Abstract
Objective:
Some treatments for systemic lupus erythematosus (SLE) can cause infertility, but the effect of SLE itself on fertility, particularly in African American women, is less clear. We examined infertility experiences in African American women with SLE compared to healthy women.
Methods:
We enrolled women ages 22–40, living in the Atlanta metropolitan area, who were diagnosed with SLE after age 17. Women ever treated with cyclophosphamide or who had a hysterectomy were excluded. African American women ages 22–40 from the same area recruited from a marketing list were used for comparison. Women were interviewed about their reproductive histories and goals. Periods of infertility were identified as times when they had regular, unprotected sex for ≥12 months without conceiving after age 20. We separately considered any period of infertility and periods of infertility when attempting pregnancy. We used Cox proportional hazards regression to examine the association between SLE and time to infertility. Models were adjusted for age, nulliparity and smoking. An age-matched analysis was also used to examine periods of infertility occurring after SLE diagnosis.
Results:
Our sample included 75 women with SLE and 154 women without SLE. SLE was associated with any infertility (adjusted hazard ratio [aHR]: 2.08; 95% CI: 1.38, 3.15), but less so with infertility when attempting pregnancy (aHR: 1.30; 95% CI: 0.62, 2.71). The matched analysis generated similar point estimates.
Conclusions:
Women with SLE may be more likely to experience episodes of infertility, but this may not translate to an inability to meet reproductive goals.
Systemic lupus erythematosus (SLE) is primarily diagnosed in women during their reproductive years. While more at risk for pregnancy complications, with proper timing and disease management, women with SLE can have good perinatal outcomes [1, 2]. However, women with SLE tend to have smaller family sizes than women without SLE [3, 4]. This may be due to a number of reasons, including personal choice, disease burden and financial issues, and increased risk of pregnancy loss [5, 6], but very few studies have examined infertility among women with SLE [5, 7, 8]. None of these studies utilized a disease-free comparison group and none included a sizeable sample of African American women, despite the fact that the incidence of SLE among African American women is 2–3 times the incidence among white women [9–12].
Certain treatments for SLE, in particular cyclophosphamide, are known to increase the risk of amenorrhea and primary ovarian insufficiency [13–15] and reduce the probability of becoming pregnant [7]. While it has not been established if women with SLE are more likely to experience infertility even if they have not been treated with cyclophosphamide, there are also potential autoimmune and/or hormonal mechanisms by which the disease itself could affect fertility. SLE is believed to potentially cause ovarian damage through autoimmune oophoritis and is also associated with menstrual dysfunction by increasing the body’s inflammatory response – both of which can affect women’s ability to become pregnant [16, 17].
Women with SLE are often advised to delay pregnancy until at least six months after a previous disease flare [18] since active disease immediately prior to conception may be associated with more flares during pregnancy and adverse birth outcomes [19, 20]. Recommending that women delay pregnancy may reduce a women’s reproductive window and prolong time to pregnancy. Thus, it is important to better understand how lupus impacts a woman’s fertility experience, particularly in African American women.
In this analysis, we examined the association between SLE and infertility in a sample of African American women, compared to a group of African American women without SLE. We hypothesized that women with SLE would be more likely to experience infertility than women without SLE. As the ultimate goal is to assess whether women with SLE are able to meet their reproductive goals, we also examined whether women with SLE were less likely to have met their desired number of children, and if so, whether this was associated with experiences of infertility.
PATIENTS AND METHODS
Study population
Women with SLE were enrolled from the Georgians Organized Against Lupus (GOAL) Cohort, an ongoing longitudinal cohort of adults with a validated diagnosis of SLE [21]. The primary source of patients in the GOAL cohort is the population-based Georgia Lupus Registry (GLR) [9], with additional patients from lupus clinics at Emory University and Grady Memorial Hospital in Atlanta and community rheumatologists from metropolitan Atlanta. All SLE cases were validated using the American College of Rheumatology (ACR) Classification criteria for SLE [22] or the GLR case definition of three ACR criteria with a final diagnosis of SLE by a board-certified rheumatologist [9].
From the existing GOAL cohort, 100 non-selected women were recruited into the pilot study Lupus Impacting the Female Experience (LIFE). Women were recruited by phone, e-mail notices and in-person at lupus clinics at Emory University and Grady Memorial Hospital. Eligible women were 22–40 years old at the time of enrollment, diagnosed with SLE at ages 18–35, without a history of cancer, never had a hysterectomy and were not receiving dialysis. Women were recruited and interviewed in-person from June through October in 2017. The in-person interview included questions about participants’ medical and reproductive histories, as well as their reproductive goals and plans. Participants’ height and weight were also taken at the time of the interview. All study procedures were approved by the Emory University Institutional Review Board. Participants provided written informed consent before the study interview and were compensated for their time and travel. For the purposes of this analysis, we restricted the sample to African American women who ever had intercourse with a male partner. In addition, because we were specifically interested in the association between SLE and infertility independent of gonadotoxic therapy, we excluded women who had ever been treated with cyclophosphamide (Figure 1).
Figure 1.
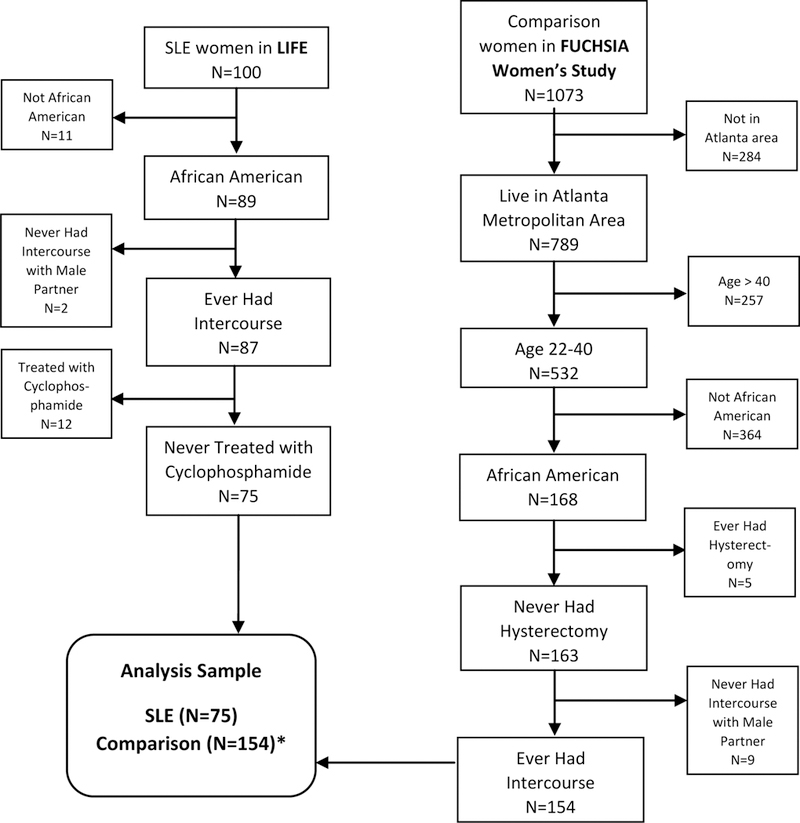
Flowchart of study sample inclusion criteria
*Four comparison women had missing data on ever experiencing infertility
Data on comparison women without SLE were taken from the Furthering Understanding of Cancer, Health, and Survivorship in Adult (FUCHSIA) Women’s Study [23]. We used the data from women that were originally recruited to represent the general population for comparison to adult female cancer survivors in the Georgia Cancer Registry (GCR). Comparison women were recruited from a marketing list and interviewed using a computer assisted telephone interview (CATI). Women provided oral informed consent at the time of interview. The Institutional Review Boards for Emory University and the Georgia Department of Public Health approved the FUCHSIA Women’s Study.
The women in the FUCHSIA Women’s Study were asked questions developed by the FUCHSIA study team about fertility and pregnancy [23]. The women in the LIFE Study were asked the same set of questions, allowing for comparisons between the two groups. The women in the FUCHSIA Women’s Study were recruited to be comparable to the statewide population of cancer survivors, but we applied additional restrictions to the population to make them comparable to the women enrolled in the LIFE Study: African-American women 22–40 years of age, who had never had a hysterectomy and lived in the metropolitan Atlanta area at the time of the interview (Figure 1).
Analysis
To examine the association between SLE and periods of infertility lasting for 12 months or longer, we used the response to the question “Has there ever been a period of time when you had unprotected sex with a male partner for six months or longer and did not get pregnant?” which was followed with “How old were you at the beginning of this time period?”, “How long did this period of time last?” and “Were you actively trying to get pregnant at this time?”. We restricted our outcome definition to periods of infertility lasting twelve months or longer after age 20 and separately considered the first period of any infertility and the first period of infertility when women were actively trying to conceive. We used Cox proportional hazards regression to examine the time to the first period of infertility after age 20. This was done as women were interviewed at different ages, and older women would have potentially have had more time to experience infertility. We first ran an unadjusted model and then a model adjusted for nulliparity and if the women reported ever being a regular smoker (average of 7 or more cigarettes/week for six months or longer). We also ran a separate set of models that only considered the first period of infertility when pregnancy was being attempted.
In addition, because our exposure of interest was a diagnosis of SLE, we were specifically interested in periods of infertility occurring after the diagnosis of SLE. Because only considering infertility after diagnosis in women with SLE would restrict the time period of interest in women with SLE but not the comparison women, we conducted a matched analysis. For example in order to assess whether a 30-year old woman who was diagnosed with SLE at age 25 is at greater risk of infertility after her SLE diagnosis than a 30-year old woman without SLE, we would only evaluate infertility that occurs after age 25 in both women in the matched analysis. This was to ensure that the risk period was the same length for both groups. Thus, each woman with SLE was randomly matched to a comparison woman in the same age group (22–29, 30–33, 34–36, 37–40), and the comparison woman was then assigned an artificial age at SLE diagnosis corresponding to the actual SLE diagnosis date of her matched SLE counterpart. Only periods of infertility occurring after this matched date of diagnosis were considered for the matched analysis. To ensure the results were robust, the 1:1 matching process was repeated 10,000 times using a SAS macro, and both unadjusted and adjusted median hazard ratios were calculated along with 95% simulation intervals. Adjusted analyses controlled for: ever gave live birth before age of diagnosis and ever been a regular smoker.
Finally, because we were interested in whether women with SLE were less likely to have met their desired family size, a potential downstream outcome of infertility, we also examined participants’ responses to the question “Including the children you are already raising, how many children would you like to raise?” and “How many children have you given birth to?”. Participants who had given birth to fewer children than they eventually wanted were categorized as not having met their desired family size at the time of interview. Because we were concerned with experiences of infertility and their association with meeting reproductive goals, we did not count any non-biological children participants were currently raising or had raised towards meeting their reproductive goals. We modeled currently having their desired number of children as a dichotomous outcome (yes/no) using logistic regression with both an unadjusted model and a model adjusted for age (22–25, 26–30, 31–35, 36–40), nulliparity and ever been a regular smoker.
RESULTS
A total of 75 women with SLE and 154 comparison women were included in the analysis (Figure 1). After other exclusions, 12 women with SLE were excluded because they had been treated with cyclophosphamide. Women treated with cyclophosphamide were younger at the time of interview than women not treated with cyclophosphamide (average age of 30.8 vs. 33.0) and were diagnosed at younger ages than women not treated with cyclophosphamide (average age of 21.8 vs. 24.8). Sixty-seven percent of women treated with cyclophosphamide had ever given birth, compared to 69% of women never treated with cyclophosphamide.
In the analysis sample, women with SLE were younger than the comparison women, with a median age of 34 (interquartile range (IQR): 29–38) compared to a median age of 36.5 (IQR: 33–38). Women with SLE were also more likely to have ever been regular smokers (22.7% vs. 6.5%) (Table 1). Over half of women with SLE were diagnosed before the age of 26. Among both women with and without SLE, about 70% of women had ever had a live birth. Although, on average, women with SLE gave birth at an earlier age to their first child (age 20.2 vs. 24.4). Eighty-seven percent of women with SLE had been pregnant before, compared with 78% of women without SLE. Among women with SLE who had been pregnant, 44.6% had pregnancies only before diagnosis, 24.6% only after diagnosis, while the remaining 30.8% had pregnancies both before and after diagnosis. Also among women who had been pregnant, 36.9% percent of women with SLE had experienced a prior pregnancy loss (miscarriage or stillbirth) compared with 29.1% of women without SLE. Women with SLE were more likely to have ever experienced any period of infertility (regular unprotected intercourse which did not result in a pregnancy) (58.7% vs. 34.7%), but when restricting just to periods where women were actively trying to conceive, these proportions were more similar (17.3% vs. 13.3%). Among women with SLE, 37.3% experienced a period of any infertility after SLE diagnosis and 9.3% experienced a period of infertility when activity trying to conceive after SLE diagnosis. Among SLE women who experienced any period of infertility, the average age of the first period of infertility was 24.2 years (standard deviation [SD]: 4.8), while among comparison women it was 25.0 years (SD: 4.7). Women with SLE experienced their first period of infertility when actively trying to conceive at a similar average age (23.5 [SD: 3.4]) while comparison women were slightly older (27.0 [SD: 4.1]).
Table 1.
Characteristics of study participants
| Characteristic | SLE (N=75) n (%) |
Comparison (N=154) n (%) |
|---|---|---|
| Age at Interview | ||
| 22–25 | 9 (12.0) | 4 (2.6) |
| 26–30 | 19 (25.3) | 11 (7.1) |
| 31–35 | 15 (20.0) | 48 (31.2) |
| 36–40 | 32 (42.7) | 91 (59.1) |
| Age at SLE Diagnosis | ||
| 18–20 | 20 (26.7) | |
| 21–25 | 26 (34.7) | |
| 26–30 | 15 (20.0) | |
| 31–35 | 14 (18.7) | |
| Parity | ||
| 0 | 23 (30.7) | 46 (29.9) |
| 1 | 18 (24.0) | 38 (24.7) |
| 2 | 16 (21.3) | 39 (25.3) |
| 3 or more | 18 (24.0) | 31 (20.1) |
| Ever been pregnant Pregnancy losses* | 65 (86.7) | 120 (78.4) |
| 0 | 41 (63.1) | 85 (70.8) |
| 1 | 18 (27.7) | 25 (20.8) |
| 2 or more | 6 (9.2) | 10 (8.3) |
| Do not want to raise any children | 2 (2.8) | 5 (3.3) |
| Ever been a regular smoker | 17 (22.7) | 10 (6.5) |
| Ever experienced infertility | 44 (58.7) | 52 (34.7) |
| Before SLE diagnosis | 21 (28.0) | |
| After SLE diagnosis | 28 (37.3) | |
| Every experienced infertility when trying | 13 (17.3) | 20 (13.3) |
| Before SLE diagnosis | 7 (9.3) | |
| After SLE diagnosis | 7 (9.3) | |
Among those who have ever been pregnant; losses include miscarriages and stillbirths
In unadjusted analyses, the hazard of any period of infertility among African-American women with SLE was about twice the hazard among African American comparison women (hazard ratio [HR]: 2.20, 95% confidence interval [CI]: 1.47, 3.30) and this association was only slightly attenuated after controlling for parity and smoking status (HR: 2.08, 95% CI: 1.38, 3.15) (Table 2). When only periods of infertility when actively trying to conceive were considered, women with SLE had a slightly greater hazard of infertility in the unadjusted model (HR: 1.53, 95% CI: 0.76, 3.09), but the association was attenuated in the adjusted analysis (HR: 1.30, 95% CI: 0.62, 2.71). The results of the matched analysis largely mirrored the results of the unmatched models, though the simulation intervals were much less precise. We were unable to run the matched adjusted model of time to infertility when actively trying to conceive due to small cell sizes. The median hazard ratios and 95% simulation intervals suggested that the hazard of infertility in women with SLE was two times that in comparison women when considering any period of infertility, but there was no association when considering only periods of infertility when women were actively trying to conceive.
Table 2.
Hazard of infertility among women with SLE (N=75) compared to women without SLE (N=154)
| Unmatched* | Matched Analysis^ | |
|---|---|---|
| Outcome | Hazard Ratio (95% Confidence Interval) |
Median Hazard Ratio (95% Simulation Interval) |
| First period of infertility (Unadjusted) | 2.20 (1.47, 3.30) | 2.43 (1.21, 6.00) |
| First period of infertility (Adjusted) | 2.08 (1.38, 3.15) | 1.93 (0.75, 6.54) |
| First period of infertility when trying to conceive (Unadjusted) | 1.53 (0.76, 3.09) | 1.50 (0.36, 8.00) |
| First period of infertility when trying to conceive (Adjusted) | 1.30 (0.62, 2.71) | † |
Adjusted models control for nulliparity and if the woman reported ever being a regular smoker
Adjusted models control for ever gave live birth prior to matched date of diagnosis and ever been a regular smoker
Results omitted due to small sample size
Both women with SLE and comparison women wanted to raise a median of two children (Table 3). However, a greater proportion of women with SLE wanted to raise more than two children. A very small proportion of both groups (~3%) stated they did not want to raise any children. After adjusting for age, smoking history and nulliparity, women with SLE were slightly more likely to have met their desired family size at the time of the interview (odds ratio: 1.34, 95% CI: 0.66, 2.64).
Table 3.
Reproductive goals of women with SLE and comparison women
| SLE (N=75) | Comparison (N=154) | |
|---|---|---|
| Number of children want to raise (Median [Interquartile Range]) | 2 (2–4) | 2 (2–3) |
| Do not want to raise any children (n [%]) | 2 (2.8) | 5 (3.3) |
| Currently met desired number of children (Odds Ratio [95% Confidence Interval]*) | 1.32 (0.66, 2.64) | REF |
Adjusted models control for age (22–25, 26–30, 31–35, 36–40), nulliparity and if the woman ever been a regular smoker
DISCUSSION
The proportion of participants with SLE in our study who reported experiencing infertility when actively trying to conceive, 17.3%, was similar to what has been reported in other studies (12–16%), although these studies did not examine periods of infertility when pregnancy was not being attempted [7, 8, 24]. Contrary to our expectations, women with SLE were not more likely to experience periods of infertility when actively trying to conceive, and were as likely to have met their desired family size as comparison women at the time of the interview when controlling for age. However, we did see that women with SLE were more likely to have experienced prior pregnancy losses, as has been found in other studies [25, 26], meaning they may have had more pregnancies to reach their desired family size.
While we did not find a difference in experiences of infertility when women were actively trying to conceive, women with SLE were more likely to experience a 12-month period of any infertility at younger ages. We would have expected that women with SLE would be more likely to experience infertility when they were actively trying to conceive, but this may reflect some ambiguity about pregnancy intentions in the SLE population. Women with SLE may believe that they are less likely to become pregnant or be preoccupied with managing their disease; they may desire pregnancies, but be less likely to plan them than disease-free women. Previous research has found a similar phenomenon among women with other chronic conditions, such as obesity and diabetes. Women with pre-pregnancy obesity and/or diabetes are also advised to plan their pregnancies and meet certain preconception health recommendations [27, 28]. However, women with obesity reported non-use of contraception more often than normal weight women [29]. In a sample of diabetic women who had a live birth, more than half of pregnancies were unplanned. Only a third of women with unplanned pregnancies reported contraceptive failure, while half believed they could not get pregnant and 70% of women with unplanned pregnancies reported being happy when they found out they were pregnant [30]. It was also not uncommon for women with chronic disease to report a lack of control in their attempts to avoid pregnancy [30, 31]. Though we could not find studies on pregnancy desires in a sample of women with SLE, women with SLE may exhibit the same ambiguous feelings about becoming pregnant. In a survey of women with SLE, more than half had reported intercourse without any form of contraception in the prior 3 months [32]. Non-use of contraception in women with SLE may also be due to safety concerns, even though hormonal contraception is generally considered safe for women with SLE, as long as they do not have certain SLE complications [33]. Potential ambiguities around pregnancy desire and planning after an SLE diagnosis may mean that women with SLE have unplanned pregnancies earlier in life when they are more fertile, which translates into meeting their reproductive goals earlier, having fewer attempted pregnancies later and therefore less infertility later.
Providing support for the hypothesis of ambiguities around pregnancy planning is the fact that the average ages at first live birth in our sample, 20.2 for women with SLE and 24.4 years for the comparison women, were fairly young. In Fulton and DeKalb counties, the two counties that comprise most of Atlanta, Georgia, the average ages of African American first-time mothers in 2017 were 25.9 and 26.2, respectively [34]. The sample of women with SLE was also younger than the comparison group at enrollment. As fertility is extremely age-dependent and women in our sample began giving birth at younger ages, it is possible they began their families early enough that infertility, attributable to SLE and/or age, did not limit their family size. If these women had waited longer to become pregnant, they may have been more likely to experience infertility.
Alternatively, SLE itself may not meaningfully affect women’s fertility. The disease’s effects on women’s ability to become pregnant may be limited to the adverse side effects of certain treatments. We could only find two studies that measure time-to-pregnancy among women with SLE and neither included a healthy comparison group. In Morel et al., 73.7% (28 out of 38 women) who wanted to become pregnant succeeded within a year, while in Skorpen et al., it was 88.7% (47 out of 53 women) [7, 8]. Although these estimates are based on small numbers, they are not vastly different from the often cited figure that in the general population, about 80% of women will become pregnant in the first year of trying [35].
This study has several limitations that should be noted. First, there is the potential for unmeasured confounding because the SLE and comparison groups were drawn from two different studies and it was apparent that the sample of women with SLE was generally younger than the comparison group. Further, we excluded women with SLE treated with cyclophosphamide, who were likely the most severe cases of SLE, so it is possible that we may have underestimated the association between SLE and infertility if SLE severity is related to infertility. However, had these women been included, we would not have been able to separate the effect of cyclophosphamide from the effect of severe SLE. The number of months of unprotected intercourse without becoming pregnant was also self-reported. Retrospectively self-reported time-to-pregnancy may be subject to more errors than prospectively collected data, but we may have limited the influence of these errors by defining the outcome as ever experienced 12 months or more of unprotected intercourse without becoming pregnant. A recent study showed 93% sensitivity and 96% specificity for self-report of longer time-to-pregnancy (>13 cycles) [36]. However, if women without SLE were more likely to plan their pregnancies, their self-reported time to pregnancy may be more accurate than women without SLE, in which case this measurement error would be differential. Further, because these were data from a pilot study, our sample included fewer than 80 women with SLE and small numbers of women experiencing certain outcomes, resulting in imprecise estimates. We were unable to conduct the matched analysis for the association between SLE and infertility when attempting to conceive due to small numbers. Finally, there may be residual confounding by age because the sample size required that we adjust for age group rather than adjusting for age more finely.
Although small, this study is still one of the largest samples of African American women with SLE to be included in a study of fertility, and our results suggest that these women with SLE who have not been exposed to cyclophosphamide may not be at an increased risk of infertility compared to women without SLE. While there are advantages to studying a prospective cohort of women attempting pregnancy, our study also is unique in that it examines experiences of infertility among women when not attempting pregnancy. Women with chronic conditions who plan pregnancy might represent a particularly select group. Therefore, our results are important as they include women who would otherwise be excluded from fertility studies.
As African American women represent the group at greatest risk of SLE, more efforts should be made to include these women in studies of SLE, fertility and pregnancy. Pregnancy desires and their relation to pregnancy planning and intentions should also be considered when examining fertility outcomes among this group.
SIGNIFICANCE AND INNOVATIONS.
African American women with SLE were more likely to experience a 12-month period of infertility, and at younger ages.
When considering only periods of infertility when pregnancy was actively being attempted, African American women with SLE were more similar to women without SLE.
Controlling for age, African American women with SLE were as likely as comparison women without SLE to have met their desired family size.
ACKNOWLEDGEMENTS
We would like to thank Lauren Kipling who wrote the matching program.
This research was supported by grant funding from the National Institutes of Health (Grant 1R01HD066059) and a Synergy Award from the Woodruff Health Sciences Center at Emory University.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Andreoli L, Bertsias GK, Agmon-Levin N, Brown S, Cervera R, Costedoat-Chalumeau N, Doria A, Fischer-Betz R, Forger F, Moraes-Fontes MF et al. : EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis 2017, 76(3):476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazzaroni MG, Dall’Ara F, Fredi M, Nalli C, Reggia R, Lojacono A, Ramazzotto F, Zatti S, Andreoli L, Tincani A: A comprehensive review of the clinical approach to pregnancy and systemic lupus erythematosus. J Autoimmun 2016, 74:106–117. [DOI] [PubMed] [Google Scholar]
- 3.Clowse ME, Chakravarty E, Costenbader KH, Chambers C, Michaud K: Effects of infertility, pregnancy loss, and patient concerns on family size of women with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2012, 64(5):668–674. [DOI] [PubMed] [Google Scholar]
- 4.Vinet E, Clarke AE, Gordon C, Urowitz MB, Hanly JG, Pineau CA, Isenberg D, Rahman A, Wallace D, Alarcon GS et al. : Decreased live births in women with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2011, 63(7):1068–1072. [DOI] [PubMed] [Google Scholar]
- 5.Clowse ME, Harward L, Criscione-Schreiber L, Pisetsky D, Copland S: Anti-Mullerian hormone: a better marker of ovarian damage from cyclophosphamide. Arthritis Rheum 2012, 64(5):1305–1310. [DOI] [PubMed] [Google Scholar]
- 6.Drenkard C, Bao G, Dennis G, Kan HJ, Jhingran PM, Molta CT, Lim SS: Burden of systemic lupus erythematosus on employment and work productivity: data from a large cohort in the southeastern United States. Arthritis Care Res (Hoboken) 2014, 66(6):878–887. [DOI] [PubMed] [Google Scholar]
- 7.Morel N, Bachelot A, Chakhtoura Z, Ghillani-Dalbin P, Amoura Z, Galicier L, Aumaitre O, Piette JC, Pourrat J, Boutin D et al. : Study of anti-Mullerian hormone and its relation to the subsequent probability of pregnancy in 112 patients with systemic lupus erythematosus, exposed or not to cyclophosphamide. J Clin Endocrinol Metab 2013, 98(9):3785–3792. [DOI] [PubMed] [Google Scholar]
- 8.Skorpen CG, Lydersen S, Gilboe IM, Skomsvoll JF, Salvesen KA, Palm O, Koksvik HSS, Jakobsen B, Wallenius M: Women with systemic lupus erythematosus get pregnant more easily than women with rheumatoid arthritis. Rheumatology 2018, 57(6):1072–1079. [DOI] [PubMed] [Google Scholar]
- 9.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C: The incidence and prevalence of systemic lupus erythematosus, 2002–2004: The Georgia Lupus Registry. Arthritis Rheumatol 2014, 66(2):357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izmirly PM, Wan I, Sahl S, Buyon JP, Belmont HM, Salmon JE, Askanase A, Bathon JM, Geraldino-Pardilla L, Ali Y et al. : The Incidence and Prevalence of Systemic Lupus Erythematosus in New York County (Manhattan), New York: The Manhattan Lupus Surveillance Program. Arthritis Rheumatol 2017, 69(10):2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somers EC, Marder W, Cagnoli P, Lewis EE, DeGuire P, Gordon C, Helmick CG, Wang L, Wing JJ, Dhar JP et al. : Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol 2014, 66(2):369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dall’Era M, Cisternas MG, Snipes K, Herrinton LJ, Gordon C, Helmick CG: The Incidence and Prevalence of Systemic Lupus Erythematosus in San Francisco County, California: The California Lupus Surveillance Project. Arthritis Rheumatol 2017, 69(10):1996–2005. [DOI] [PubMed] [Google Scholar]
- 13.McDermott EM, Powell RJ: Incidence of ovarian failure in systemic lupus erythematosus after treatment with pulse cyclophosphamide. Ann Rheum Dis 1996, 55(4):224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harward LE, Mitchell K, Pieper C, Copland S, Criscione-Schreiber LG, Clowse ME: The impact of cyclophosphamide on menstruation and pregnancy in women with rheumatologic disease. Lupus 2013, 22(1):81–86. [DOI] [PubMed] [Google Scholar]
- 15.Alarfaj AS, Khalil N: Fertility, ovarian failure, and pregnancy outcome in SLE patients treated with intravenous cyclophosphamide in Saudi Arabia. Clin Rheumatol 2014, 33(12):1731–1736. [DOI] [PubMed] [Google Scholar]
- 16.Hickman RA, Gordon C: Causes and management of infertility in systemic lupus erythematosus. Rheumatology (Oxford) 2011, 50(9):1551–1558. [DOI] [PubMed] [Google Scholar]
- 17.Oktem O, Yagmur H, Bengisu H, Urman B: Reproductive aspects of systemic lupus erythematosus. J Reprod Immunol 2016, 117:57–65. [DOI] [PubMed] [Google Scholar]
- 18.Lateef A, Petri M: Managing lupus patients during pregnancy. Best Pract Res Clin Rheumatol 2013, 27(3):435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakravarty EF, Colon I, Langen ES, Nix DA, El-Sayed YY, Genovese MC, Druzin ML: Factors that predict prematurity and preeclampsia in pregnancies that are complicated by systemic lupus erythematosus. American Journal of Obstetrics and Gynecology 2005, 192(6):1897–1904. [DOI] [PubMed] [Google Scholar]
- 20.Buyon JP, Kim MY, Guerra MM, Laskin CA, Petri M, Lockshin MD, Sammaritano L, Branch DW, Porter TF, Sawitzke A et al. : Predictors of Pregnancy Outcomes in Patients With Lupus: A Cohort Study. Ann Intern Med 2015, 163(3):153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drenkard C, Rask KJ, Easley KA, Bao G, Lim SS: Primary preventive services in patients with systemic lupus erythematosus: study from a population-based sample in Southeast U.S. Semin Arthritis Rheum 2013, 43(2):209–216. [DOI] [PubMed] [Google Scholar]
- 22.1997. Update of the 1982 American College of Rheumatology Revised Criteria for Classification of Systemic Lupus Erythematosus [https://www.rheumatology.org/Portals/0/Files/1997%20Update%20of%201982%20Revised.pdf] [DOI] [PubMed]
- 23.Chin HB, Johnson CY, Kim KH, Knight JH, Mertens AC, Mink PJ, Simeone RM, Woodard JJ, Howards PP: Piloting a computer assisted telephone interview: the FUCHSIA Women’s Study. BMC Womens Health 2014, 14:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clowse ME, Grotegut C: Racial and Ethnic Disparities in the Pregnancies of Women With Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken) 2016, 68(10):1567–1572. [DOI] [PubMed] [Google Scholar]
- 25.Barnado A, Wheless L, Meyer AK, Gilkeson GS, Kamen DL: Pregnancy outcomes among African-American patients with systemic lupus erythematosus compared with controls. Lupus Sci Med 2014, 1(1):e000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling N, Lawson E, von Scheven E: Adverse pregnancy outcomes in adolescents and young women with systemic lupus erythematosus: a national estimate. Pediatr Rheumatol Online J 2018, 16(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ACOG Practice Bulletin No 156: Obesity in Pregnancy. Obstet Gynecol 2015, 126(6):e112–126. [DOI] [PubMed] [Google Scholar]
- 28.Bulletins ACoP: ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Number 60, March 2005. Pregestational diabetes mellitus. Obstet Gynecol 2005, 105(3):675–685. [DOI] [PubMed] [Google Scholar]
- 29.Chuang CH, Chase GA, Bensyl DM, Weisman CS: Contraceptive use by diabetic and obese women. Womens Health Issues 2005, 15(4):167–173. [DOI] [PubMed] [Google Scholar]
- 30.Holing EV, Beyer CS, Brown ZA, Connell FA: Why don’t women with diabetes plan their pregnancies? Diabetes Care 1998, 21(6):889–895. [DOI] [PubMed] [Google Scholar]
- 31.Chuang CH, Velott DL, Weisman CS: Exploring knowledge and attitudes related to pregnancy and preconception health in women with chronic medical conditions. Matern Child Health J 2010, 14(5):713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarz EB, Manzi S: Risk of unintended pregnancy among women with systemic lupus erythematosus. Arthritis Rheum 2008, 59(6):863–866. [DOI] [PubMed] [Google Scholar]
- 33.Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ, Whiteman MK: US Medical Eligibility Criteria for Contraceptive Use, 2016. Mmwr Recommendations and Reports 2016, 65(3):1–103. [DOI] [PubMed] [Google Scholar]
- 34.CDC Wonder: Natality, 2007-2017. URL: https://wonder.cdc.gov.
- 35.te Velde ER, Eijkemans R, Habbema HD: Variation in couple fecundity and time to pregnancy, an essential concept in human reproduction. Lancet 2000, 355(9219):1928–1929. [DOI] [PubMed] [Google Scholar]
- 36.Jukic AM, McConnaughey DR, Weinberg CR, Wilcox AJ, Baird DD: Long-term Recall of Time to Pregnancy. Epidemiology 2016, 27(5):705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]


