ABSTRACT
Background
Nonalcoholic fatty liver disease (NAFLD), considered a “barometer” of metabolic health, is the leading cause of liver disease in the United States. Despite established associations between food insecurity and obesity, hypertension, and diabetes, little is known about the relation between food insecurity and NAFLD.
Objective
We sought to evaluate the association of food insecurity with NAFLD among low-income adults in the United States.
Methods
We conducted a cross-sectional analysis of a nationally representative sample of adults from the NHANES (2005–2014 waves). Participants included adults in low-income households (≤200% of the federal poverty level) without chronic viral hepatitis or self-reported heavy alcohol use. Food insecurity was measured using the Household Food Security Survey. Our primary outcome was NAFLD, as estimated by the US Fatty Liver Index, and our secondary outcome was advanced fibrosis, as estimated by the NAFLD fibrosis score. The association between food insecurity (defined as low and very low food security) and hepatic outcomes was assessed using multivariable logistic regression, adjusting for sociodemographic factors.
Results
Among 2627 adults included in the analysis, 29% (95% CI: 26%, 32%) were food insecure. The median age was 43 y, 58% were female, and 54% were white. The weighted estimated prevalence of NAFLD did not differ significantly by food security status (food secure 31% compared with food insecure 34%, P = 0.21). In the multivariable model, food-insecure adults were more likely to have NAFLD (adjusted OR: 1.38; 95% CI: 1.08, 1.77) and advanced fibrosis (adjusted OR: 2.20; 95% CI: 1.27, 3.82) compared with food-secure adults.
Conclusions
Food insecurity may be independently associated with NAFLD and advanced fibrosis among low-income adults in the United States. Future strategies should assess whether improved food access, quality, and healthy eating habits will decrease the growing burden of NAFLD-associated morbidity and mortality among at-risk adults.
Keywords: disparities, nutrition, underserved populations, urban health, food insecurity, vulnerable populations
Introduction
Food insecurity, defined as “a household-level economic and social condition of limited or uncertain access to adequate food” (1), affects more than 12% of US households and 35% of low-income households (2). An emerging body of evidence demonstrates an association between adults living in food-insecure households and increased cardiometabolic risk, including diabetes (3, 4), hypertension (5), obesity (among women) (6, 7), and cardiovascular disease (8).
Nonalcoholic fatty liver disease (NAFLD), considered a hepatic manifestation of metabolic disease, affects 1 in 3 adults in the United States (9). As the fastest growing cause of chronic liver disease, NAFLD will likely be the leading cause of liver transplantation in the United States by 2020 (10, 11). NAFLD represents a wide spectrum of histopathology, from simple steatosis (nonalcoholic fatty liver) to nonalcoholic steatohepatitis, which is characterized by steatosis associated with inflammation and, often times, fibrosis. Fibrosis is independently associated with progression to cirrhosis and hepatocellular carcinoma, as well as mortality. It is the most important predictor of adverse outcomes in individuals with NAFLD (11).
Cardiometabolic risk factors are considered key predictors of NAFLD (10), with diabetes and hypertension linked to poor NAFLD outcomes (12–14). Emerging evidence supports a bidirectional relation between NAFLD, diabetes, and hypertension (15). Food insecurity may trigger a complex cycle of poorer dietary intake and compensatory overconsumption of poor-quality foods, predisposing an individual to increased metabolic risk. Furthermore, food insecurity increases household stress, forces trade-offs between food and medical care, increases risk of medication nonadherence, and leads to poorer chronic disease self-management, all important pathways toward worse metabolic and chronic disease outcomes (16, 17).
Despite the reported associations between 1) food insecurity and cardiometabolic risk and 2) cardiometabolic risk and NAFLD, no prior studies have examined the association of food insecurity with NAFLD. There are several plausible mechanisms for how food insecurity may heighten risk of fatty liver and progressive fibrosis. Cumulative episodes of diet disruption and hunger may increase systemic inflammation and promote central adiposity, insulin resistance (18), and liver injury (11, 19). Furthermore, changes in diet quality during food-insecure periods may alter gut microbiota (20, 21) and promote hepatic inflammation and scarring (22).
Current individual-level strategies to mitigate the growing NAFLD burden, such as lifestyle change to promote weight loss (11), may be limited in their impact among adults living in low-income households since they may not address upstream, structural drivers of NAFLD (20). Understanding the relation between food insecurity and NAFLD can potentially inform how these structural factors increase risk of NAFLD and subsequently help develop more effective interventions for this at-risk group. We therefore sought to evaluate the association of food insecurity with NAFLD among low-income adults in the United States. We hypothesized that food insecurity would be associated with an increased odds of NAFLD among adults in low-income households.
Methods
Study design and population
We used data from the continuous NHANES from 2005 to 2014. NHANES is a complex multistage, stratified, clustered probability sample representative of the noninstitutionalized population of the United States consisting of cross-sectional interview, examination, and laboratory data (23). For this analysis, participants were included if they were 20 y or older and attended a medical examination at a mobile center and participated in a fasting examination. To restrict our analysis to metabolic liver disease, we excluded participants who had serologic evidence of viral hepatitis infection (i.e., hepatitis B surface antigen, hepatitis C antibody, or hepatitis C viral load), self-reported heavy alcohol use (defined as ≥3 drinks or 36 g of ethanol per day for men, ≥2 drinks or 28 g of ethanol per day for women) (24), or tested positive for pregnancy. We restricted our sample to low-income adults to reduce residual confounding of household wealth. In addition, our primary exposure of food insecurity predominantly affects adults with limited resources. We chose the 200% federal poverty level threshold since individuals living in households reporting >185% federal poverty level have a prevalence of food insecurity of <6% (25). This approach is consistent with other previously cited studies exploring food insecurity and metabolic outcomes that also use the same income poverty threshold (2, 4, 26). The Census Bureau identifies the federal poverty annually based on household income, size, and number of adults and children.
Independent variable
Food insecurity was measured within NHANES using the US Department of Agriculture's Core Food Security Module, which assesses food security status for households using an 18-item questionnaire (1). The module is a widely validated household scale that captures anxiety around food procurement, poor diet quality, and insufficient food quantity over the past 12 mo. Using standard scoring, decreasing numbers of affirmative responses indicate very low, low, marginal, and high food security (27). In our primary analysis, food insecurity was defined as food secure (“high” or “marginal” food security) or food insecure (“low” or “very low” food security) based on official USDA definitions and similar to prior studies linking food insecurity to health outcomes (3, 4).
Dependent variables
Our primary outcome was NAFLD, estimated using the US Fatty Liver Index (USFLI; see Supplemental Data for equation). The USFLI was derived in NHANES using ultrasound-assessed NAFLD defined as “mild,” “moderate,” or “severe.” The analysis that derived USFLI combined the “moderate” and “severe” categories to estimate the presence/absence of NAFLD as the outcome (binary), using a cutoff score of ≥30 (9). The index incorporates race/ethnicity, age, waist circumference, and laboratory values for γ-glutamyltransferase, fasting insulin, and fasting glucose.
Our secondary outcome was advanced liver fibrosis, as estimated using the NAFLD Fibrosis Score (NFS; see Supplemental Data for equation). We used a cutoff score of >0.675 for estimating presence of advanced fibrosis based on prior validation studies using biopsy-confirmed advanced fibrosis (28). Biopsy advanced fibrosis has been shown to predict liver-related morbidity and mortality in a similar sample of NHANES patients with NAFLD (29–31). The score uses age, BMI, diabetes/impaired fasting glucose, aspartate aminotransferase, alanine aminotransferase, platelets, and albumin. Both of these noninvasive measures incorporate fasting blood tests to estimate liver disease. The fasting subsample excluded participants if they self-reported taking oral hypoglycemic medications and/or insulin.
Given the overlap of cardiometabolic disease with NAFLD and fibrosis, we also examined the association between food insecurity with diabetes and obesity. We developed a conceptual model based on previous literature that links food insecurity to cardiometabolic risk factors (17, 20) and cardiometabolic risk to NAFLD [(10, 11) see Supplemental Figure 1]. Cardiometabolic diseases were defined by both self-report and clinical diagnosis (see Supplemental Methods).
Covariates
We included covariates known to be associated with cardiometabolic disease and food insecurity, including sociodemographic factors: age, sex, race/ethnicity (self-reported non-Hispanic black, non-Hispanic white, Mexican, non-Mexican Hispanic, or other/multiple), household income (< 10,000,
10,000,  10,000–
10,000– 19,999,
19,999,  20,000–
20,000– 24,999, ≥
24,999, ≥ 25,000), household size, and self-reported educational attainment (less than high school, high school degree, or more than high school degree). Health behavior measurements included daily alcohol intake (any compared with none), smoking consumption (any compared with none), and prior intravenous drug use (“ever use a needle to inject illegal drug”) by self-reported questionnaire. We examined if NAFLD and liver fibrosis varied by NHANES survey year and found no change in our model outputs after adjusting for survey cycle.
25,000), household size, and self-reported educational attainment (less than high school, high school degree, or more than high school degree). Health behavior measurements included daily alcohol intake (any compared with none), smoking consumption (any compared with none), and prior intravenous drug use (“ever use a needle to inject illegal drug”) by self-reported questionnaire. We examined if NAFLD and liver fibrosis varied by NHANES survey year and found no change in our model outputs after adjusting for survey cycle.
Statistical analysis
All estimated percentages and ORs were calculated using sampling weights with the survey commands in Stata 15.0 (StataCorp) to properly account for the NHANES complex survey design. We used 10-y examination and fasting weights for mobile examination and self-reported data. We considered P values < 0.05 as significant.
We calculated population-based prevalence estimates using the design-based F statistic. We conducted descriptive analyses of sociodemographic and metabolic characteristics using proportions (%) for categorical variables and means for continuous variables. To examine bivariate associations between categorical or dichotomous variables and liver outcomes, we used the Wald chi-square (χ2) test adjusting for the complex survey design. We used both unadjusted and multivariable adjusted logistic regression models for each binary outcome (primary: NAFLD; secondary: advanced fibrosis), where the primary independent variable was food insecurity status, controlling for potential confounders: age (per 5 y), sex, household income, household size, education status, race/ethnicity, smoking (current and/or past), and alcohol intake (any). Results are reported as adjusted ORs (AORs) with 95% CIs.
Sensitivity analyses
Recognizing that marginal food security among adults is a risk factor for adverse health outcomes (32), we repeated our analysis with food insecurity categorized by the 4 groups (high, marginal, low, and very low). We then used the margins command in Stata to estimate the model-adjusted predicted prevalence of metabolic outcomes by food security category. To test the robustness of our results in the higher income population, we repeated our analysis including adults from households at all income levels. Since our liver outcome measurement (USFLI and NFS) calculations incorporate cardiometabolic risk factors and thus impede our ability to explore the direct effect of food insecurity with NAFLD with a mediation analysis, we also performed a subgroup analysis assessing the association of food insecurity with NAFLD among adults with diabetes and obesity. Last, we compared estimates by the NAFLD fibrosis score to advanced fibrosis using the aspartate aminotransferase to platelet index ratio index (cutoff ≥0.7 significant fibrosis) (33) and the Fibrosis-4 score (≥3.25 advanced fibrosis) (34) to corroborate our measurement of advanced fibrosis.
Ethics statement
This secondary data analysis did not require institutional review board exemption or approval per the University of California San Francisco Human Research Protection Program. The NHANES surveys are approved by the National Center for Health Statistics ethics board (35).
Results
The 2005–2014 waves of NHANES include 50,965 participants, of whom 13,286 participated in an overnight fasting examination. Among the fasting subsample, 2995 participants met inclusion criteria, of whom 2627 had complete fasting data and were included in the analysis (see Supplemental Figure 2). Weighted overall characteristics of the 2627 included in the analysis are summarized in Table 1. The estimated prevalence of NAFLD was 32%, and estimated prevalence of advanced fibrosis was 5% among low-income adults. Approximately 1 in 3 adults (29%) lived in food-insecure households. The majority of the sample was female (58%). The median age was 43 y (IQR 30–62), although adults reporting household food insecurity were significantly younger [39 y (IQR 29–53) compared with 46 y (IQR 30–65), P < 0.01]. More than 1 in 3 adults (38%) were obese, 13% had diabetes, and 23% had hypertension.
TABLE 1.
Weighted population characteristics among adults in low-income households (n = 2627)1
| Characteristic | n | % (95% CI) |
|---|---|---|
| Food insecure | 795 | 29 (26, 32) |
| Liver disease | ||
| NAFLD | 911 | 32 (30, 35) |
| Advanced fibrosis | 173 | 5 (4, 6) |
| Demographics | ||
| Age | ||
| 20–39 y | 1143 | 51 (48, 54) |
| 40–59 y | 737 | 28 (25, 30) |
| >60 y | 747 | 22 (20, 24) |
| Sex (female) | 1541 | 58 (56, 60) |
| Race/ethnicity | ||
| African American | 533 | 16 (14, 19) |
| White | 1007 | 54 (48, 59) |
| Mexican | 592 | 16 (13, 18) |
| Non-Mexican Hispanic | 288 | 8 (6, 10) |
| Other2 | 207 | 7 (5, 9) |
| Household income | ||
< 10,000 10,000 |
326 | 11 (10, 13) |
 10,000– 10,000– 19,999 19,999 |
791 | 28 (26, 31) |
 20,000– 20,000– 24,999 24,999 |
467 | 18 (16, 21) |
> 25,000 25,000 |
1043 | 42 (39, 46) |
| Education | ||
| Less than high school | 925 | 30 (27, 33) |
| High school | 669 | 26 (24, 28) |
| More than high school | 1033 | 44 (41, 48) |
| Behavior | ||
| Current smoking | 587 | 25 (22, 27) |
| Any smoker | 1060 | 42 (40, 45) |
| Alcohol3 | 1920 | |
| Light | 1670 | 66 (63, 69) |
| Moderate | 247 | 10 (9, 11) |
| Metabolic | ||
| Obesity | 1001 | 38 (36, 40) |
| Diabetes | 418 | 13 (11, 14) |
| Hypertension | 673 | 23 (21, 26) |
12005–2014 NHANES fasting subsample (23). Excluded currently pregnant, evidence of chronic hepatitis B virus or hepatitis C virus infection, heavy alcohol consumption, and household income >200% federal poverty line. Subsample by question or examination type: all weights based on examination sampling weight divided by proportion of cycle years (for example, 10 y of data weight constructed by the following equation: WTMEC10YR = 1/5 × WTMEC2YR) (36). NAFLD, nonalcoholic fatty liver disease.
2Includes Asian, Pacific Islander, Native American, Alaskan, and other study participants.
3Light <1 drink per day; moderate 1–2 drinks per day for women, 1–3 drinks per day for men.
Estimated prevalence of NAFLD and advanced fibrosis by food security status
Comparing food-secure with food-insecure adults, the unadjusted, estimated NAFLD prevalence (food secure 31% compared with food insecure 34%, P = 0.21) and unadjusted, estimated advanced fibrosis prevalence did not significantly differ (food secure 6% compared with food insecure 5%, P = 0.67).
Association of food insecurity with odds of estimated NAFLD, advanced fibrosis, and cardiometabolic disease
In models adjusted for demographic, socioeconomic, and behavioral health characteristics (Table 2, Figure 1), food insecurity was associated with higher odds of estimates of NAFLD (AOR: 1.38; 95% CI: 1.08, 1.77; P < 0.01) and advanced fibrosis (AOR: 2.20; 95% CI: 1.27, 3.82; P < 0.01) compared with living in a food-secure household. Food insecurity was also associated with higher odds of obesity (AOR: 1.32; 95% CI: 1.06, 1.66; P < 0.05), diabetes (AOR: 1.41; 95% CI: 0.999, 1.982; P = 0.05), and hypertension (AOR: 1.26; 95% CI: 0.96, 1.65; P = 0.09), although the latter association did not reach significance (Figure 1).
TABLE 2.
Association of food insecurity with NAFLD and fibrosis among adults in low-income households (2005–2014 NHANES)1
| NAFLD (n = 911) | Advanced fibrosis (n = 173) | |||
|---|---|---|---|---|
| Characteristic | Crude OR (95% CI) | AOR (95% CI)2 | Crude OR (95% CI) | AOR (95% CI)2 |
| Food insecure (reference: food secure) | 1.14 (0.93, 1.40) | 1.38 (1.08, 1.77)** | 0.91 (0.57, 1.45) | 2.20 (1.27, 3.82)**3 |
| Age (per 5 y) | 1.09 (1.06, 1.13)*** | 1.14 (1.10, 1.18)*** | 1.52 (1.45, 1.59)*** | 1.57 (1.47, 1.68)*** |
| Sex (reference: male) | 0.78 (0.64, 0.96)* | 0.67 (0.54, 0.83)** | 1.49 (1.05, 2.11)* | 0.70 (0.48, 1.03) |
| Race/ethnicity (reference: white) | ||||
| African American | 0.41 (0.41, 0.56)*** | 0.42 (0.31, 0.58)*** | 0.60 (0.38, 0.93)* | 1.00 (0.60, 1.67) |
| Mexican | 1.85 (1.40, 1.45)*** | 2.03 (1.50, 2.74)*** | 0.59 (0.37, 0.94)** | 1.46 (0.78, 2.70) |
| Non-Mexican Hispanic | 0.82 (0.61, 1.11) | 0.93 (0.68, 1.27) | 0.60 (0.38, 0.93)* | 0.32 (0.16, 0.66)* |
| Other3 | 0.54 (0.35, 0.83)** | 0.50 (0.32, 0.77)** | 0.37 (0.14, 0.97)* | 0.44 (0.16, 1.18) |
Household income (reference: ≥ 25,000) 25,000) | ||||
< 10,000 10,000 |
0.88 (0.64, 1.18) | 1. 08 (00.76, 1.55) | 2.85 (1.57, 5.20)** | 2.29 (0.99, 5.32) |
 10,000– 10,000– 19,999 19,999 |
1.02 (0.84, 1.24) | 0.99 (0.76, 1.29) | 3.57 (1.94, 6.55)*** | 1.58 (0.77, 3.22) |
 20,000– 20,000– 24,999 24,999 |
0.95 (0.70, 1.29) | 0.87 (0.62, 1.20) | 2.30 (1.26, 4.18)** | 1.31 (0.66, 2.60) |
| Education (reference: >high school) | ||||
| <High school | 1.28 (0.99, 1.64) | 0.82 (0.62, 1.08) | 1.38 (0.85, 2.25) | 0.79 (0.43, 1.44) |
| High school | 1.10 (0.84, 1.42) | 0.89 (0.67, 1.18) | 1.15 (0.70, 1.88) | 0.70 (0.40, 1.23) |
| Any alcohol | 0.80 (0.65, 0.98)* | 0.83 (0.66, 1.05) | 0.36 (0.24, 0.54)*** | 0.84 (0.49, 1.44) |
| Any smoker | 1.02 (0.86, 1.20) | 1.04 (0.84, 1.28) | 0.70 (0.48, 1.02) | 0.74 (0.46, 1.17) |
1Fasting subsample (n = 2627 fasting examination). P values were generated using multivariable logistic regression, adjusted with the Wald F test. *P < 0.05. **P < 0.01. ***P < 0.001. AOR, adjusted OR; NAFLD, nonalcoholic fatty liver disease.
2Adjusted for age, sex, household income (4 categorical intervals), household size, ethnicity (Mexican, non-Mexican Hispanic, other, white, African American), education (less than high school, high school diploma/GED, greater than high school), and any prior alcohol and smoking history.
3Estimates have a relative standard error (RSE) of 35%. RSEs over 30% may not be statistically reliable per NHANES analytic guidelines.
FIGURE 1.
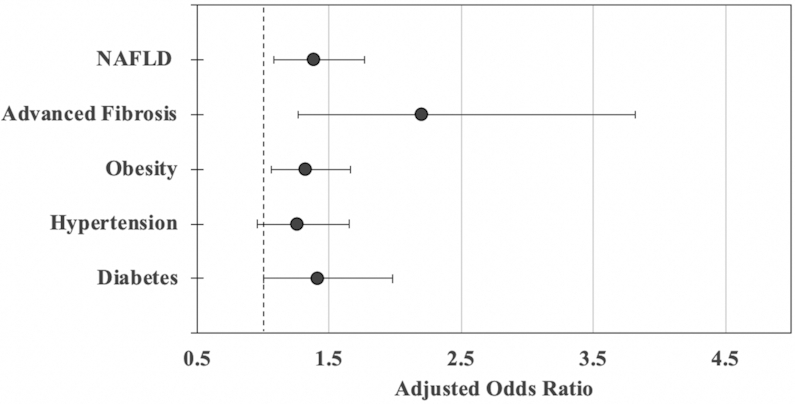
Adjusted OR (AOR) of food insecurity with cardiometabolic diseases, nonalcoholic fatty liver disease (NAFLD), and fibrosis among adults in low-income households. Adjusted for age, sex, household income (4 categorical intervals), household size, ethnicity (Mexican, non-Mexican Hispanic, other, white, African American), education (less than high school, high school diploma/GED, greater than high school), and any prior alcohol and smoking history. Includes fasting subsample (n = 2627 fasting examination). Error bars represent 95% CI. The dashed reference line reflects an OR of 1.0 (no difference in food insecurity and outcome).
Sensitivity analyses
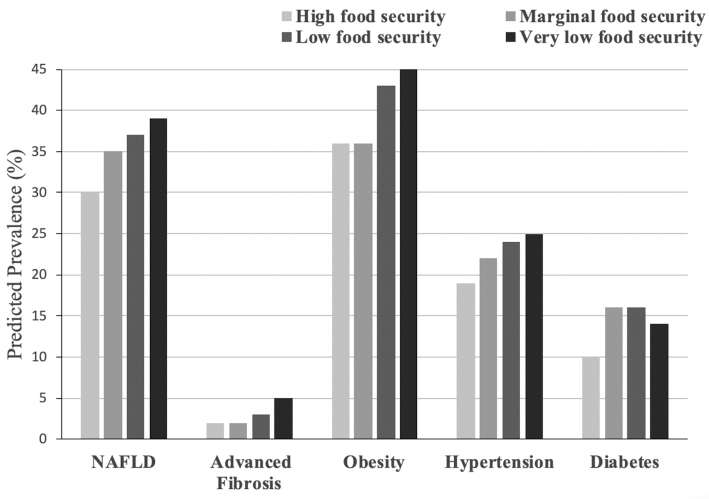
When food security was examined as a categorical variable, there was a dose-response relation between increasing severity of food insecurity and both NAFLD and advanced fibrosis (Table 3). We present the fully adjusted predicted prevalence of obesity, hypertension, diabetes, NAFLD, and advanced fibrosis by food security category (Figure 2). The adjusted predicted prevalence of NAFLD among adults in low-income households increased by worsening food security status, approaching nearly 40% among very low food-secure households (30% increase compared to high food-secure households, P < 0.05). There was a similar increased adjusted predicted prevalence of advanced fibrosis among very low food-secure households (5% adjusted prevalence, 118% increase compared to high food-secure households, P < 0.01).
TABLE 3.
Association of food insecurity with NAFLD in sensitivity analysis among adults in low-income households (2005–2014 NHANES)1
| NAFLD (n = 911) | Advanced fibrosis (n = 173) | |||
|---|---|---|---|---|
| Characteristic | n | AOR (95% CI) | n | AOR (95% CI)2 |
| Food insecurity definition | ||||
| Low/very low food security (reference: high/marginal) | 291 | 1.38 (1.08, 1.77)** | 44 | 2.20 (1.27, 3.82)** |
| Food insecurity by category (reference: high) | ||||
| Marginal food security | 150 | 1.11 (0.81, 1.52) | 15 | 0.95 (0.51, 1.82) |
| Low food security | 189 | 1.36 (1.02, 1.83)* | 27 | 1.39 (0.78, 2.51) |
| Very low food security | 102 | 1.52 (1.05, 2.18)* | 17 | 3.64 (1.53, 8.65)** |
1Adjusted for age, sex, household income (4 categorical intervals), household size, ethnicity (Mexican, non-Mexican Hispanic, other, white, African American), education (less than high school, high school diploma/GED, greater than high school), and any prior alcohol and smoking history. P values were generated using multivariable logistic regression, adjusted with the Wald F test. Fasting subsample (n = 2627 fasting examination). *P < 0.05. **P < 0.01. AOR, adjusted OR; NAFLD, nonalcoholic fatty liver disease.
2Estimates have a relative standard error (RSE) of 35%. RSEs over 30% may not be statistically reliable per NHANES analytic guidelines.
FIGURE 2.
Predicted prevalence of cardiometabolic and liver diseases by food security status in adults from low-income households. Adjusted for age, sex, household income (4 categorical intervals), household size, ethnicity (Mexican, non-Mexican Hispanic, other, white, African American), education (less than high school, high school diploma/GED, greater than high school), and any prior alcohol and smoking history. Includes fasting subsample (n = 2627 fasting examination). Each darker shaded bar represents worsening food security status. NAFLD, nonalcoholic fatty liver disease.
When we expanded the sample to include all federal poverty levels, the models did not change substantively compared with the original models [n = 6532, NAFLD (AOR: 1.36; 95% CI: 1.12, 1.65; P < 0.01) and advanced fibrosis (AOR: 2.18; 95% CI: 1.37, 3.47; P < 0.01)]. Our model estimates of food insecurity with advanced fibrosis remained significant after limiting our analysis to adults with diabetes [n = 418, advanced fibrosis (AOR: 2.40; 95% CI: 1.02, 5.67; P < 0.05)] and obesity [n = 1001, advanced fibrosis (AOR: 1.97; 95% CI: 1.09, 3.56; P < 0.05)]. When we examined the association of food insecurity with advanced fibrosis defined using the aspartate aminotransferase to platelet ratio index, the estimate was similar to when we used the NFS but did not reach statistical significance (AOR: 2.65; 95% CI: 0.97, 7.27; P = 0.06; Supplemental Table 1).
Discussion
In this sample of adults in low-income households in the United States, there was an increased association of estimated NAFLD among adults living in food-insecure households, after adjusting for sociodemographic and behavioral factors. The magnitude of association between food insecurity and estimate of advanced liver disease was similar to associations with traditional cardiometabolic diseases, such as obesity and diabetes. These findings suggest that food insecurity may be a contributor to the burgeoning prevalence of metabolic-related liver disease in the United States.
One potential explanation for the observed association between food insecurity and NAFLD is that adults living in food-insecure households have a greater burden of cardiometabolic disease, and subsequent liver disease is mediated by these cardiometabolic risk factors. Prior NHANES analyses found similar associations between food insecurity and diabetes, hypertension, and obesity (2, 4, 5, 7, 8). In addition, we found a “dose-response” relation between severity of food insecurity and NAFLD that mimics similar trends observed in hypertension, diabetes, and obesity (2, 5). Weiser et al. (20) and Seligman and Schillinger (17) offer conceptual models to explain how worsening food insecurity may cause increased cardiometabolic disease. For example, food-insecure adults may have increased rates of obesity due to greater affordability of energy-dense, high-fat foods (37) and an overall decline in dietary quality (38). Food-insecure individuals also have difficulty adhering to their medications and care plans that may be related to financial trade-offs and skipping meals (39). For example, in diabetes, studies have shown that food insecurity is an independent risk factor for poor glycemic control, low self-efficacy (40), and hospitalizations (41, 42). Since NAFLD has traditionally been thought to be a consequence of metabolic disease (11), the increased odds of NAFLD among food-insecure adults may be due to the higher prevalence of metabolic disease among adults in food-insecure households.
Our finding of an increased odds of NAFLD among food insecure adults with diabetes and obesity suggests that food insecurity exacerbates known metabolic risk factors (effect modification) and/or directly drives liver injury (true causal relation). First, insulin resistance may be an evolutionary adaptation to conserve muscle mass during hunger and starvation states, a protective mechanism that may be inappropriately activated during episodes of food inadequacy accompanying food insecurity (3). The resulting hyperinsulinemia can trigger fat breakdown, increasing free fatty acid absorption by hepatocytes and promoting lipotoxicity and inflammation (11, 19). Second, the stress created by inadequate access to food may increase cortisol production and subsequent systemic inflammation (18), creating complex interrelations between central obesity, insulin resistance, and abnormalities in fatty acid metabolism associated with NAFLD (43). Finally, diet quality (high-fat and high-fructose diets) and caloric disruption alter the gut microbiota in ways that could both promote hepatic inflammation and promote gut bacterial translocation that independently drives hepatic injury (20–22, 44–46). These pathways suggest that increased duration and severity of food insecurity may worsen NAFLD outcomes.
An alternate explanation of our findings is that worsened health status (i.e., NAFLD) causes food insecurity, rather than food insecurity causing worsened health status (reverse causality). This “vicious cycle” is well documented in HIV, where illness frequently hampers the ability of the most productive adults to work, drawing resources through caretaking needs and diminishing economic household potential (20, 47, 48). Furthermore, adults living in food-insecure households have significantly greater annual health care expenditures, which may further divert household resources (49). Similar to other metabolic diseases, adults with NAFLD accrue significant medical costs, health care visits (50), and decreased physical function (51) that may diminish their economic productivity and increase their caretaking needs. Longitudinal studies are needed to better understand the temporal associations and directionality between food insecurity and NAFLD.
Whether food insecurity is a cause or a consequence of NAFLD, these findings could have important implications for policy and interventions. Adults with NAFLD, from steatosis to advanced fibrosis, can be screened for food insecurity using validated tools such as the 2-item “Hunger Vital Sign” (52). Targeted interventions to support at-risk adults may attenuate fibrosis progression and offset the annual direct medical costs of over  100 billion attributable to NAFLD in the United States (53). Effective linkage to the Supplemental Nutrition Assistance Program could improve food access and decrease food insecurity (54, 55). Community-partnered programs, such as urban gardens (56) and integrated tailored nutrition and chronic disease programs (57), may both improve and maintain quality dietary intake. Policy strategies, including soda tax (58) and fresh food vouchers (59), may provide the structural backbone to improve access and intake of a nutrient-rich diet among low-income adults with NAFLD.
100 billion attributable to NAFLD in the United States (53). Effective linkage to the Supplemental Nutrition Assistance Program could improve food access and decrease food insecurity (54, 55). Community-partnered programs, such as urban gardens (56) and integrated tailored nutrition and chronic disease programs (57), may both improve and maintain quality dietary intake. Policy strategies, including soda tax (58) and fresh food vouchers (59), may provide the structural backbone to improve access and intake of a nutrient-rich diet among low-income adults with NAFLD.
Our study had several limitations. As a cross-sectional study, we are unable to assess the causal nature of the association between food insecurity and adverse liver outcomes. These liver disease estimates are indirect measures of NAFLD and advanced fibrosis. In addition, our novel use of noninvasive liver measures that incorporate fasting values limited our analysis to adults who participated in the fasting examination. This excluded participants who self-reported oral hypoglycemic medications and/or insulin. Last, our liver outcome measurement (USFLI and NFS) calculations incorporate cardiometabolic risk factors (diabetes, BMI, waist circumference, impaired fasting glucose), and thus we were limited in our ability to conduct a mediation analysis. Furthermore, because age and race/ethnicity are used in calculating the USFLI score, there is by definition an association of these factors with the presence of NAFLD defined using a cutoff value of USFLI. The associations we found therefore cannot be considered to independently corroborate the influence of these factors on risk of NAFLD. We have controlled for them to reduce possible confounding of the other potential risk factors that we have examined, as has been done in previous studies (60–63). As transient elastography monitoring becomes more widespread, improved measurements of NAFLD and advanced fibrosis can help better characterize the relation between food insecurity and liver disease. Despite these limitations, this study is the first large-scale examination of liver disease and its association with food insecurity and suggests an increased burden of NAFLD among food-insecure adults in the United States.
Conclusions
Food insecurity may be independently associated with NAFLD and advanced fibrosis among adults living in low-income households, after adjusting for sociodemographic and behavioral factors. Programs addressing the growing burden of NAFLD-associated morbidity and mortality should consider strategies that improve food access, quality, and healthy eating habits among low-income adults.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—IG, PCT, JCP, and SDW: designed research; IG and LS: conducted research; IG, LS, PCT, JCP, and SDW: analyzed and interpreted the data; IG and SDW: drafted the article; IG, PCT, JCP, LS, HS, and SDW: critically revised the article. All authors read and approved the final manuscript.
Notes
This work has been supported by the National Institutes of Health (NIH) T32 HP19025 award. SDW receives salary support from NIH NIAID K24 AI134326-01.
Author disclosures: IG, LS, and SDW, no conflicts of interests. PCT: Grant support from Merck and Theratechnologies. JCP: Grant support from Gilead Sciences and Merck and ownership interest in Bristol-Myers Squibb, Johnson and Johnson, Merck, and Abbvie. HS: Senior medical advisor for Feeding America, a 501(c)3 organization that works to end hunger in the United States.
Supplemental Data, Supplemental Figures 1 and 2, Supplemental Methods, and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations: AOR, adjusted OR; NAFLD, nonalcoholic fatty liver disease; NFS, NAFLD Fibrosis Score; USFLI, US Fatty Liver Index.
References
- 1. US Department of Agriculture ERS Definitions of Food Security. [Internet]. 2016. [cited 2018 Mar]. Available from: https://www.ers.usda.gov/topics/food-nutrition-assistance/food-security-in-the-us/definitions-of-food-security.aspx. [Google Scholar]
- 2. Gregory C, Coleman-Jensen A. Food insecurity, chronic disease and health among working-age adults. Washington (DC): US Department of Agriculture ERS; 2017. Economic Research Report Number 235. [Google Scholar]
- 3. Seligman HK, Bindman AB, Vittinghoff E, Kanaya AM, Kushel MB. Food insecurity is associated with diabetes mellitus: results from the National Health Examination and Nutrition Examination Survey (NHANES) 1999–2002. J Gen Intern Med. 2007;22:1018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140:304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berkowitz SA, Berkowitz TSZ, Meigs JB, Wexler DJ. Trends in food insecurity for adults with cardiometabolic disease in the United States: 2005–2012. PLoS One. 2017;12:e0179172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morales ME, Berkowitz SA. The relationship between food insecurity, dietary patterns, and obesity. Curr Nutr Rep. 2016;5:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sirotin N, Hoover DR, Shi Q, Anastos K, Weiser SD. Food insecurity with hunger is associated with obesity among HIV-infected and at risk women in Bronx, NY. PLoS One. 2014;9:e105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ford ES. Food security and cardiovascular disease risk among adults in the United States: findings from the National Health and Nutrition Examination Survey, 2003–2008. Prev Chronic Dis. 2013;10:E202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2015;41:65–76. [DOI] [PubMed] [Google Scholar]
- 10. Pappachan JM, Babu S, Krishnan B, Ravindran NC. Non-alcoholic fatty liver disease: a clinical update. J Clin Transl Hepatol. 2017;5:384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063–72. [DOI] [PubMed] [Google Scholar]
- 12. Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–8. [DOI] [PubMed] [Google Scholar]
- 13. Pappachan JM, Antonio FA, Edavalath M, Mukherjee A. Non-alcoholic fatty liver disease: a diabetologist's perspective. Endocrine. 2014;45:344–53. [DOI] [PubMed] [Google Scholar]
- 14. Loria P, Lonardo A, Anania F. Liver and diabetes: a vicious circle. Hepatol Res. 2013;43:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence?. J Hepatol. 2018;68(2):335–52. [DOI] [PubMed] [Google Scholar]
- 16. Berkowitz SA, Seligman HK, Choudhry NK. Treat or eat: food insecurity, cost-related medication underuse, and unmet needs. Am J Med. 2014;127:303–10.e3. [DOI] [PubMed] [Google Scholar]
- 17. Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363:6–9. [DOI] [PubMed] [Google Scholar]
- 18. Gowda C, Hadley C, Aiello AE. The association between food insecurity and inflammation in the US adult population. Am J Public Health. 2012;102:1579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petta S, Gastaldelli A, Rebelos E, Bugianesi E, Messa P, Miele L, Svegliati-Baroni G, Valenti L, Bonino F. Pathophysiology of non alcoholic fatty liver disease. Int J Mol Sci. 2016;17(12):2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weiser SD, Palar K, Hatcher AM, Young SL, Frongillo EA. Food insecurity and health: a conceptual framework. In: Ivers L.editor. Food insecurity and public health. Boca Raton (FL): CRC Press; 2015. p. 23–50. [Google Scholar]
- 21. Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12:5–9. [DOI] [PubMed] [Google Scholar]
- 22. Vos MB, Lavine JE. Dietary fructose in nonalcoholic fatty liver disease. Hepatology. 2013;57:2525–31. [DOI] [PubMed] [Google Scholar]
- 23. NCHS About the National Health and Nutrition Examination Survey. [Internet]. 2017. [cited 2018 Mar]. Available from: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. [Google Scholar]
- 24. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57. [DOI] [PubMed] [Google Scholar]
- 25. US Department of Agriculture ERS Household food security in the United States in 2017. [Internet]. 2018. [cited 2018 Mar]. Available from: https://www.ers.usda.gov/webdocs/publications/90023/err-256.pdf?v = 0. [Google Scholar]
- 26. Banerjee T, Crews DC, Wesson DE, Dharmarajan S, Saran R, Rios Burrows N, Saydah S, Powe NR; Team CCS . Food insecurity, CKD, and subsequent ESRD in US adults. Am J Kidney Dis. 2017;70:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bickel G NM, Price C, Hamilton W, Cook J. Guide to measuring household food security. [Internet]. 2000. [cited 2018 Mar]. Available from: https://www.fns.usda.gov/guide-measuring-household-food-security-revised. [Google Scholar]
- 28. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP et al.. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–54. [DOI] [PubMed] [Google Scholar]
- 29. Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A et al.. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–97. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, Haflidadottir S, Day CP, George J. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:782–9.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Unalp-Arida A, Ruhl CE. Liver fibrosis scores predict liver disease mortality in the United States population. Hepatology. 2017;66:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cook JT, Black M, Chilton M, Cutts D, Ettinger de Cuba S, Heeren TC, Rose-Jacobs R, Sandel M, Casey PH, Coleman S et al.. Are food insecurity's health impacts underestimated in the U.S. population? Marginal food security also predicts adverse health outcomes in young U.S. children and mothers. Adv Nutr. 2013;4:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–36. [DOI] [PubMed] [Google Scholar]
- 34. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, Sulkowski MS, Torriani FJ, Dieterich DT, Thomas DL et al.. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- 35. NCHS NCHS Research Ethics Review Board (ERB) Approval. [Internet]. 2017. [cited 2018 Mar]. Available from: https://www.cdc.gov/nchs/nhanes/irba98.htm. [Google Scholar]
- 36. NCHS Task 2: When and how to construct weights when combining survey cycles. [Internet]. 2013. [cited 2018 Mar]. Available from: https://www.cdc.gov/nchs/tutorials/NHANES/SurveyDesign/Weighting/Task2.htm. [Google Scholar]
- 37. Drewnowski A, Darmon N. The economics of obesity: dietary energy density and energy cost. Am J Clin Nutr. 2005;82:265S–73S. [DOI] [PubMed] [Google Scholar]
- 38. Bhattacharya J, Currie J, Haider S. Poverty, food insecurity, and nutritional outcomes in children and adults. J Health Econ. 2004;23:839–62. [DOI] [PubMed] [Google Scholar]
- 39. Heerman WJ, Wallston KA, Osborn CY, Bian A, Schlundt DG, Barto SD, Rothman RL. Food insecurity is associated with diabetes self-care behaviours and glycaemic control. Diabet Med. 2016;33:844–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seligman HK, Jacobs EA, Lopez A, Tschann J, Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012;35:233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seligman HK, Bolger AF, Guzman D, Lopez A, Bibbins-Domingo K. Exhaustion of food budgets at month's end and hospital admissions for hypoglycemia. Health Aff (Millwood). 2014;33:116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Basu S, Berkowitz SA, Seligman H. The monthly cycle of hypoglycemia: an observational claims-based study of emergency room visits, hospital admissions, and costs in a commercially insured population. Med Care. 2017;55:639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Machado MV, Cortez-Pinto H. Diet, microbiota, obesity, and NAFLD: a dangerous quartet. Int J Mol Sci. 2016;17:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kirpich IA, Marsano LS, McClain CJ. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin Biochem. 2015;48:923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Federico A, Dallio M, Godos J, Loguercio C, Salomone F. Targeting gut-liver axis for the treatment of nonalcoholic steatohepatitis: translational and clinical evidence. Transl Res. 2016;167:116–24. [DOI] [PubMed] [Google Scholar]
- 47. Tarasuk V, Mitchell A, McLaren L, McIntyre L. Chronic physical and mental health conditions among adults may increase vulnerability to household food insecurity. J Nutr. 2013;143:1785–93. [DOI] [PubMed] [Google Scholar]
- 48. McIntyre D, Thiede M, Dahlgren G, Whitehead M. What are the economic consequences for households of illness and of paying for health care in low- and middle-income country contexts?. Soc Sci Med. 2006;62:858–65. [DOI] [PubMed] [Google Scholar]
- 49. Berkowitz SA, Basu S, Meigs JB, Seligman HK. Food insecurity and health care expenditures in the United States, 2011–2013. Health Serv Res. 2018;53(3):1600–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Younossi ZM, Zheng L, Stepanova M, Henry L, Venkatesan C, Mishra A. Trends in outpatient resource utilizations and outcomes for Medicare beneficiaries with nonalcoholic fatty liver disease. J Clin Gastroenterol. 2015;49:222–7. [DOI] [PubMed] [Google Scholar]
- 51. Younossi ZM, Henry L. Economic and quality-of-life implications of non-alcoholic fatty liver disease. Pharmacoeconomics. 2015;33:1245–53. [DOI] [PubMed] [Google Scholar]
- 52. Gundersen C, Engelhard EE, Crumbaugh AS, Seligman HK. Brief assessment of food insecurity accurately identifies high-risk US adults. Public Health Nutr. 2017;20(8):1367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, Hunt S, Beckerman R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–86. [DOI] [PubMed] [Google Scholar]
- 54. Swann CS. Household history, SNAP participation, and food insecurity. Food Policy. 2017;73:1–9. [Google Scholar]
- 55. Gundersen C Kreider B, Pepper J. Partial identification methods for evaluating food assistance programs: a case study of the causal impact of SNAP on food insecurity. Am J Agric Econ. 2017;99:875–94. [Google Scholar]
- 56. Derose KP, Palar K, Farias H, Adams J, Martinez H. Developing pilot interventions to address food insecurity and nutritional needs of people living with HIV in Latin America and the Caribbean: an interinstitutional approach using formative research. Food Nutr Bull. 2018;39:549–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ippolito MM, Lyles CR, Prendergast K, Marshall MB, Waxman E, Seligman HK. Food insecurity and diabetes self-management among food pantry clients. Public Health Nutr. 2017;20:183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee MM, Falbe J, Schillinger D, Basu S, McCulloch CE, Madsen KA. Sugar-sweetened beverage consumption 3 years after the Berkeley, California, sugar-sweetened beverage tax. Am J Public Health. 2019;109:637–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Basu S. Designing food voucher programs to reduce disparities in healthy diets (CHIVES). San Francisco (CA): National Institutes of Health; 2016–2021. [Google Scholar]
- 60. Wong RJ, Liu B, Bhuket T. Significant burden of nonalcoholic fatty liver disease with advanced fibrosis in the US: a cross-sectional analysis of 2011–2014 National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2017;46:974–80. [DOI] [PubMed] [Google Scholar]
- 61. Yim JY, Kim J, Kim D, Ahmed A. Serum testosterone and non-alcoholic fatty liver disease in men and women in the US. Liver Int. 2018;38:2051–9. [DOI] [PubMed] [Google Scholar]
- 62. Martinez LA, Larrieta E, Kershenobich D, Torre A. The expression of PNPLA3 polymorphism could be the key for severe liver disease in NAFLD in Hispanic population. Ann Hepatol. 2017;16:909–15. [DOI] [PubMed] [Google Scholar]
- 63. Bae JC, Rhee EJ, Lee WY, Park SE, Park CY, Oh KW, Park SW, Kim SW. Combined effect of nonalcoholic fatty liver disease and impaired fasting glucose on the development of type 2 diabetes: a 4-year retrospective longitudinal study. Diabetes Care. 2011;34:727–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.