared to their corresponding parental cells (Fig. 1A, Supplementary Fig. S2A). We also treated PEO1 and Kuramochi cells with olaparib for a short time, and found that olaparib treatment is able to expand the fraction of ALDHbr cells as well (Fig. 1B, Supplementary Fig. S2B). The enrichment of ALDHbr cells can be achieved by activating the ALDH activity in all cells, or/and by selectively killing fraction of ALDH-dim (ALDHdim) cells by olaparib. To determine whether olaparib can activate ALDH activity in EOC cells, we isolated ALDHdim cells from both Kuramochi and PEO1 cells (Fig. 1C, D), treated them with olaparib or vehicle control for 7 days, and analyzed ALDH activity again. We found that ALDHdim cells can spontaneously convert to ALDHbr cells during culture, particularly in Kuramochi cells (Fig. 1E-H), as we previously reported (30). Most importantly, olaparib treatment significantly enhanced this ALDHdim cell-to-ALDHbr cell conversion (Fig. 1E-H). Taken together, these data indicate that olaparib resistant cells possess highly activated ALDH; olaparib treatment can enhance the ALDH activity in EOC cells, promote the conversion of ALDHdim cells to ALDHbr cells, and eventually expand the ALDHbr cell subpopulation.
Figure 1.
Olaparib treatment expands the ALDHbr cell population by promoting the conversion from ALDHdim to ALDHbr cells in EOC cells. A, Olaparib-resistant EOC cells possess increased ALDHbr cells. The ALDH activity in olaparib-resistant EOC cell line PEO1-R and Kura-R, as well as their corresponding parental cells was analyzed using the ALDEFLUOR assay by flow cytometry. DEAB was used as a negative control. N=3, Bar: SD, **: P < 0.01, compared with their corresponding parental cells. B, Olaparib treatment increases ALDHbr cells in EOC cell lines. PEO1 and Kuramochi cells were treated with olaparib (4 μM) for 7 days, The ALDH activity was analyzed, and ALDHbr cells were determined. N=3, Bar: SD, **: P < 0.01, compared with DMSO treated control cells. C and D, Olaparib treatment increases the conversion from ALDHdim to ALDHbr cells in EOC cells. ALDHdim cells were sorted from Kuramochi (C) and PEO1 (D) cells using FACS. E-H, The ALDHdim Kuramochi cells (E) and ALDHdim PEO1 cells (F) were treated with olaparib (4 μM) for 7 days, and the ALDH activity was analyzed by the ALDEFLUOR assay. DEAB was used as a negative control to define ALDHbr cells. The percentage of ALDHbr cells after treatment in Kuramochi (G) and PEO1 cells (H) was plotted. N = 3, Bar: SD, **: P < 0.01.
ALDH1A1 confers BRCA2 mutated EOC cells resistance to olaparib
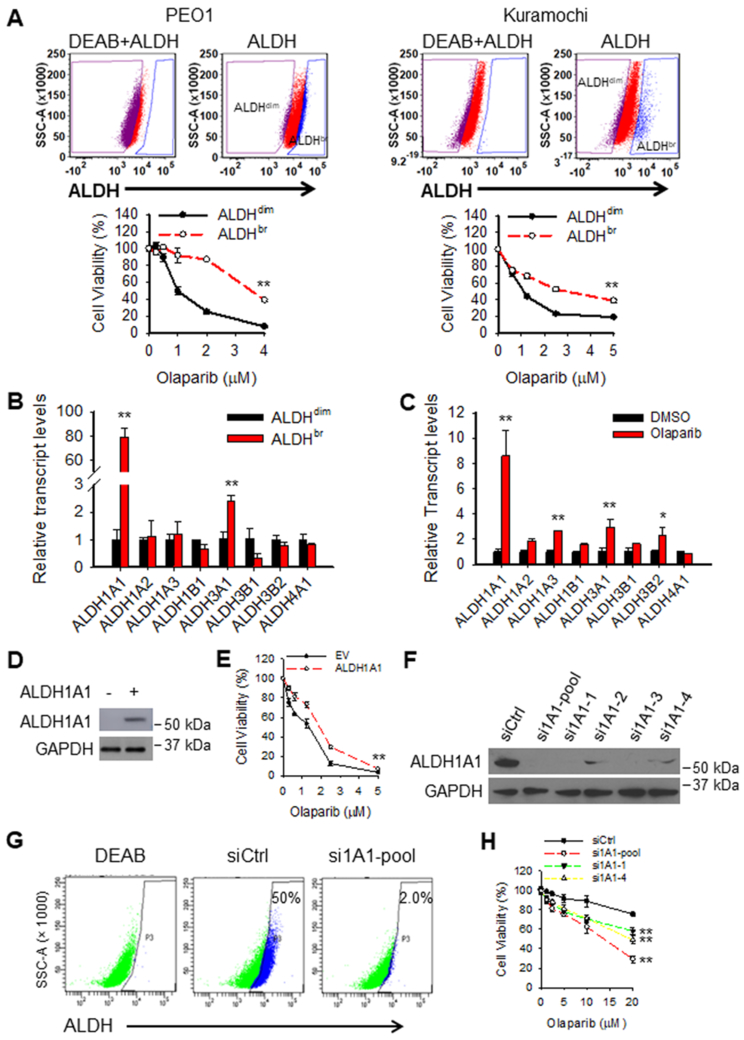
It has been reported that high ALDH activity renders cancer cells resistance to chemotherapy (19). To determine whether ALDH activity plays a role in PARPi resistance in EOC cells, we sorted ALDHdim and ALDHbr cells from both PEO1 and Kuramochi cells, and determined their sensitivity to olaparib. Consistent with the previous study, ALDHbr EOC cells are more resistant to olaparib than ALDHdim cells (Fig. 2A). To further identify which ALDH family gene contributes to the high ALDH activity in ALDHbr EOC cells, we analyzed the mRNA level of 8 most studied ALDH family genes in ALDHdim and ALDHbr PEO1 cells. We found that ALDH1A1 is the most upregulated ALDH family gene in ALDHbr cells compared to ALDHdim PEO1 cells (~80 folds). In addition, ALDH3A1 also increased more than 2 folds in ALDHbr cells than that in ALDHdim PEO1 cells (Fig. 2B). Similarly, ALDH1A1 was also found to be one of the most upregulated ALDH isoforms in ALDHbr cells compared to ALDHdim Kuramochi cells (Supplementary Fig. S3A). We also found that ALDH1A1 is the most induced ALDH family gene in PEO1 cells but not in Kuramochi cells after short-term olaparib treatment (Fig. 2C, Supplementary Fig. S3B). We further analyzed expression of various ALDH isoforms in PARPi-resistant PEO1-R and Kura-R cells, and confirmed that ALDH1A1 is one of the most upregulated ALDH isoforms in these PARPi-resistant EOC cells (Supplementary Fig. S4). These data indicate that ALDH1A1 is the primary isozyme in the ALDH family that is induced by olaparib and contributes to the high ALDH activity in ALDHbr cells. To further determine whether ALDH1A1 is the key ALDH isozyme that renders ALDHbr cells resistance to olaparib, we overexpressed ALDH1A1 in PEO1 cells, and found that ALDH1A1 overexpression significantly reduced the sensitivity of PEO1 cells to olaparib (Fig. 2D and E). We then knocked down the expression of ALDH1A1 in PARPi-resistant PEO1-R cells (Fig. 2F), and found that downregulation of ALDH1A1 can significantly reduce the portion of ALDHbr cells (Fig. 2G) and sensitize these cells to olaparib (Fig. 2H). In addition, knockdown of ALDH1A1 in Kura-R cells also dramatically limited the ALDHbr cell subpopulation and sensitized these cells to olaparib (Supplementary Fig. S5), further suggesting that ALDH1A1 is the major ALDH isoforms contributing to the enhanced ALDH activity in PARPi-resistant cells.
Figure 2.
ALDH1A1 enhances resistance of EOC cells to olaparib. A, ALDHbr cells exhibit resistance to olaparib. ALDHdim and ALDHbr cells were sorted from PEO1 and Kuramochi cells, treated with olaparib at various doses for 7 days, cell viability was determined using methylene blue staining (IC50: PEO1-ALDHdim: 1.43 μM, PEO1-ALDHbr: 3.52 μM; Kura-ALDHdim: 0.82 μM, Kura-ALDHbr: 2.78 μM). N = 3, Bar: SD, **: P < 0.01 compared to the ALDHdim group. B and C, ALDH1A1 is the major ALDH family gene contributes to the ALDH activity in ALDHbr cells and olaparib-induced high ALDH activity in EOC cells. Expression of various ALDH family genes in ALDHdim and ALDHbr cells sorted from PEO1 cells were analyzed using qRT-PCR (B). PEO1 cells were treated with olaparib for 7 days, expression of various ALDH family genes in these cells were determined using qRT-PCR (C). N = 3, Bar: SD, *: P < 0.05; **: P < 0.01 compared to the ALDHdim group and the DMSO group, respectively. D and E, ALDH1A1 overexpression decreased the sensitivity of EOC cells to olaparib. PEO1 cells were transfected with ALDH1A1 expressing plasmids for 48 h, treated with olaparib at various doses for 7 days. Immunoblotting was conducted to determine the expression level of ALDH1A1 after 48 h of transfection (D). Cell viability after olaparib treatment was determined using methylene blue staining (IC50: EV: 1.45 μM, ALDH1A1: 1.9 μM) (E). N = 4, Bar: SD, **: P < 0.01 compared to the EV group. F-H, Knockdown of ALDH1A1 sensitizes EOC cells to olaparib. PEO1-R cells were transfected with ALDH1A1 siRNA for 48 h, treated with olaparib at various doses for 7 days. Immunoblotting was conducted to determine the expression level of ALDH1A1 after 48 h of transfection (F). The ALDEFLUOR assay was used to determine the ALDH activity in these cells after 48 h of transfection (G). Cell viability after olaparib treatment was determined using methylene blue staining (IC50: siCtrl: 65.3 μM, si1A1-pool: 12.5 μM, si1A1-1: 23.3 μM, si1A1-4: 20.8 μM) (H). N = 4, Bar: SD, **: P < 0.01 compared with the siCtrl group.
However, downregulation of ALDH1A1 does not appear to be highly effective at sensitizing to olaparib. Given that ALDH1A2, ALDH3B2, ALDH1A3, and ALDH3A1 are also upregulated in PARPi-resistant EOC cells (Supplementary Fig. S4), it is possible that these ALDH isoforms may also play a critical role in enhancing PARPi resistance, and sole downregulation of ALDH1A1 may not exhibit a highly effective effect on sensitizing cells to PARPi. Taken together, these data indicate that high ALDH activity correlates with olaparib resistance and ALDH1A1 is a major contributor to the enhanced ALDH activity in olaparib-resistant EOC cells, and plays an important role in olaparib resistance in these BRCA2 mutated EOC cells.
PARPi induces ALDH activity via enhancing BRD4 expression
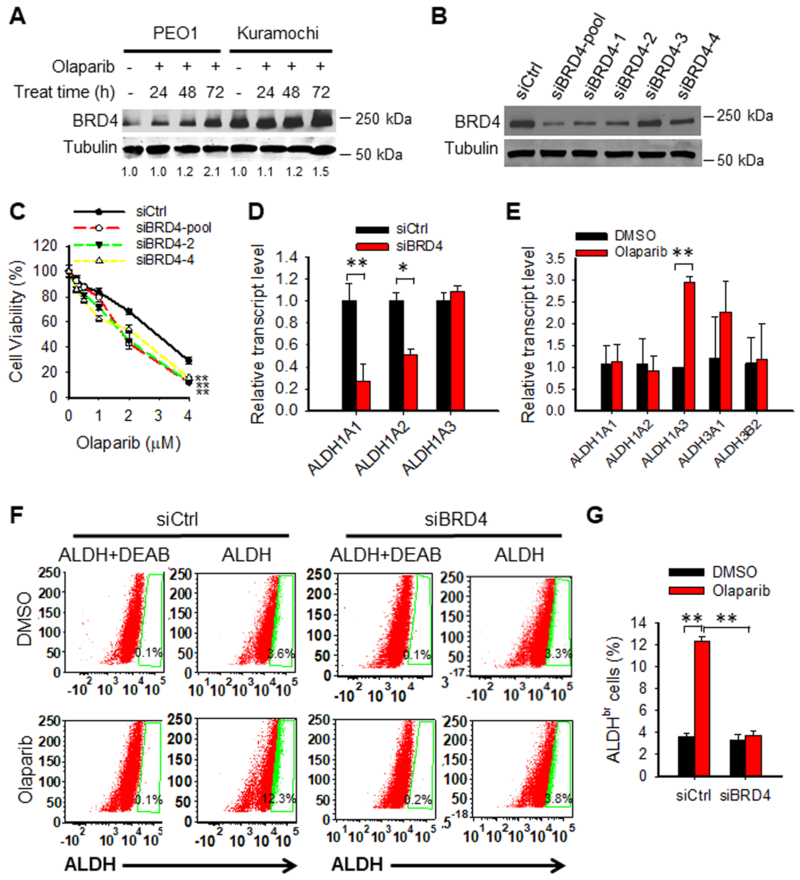
Recent studies have shown that ALDH activity is positively regulated by the bromodomain and extraterminal (BET) family protein BRD4, which is able to upregulate ALDH1A1 transcription through a super-enhancer element (31). In addition, a previous transcriptome analysis has indicated that olaparib can increase the expression of BRD4 (32). Therefore, we hypothesized that olaparib-induced BRD4 enhances the expression of ALDH1A1, which render olaparib resistance to EOC cells. In support of this hypothesis, we found that olaparib treatment induced the BRD4 protein level in PEO1 and Kuramochi cells (Fig. 3A). Downregulation of BRD4 in PEO1 cells sensitized these cells to olaparib (Fig. 3B, C). In addition, we found that BRD4 can positively regulate the expression of ALDH1A1 and ALDH1A2 in EOC cells (Fig. 3D); Downregulation of BRD4 can inhibit olaparib-induced expression of ALDH1A1 (Fig. 3E), and downregulation of BRD4 also antagonize olaparib-induced expansion of ALDHbr cells (Fig. 3F, G). It is noteworthy that the expression of ALDH1A3 is not regulated by BRD4 (Fig. 3D), and knockdown of BRD4 was unable to inhibit olaparib-induced expression of ALDH1A3 in PEO1 cells (Fig. 3E). Therefore, although the cellular ALDH activity can be inhibited by BRD4 knockdown (Fig. 3F, G), olaparib-induced ALDH1A3 may still play a role in protecting cells from killing by olaparib, and this could be a reason that knockdown of BRD4 only exhibited a marginal protective effect on olaparib-induced cell death. In summary, these data indicate that olaparib-induced increase in BRD4 protein plays an important role in the induction of ALDH activity in EOC cells after olaparib treatment. Downregulation of BRD4 can sensitize BRCA2 mutated EOC cells to olaparib, probably via inhibiting ALDH1A1 expression.
Figure 3.
Olaparib enhances the ALDH activity by increasing BRD4 expression in EOC cells. A, BRD4 expression is induced by olaparib. PEO1 and Kuramochi cells were treated with olaparib (4 μM) for various time periods, BRD4 expression was determined using immunoblotting, and the relative amounts of BRD4 were quantified relative to the respective untreated sample and normalized by tubulin. B and C, Downregulation of BRD4 sensitizes EOC cells to olaparib treatment. PEO1 cells were transfected with control or BRD4 siRNA for 24 h, treated with olaparib at the indicated doses for 7 days. Immunoblotting was conducted to determine BRD4 protein level after 48 h of transfection (B). Methylene blue assay was conducted to determine cell viability after treatment for 7 days (IC50: siCtrl: 2.76 μM, siBRD4-pool: 1.89 μM, siBRD4-2: 1.88 μM, siBRD4-4: 2.00 μM) (C). N = 4, Bar: SD, **: P < 0.01 compared to the siCtrl group. D, Downregulation of BRD4 reduces expression of ALDH1A1 and ALDH1A2. PEO1 cells were transfected with siCtrl or siBRD4 for 48 h, expression of ALDH1 subfamily genes in these cells were determined using qRT-PCR. N = 3, Bar: SD, *: P < 0.05; **: P < 0.01. E, Downregulation of BRD4 antagonizes olaparib-induced expression of ALDH1A1. PEO1 cells were transfected with siBRD4 for 24 h, treated with olaparib (4 μM) for 7 days. Expression of ALDH family genes in these cells were determined using qRT-PCR. N = 3, Bar: SD, **: P < 0.01. F and G, Downregulation of BRD4 compromises olaparib-induced ALDH activity. PEO1 cells were transfected with siCtrl or siBRD4 for 24 h, treated with olaparib (4 μM) for 7 days. The ALDH activity was analyzed with the ALDEFLUOR assay using flow cytometry. N = 3, Bar: SD, **: P < 0.01 compared to the corresponding DMSO group.
ALDH1A1 differentially modulates DNA repair capabilities in BRCA2 mutated EOC cells
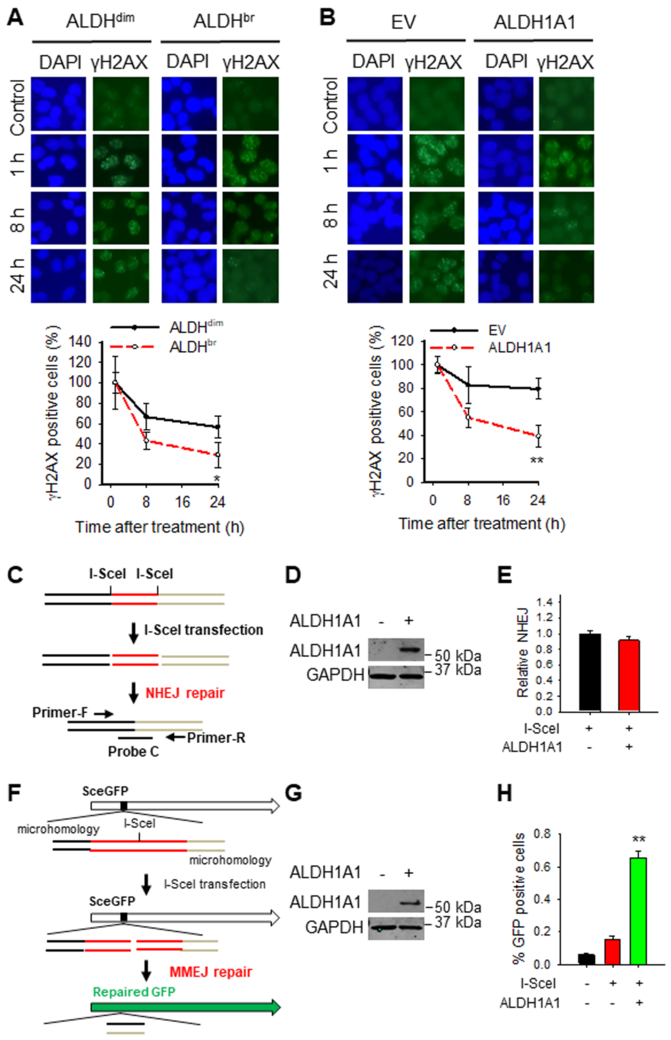
One of the mechanisms underlying PARPi resistance is the restoration of DNA repair capability, including treatment-induced reverse mutation in the defective BRCA1/2 gene (24, 33-35). More importantly, it has also been reported that ALDH1A1 can alter DNA repair networks in ovarian cancer cells (23). Given that ALDHbr cells exhibit increased resistance to olaparib compared to ALDHdim cells (Fig. 2A), we first investigated whether ALDHbr cells possess enhanced DNA repair capability. ALDHdim and ALDHbr cells were sorted from HR-deficient PEO1 cells, treated with H2O2 to induce DNA damage, and γH2AX foci in these cells were analyzed at different time points to evaluate the DNA repair capability. It is clear that ALDHbr cells exhibit enhanced DNA repair capacity compared to ALDHdim cells, reflected by faster disappearance of γH2AX foci in ALDHbr cells (Supplementary Fig. S6). We then treated ALDHdim and ALDHbr cells isolated from PEO1 cells with olaparib, or overexpressed ALDH1A1 in PEO1 cells, and treated them with olaparib to analyze the disappearance of γH2AX foci in these cells. Once again, we found γH2AX foci disappeared faster in ALDHbr cells than in ALDHdim cells (Fig. 4A), and faster in ALDH1A1 overexpressed cells than empty vector transfected cells (Fig. 4B). These data indicate that high ALDH activity, mainly due to high expression of ALDH1A1, can enhance DNA repair capacity in HR-deficient EOC cells.
Figure 4.
ALDHbr cells exhibit enhanced DNA repair capacity due to high expression of ALDH1A1. A, ALDHdim and ALDHbr cells were sorted from PEO1 cells, treated with olaparib (10 μM) for 1 h, further cultured in the drug-free medium for the indicated time periods. Immunofluorescence was conducted to visualize γH2AX foci. γH2AX positive cells (> 5 foci/cell) were quantified. N = 6, Bar: SD, **: P < 0.01 compared to the ALDHdim group. B, PEO1 cells were transfected with either empty vector (EV) or ALDH1A1 expressing vector for 24 h, treated with olaparib (10 μM) for 1 h, further cultured in the drug-free medium for the indicated time periods. Immunofluorescence was conducted to visualize γH2AX foci. γH2AX positive cells (> 5 foci/cell) were quantified. N = 6, Bar: SD, **: P < 0.01 compared to the EV transfected group. C-E, ALDH1A1 overexpression does not affect NHEJ activity. HEK293-pPHW1 cells containing a NHEJ reporter plasmid pPHW1 were transfected with either empty vector or ALDH1A1 expression vector, along with I-SceI expression vector. Schematic of the NHEJ reporter assay was illustrated on the left (C). ALDH1A1 was determined using immunoblotting (D); the NHEJ activity was determined using quantitative real-time PCR (E). N = 3, Bar: SD. F-H, ALDH1A1 overexpression enhances the MMEJ activity. H1299-pCMV-1810 cells containing a MMEJ reporter vector pCMV/I-SceI/GFP were transfected with either empty vector or ALDH1A1 expression vector, along with I-SceI expression vector. Schematic of the MMEJ reporter assay was illustrated on the left (F). ALDH1A1 was determined using immunoblotting (G); GFP-positive cells indicating successful MMEJ repair were detected by flow cytometry (H). N = 3, Bar: SD, **: P < 0.01 compared to the EV group transfected with I-SceI.
Both H2O2 and olaparib can induce DSBs, which are mainly repaired by HR to allow cell survival. Given that PEO1 cells possess mutated BRCA2, and thus are HR deficient, we first determined whether ALDHbr cells have restored HR capability, or whether overexpression of ALDH1A1 can restore HR. RAD51 immunofluorescence analysis in PEO1 cells showed few RAD51 foci after olaparib treatment, and there was no difference in the formation and disappearance of RAD51 foci between ALDHdim cells and ALDHbr cells, neither between empty vector and ALDH1A1 transfected PEO1 cells (Supplementary Fig. S7), indicating that ALDHbr BRCA2 mutated cells do not have enhanced HR, and ALDH1A1 does not enhance DNA repair by restoration of HR in BRCA2 mutated cells. Besides HR, DSBs can also be repaired by NHEJ, including c-NHEJ and alt-NHEJ (MMEJ) (36). By using a c-NHEJ reporter assay, in which, a specific DNA sequence corresponding to the accurate relegation product can only be generated by I-SceI cleavage and subsequent repair by c-NHEJ in the NHEJ reporter plasmid pPHW1, and can be determined using qRT-PCR, we found that ALDH1A1 overexpression did not change the c-NHEJ activity (Fig. 4C-E). In contrast, overexpression of ALDH1A1 can significantly promote the MMEJ activity, demonstrated by using an intra-chromosomal MMEJ reporter, in which, functional GFP is only generated after I-SceI cleavage and subsequent repair by MMEJ (Fig. 4F-H). These data suggest that ALDH1A1 is able to enhance the repair of DSBs in HR-deficient cells via augmenting MMEJ.
ALDH1A1 inhibitor sensitizes BRCA2 mutated EOC cells to olaparib treatment
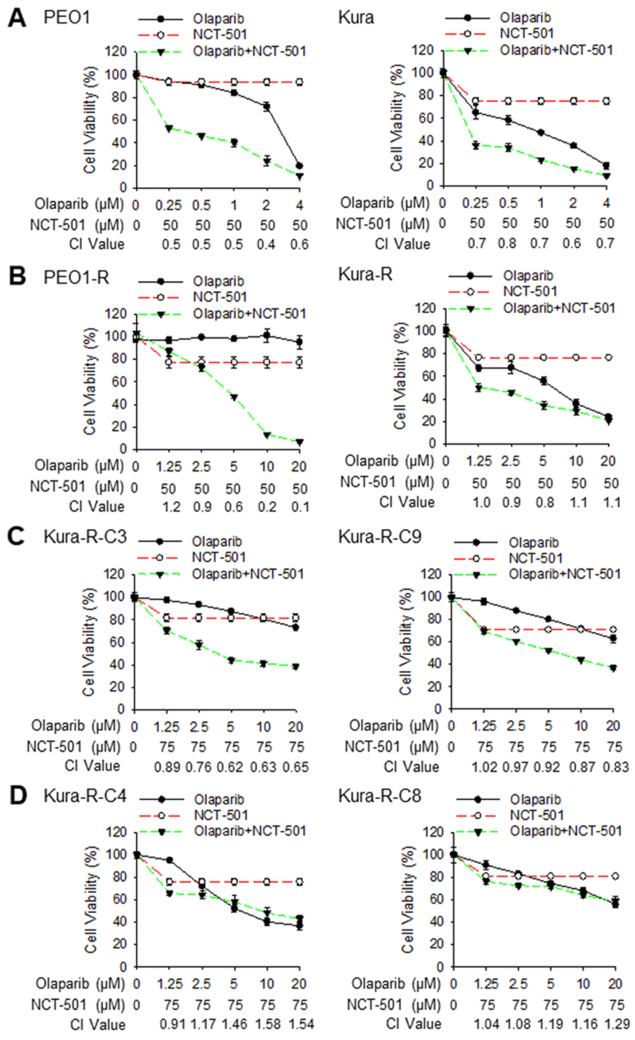
Given that ALDH1A1 can be induced by olaparib and contribute to PARP resistance, we sought to investigate whether inhibition of ALDH1A1 can enhance the sensitivity of EOC cells to olaparib. NCT-501 is a potent and selective ALDH1A1 inhibitor (37). We demonstrated that 50 μM of NCT-501 can significantly inhibit the ALDH activity in both PARPi-sensitive and –resistant PEO1 and Kuramochi cells without affecting the expression of ALDH1A1, but only induces about 20-30% cell deaths (Supplementary Fig. S8A-C). In addition, our previous study has shown that NCT-501 is able to inhibit the sphere formation ability and tumorigenicity of EOC cells (30). In combination with olaparib, NCT-501 at 50 μM displayed a synergistic effect (CI < 0.9) with olaparib in killing olaparib-sensitive EOC cells (Fig. 5A). In addition, a synergistic effect was also found on killing olaparib-resistant PEO1 cells, but only a marginal synergistic effect was found in killing Kura-R cells (olaparib at 5 μM + NCT-501 at 50 μM) (Fig. 5B). It has been shown that long-term PARPi treatment can induce reverse mutation in the defective BRCA2 gene. The secondary mutations could restore the open reading frame of the mutant BRCA2, and restore HR repair, leading to resistance for HR-deficiency therapy (36). We have found that PEO1-R cells did not show an obvious BRCA2 expression, while Kura-R cells showed a clear BRCA2 protein expression (Supplementary Fig. S9), indicating that Kura-R cells must have undergone secondary reverse mutation in the defective BRCA2, and this could be a reason that NCT-501 and olaparib have only a marginal synergistic effect in Kura-R cells. To understand whether the BRCA2 gene status affects the synergistic effect of NCT-501 and olaparib on survival of olaparib-resistant EOC cells, we selected 6 single cell clones from Kura-R cells using limiting dilution, and determined the BRCA2 gene status in these cells. The BRCA2 mutation in Kuramochi cells is c.6952C>T (25). We found that 2 clones still carry BRCA2 c.6952T, while 4 clones carry BRCA2 c.6952C, which is wild type, indicating that 2/3 of olaparib-resistant Kura-R cells have secondary reverse BRCA2 mutation. We further determined the combination effect of olaparib and NCT-501 on the survival of these clones. The BRCA2 mutated C3 and C9 clones exhibited the synergistic effect (Fig. 5C), while the BRCA2 restored C4 and C8 clones exhibited the additive or antagonistic effect (Fig. 5D) on cell survival when treated with olaparib and NCT-501 simultaneously. Furthermore, the BRCA2-restored PEO4 cells (Supplementary Fig. S9) also displayed an additive effect when treated with olaparib and NCT-501 (Supplementary Fig. S10). In addition, we also found that NCT-501 can reduce DNA repair capacity of olaparib-resistant EOC cells, reflected by prolonged persistence of γH2AX foci in these cells after 1 h of olaparib treatment (Supplementary Fig. S11). These data indicate that the ALDH1A1 inhibitor can synergistically enhance the efficacy of olaparib in killing EOC cells carrying BRCA2 mutation.
Figure 5.
Inhibition of ALDH1A1 synergistically increases the efficacy of olaparib in treating BRCA2-deficient EOC cells. A, The ALDH1A1 inhibitor NCT-501 synergistically augments the cytotoxicity of olaparib in BRCA2-deficient EOC cell lines. PEO1 and Kuramochi cells were treated with olaparib, NCT-501, or olaparib+NCT-501 for 7 days, cell viability was determined using the methylene blue assay (IC50: PEO1-Olaparib: 2.61 μM, PEO1-Olaparib+NCT-501: 0.8 μM; Kura-Olaparib: 1.36 μM, Kura-Olaparib+NCT-501: 0.24 μM). The CI value was calculated. CI<0.9: synergism; 0.9-1.1: additive effect; >1.1: antagonism. N = 4, Bar: SD. B, NCT-501 rescues the sensitivity of PEO1-R cells to olaparib. PARPi-resistant PEO1-R and Kura-R cells were treated with olaparib, NCT-501, or olaparib+NCT-501 for 7 days, cell viability was determined using the methylene blue assay (IC50: PEO1-R-Olaparib: >50 μM, PEO1-R-Olaparib+NCT-501: 4.4 μM; Kura-R-Olaparib: 5.79 μM, Kura-R-Olaparib+NCT-501: 2.95 μM). CI values were calculated as aforementioned. N = 4, Bar: SD. C and D, NCT-501 rescues the sensitivity of Kura-R cells without reverse BRCA2 mutation to olaparib treatment. Multiple single clones were selected from Kura-R cells; the BRCA2 gene was sequenced to identify secondary reverse mutation. C3 and C9 clones without secondary mutation (C), as well as C4 and C8 clones possessing secondary mutation (D), were treated with olaparib, NCT-501, or olaparib+NCT-501 for 7 days, cell viability was determined using the methylene blue assay (IC50: C3-Olaparib: 62.3 μM, C3-Olaparib+NCT-501: 6.19 μM; C9-Olaparib: 34.1 μM, C9-Olaparib+NCT-501: 7.16 μM; C4-Olaparib: 7.81 μM, C4-Olaparib+NCT-501: 7.87 μM; C8-Olaparib: 25.7 μM, C8-Olaparib+NCT-501: 33.4 μM). CI values were calculated as aforementioned. N = 3, Bar: SD.
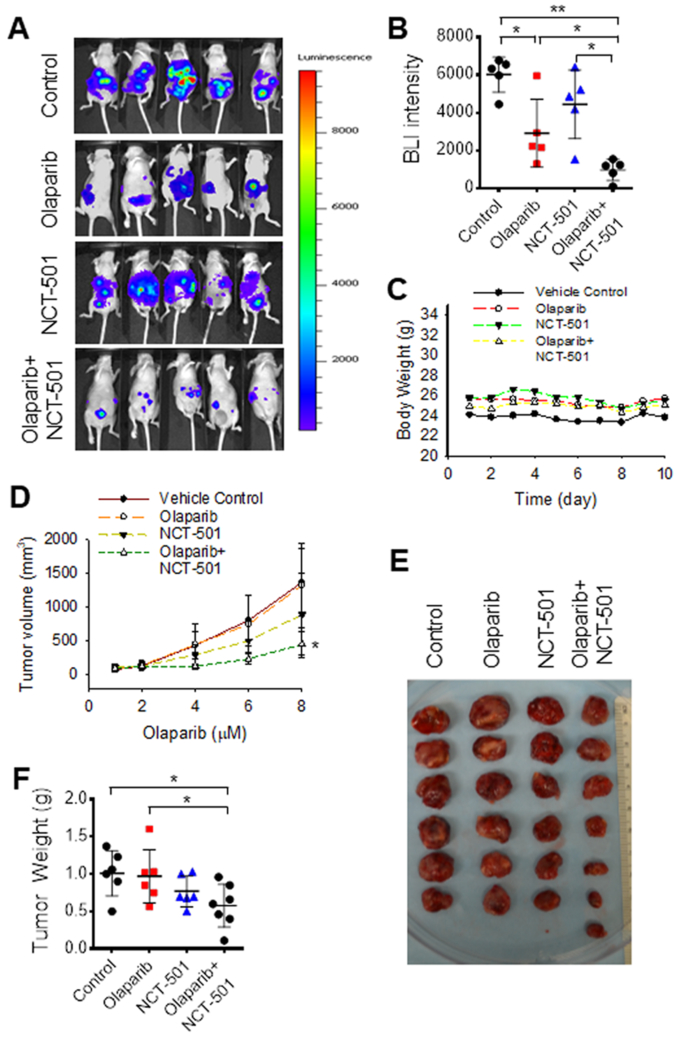
Finally, we generated ovarian xenografts by injecting PEO1-Luc cells into nude mice intraperitoneally, and injecting PEO1-R cells into nude mice subcutaneously, treated xenograft-bearing mice with either olaparib or/and NCT-501 for 10 or 8 days, respectively. It is clear that in the PARPi-sensitive PEO1 xenograft model, olaparib significantly impedes the growth of xenografts, while NCT-501 does not show a significant effect on tumor growth. However, combination treatment with both olaparib and NCT-501 exhibits a synergistic effect on the inhibition of tumor growth (Fig. 6A, B). Furthermore, olaparib and olaparib+NCT-501 did not cause obvious toxicity, as weights of mice did not change (Fig. 6C). In the PARPi-resistant PEO1-R xenograft model, neither olaparib nor NCT-501 affects the growth of xenografts, while the combined treatment with both olaparib and NCT-501 can significantly inhibit the growth of tumor (Fig. 6D-F), indicating that NCT-501 can not only sensitize PEO1-derived xenografts to olaparib, but also reverse PARPi resistance in PEO1-R-derived xenografts. Taken together, these in vitro and in vivo data indicate that selective inhibition of ALDH1A1 could enhance the efficacy of olaparib in treating both sensitive and resistant EOCs carrying BRCA2 mutation.
Figure 6.
Inhibition of ALDH1A1 synergistically increases the efficacy of olaparib in treating BRCA2-deficient EOC cell-derived xenografts. A-C, PEO1 cells containing luciferase expression vector (2 × 106) were injected into nude mice intraperitoneally to generate xenografts, treated with olaparib (50 mg/kg, once a day) or/and NCT-501 (10 mg/kg, once a day) intraperitoneally for 10 days. Tumor volumes were determined using BLI (A). BLI intensity was plotted (B). Mice weights were monitored every day (C). N = 5, *: P < 0.05, **: P < 0.01. D-F, PEO1-R cells (2 × 106) were injected into nude mice subcutaneously to generate xenografts, treated with olaparib (50 mg/kg, once a day) or/and NCT-501 (10 mg/kg, once a day) intraperitoneally for 8 days. Tumor volumes were determined using caliber every other day (D); tumors were removed from mice at the end of experiment and weighed (E, F). N = 6 or 7, *: P < 0.05 compared to either vehicle control or olaparib group.
Discussion
PARP inhibitors are an exciting and promising new class of anticancer drugs, which selectively kill BRCA1/2-deficient cancer cells based on synthetic lethality. However, acquisition of PARPi resistance in these patients remains a clinical hurdle. Although secondary “revertant” mutations within the BRCA1 or BRCA2 genes to restore HR has been demonstrated to be a common mechanism underlying PARPi resistance in patients carrying BRCA2 mutation (24, 33-35), other mechanisms also exist because reverse mutation does not occur in all PARPi resistant BRCA2 mutated tumors (38). Here, we reveal a new mechanism that PARPi treatment increases ALDH1A1 expression, which further augments the MMEJ pathway and promotes cell survival after PARPi treatment. Selective inhibition of ALDH1A1 is able to efficiently sensitize BRCA2 mutated EOC cells, as well as rescue the sensitivity of PARPi resistant BRCA2 mutated EOC cells to olaparib.
ALDH1A1 is upregulated in taxane-resistant ovarian cancer cells, cisplatin-resistant lung cancer cells (19), and high grade serous ovarian carcinoma tissues after chemotherapy (platinum + taxane) (20). However, it remains unclear how ALDH1A1 is induced. It has been reported that ALDH activity is positively regulated by the BET family protein BRD4, which is able to upregulate ALDH1A1 transcription through a super-enhancer element (31). In our study, we showed that olaparib treatment was able to increase BRD4 expression, and downregulation of BRD4 antagonized olaparib-induced ALDH activity in EOC cells. BET plays an important role in modulating the sensitivity of EOC cells to PARPi. The BET inhibitor JQ1 can synergize with olaparib in suppressing the growth of BRCA1/2 wild-type EOC cells by downregulating TOPBP1 and WEE1, which are involved in DNA damage responses (39). We further demonstrated that downregulation of BRD4 also sensitized BRCA2 mutated EOC cells to olaparib by compromising olaparib-induced ALDH1A1 expression. Thus, BET regulates olaparib sensitivity by multiple mechanisms, and BET inhibitors could be used to enhance the efficacy of PARPi in both BRCA2 wild type and mutated EOC cells.
The high ALDH activity is considered a marker of CSCs (14); ALDH-mediated DNA repair has also been reported to contribute to chemoresistance in CSCs (23). However, it is still unclear which DNA repair pathway is regulated by ALDH. In this study, we found that inhibition of ALDH1A1 only synergized olaparib in killing BRCA2 mutated EOC cells, but not BRCA2 wild-type EOC cells. HR is the major DNA repair pathway to repair DSBs after olaparib treatment to rescue cells. BRCA2 plays a critical role in HR by facilitating loading of RAD51 onto the DSBs. Thus, cells carrying BRCA2 mutation have deficient HR. Given that ALDH1A1 increases DNA repair after olaparib and H2O2 treatment, but not through restoration of HR capability in BRCA2 mutated EOC cells, other DNA repair machinery must be enhanced by ALDH1A1. NHEJ is another important DSB repair pathway that directly joins broken ends of DNA with little or no regard for sequence homology. However, enhanced c-NHEJ does not rescue HR-deficient cells from PARPi treatment. Instead, it promotes the cytotoxicity of HR-deficient cells treated with PARPi (11). Most importantly, we demonstrated that the c-NHEJ activity is not affected by ALDH1A1 overexpression, indicating that c-NHEJ is not involved in the synergistic effect of ALDH1A1 inhibition and olaparib treatment on killing BRCA2 mutated EOC cells. In contrast, MMEJ has been reported to be enhanced in HR-deficient cells and promotes the survival of HR-deficient cells following PARPi treatment (7). Distinguished from c-NHEJ, MMEJ uses 5-25 base pair microhomologous sequences to align the broken strands before joining (8), and this repair pathway requires DNA polymerase θ (7). By using the MMEJ cell reporter assay, we demonstrated that overexpression of ALDH1A1 is able to enhance the MMEJ activity. Thus, it is very likely that olaparib-induced ALDH1A1 renders olaparib resistance to BRCA2 mutated EOC cells by enhancing the MMEJ activity, and inhibition of ALDH1A1 sensitizes BRCA2 mutated EOC cells to olaparib by compromising the MMEJ activity. Given that HR is the predominant DSB repair mechanism, ALDH1A1-enhanced MMEJ may not significantly increase the repair of DSBs in HR-proficient cells, and thus, ALDH1A1 inhibition is unable to sensitize HR-proficient EOC cells, e.g., Kura-R-C4, Kura-R-C8 (Fig. 6D), and PEO4 (Supplementary Fig.S10), to olaparib.
Given that ALDH activity is critical to chemoresistance, ALDH has been regarded as a target for treatment. Broad ALDH inhibitors such as DEAB and disulfiram (DSF) have been used to investigate the role of ALDH in chemotherapy resistance (22, 40, 41). In addition, an ALDH1A selective inhibitor has been reported to deplete the CSC pool and synergize with cisplatin in killing EOC cell lines (42). Furthermore, we have shown that a potent and selective Theophylline-based inhibitor of ALDH1A1, NCT-501 (37), is able to reduce the growth of xenografts derived from EOC cell line with low DDB2 expression (30). In this study, we further demonstrated that NCT-501 can not only synergize with PARPi in treating BRCA2 mutated EOC cells, but also rescue the sensitivity of BRCA2 mutated PARPi-resistant EOC cells to PARPi treatment. Thus, targeting ALDH1A1 can be exploited for overcoming acquired PARPi resistance in EOC patients carrying BRCA2 mutation.
In summary, although patients with and without HR deficiencies benefit from PARPi maintenance treatment, the greatest benefit of PARPi is seen in patients with somatic or germline BRCA mutations (12). Thus, preventing and overcoming PARPi resistance in patients carrying BRCA mutations would dramatically improve the outcome of these patients. Given that ALDH1A1 can be induced by olaparib, and contributes to PARPi resistance in BRCA2 mutated EOC cells by augmenting MMEJ to repair DSBs, selective inhibition of ALDH1A1, e.g., via NCT-501, could be used to prevent and even overcome PARPi resistance.
Supplementary Material
Acknowledgements
We thank Dr. Thomas C. Hamilton (Fox Chase Cancer Center), Dr. Adam Karpf (University of Nebraska Medical Center), and Dr. Kay Huebner (The Ohio State University) for kindly providing cell lines. This work was supported by NIH/NCI R01CA211175 (Q.E. Wang), NCI Shared Resources Grant P30CA016058 (OSUCCC), and OSUCCC Pelotonia Idea Award (Q.E. Wang).
Financial Information: This work was supported by NIH/NCI R01CA211175 (Q.E. Wang), NCI Shared Resources Grant P30CA016058 (OSUCCC), and OSUCCC Pelotonia Idea Award (Q.E. Wang).
Footnotes
Conflict of interests: The authors declare no potential conflicts of interest.
References
- 1.Siegel RL, Miller KD, and Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, and Ronnett BM. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol. 2004;23(1):41–4. [DOI] [PubMed] [Google Scholar]
- 3.Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer. 2010;10(11):803–8. [DOI] [PubMed] [Google Scholar]
- 4.Timmermans M, Sonke GS, Van de Vijver KK, van der Aa MA, and Kruitwagen RFPM. No improvement in long-term survival for epithelial ovarian cancer patients: A population-based study between 1989 and 2014 in the Netherlands. Eur J Cancer. 2018;88:31–7. [DOI] [PubMed] [Google Scholar]
- 5.Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012;72(21):5588–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma W, Halweg CJ, Menendez D, and Resnick MA. Differential effects of poly(ADP-ribose) polymerase inhibition on DNA break repair in human cells are revealed with Epstein-Barr virus. Proc Natl Acad Sci U S A. 2012;109(17):6590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceccaldi R, Liu JC, Amunugama R, Hajdu I, Primack B, Petalcorin MI, et al. Homologous-recombination-deficient tumours are dependent on Poltheta-mediated repair. Nature. 2015;518(7538):258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sallmyr A, and Tomkinson AE. Repair of DNA double-strand breaks by mammalian alternative end-joining pathways. J Biol Chem. 2018;293(27):10536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–7. [DOI] [PubMed] [Google Scholar]
- 10.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21. [DOI] [PubMed] [Google Scholar]
- 11.Patel AG, Sarkaria JN, and Kaufmann SH. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc Natl Acad Sci U S A. 2011;108(8):3406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. 2018;379(26):2495–505. [DOI] [PubMed] [Google Scholar]
- 13.Koppaka V, Thompson DC, Chen Y, Ellermann M, Nicolaou KC, Juvonen RO, et al. Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol Rev. 2012;64(3):520–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcato P, Dean CA, Giacomantonio CA, and Lee PW. Aldehyde dehydrogenase: its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle. 2011;10(9):1378–84. [DOI] [PubMed] [Google Scholar]
- 15.Croker AK, and Allan AL. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44(+) human breast cancer cells. Breast Cancer Res Treat. 2012;133(1):75–87. [DOI] [PubMed] [Google Scholar]
- 16.Awad O, Yustein JT, Shah P, Gul N, Katuri V, O’Neill A, et al. High ALDH activity identifies chemotherapy-resistant Ewing’s sarcoma stem cells that retain sensitivity to EWS-FLI1 inhibition. PLoS One. 2010;5(11):e13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magni M, Shammah S, Schiro R, Mellado W, Dalla-Favera R, and Gianni AM. Induction of cyclophosphamide-resistance by aldehyde-dehydrogenase gene transfer. Blood. 1996;87(3):1097–103. [PubMed] [Google Scholar]
- 18.Silva IA, Bai S, McLean K, Yang K, Griffith K, Thomas D, et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71(11):3991–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landen CN Jr., Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, et al. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010;9(12):3186–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steg AD, Bevis KS, Katre AA, Ziebarth A, Dobbin ZC, Alvarez RD, et al. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin Cancer Res. 2012;18(3):869–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobbin ZC, Katre AA, Steg AD, Erickson BK, Shah MM, Alvarez RD, et al. Using heterogeneity of the patient-derived xenograft model to identify the chemoresistant population in ovarian cancer. Oncotarget. 2014;5(18):8750–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raha D, Wilson TR, Peng J, Peterson D, Yue P, Evangelista M, et al. The cancer stem cell marker aldehyde dehydrogenase is required to maintain a drug-tolerant tumor cell subpopulation. Cancer Res. 2014;74(13):3579–90. [DOI] [PubMed] [Google Scholar]
- 23.Meng E, Mitra A, Tripathi K, Finan MA, Scalici J, McClellan S, et al. ALDH1A1 maintains ovarian cancer stem cell-like properties by altered regulation of cell cycle checkpoint and DNA repair network signaling. PloS one. 2014;9(9):e107142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakai W, Swisher EM, Jacquemont C, Chandramohan KV, Couch FJ, Langdon SP, et al. Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinoma. Cancer Res. 2009;69(16):6381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ihnen M, zu EC, Kolarova T, Qi JW, Manivong K, Chalukya M, et al. Therapeutic potential of the poly(ADP-ribose) polymerase inhibitor rucaparib for the treatment of sporadic human ovarian cancer. Mol Cancer Ther. 2013;12(6):1002–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong X, Du Z, Wang Y, Feng Z, Fan P, Yan C, et al. 53BP1 promotes microhomology-mediated end-joining in G1-phase cells. Nucleic acids research. 2015;43(3):1659–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–81. [DOI] [PubMed] [Google Scholar]
- 28.Yun MH, and Hiom K. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature. 2009;459(7245):460–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhuang J, Jiang G, Willers H, and Xia F. Exonuclease function of human Mre11 promotes deletional nonhomologous end joining. J Biol Chem. 2009;284(44):30565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui T, Srivastava AK, Han C, Wu D, Wani N, Liu L, et al. DDB2 represses ovarian cancer cell dedifferentiation by suppressing ALDH1A1. Cell Death Dis. 2018;9(5):561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokoyama Y, Zhu H, Lee JH, Kossenkov AV, Wu SY, Wickramasinghe JM, et al. BET Inhibitors Suppress ALDH Activity by Targeting ALDH1A1 Super-Enhancer in Ovarian Cancer. Cancer Res. 2016;76(21):6320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin KA, Cesaroni M, Denny MF, Lupey LN, and Tempera I. Global Transcriptome Analysis Reveals That Poly(ADP-Ribose) Polymerase 1 Regulates Gene Expression through EZH2. Mol Cell Biol. 2015;35(23):3934–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards SL, Brough R, Lord CJ, Natrajan R, Vatcheva R, Levine DA, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451(7182):1111–5. [DOI] [PubMed] [Google Scholar]
- 34.Norquist B, Wurz KA, Pennil CC, Garcia R, Gross J, Sakai W, et al. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J Clin Oncol. 2011;29(22):3008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barber LJ, Sandhu S, Chen L, Campbell J, Kozarewa I, Fenwick K, et al. Secondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitor. J Pathol. 2013;229(3):422–9. [DOI] [PubMed] [Google Scholar]
- 36.Ceccaldi R, Rondinelli B, and D’Andrea AD. Repair Pathway Choices and Consequences at the Double-Strand Break. Trends Cell Biol. 2016;26(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang SM, Yasgar A, Miller B, Lal-Nag M, Brimacombe K, Hu X, et al. Discovery of NCT-501, a Potent and Selective Theophylline-Based Inhibitor of Aldehyde Dehydrogenase 1A1 (ALDH1A1). J Med Chem. 2015;58(15):5967–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin KK, Harrell MI, Oza AM, Oaknin A, Ray-Coquard I, Tinker AV, et al. BRCA Reversion Mutations in Circulating Tumor DNA Predict Primary and Acquired Resistance to the PARP Inhibitor Rucaparib in High-Grade Ovarian Carcinoma. Cancer Discov. 2019;9(2):210–9. [DOI] [PubMed] [Google Scholar]
- 39.Karakashev S, Zhu H, Yokoyama Y, Zhao B, Fatkhutdinov N, Kossenkov AV, et al. BET Bromodomain Inhibition Synergizes with PARP Inhibitor in Epithelial Ovarian Cancer. Cell Rep. 2017;21(12):3398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kast RE, and Belda-Iniesta C. Suppressing glioblastoma stem cell function by aldehyde dehydrogenase inhibition with chloramphenicol or disulfiram as a new treatment adjunct: an hypothesis. Curr Stem Cell Res Ther. 2009;4(4):314–7. [DOI] [PubMed] [Google Scholar]
- 41.Lin J, Haffner MC, Zhang Y, Lee BH, Brennen WN, Britton J, et al. Disulfiram is a DNA demethylating agent and inhibits prostate cancer cell growth. Prostate. 2011;71(4):333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huddle BC, Grimley E, Buchman CD, Chtcherbinine M, Debnath B, Mehta P, et al. Structure-Based Optimization of a Novel Class of Aldehyde Dehydrogenase 1A (ALDH1A) Subfamily-Selective Inhibitors as Potential Adjuncts to Ovarian Cancer Chemotherapy. J Med Chem. 2018;61(19):8754–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.