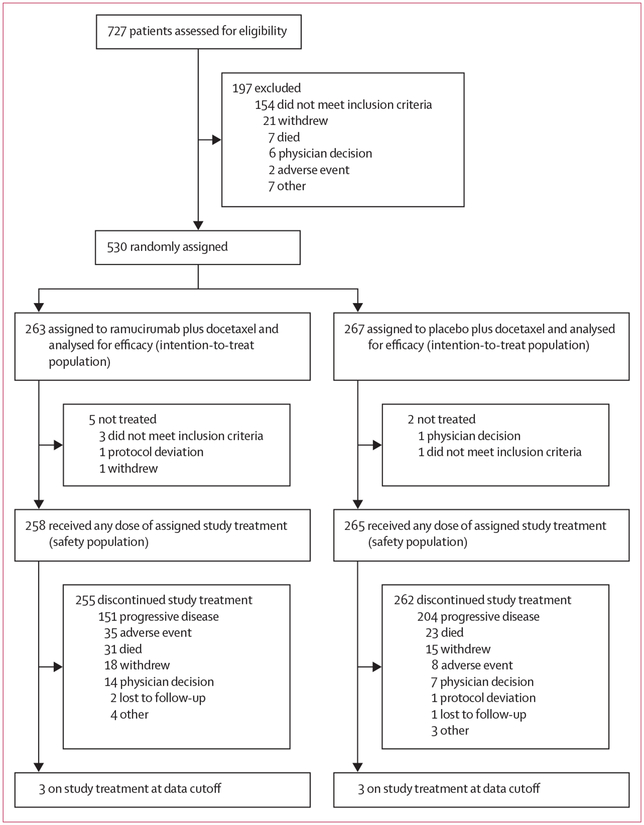
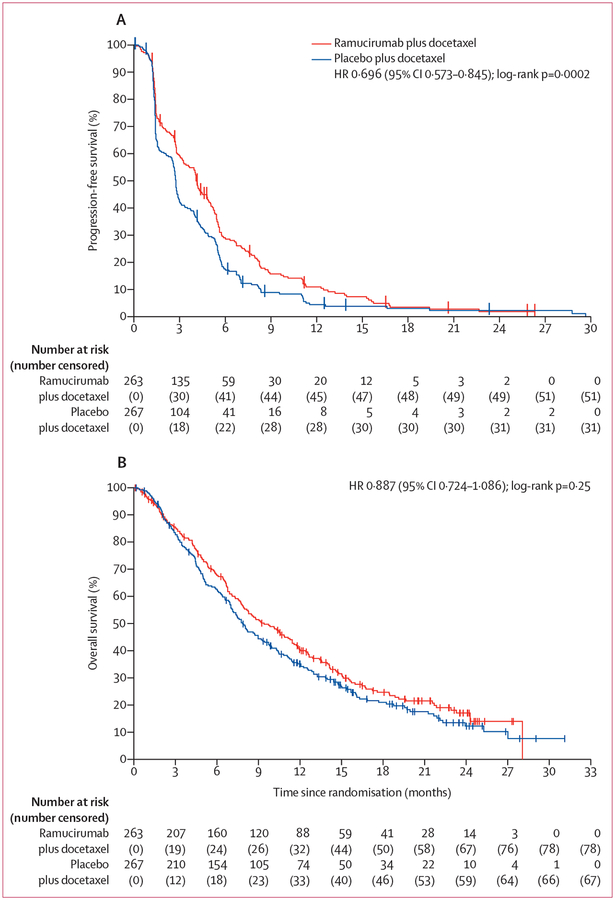
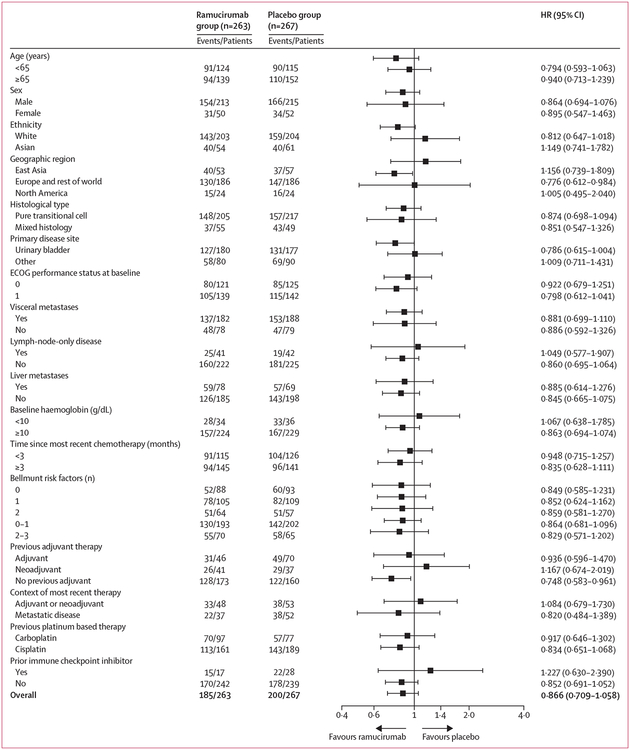
Daniel P Petrylak
Daniel P Petrylak
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Ronald de Wit
Ronald de Wit
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Kim N Chi
Kim N Chi
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Alexandra Drakaki
Alexandra Drakaki
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Cora N Sternberg
Cora N Sternberg
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,*,
Hiroyuki Nishiyama
Hiroyuki Nishiyama
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Daniel Castellano
Daniel Castellano
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Syed A Hussain
Syed A Hussain
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Aude Fléchon
Aude Fléchon
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Aristotelis Bamias
Aristotelis Bamias
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Evan Y Yu
Evan Y Yu
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Michiel S van der Heijden
Michiel S van der Heijden
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Nobuaki Matsubara
Nobuaki Matsubara
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Boris Alekseev
Boris Alekseev
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Andrea Necchi
Andrea Necchi
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Lajos Géczi
Lajos Géczi
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Yen-Chuan Ou
Yen-Chuan Ou
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Hasan Senol Coskun
Hasan Senol Coskun
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Wen-Pin Su
Wen-Pin Su
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Jens Bedke
Jens Bedke
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Georgios Gakis
Georgios Gakis
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Ivor J Percent
Ivor J Percent
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Jae-Lyun Lee
Jae-Lyun Lee
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Marcello Tucci
Marcello Tucci
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Andrey Semenov
Andrey Semenov
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Fredrik Laestadius
Fredrik Laestadius
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Avivit Peer
Avivit Peer
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Giampaolo Tortora
Giampaolo Tortora
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,†,
Sufia Safina
Sufia Safina
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Xavier Garcia del Muro
Xavier Garcia del Muro
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Alejo Rodriguez-Vida
Alejo Rodriguez-Vida
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Irfan Cicin
Irfan Cicin
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Hakan Harputluoglu
Hakan Harputluoglu
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Scott T Tagawa
Scott T Tagawa
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Ulka Vaishampayan
Ulka Vaishampayan
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Jeanny B Aragon-Ching
Jeanny B Aragon-Ching
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Oday Hamid
Oday Hamid
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Astra M Liepa
Astra M Liepa
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Sameera Wijayawardana
Sameera Wijayawardana
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Francesca Russo
Francesca Russo
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Richard A Walgren
Richard A Walgren
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Annamaria H Zimmermann
Annamaria H Zimmermann
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Rebecca R Hozak
Rebecca R Hozak
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Katherine M Bell-McGuinn
Katherine M Bell-McGuinn
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1,
Thomas Powles
Thomas Powles
1Yale School of Medicine, Yale University, New Haven, CT, USA (Prof D P Petrylak MD); Erasmus MC Cancer Institute, Rotterdam, Netherlands (Prof R de Wit PhD); British Columbia Cancer Agency, Vancouver, BC, Canada (Prof K N Chi MD); David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA (A Drakaki MD); San Camillo and Forlanini Hospitals, Rome, Italy (Prof C N Sternberg MD); University of Tsukuba, Tsukuba, Ibaraki, Japan (Prof H Nishiyama PhD); Hospital Universitario 12 de Octubre (CiberOnc), Madrid, Spain (D Castellano MD); Department of Oncology and Metabolism, Medical School, University of Sheffield, Sheffield, UK (Prof S A Hussain MD); Centre Léon Bérard, Lyon, France (A Fléchon MD); National and Kapodistrian University of Athens, Athens, Greece (Prof A Bamias PhD); University of Washington, Seattle, WA, USA (Prof E Y Yu MD); Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands (M S van der Heijden PhD); National Cancer Center Hospital East, Chiba, Japan (N Matsubara MD); P.A. Herzen Moscow Oncological Research Institute, Moscow, Russia (Prof B Alekseev PhD); Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (A Necchi MD); National Institute of Oncology, Budapest, Hungary (L Géczi PhD); Tungs’ Taichung Metro Harbor Hospital, Taichung, Taiwan (Prof Y-C Ou PhD); Akdeniz University School of Medicine, Antalya, Turkey (Prof H S Coskun MD); Institute of Clinical Medicine, College of Medicine, National Cheng Kung University & Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan (Prof W-P Su PhD); Department of Urology, University of Tübingen, Tübingen, Germany (Prof J Bedke MD, Prof G Gakis MD); Pediatric Urology, Julius Maximillians University, Würzburg, Germany (Prof G Gakis); Florida Cancer Specialists, Port Charlotte, FL, USA (I J Percent MD); Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea (Prof J-L Lee PhD); Division of Medical Oncology, Department of Oncology, University of Turin, San Luigi Gonzaga Hospital, Turin, Italy (M Tucci MD); RBHI Ivanovo Regional Oncology Dispensary, Ivanovo, Russia (A Semenov PhD); Centre Oscar Lambret, Lille, France (F Laestadius MD); Rambam Health Care Campus, Haifa, Israel (A Peer MD); University of Verona and Azienda Ospedaliera Universitaria Integrata, Verona, Italy (Prof G Tortora PhD); Tatarstan Regional Cancer Center, Kazan, Russia (S Safina MD); Institut Català d’Oncologia L’Hospitalet, Institut d’Investigacio Biomedica de Bellvitge, University of Barcelona, Barcelona, Spain (X Garcia del Muro PhD); Hospital del Mar, Barcelona, Spain (A Rodriguez-Vida MD); Trakya University, Edirne, Turkey (Prof I Cicin MD); Inonu University, Malatya, Turkey (Prof H Harputluoglu MD); New York-Presbyterian/Weill Cornell Medical Center, New York, NY, USA (S T Tagawa MD); Karmanos Cancer Institute, Detroit, MI, USA (Prof U Vaishampayan MD); Inova Schar Cancer Institute, Fairfax, VA, USA (J B Aragon-Ching MD); Eli Lilly and Company, Indianapolis, IN, USA (O Hamid PharmD, A M Liepa PharmD, S Wijayawardana PhD, F Russo MD, R A Walgren MD, A H Zimmermann MS, R R Hozak PhD, K M Bell-McGuinn MD); and Barts Cancer Institute, Queen Mary University of London, London, UK (Prof T Powles MD)
1;
RANGE study investigators1