ABSTRACT
Background
The role of diet on hypertensive disorders of pregnancy (HDPs), including preeclampsia and gestational hypertension (GHTN), remains unclear.
Objectives
We evaluated whether adherence during pregnancy to dietary recommendations that reduce cardiovascular disease (CVD) in the general population is related to the risk of HDPs.
Methods
We followed 66,651 singleton pregnancies from 62,774 women participating in the Danish National Birth Cohort. Diet was assessed during week of gestation 25 with an FFQ from which we created 2 dietary pattern scores: 1) AHA, based on the diet recommendations from the AHA 2020 Strategic Impact Goals; and 2) the Dietary Approaches to Stop Hypertension (DASH) diet. Cases of HDPs were identified through linkage with the Danish National Patient Registry. RRs and 95% CIs of HDPs were estimated by increasing quintiles of adherence to the AHA and DASH scores using log-Poisson regression models with generalized estimating equations—to account for repeated pregnancies per woman—while adjusting for potential confounders.
Results
We identified 1809 cases of HDPs: n = 1310 preeclampsia (n = 300 severe preeclampsia) and n = 499 cases of GHTN. Greater adherence to AHA or DASH scores was not related to the risk of HDPs. However, when each component of the scores was separately evaluated, there were positive linear relations of sodium intake with HDPs (P-linearity < 0.01). Women with the highest sodium intake [median 3.70 g/d (range: 3.52, 7.52 g/d)] had 54% (95% CI:16%, 104%) higher risk of GHTN and 20% (95% CI:1%, 42%) higher risk of preeclampsia than women with the lowest intake [median 2.60 g/d (range: 0.83, 2.79 g/d)]. In addition, intake of whole grains was positively related to the risk of GHTN but not to preeclampsia ( P-heterogeneity = 0.002).
Conclusion
Sodium intake during pregnancy, but no other diet recommendations to prevent CVD among nonpregnant adults, is positively related to the occurrence of HDPs among pregnant Danish women.
Keywords: preeclampsia, sodium, AHA 2020 goals, DASH, pregnancy, gestational hypertension, dietary patterns
Introduction
Hypertensive disorders of pregnancy (HDPs), including preeclampsia and gestational hypertension (GHTN), are responsible for 10–15% of maternal deaths worldwide (1). In addition, women with a history of HDP are at increased risk of type 2 diabetes mellitus (2–4) and cardiovascular disease (CVD) later in life (5). In the general adult population, diet has a major role in the prevention and management of hypertension and CVD (6). Current nutritional guidance for pregnant women focuses on micronutrient supplementation for the prevention of neural tubal defects and anemia, broad guidelines of gestational weight gain, and recommendations on the avoidance of alcohol and caffeine during pregnancy (7). As a result, healthcare providers that counsel pregnant women rarely give specific dietary recommendations to promote better pregnancy outcomes and women report receiving inadequate nutrition information from healthcare professionals during pregnancy (8).
Calcium supplementation plays an important role in the prevention of preeclampsia among women with deficient intakes (9, 10). However, in populations without nutritional deficiencies, the findings have been largely inconclusive when supplementation with antioxidants (vitamins E and C) (11), fish oil (12), or sodium restriction (13) were investigated in clinical trials. Furthermore, little is known about the relation of dietary patterns and the risk of HDPs (14–17). As a result, no specific dietary recommendations for the prevention of HDPs exist among women without micronutrient deficiencies, nor is it known to what extent dietary recommendations for the general population may also benefit pregnant women. In fact, the American College of Obstetricians and Gynecologists (ACOG) discourages sodium restriction during pregnancy for the prevention of preeclampsia (7) based on null results from salt restriction in clinical trials. For these reasons, we evaluated whether greater adherence during pregnancy to dietary recommendations aimed at preventing CVD in the general population—the diet recommendations from the AHA 2020 Impact Strategic Goals (18) and the Dietary Approaches to Stop Hypertension (DASH) (19)—is related to HDPs among Danish pregnant women. We hypothesize that greater adherence to these dietary patterns will be inversely related to the risk of preeclampsia and GHTN.
Methods
Study population
The Danish National Birth Cohort (DNBC) is a pregnancy cohort that evaluated multiple exposures during women's pregnancies, including diet, and their impact on pregnancy complications and the health of their offspring (20, 21). All Danish women presenting for their first prenatal visit (weeks of gestation 6–12) between January 1996 and October 2002 were invited to participate in the study. In total, 101,033 pregnancies from 91,827 women were included in the study. For each pregnancy, all participants gave written informed consent and completed 4 telephone interviews at weeks of gestations 12 and 30, and 6 and 18 mo postpartum. Participants were also mailed a semi-quantitative FFQ at week of gestation 25, that was completed and returned within 1 wk of mailing by 77% women. The Danish Committee of Ethics and the Danish Data Protection Agency approved the DNBC.
For this study, we included women with singleton pregnancies (2656 multifetal pregnancies excluded), without history of HDPs (56 pregnancies with recurrent HDPs excluded) and who completed the diet assessment (30,859 pregnancies excluded). We then excluded women who completed the FFQ after receiving a HDP diagnosis (458 pregnancies excluded). Of the 67,013 remaining pregnancies, we excluded 361 women with implausible dietary intake (<4.20 MJ/d or >2.50 MJ/d) and 1 woman of unknown maternal age at birth, leaving 66,651 pregnancies from 62,774 women available for analysis, of whom 58,831 women contributed 1 pregnancy, 3895 contributed 2 pregnancies, and 48 contributed 3 pregnancies (Supplemental Figure 1). Completing a diet assessment as part of this study was unrelated to the risk of preeclampsia [RR = 0.97 (95% CI: 0.88, 1.06)] or GHTN [RR = 0.96 (95% CI: 0.82, 1.12)].
Dietary assessment and dietary pattern scores
Diet was assessed with an FFQ that included questions on ∼360 food items and dietary supplements, which had been specifically developed for pregnant Danish women (22). Women were asked to report how often they had consumed each of the listed foods and beverages in the previous 4 wk, to reflect diet around week of gestation 20. Questions included 7–11 response categories for frequency of intake, which ranged from never to ≥8 times/d. Nutrient intakes were estimated by summing the nutrient contribution of each food item in the questionnaire, taking into consideration the brand and type of fats used for cooking or added to food. Nutrient contents of each food and standard portion size were obtained from the Danish Food Tables (20). In a study that specifically evaluated fruit and vegetable intake among 88 pregnant women who participated in DNBC, strong correlations between intake from the FFQ and 7-d weighed food diaries were reported for fruit (ρ = 0.66), vegetables (ρ = 0.32), and fruits and vegetables (ρ = 0.57). In addition, 3 biomarkers that reflect fruit and vegetable intake also had reasonable correlations with the FFQ (22).
We constructed 2 dietary pattern scores: 1) the AHA score based on the diet recommendations from the AHA 2020 Impact Strategic Goals (18), and 2) the DASH diet (23) (Supplemental Table 1). For the AHA score, intake of each component was assigned a continuous score based on methods previously described (24) with predetermined cutoffs that assign 10 points when each component was at or greater than the target level for encouraged foods (fruits and vegetables, whole grains, fish, nuts, and legumes) or at or less than the target level for discouraged foods/nutrients [sodium, sugar-sweetened beverages (SSBs), processed meat, and saturated fat]. Intermediate intakes were scored linearly between 0 and 10. The DASH diet encourages intake of fruits, vegetables, low-fat dairy products, whole grains, poultry, fish, and nuts; and discourages intake of saturated fat, red meat, and SSBs. The DASH score allocates 1–5 points for each food group based on quintiles of intake within the study population for intakes of all components. Scoring was reversed for red and processed meats, SSBs, and sodium, receiving more points for less consumption. For both scores, sodium intake (g/d) was energy adjusted with the residual method and saturated fat intake as densities (percentage of total energy/d). The remaining food groups represent servings/d.
HDP ascertainment
Preeclampsia or GHTN diagnoses were obtained via linkage to the Danish National Patient Registry (NPR) in which outcomes are defined following International Classification of Diseases-10 codes (25) for preeclampsia (DO140, DO141, DO142, DO149, DO150, DO151, DO152, and DO159) and GHTN (DO130, DO131, DO133, DO134, DO135, DO139). In a validation study among DNBC participants comparing registry diagnosis to medical record reviews, preeclampsia diagnosis had a specificity of 99% and a sensitivity of 69% (26). GHTN diagnosis was limited to those registered in the NPR from outpatient and inpatient records, which may have a more severe clinical presentation compared to blood pressure measurements from medical records.
Assessment of covariates
Women reported age at birth, education, weight, height, socioeconomic status, cohabitation status, homeownership, education, and supplement use in the first telephone interview (week of gestation 12). Height and weight were used to estimate prepregnancy BMI (kg/m2). Information on smoking status, and fish oil supplement use was obtained from the second telephone interview (week of gestation 30). Other relevant health information, including infertility diagnosis and treatment, and history of pregnancy outcomes, were obtained from the NPR.
Statistical analysis
Differences in baseline characteristics across quintiles of the AHA and DASH scores were compared and we computed the Spearman correlation between the 2 patterns to assess the similarity of exposures. We estimated the RR and 95% CI of incident HDPs, overall and separately for preeclampsia or GHTN, using log-Poisson (27) generalized estimating equation models (28) with an exchangeable working correlation structure to account for within-woman correlations across pregnancies. Tests for linear trend were conducted with use of the median values of intake in each quintile (29). To adjust for confounding, we used directed acyclic graphs considering their prior biological association to nutrient intake or whether they were known predictors of preeclampsia in this population (30). The adjusted models included total energy intake (MJ/d), age (<30 y, 30–34 y, and ≥35 y), prepregnancy BMI (<18.5, 18.5–24.9, 25–29.9, ≥30), parity (nulliparous, 1, 2, ≥3), smoking (nonsmoker, smokers: occasional, daily: <15cigarettes/d, ≥15 cigarettes/d), concomitant gestational diabetes (yes, no), height (<165 cm, 165–168 cm, 169–172 cm, ≥173 cm, and unknown), region in Denmark (Copenhagen, others), education (high school, other), and intakes of vitamin C and vitamin E (g/d) (31). Finally, to estimate the proportion contributed by each food group to a specific nutrient, we fitted stepwise linear regression models to estimate R2 values from the FFQ (32).
We first evaluated the risk of preeclampsia, including severe preeclampsia, and GHTN according to increasing adherence to AHA and DASH scores by comparing women with the highest adherence with women with the lowest score adherence. We also considered each of the components of the overall recommendation and estimated the RR (95% CI) of preeclampsia or GHTN per unit increase, while adjusting for the remaining components of the overall recommendations. To check the adequacy of the model we examined the possibility of a nonlinear relation between nutrient intakes and all HDPs with restricted cubic splines (33). To assess nonlinearity, we used the likelihood ratio test comparing the model with only the linear term to the model with the linear and the cubic spline terms.
In sensitivity analyses, we excluded all women with a diagnosis of hypertension before pregnancy and when preeclampsia is the main outcome of interest, we excluded those with a diagnosis of GHTN from the study population, and vice versa, making normotensive women the comparison group. Lastly, to assess effect modification, we used cross-product terms of the AHA primary and DASH scores, intakes of sodium and whole grain with age (<30 y, ≥30 y), prepregnancy BMI (<25, ≥25), parity (nulliparous, parous), and smoking during pregnancy (yes, no) with the risk of preeclampsia or GHTN. To assess differences for each AHA secondary score component between pregnancies with preeclampsia and pregnancies with GHTN, we calculated pairwise tests (P-heterogeneity) by employing multivariable generalized estimating equation models (i.e., sodium intake in pregnancies with preeclampsia compared with pregnancies with GHTN). Thus, for these models we only included women with any HDPs diagnoses (n = 1809). All statistical analyses for this paper were generated by SAS software, Version 9.4 released in 2013 (SAS Institute Inc.).
Results
We documented 1809 cases of HDPs (2.71%) among the 66,651 pregnancies [n = 1310 cases of preeclampsia (1.98%) and n = 499 (0.75%) cases of GHTN]. The mean ± SD age at pregnancy was 30 ± 4 y, prepregnancy BMI was 24 ± 4, and height was 169 ± 6 cm. The AHA primary score had a moderate positive correlation with the DASH score (ρ = 0.66), which was even stronger for the AHA secondary score (ρ = 0.77). Women with greater adherence to the diet recommendations from the AHA 2020 Strategic Impact Goals and the DASH diet were more likely to have higher education, be nonsmokers, drink more water, and were less likely to drink alcohol during pregnancy. Nutritional characteristics according to adherence scores followed the expected pattern based on the intent of the recommendations ( Table 1).
TABLE 1.
Baseline demographic characteristics according to second trimester of pregnancy adherence to the diet recommendations from the AHA 2020 Impact Strategic Goals (18) and DASH (19) diet among 66,651 singleton pregnancies from the Danish National Birth Cohort (1996–2002)1
| AHA primary score | AHA secondary score | DASH score | ||||
|---|---|---|---|---|---|---|
| Q1 | Q5 | Q1 | Q5 | Q1 | Q5 | |
| Median score, points (IQR) | 23 (20, 25) | 41 (40, 43) | 35 (31, 37) | 62 (60, 65) | 19 (17, 20) | 30 (28, 31) |
| n pregnancies | 13,942 | 14,056 | 12,604 | 14,015 | 14,734 | 14,665 |
| Demographic characteristics | ||||||
| Maternal age at birth, y | 29 ± 4 | 31 ± 42 | 29 ± 4 | 31 ± 42 | 30 ± 4 | 31 ± 42 |
| Prepregnancy BMI, kg/m2 | 24.3 ± 4.69 | 22.8 ± 3.662 | 24.2 ± 4.65 | 22.9 ± 3.652 | 24.4 ± 4.66 | 22.7 ± 43.612 |
| Height, cm | 168 ± 6 | 169 ± 62 | 168 ± 6 | 169 ± 62 | 168 ± 6 | 169 ± 62 |
| High school graduate, % | 32 | 482 | 30 | 502 | 33 | 472 |
| Never smoker, % | 67 | 822 | 65 | 842 | 68 | 812 |
| Diagnosis of hypertension before pregnancy, % | 1.02 | 0.822 | 1.18 | 0.762 | 1.22 | 0.68 |
| Diet characteristics | ||||||
| Total energy, MJ/d | 9.37 ± 2.73 | 10.6 ± 2.442 | 10.1 ± 2.97 | 10.2 ± 2.352 | 9.2 ± 2.53 | 11.0 ± 2.692 |
| Protein, %E/d | 15 ± 3 | 15 ± 22 | 15 ± 3 | 16 ± 22 | 15 ± 2 | 15 ± 22 |
| Carbohydrate, %E/d | 54 ± 6 | 55 ± 62 | 51 ± 6 | 57 ± 52 | 52 ± 6 | 56 ± 62 |
| Total fat, %E/d | 31 ± 6 | 29 ± 62 | 34 ± 6 | 26 ± 52 | 32 ± 6 | 28 ± 62 |
| Saturated fat, %E/d | 13 ± 3 | 12 ± 32 | 15 ± 3 | 10 ± 32 | 14 ± 3 | 11 ± 32 |
| Drank alcohol, % | 50 | 382 | 49 | 392 | 47 | 412 |
| Water, glass 3/d | 3 ± 2 | 5 ± 22 | 3 ± 2 | 5 ± 22 | 4 ± 2 | 5 ± 22 |
| Vitamin C, g/d | 0.01 ± 0.08 | 0.17 ± 0.092 | 0.11 ± 0.07 | 0.18 ± 0.092 | 0.09 ± 0.05 | 0.19 ± 0.112 |
| Vitamin E, g/d | 0.01 ± 0.03 | 0.09 ± 0.032 | 0.01 ± 0.00 | 0.01 ± 0.032 | 0.07 ± 0.03 | 0.09 ± 0.032 |
| Calcium, g/d | 1.32 ± 0.57 | 1.52 ± 0.522 | 1.35 ± 0.56 | 1.49 ± 0.532 | 1.17 ± 0.48 | 1.78 ± 0.562 |
| Sodium, g/d | 3.15 ± 0.51 | 3.07 ± 0.392 | 3.21 ± 0.49 | 3.06 ± 0.432 | 3.32 ± 0.48 | 3.00 ± 0.402 |
| Supplements | ||||||
| Vitamin C, g/d | 0.09 ± 0.11 | 0.10 ± 0.142 | 0.88 ± 0.99 | 0.10 ± 0.142 | 0.09 ± 0.01 | 0.10 ± 0.142 |
| Vitamin E, g/d | 0.01 ± 0.03 | 0.01 ± 0.032 | 0.01 ± 0.04 | 0.01 ± 0.032 | 0.01 ± 0.03 | 0.01 ± 0.032 |
| Calcium, g/d | 0.21 ± 0.48 | 0.19 ± 0.452 | 0.19 ± 0.47 | 0.21 ± 0.482 | 0.23 ± 0.52 | 0.16 ± 0.422 |
1Values are presented as means ± SDs or percentage (%). DASH, Dietary Approaches To Stop Hypertension; Q, quintile of adherence; %E, percentage of total energy.
2 P value < 0.05 for differences across quintiles from Kruskal-Wallis test for continuous variables and chi-square tests for categorical variables.
31 glass = 237 mL.
Greater adherence to the AHA or DASH dietary pattern scores during the second trimester of pregnancy was not associated with risk of preeclampsia (Table 2), including severe preeclampsia (Supplemental Table 2), or GHTN (Supplemental Table 3). Results were unchanged when preeclampsia or GHTN were compared exclusively to normotensive pregnancies (Table 2 and Supplemental Table 3). Neither adjusting for history of chronic HTN (Supplemental Tables 4 and 5) nor excluding women with a history of chronic HTN (Table 2, Supplemental Tables 4 and 5) from the analyses changed the results. Quantitative changes in the estimates were minimal and the interpretation of the findings was unaffected.
TABLE 2.
Association of second trimester adherence to the dietary recommendations from the AHA 2020 Impact Strategic Goals (18) and the DASH (19) diet with risk of preeclampsia (n = 66,651 pregnancies), the Danish National Birth Cohort (1996–2002)1
| Relative risk (95% CI) of preeclampsia by quintiles of adherence | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | P-trend | |
| AHA primary score | ||||||
| Median, (range) | 23 (4, 26) | 29 (27, 30) | 35 (31, 37) | 36 (35, 38) | 41 (39, 49) | — |
| n cases | 311 | 262 | 278 | 231 | 228 | — |
| n pregnancies | 14,258 | 11,911 | 14,000 | 12,937 | 13,545 | — |
| Age and energy2 | 1.00 | 1.02 (0.86, 1.19) | 0.92 (0.79, 1.09) | 0.83 (0.70, 0.98) | 0.79 (0.66, 0.94) | <0.01 |
| Multivariable3 | 1.00 | 1.08 (0.92, 1.27) | 1.01 (0.86, 1.18) | 0.92 (0.77, 1.10) | 0.90 (0.75, 1.08) | 0.13 |
| Multivariable vs. normotensive4 | 1.00 | 1.08 (0.92, 1.27) | 1.01 (0.85, 1.18) | 0.92 (0.78, 1.10) | 0.90 (0.75, 1.08) | 0.14 |
| Multivariable excluding HTN before pregnancy5 | 1.00 | 0.98 (0.83, 1.17) | 1.00 (0.85, 1.18) | 0.90 (0.75, 1.08) | 0.88 (0.73, 1.06) | 0.14 |
| AHA secondary score | ||||||
| Median, (range) | 35 (9, 39) | 43 (40, 46) | 49 (47, 51) | 54 (52, 57) | 62 (58, 79) | — |
| n cases | 271 | 289 | 218 | 268 | 254 | — |
| n pregnancies | 12,604 | 14,708 | 12,095 | 13,229 | 14,015 | — |
| Age and energy2 | 1.00 | 0.91 (0.78, 1.08) | 0.84 (0.70, 1.00) | 0.94 (0.80, 1.12) | 0.88 (0.74, 1.04) | 0.21 |
| Multivariable3 | 1.00 | 0.95 (0.81, 1.12) | 0.90 (0.75, 1.08) | 1.02 (0.86, 1.22) | 0.95 (0.79, 1.14) | 0.78 |
| Multivariable vs. normotensive4 | 1.00 | 0.95 (0.81, 1.12) | 0.90 (0.75, 1.08) | 1.02 (0.86, 1.21) | 0.95 (0.79, 1.14) | 0.77 |
| Multivariable excluding HTN before pregnancy5 | 1.00 | 0.94 (0.80, 1.12) | 0.95 (0.80, 1.13) | 1.00 (0.83, 1.20) | 0.96 (0.79, 1.15) | 0.82 |
| DASH score | ||||||
| Median, (range) | 19 (9, 20) | 22 (21, 22) | 24 (23, 25) | 26 (26, 27) | 29 (28, 39) | — |
| n cases | 310 | 246 | 281 | 232 | 241 | — |
| n pregnancies | 13,942 | 11,671 | 13,941 | 13,041 | 14,056 | — |
| Age and energy2 | 1.00 | 0.97 (0.81, 1.15) | 0.97 (0.84, 1.14) | 0.88 (0.73, 1.06) | 0.91 (0.77, 1.08) | 0.19 |
| Multivariable3 | 1.00 | 0.99 (0.83, 1.19) | 1.02 (0.87, 1.20) | 0.93 (0.77, 1.12) | 0.96 (0.80, 1.15) | 0.57 |
| Multivariable vs. normotensive4 | 1.00 | 0.99 (0.82, 1.18) | 1.02 (0.87, 1.20) | 0.93 (0.77, 1.12) | 0.96 (0.80, 1.15) | 0.56 |
| Multivariable excluding HTN before pregnancy5 | 1.00 | 1.02 (0.85, 1.23) | 1.03 (0.88, 1.22) | 0.97 (0.79, 1.17) | 0.99 (0.82, 1.20) | 0.83 |
1DASH, Dietary Approaches to Stop Hypertension; GHTN, gestational hypertension; HTN, hypertension; ICD-10, International Classification of Diseases, 10th revision; Q, quintile of adherence.
2Model adjusted for total energy intake and maternal age at pregnancy.
3Model adjusted for total energy intake, age, prepregnancy BMI, parity, smoking, concurrent gestational diabetes, height, Denmark demographic regions, education, vitamin C and vitamin E intake.
4Excluded 499 cases of GHTN (n = 65,152 pregnancies).
5Excluded 589 pregnancies with diagnosis of chronic hypertension before pregnancy (ICD-10: I10, I11, I12, I13, I15, I16).
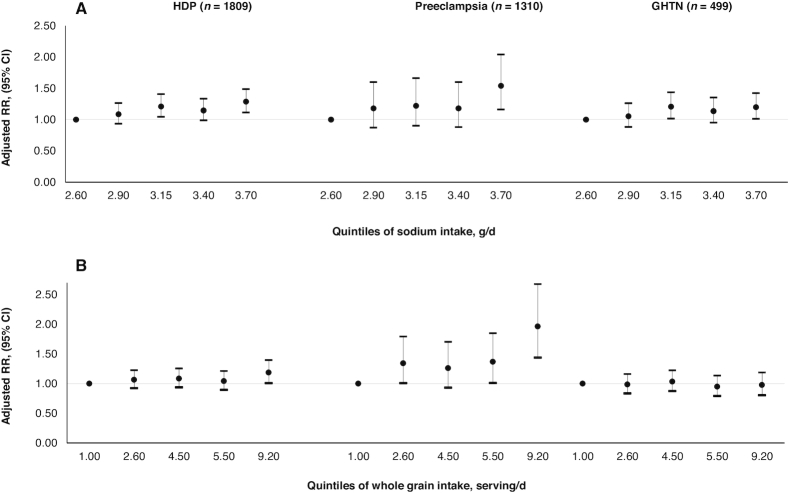
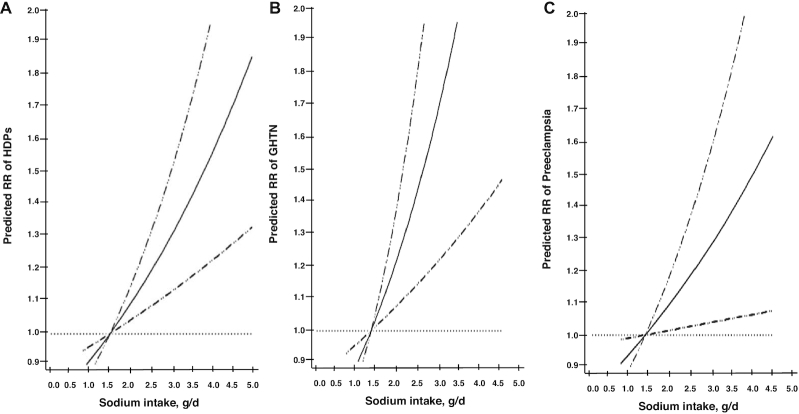
We then evaluated each component of the recommendation independently while considering the intake of the remaining components of the AHA and DASH scores. In these analyses, sodium intake was positively associated to HDPs overall (Figure 1) and to preeclampsia, including severe preeclampsia, and GHTN separately (Supplemental Figure 2). When we modeled intakes using quintiles of intake, we found that women in the highest quintile of sodium intake [median 3.70 g/d (range: 3.52, 7.52 g/d)] had a higher risk of HDPs overall [RR 1.29 (95% CI: 1.11, 1.49)], GHTN [RR 1.54 (95% CI: 1.16, 2.04)], and preeclampsia [RR 1.20 (95% CI: 1.01, 1.42)] than women in the lowest quintile [median 2.60 g/d (range: 0.83, 2.73 g/d)]. Whole grain intake was only associated with higher risk of GHTN, but not with preeclampsia or HDPs (Figure 2). No other individual components were related to HDPs. When intake was modeled continuously using a cubic spline, sodium intake also had a positive dose-response relation with all HDPs without evidence of departure from linearity (P-linearity < 0.01 for HDP, 0.02 for preeclampsia, and 0.02 for GHTN) (Figure 3).
FIGURE 1.
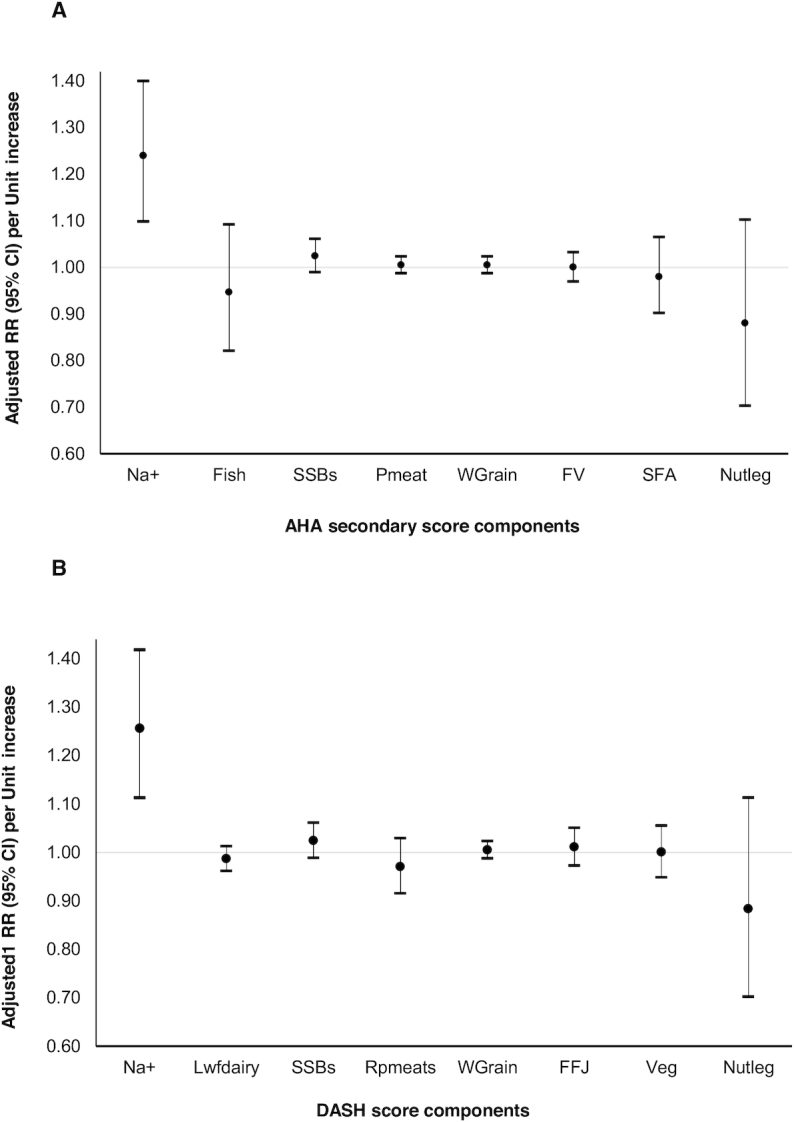
Association of adherence to individual components of the AHA (A) and DASH (B) dietary pattern scores during the second trimester of pregnancy with the risk of HDPs ( n = 66,651 pregnancies). Units for each score component were: an increase from the 10th to the 90th percentile for sodium intake (1.13 g/d), a 5% increase in E%/d for saturated fat, and serving/d for all the remaining components (fish, SSBs, WG, FV, Pmeat, SFA, and Nutleg). DASH, Dietary Approaches to Stop Hypertension; E%, percentage of total energy; FFJ, fruits and fruit juices; GHTN, gestational hypertension; hypertensive disorders of pregnancy; Lwfdairy, low-fat dairy; Na+, sodium; Nutleg, nuts and legumes; Pmeat, processed meats; RPmeats, red and processed meats; SFA, saturated fatty acids; SSB, sugar-sweetened beverage; Wgrain, whole-grain.
FIGURE 2.
Association between increasing quintiles of sodium (A) and whole grain (B) intakes during the second trimester of pregnancy with the risk of HDPs ( n = 66,651 pregnancies). HDP, hypertensive disorder of pregnancy.
FIGURE 3.
Association betw een sodium intake with different hypertensive disorders of pregnancy phenotypes ( n = 66,651 pregnancies). The relative risks of HDPs were estimated nonparametrically with spline function polynomials with the reference value for sodium 1.50 g/d. P-linearity represents the test for linear relation. (A) HDPs (P-linearity < 0.01). (B) GHTN (P-linearity = 0.02). (C) Preeclampsia (P-linearity = 0.02). GHTN, gestational hypertension; HDP, hypertensive disorder of pregnancy.
We then evaluated whether the association of each component differed significantly from each other comparing the 2 HDP phenotypes (Supplemental Figure 2). The association between intake of whole grains with GHTN was significantly different from the association of whole grain intake with preeclampsia (P-heterogeneity = 0.02). We found no evidence that the association of all other individual score components related differently to preeclampsia or GHTN (Supplemental Figure 2). The linear associations remained unchanged when we compared separate models adjusting for the AHA scores (excluding sodium points) and its individual components, and whole grain only (Supplemental Figure 3).
Lastly, we examined whether participant characteristics modified the relation between adherence to AHA or DASH scores, sodium, and whole grain with the risk of preeclampsia or GHTN. Among women with GHTN, there was evidence of effect modification between smoking during pregnancy with the AHA primary and the DASH score, as well as prepregnancy BMI (<25, ≥25) with the DASH score and sodium intake, that was not replicated among women with preeclampsia. There was no evidence of significant heterogeneity for maternal age at birth (<30 y, ≥30 y) and parity (nulliparous or parous) with any HDP phenotype (Supplemental Table 6).
Discussion
We examined women's second trimester adherence to the AHA and the DASH dietary pattern scores in relation to risk of HDPs among participants of the DNBC. Although we found no association between increased adherence to these diet patterns and risks of HDPs, GHTN, or preeclampsia, we found that lower sodium intake—a component of both AHA and DASH—was related to a lower risk of HDPs. Our findings suggest that while overall recommendations for the management and prevention of heart disease in the general population may not directly translate to the prevention of HDPs, sodium reduction, a cornerstone of these recommendations, may have a role in preventing HDPs.
The positive association we observed between sodium intake with the risk of HDPs, is consistent with findings in animal models and a few observational studies. For example, feeding aldosterone knockout mice a high salt diet resulted in reduced litter numbers and higher rates of intrauterine growth restriction (34). Both of these are signs with placental hypoperfusion typically seen in preeclampsia, with the limitation that preeclampsia is a disease unique to humans and is roughly replicated in animal models (35). Third trimester 24-h urinary sodium excretion among women with preeclampsia was related to higher systolic and diastolic blood pressure (36). The same study found that women with lower sodium excretion were less likely to have severe preeclampsia and kidney damage, compared to those with higher sodium excretion. In a study in Bangladesh, drinking water with sodium levels of 517 mg/L increased the odds of preeclampsia and GHTN (37).
Conversely, a handful of observational studies have proposed that sodium supplementation during pregnancy may reduce the development of preeclampsia, whereas others have found null or mixed results (38). It is worth noting that some of these studies date back to the 1900s, and most lacked randomization or any type of control for confounding. Furthermore, a recent meta-analysis that included 2 randomized clinical trials with 603 pregnant women found that salt restriction did not prevent HDPs (pooled RR of 1.11 (95% CI: 0.46, 2.66) (13), thus ACOG does not consider salt restriction in their diet recommendations for the prevention of preeclampsia based on the evidence of these studies.
Sodium intake in early stages of pregnancy is pivotal for physiologic extracellular volume expansion, which regulates maternal blood pressure and uteroplacental circulation (39,40). However, it is not entirely clear whether dietary salt has a causal association with risk of HDPs. It is also unknown whether placental sodium metabolism is responsible for the lower volume expansion coupled with higher urinary sodium excretion observed in preeclampsia (41). Clearly, further research into the role of sodium intake during pregnancy on HDPs is warranted.
We also found relations with whole grain intake that were not consistent across different HDPs and modeling strategies. Specifically, intake of whole grains was related to higher risk of GHTN but not of preeclampsia, which may suggest that GHTN has other diet risk factors than those in preeclampsia. The association between whole grain intake and higher risk of GHTN in this population is likely a result of residual confounding. Whole grains, and in particular rye bread, were a major source of the estimated sodium intake in this population at the time of the diet assessment (1996–2002). From the 360 food items listed in the FFQ, rye bread (with butter and with other food topping) had the highest contribution to sodium intake (R2 = 0.18). In addition, 89% of the total whole grain intake was from rye bread in this population of Danish pregnant women. These facts, along with known difficulties in estimating sodium intake using FFQs, make this study highly susceptible to residual confounding.
Our study has several limitations inherent in observational data such as confounding and measurement error. First, diet was assessed around week of gestation 25 and participants were asked to report on their intake during the previous 4 wk, thus timing of the questionnaire may not adequately capture the relevant exposure window for preeclampsia, which may be at the time of placental formation. However, excluding diagnoses of preeclampsia before completion of the FFQ as well as excluding women with prior preeclampsia diagnosis and adjusting the multivariable model for parity and prepregnancy BMI, allowed us to limit the chance of reverse causation. It is also not possible to know to what extent our findings may be generalizable to non-European women. The accuracy of measuring sodium intake with FFQs is not well understood (42), and, overall, correlation with measures of 24-h urinary sodium (gold-standard) was reported to be low in a meta-analysis of 19 observational studies (43). This is a limitation in our study, particularly because sodium-specific validation was not done for the FFQ used in DNBC. Another limitation is the sensitivity of 69% reported in the validation study. Because preeclampsia diagnosis was highly specific (99%), we are confident that among women presenting with symptoms of preeclampsia, noncases were correctly excluded. Strengths of our study include the prospective design, the use of a previously validated FFQ, the high specificity of case assessment through linkage with a National Disease Registry, dietary patterns based on clinical recommendations which increase generalizability of our results, and the large sample size that gave us sufficient cases necessary for the estimation of relative risks with significant statistical power and to examine different effects for preeclampsia or GHTN.
In summary, we found that lower sodium intake during pregnancy among Danish women is related to a lower risk of HDP. Other specific dietary recommendations, such as adherence to the AHA and DASH scores, did not seem to provide benefits during pregnancy among our study population. Before considering changes to recommendations regarding sodium intake during pregnancy, additional studies are needed to confirm or refute our findings.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—SFO and JEC: designed the research study; MA: analyzed data; SFO and TIH: contributed to the acquisition of data; MA: wrote the manuscript; AAB, TIH, SFO, AJG, JWR-E, BAR, and JEC: critically revised the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by grants from the March of Dimes Foundation (6-FY96-0240, 6-FY97-0553, 6-FY97-0521, 6-FY00-407), the European Union (QLK1-CT-2000-00083), Danish National Research Foundation, Danish Medical Research Council (9601842 and 22-03-0536), Danish Health Foundation (11/263-96), Danish Heart Foundation (96-2-4-83-22450), and the US National Institutes of Health (5P30DK46200-18, DK007703-16).
Author disclosures: MA, AAB, MTBM, CG, TIH, SFO, AJG, JWR-E, BAR, and JEC, no conflicts of interest.
Supplemental Figures 1–3 and Supplemental Tables 1–6 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Data sharing: Data described in the manuscript, codebook, and analytic code will be made available; code is available upon request without constraints. Access to the data is contingent upon approval by the Danish Committee of Ethics and the Danish Data Protection Agency. To initiate authorization for data access, the investigators must contact the corresponding author.
Abbreviations used: ACOG, American College of Obstetricians and Gynecologists; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; DNBC, Danish National Birth Cohort; GHTN, gestational hypertension; HDP, hypertensive disorder of pregnancy; NPR, National Patient Registry; SSB, sugar-sweetened beverage.
References
- 1. Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PFA. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–74. [DOI] [PubMed] [Google Scholar]
- 2. Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53:944–51. [DOI] [PubMed] [Google Scholar]
- 3. Callaway LK, Lawlor DA, O'Callaghan M, Williams GM, Najman JM, McIntyre HD. Diabetes mellitus in the 21 years after a pregnancy that was complicated by hypertension: findings from a prospective cohort study. Am J Obstet Gynecol. 2007;197:492.e1-7. [DOI] [PubMed] [Google Scholar]
- 4. Engeland A, Bjørge T, Daltveit AK, Skurtveit S, Vangen S, Vollset SE, Furu K. Risk of diabetes after gestational diabetes and preeclampsia. A registry-based study of 230,000 women in Norway. Eur J Epidemiol. 2011;26:157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women's future cardiovascular health: an underused opportunity to improve women's health?. Epidemiol Rev. 2014;36:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT et al.. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- 7. ACOG Practice Bulletin No. 202: Gestational hypertension and preeclampsia. Obstet Gynecol [Internet] 2019;133:e1–25. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30575675. [DOI] [PubMed] [Google Scholar]
- 8. Lucas C, Charlton KE, Yeatman H. Nutrition advice during pregnancy: do women receive it and can health professionals provide it?. Matern Child Health J. 2014;18:2465–78. [DOI] [PubMed] [Google Scholar]
- 9. Khaing W, Vallibhakara SA-O, Tantrakul V, Vallibhakara O, Rattanasiri S, McEvoy M, Attia J, Thakkinstian A. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. 2017;9:E1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92:3517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rumbold A, Duley L, Crowther CA, Haslam RR. Antioxidants for preventing pre-eclampsia. Cochrane Database Syst Rev. 2008;CD004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Makrides M, Duley L, Olsen SF. Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre-eclampsia or intrauterine growth restriction. Cochrane Database Syst Rev. 2006;CD003402. [DOI] [PubMed] [Google Scholar]
- 13. Duley L, Henderson-Smart D, Meher S. Altered dietary salt for preventing pre-eclampsia, and its complications. Cochrane Database Syst Rev. 2005;Cd005548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schoenaker D, Soedamah-Muthu S, Callaway L, Mishra G. Prepregnancy dietary patterns and risk of developing hypertensive disorders of pregnancy: results from the Australian Longitudinal Study on Women's Health. Am J Clin Nutr. 2015;102:94–101. [DOI] [PubMed] [Google Scholar]
- 15. Fulay AP, Rifas-Shiman SL, Oken E, Perng W. Associations of the dietary approaches to stop hypertension (DASH) diet with pregnancy complications in Project Viva. Eur J Clin Nutr. 2018;72:1385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ikem E, Halldorsson TI, Birgisdóttir BE, Rasmussen MA, Olsen SF, Maslova E. Dietary patterns and the risk of pregnancy-associated hypertension in the Danish National Birth Cohort: a prospective longitudinal study. BJOG. 2019;126:663–73. [DOI] [PubMed] [Google Scholar]
- 17. Gicevic S, Gaskins AJ, Fung TT, Rosner B, Tobias DK, Isanaka S, Willett WC. Evaluating pre-pregnancy dietary diversity vs. dietary quality scores as predictors of gestational diabetes and hypertensive disorders of pregnancy. PLoS One. 2018;13:e0195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF et al.. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 19. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM et al.. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
- 20. Olsen J, Melbye M, Olsen SF, Sørensen TI, Aaby P, Andersen AM, Taxbøl D, Hansen KD, Juhl M, Schow TB et al.. The Danish National Birth Cohort—its background, structure and aim. Scand J Public Health. 2001;29:300–7. [DOI] [PubMed] [Google Scholar]
- 21. Olsen SF, Mikkelsen TB, Knudsen VK, Orozova-Bekkevold I, Halldórsson TI, Strøm M, Osterdal ML. Data collected on maternal dietary exposures in the Danish National Birth Cohort. Paediatr Perinat Epidemiol. 2007;21:76–86. [DOI] [PubMed] [Google Scholar]
- 22. Mikkelsen TB, Olsen SF, Rasmussen SE, Osler M. Relative validity of fruit and vegetable intake estimated by the food frequency questionnaire used in the Danish National Birth Cohort. Scand J Public Health. 2007;35:172–9. [DOI] [PubMed] [Google Scholar]
- 23. Moore TJ, Vollmer WM, Appel LJ, Sacks FM, Svetkey LP, Vogt TM, Conlin PR, Simons-Morton DG, Carter-Edwards L, Harsha DW. Effect of dietary patterns on ambulatory blood pressure: results from the Dietary Approaches to Stop Hypertension (DASH) Trial. DASH Collaborative Research Group. Hypertension. 1999;34:472–7. [DOI] [PubMed] [Google Scholar]
- 24. Rehm CD, Peñalvo JL, Afshin A, Mozaffarian D. Dietary intake among US adults, 1999–2012. JAMA. 2016;315:2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization ICD-10: international statistical classification of diseases and related health problems: tenth revision, 2nd ed. Geneva: World Health Organization; 2004. [Google Scholar]
- 26. Klemmensen AK, Olsen SF, Osterdal ML, Tabor A. Validity of preeclampsia-related diagnoses recorded in a national hospital registry and in a postpartum interview of the women. Am J Epidemiol. 2007;166:117–24. [DOI] [PubMed] [Google Scholar]
- 27. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- 28. Fitzmaurice G., Laird N., Ware J. Applied longitudinal analysis. 2nd edition. Wiley series in probability and statistics. Hoboken (NJ): Wiley; 2011. 1309p. [Google Scholar]
- 29. Rosner B. Fundamentals of biostatistics. 5th ed Pacific Grove (California: ): Doxbury Press; 2005. [Google Scholar]
- 30. Weng H-Y, Hsueh Y-H, Messam LLM, Hertz-Picciotto I. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol. 2009;169:1182–90. [DOI] [PubMed] [Google Scholar]
- 31. Klemmensen A, Tabor A, Østerdal ML, Knudsen VK, Halldorsson TI, Mikkelsen TB, Olsen SF. Intake of vitamin C and E in pregnancy and risk of pre-eclampsia: prospective study among 57 346 women. BJOG. 2009;116:964–74. [DOI] [PubMed] [Google Scholar]
- 32. Willett W. Nutritional epidemiology. Oxford University Press; 1998. 514 p. [Google Scholar]
- 33. Durrleman S, Simon R.. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 34. Todkar A, Di Chiara M, Loffing-Cueni D, Bettoni C, Mohaupt M, Loffing J, Wagner CA. Aldosterone deficiency adversely affects pregnancy outcome in mice. Pflugers Arch. 2012;464:331–43. [DOI] [PubMed] [Google Scholar]
- 35. Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. [DOI] [PubMed] [Google Scholar]
- 36. Yılmaz ZV, Akkaş E, Türkmen GG, Ö Kara, Yücel A, Uygur D. Dietary sodium and potassium intake were associated with hypertension, kidney damage and adverse perinatal outcome in pregnant women with preeclampsia. Hypertens Pregnancy. 2017;36:77–83. [DOI] [PubMed] [Google Scholar]
- 37. Khan AE, Scheelbeek PFD, Shilpi AB, Chan Q, Mojumder SK, Rahman A, Haines A, Vineis P. Salinity in drinking water and the risk of (pre)eclampsia and gestational hypertension in coastal Bangladesh: a case-control study. PLoS One. 2014;9:e108715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steegers EA, Eskes TK, Jongsma HW, Hein PR. Dietary sodium restriction during pregnancy; a historical review. Eur J Obstet Gynecol Reprod Biol. 1991;40:83–90. [DOI] [PubMed] [Google Scholar]
- 39. Scaife PJ, Mohaupt MG. Salt, aldosterone and extrarenal Na+ - sensitive responses in pregnancy. Placenta. 2017;56:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Asayama K, Imai Y. The impact of salt intake during and after pregnancy. Hypertens Res. 2018;41:1–5. [DOI] [PubMed] [Google Scholar]
- 41. Gallery ED, Brown MA. Control of sodium excretion in human pregnancy. Am J Kidney Dis. 1987;9:290–5. [DOI] [PubMed] [Google Scholar]
- 42. Strazzullo P, D'Elia L, Kandala N-B, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McLean RM, Farmer VL, Nettleton A, Cameron CM, Cook NR, Campbell NRC, TRUE Consortium (International Consortium for Quality Research on Dietary Sodium/Salt) . Assessment of dietary sodium intake using a food frequency questionnaire and 24-hour urinary sodium excretion: a systematic literature review. J Clin Hypertens. 2017;19:1214–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.