Abstract
Objectives:
Chest radiographs (CXRs) are often performed in children with respiratory illness to inform the decision to prescribe antibiotics. Our objective was to determine the factors associated with clinicians’ plans to treat with antibiotics prior to knowledge of CXR results, and the associations between pre-radiograph plans with antibiotic prescription and return to medical care.
Methods:
Previously healthy children aged 3 months to 18 years with a CXR for suspected pneumonia were enrolled in a prospective cohort study in the ED. Our primary outcomes were antibiotic prescription or administration in the ED and medical care sought within 7–15 days’ post discharge. Inverse probability treatment weighting (IPTW) was used to limit bias due to treatment selection. IPTW was included in a logistic regression model estimating the association between the intention to give antibiotics and outcomes.
Results:
Providers planned to prescribe antibiotics prior to CXR in 68 (34.9%) children. There was no difference in the presence of radiographic pneumonia between those with and without a plan for antibiotics. Children who had a plan for antibiotics were more likely to receive antibiotics than those without (OR 6.39, 95% CI 3.7–11.0). This association was stronger than the association between radiographic pneumonia and antibiotic receipt (OR 3.49, 95% CI 1.98–6.14). Children prescribed antibiotics were more likely to seek care after discharge than children who were not (OR 1.85, 95% CI 1.13–3.05).
Conclusions:
Intention to prescribe antibiotics based on clinical impression was the strongest predictor of antibiotic prescription in our study. Prescribing antibiotics may lead to subsequent medical care after controlling for radiographic pneumonia.
INTRODUCTION
The decision to prescribe antibiotics in children suspected of community-acquired pneumonia (CAP) can be challenging, particularly given the lack of reliable methods to distinguish patients with bacterial pneumonia from those with viral illness.1 Pneumonia is commonly caused by viral pathogens, especially in younger patients, yet most children diagnosed with pneumonia are treated empirically with antibiotics.2, 3
The role of chest radiographs (CXR) for the diagnosis of pneumonia is controversial. The most recent national guidelines on the management of pediatric CAP recommend foregoing routine chest radiographs in children who can be managed as outpatients.4 However, given the unreliability of physical exam findings for the diagnosis of pneumonia, chest radiographs are still commonly used in the evaluation of respiratory illness in the emergency department (ED).5–8 There is an increasing body of evidence suggesting that chest radiographs do little to change provider behavior in suspected pneumonia.9–11 Nelson et al. demonstrate that physicians rely mainly on their pre-radiograph impressions rather than radiograph results when prescribing antibiotics for suspected pneumonia, especially when the pre-radiograph suspicion for pneumonia is relatively high.11 However, the factors clinicians use to formulate their suspicions for pneumonia are still incompletely understood. In addition, the downstream effects of various diagnostic strategies for pneumonia are unknown.
The objectives of this study were (1) to determine the clinical factors associated with a clinician’s plan to prescribe antibiotics for pneumonia prior to knowing CXR results, (2) the association between clinicians’ plans to prescribe antibiotics and actual antibiotic prescription in the ED, and (3) the association between actual antibiotic prescribing and return to medical care (i.e. outpatient or ED) within 7–15 days of discharge.
MATERIALS AND METHODS
Study Design
This was a planned secondary analysis of data from a prospective cohort study, “Catalyzing Ambulatory Research in Pneumonia Etiology and Diagnostic Innovations in Emergency Medicine” (CARPE DIEM). The objective of CARPE DIEM was to improve risk stratification and etiologic classification in children presenting to the ED with CAP using predictive tools and novel biomarkers. Children 3 months to 18 years of age who presented to a tertiary urban children’s hospital ED with suspicion for CAP between July 2013 and December 2017 were eligible. The study was approved by the Cincinnati Children’s Hospital Medical Center (CCHMC) Institutional Review Board. Informed consent was obtained from all legal guardians of patients and assent was obtained for children ≥ 11 years of age.
Study Population
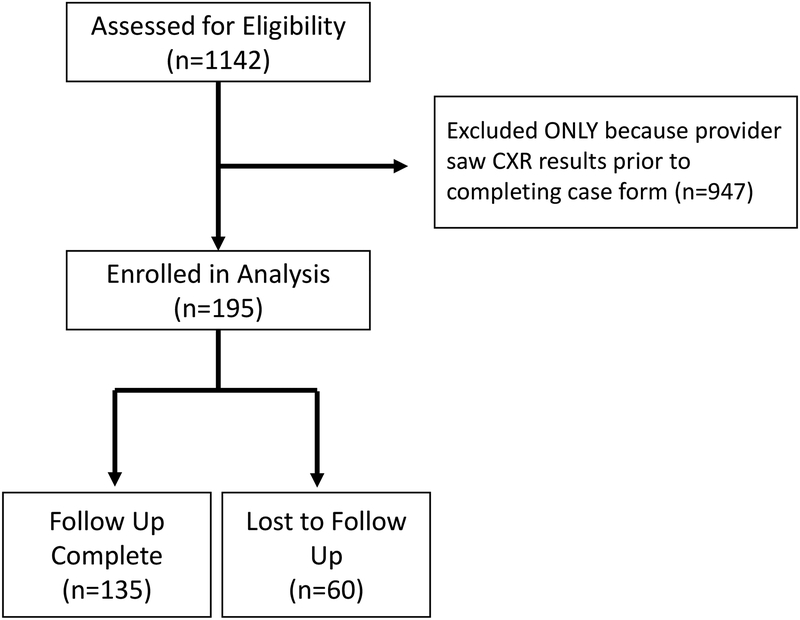
Children 3 months to 18 years of age with signs and symptoms of lower respiratory tract infections (LRTI) who received a CXR for suspicion of CAP were eligible for study enrollment. Signs and symptoms of LRTI were defined as one or more of the following items: cough, sputum, chest pain, shortness of breath, tachypnea, or abnormal findings (e.g. rales/crackles, wheezing) on physical examination.8, 12 The intent of the study was to investigate pneumonia in otherwise healthy children; therefore children were excluded if they had underlying chronic complex conditions (e.g. immunodeficiency, chronic corticosteroid use, congenital heart disease, tracheostomy dependence, neuromuscular disease affecting the lungs, chronic lung disease, sickle cell disease, cystic fibrosis), were hospitalized within the prior 14 days, or had a history of aspiration pneumonia.13 Children with a history of asthma were included in the study. For this analysis, only children for whom the clinician completed the study case report form prior to reviewing the CXR images or radiologist impression were included (Figure 1).
Figure 1:
Flow Diagram of Study Enrollment
Study Procedure:
Children with suspected CAP were screened 7 days per week for 12–16 hours per day. The current study was composed of a convenience sample of children that were seen by a clinician who had completed a standardized case report form prior to reviewing the CXR images or radiologist impression. The clinicians approached included attending physicians, pediatric emergency medicine fellows, or nurse practitioners. The clinician completed a standardized case report form that included physical exam findings, perceived severity of illness, pre-CXR plans for antibiotic therapy, and planned disposition.
Exposure Measurements
The primary exposure was the clinician’s plan to give antibiotic therapy prior to reviewing the CXR images or radiologist impression. Clinicians were asked “Based on your overall clinical impression and without knowing the CXR results, would you start this participant on antibiotics for pneumonia?”, with possible answers of yes and no.
Covariates included age, sex, race, ethnicity, insurance status, highest level of maternal education and prematurity. Aspects of clinical history, physical examination, and clinician impression were all included as covariates. Clinical history included duration of illness, fever, cough, difficulty breathing, wheezing, rapid breathing, and previous treatment for CAP. Physical exam findings included general appearance (well, mildly ill, moderately ill, or severely ill), cool extremities, nasal flaring, cough, head bobbing, grunting, retractions, abdominal pain, wheeze, crackles, rhonchi, decreased breath sounds, oxygen saturation, tachypnea, respiratory rate, skin color, capillary refill time, and patient behavior (playing and appropriate, quiet but appropriate, sleeping but easily arousable, sleeping but not easily arousable, fussy but consolable, irritable, lethargic, or unresponsive). Vital signs taken closest to the time of clinical examination were utilized. Clinicians were asked “What is your overall assessment of the clinical severity of this patient?” and were given 4 options: mild, moderate, severe, and very severe. They were also asked “What is the probability of this participant having pneumonia on chest x-ray?” and were given 7 options: <1%, 1–5%, 5–10%, 10–25%, 25–50%, 50–75% and 75–100%, as well as; “Based on your overall clinical impression and without knowing the CXR results, would you start this participant on antibiotics for pneumonia (yes/no)?” The CXR interpretation included in the final analysis was based on the review of two independent study radiologists blinded to clinical information. Radiograph results were classified as “normal,” “definite/probable atelectasis,” “atelectasis vs. pneumonia,” or “definite/probable pneumonia.” Radiologic pneumonia was defined as “definite/probable pneumonia.” In case of disagreement between the two radiologists, the attending radiologist’s read from the ED visit was used as a tiebreaker. If there was persistent disagreement between the clinical read and the two blinded radiologist’s reads, the radiologists came to consensus on a final interpretation during an in-person consensus meeting.
Primary Outcome
The primary outcome was the receipt of antibiotic therapy during the ED visit or a prescription by the ED provider upon discharge from the ED. The secondary outcome was medical care (e.g. outpatient visit, urgent care, ED) sought within 7–15 days post discharge, as measured using follow-up phone calls made to the legal guardians by study staff. Seven days was chosen as the minimal interval for follow up due to the likelihood that that any clinically significant complications would occur by this time. The 7 to 15 day range for follow up accounted for the potential of not making contact with participants on first attempt and allowing for additional contact attempts.
Data Analysis
Continuous variables were described using median and interquartile ranges (IQR) due to non-normal distributions. The Wilcoxon Rank Sum test was used to compare outcomes between those with a plan to receive antibiotic therapy before CXR and those without a plan to receive antibiotic therapy. Categorical variables were described using counts and percentages and compared between groups using Fisher Exact test or Chi-square test, as appropriate.
Propensity scores were developed using logistic regression predicting the propensity of having a plan for receiving antibiotic therapy.14 Variables included a priori in the propensity score model were age, fever, and overall clinical impression. Additional variables included based on statistical significance (p-value <0.05) were crackles, decreased breath sounds, and tachypnea. Inverse probability treatment weighting (IPTW) based on propensity score was used in a multivariable logistic regression model that adjusted for radiographic pneumonia. IPTW assigns weights to each subject in the analysis to create a distribution of propensity scores which is not dependent on the exposure, thus not excluding cases in the analysis as would occur using propensity score matching and obtaining a treatment effect that is independent of baseline characteristics.15 All analyses were performed using SAS v9.3 (SAS Institute, Cary, NC).
RESULTS
Study Population
A total of 1142 children were enrolled, 195 of which were included in the analysis. Of these, 34.9% (n=68) of children had a pre-CXR plan to receive antibiotic therapy. The median age of the cohort was 3.5 years (IQR: 1.7, 8.2). There were no statistical differences in demographics or past medical history, including immunization status, history of prematurity, history of asthma, or previous pneumonia history between children with and without a pre-CXR plan for antibiotics (Table 1). Compared to missed eligible children and children whose caregivers declined participation, children enrolled in the study were similar in terms of gender, ethnicity, and level of care if hospitalized (floor vs. ICU). Children who were missed eligible or declined participation had a lower rate of admission, self-identified white race, and commercial insurance than enrolled children. Children who were missed eligible or declined participation had a lower rate of admission than enrolled children.
Table 1:
Demographics and Medical History
| Overall (n=195) |
Plan to Prescribe Antibiotics (n=68) |
Do Not Plan to Prescribe Antibiotics (n=127) |
p-value | |
|---|---|---|---|---|
| Age (median in yrs, IQR) | 3.5 (1.7, 8.2) | 3.30 (1.3, 8.3) | 3.75 (2.0, 7.95) | 0.23 |
| Male (n, %) | 108 (55.4) | 37 (54.4) | 31 (45.6) | 0.84 |
| Race (n, %) | ||||
| White | 131 (67.2) | 49 (72.1) | 82 (64.6) | 0.28 |
| Black | 70 (30.9) | 21 (30.9) | 49 (38.6) | 0.29 |
| Other | 5 (2.6) | 2 (2.94) | 3 (2.36) | 0.34 |
| Ethnicity (n, %) | 0.99 | |||
| Hispanic | 7 (3.6) | 2 (2.94) | 5 (3.94) | |
| Non-Hispanic | 187 (95.9) | 66 (97.1) | 121 (95.3) | |
| History of Asthma (n, %) | 65 (33.3) | 24 (35.3) | 41 (32.3) | 0.75 |
| History of Pneumonia (n, %) | 47 (24.1) | 21 (30.9) | 26 (20.5) | 0.15 |
| Immunizations Up to Date (n, %) | 184 (94.4) | 65 (95.6) | 119 (93.7) | 0.99 |
| Premature Infant (n, %) | 33 (16.2) | 11 (16.2) | 22 (17.3) | 0.84 |
Clinical Presentation and Physical Exam Findings
Clinical presentation and physical exam findings of children with and without a plan to receive antibiotics are listed in Table 2. A significantly higher proportion of children with a pre-CXR plan for antibiotics had history of difficulty breathing, crackles, decreased breath sounds, and tachypnea, compared with children for whom the clinician did not intend to provide antibiotics. The children with a pre-CXR plan for antibiotics had a slightly higher median oxygen saturation (98% vs. 96%), it is unclear whether this difference is clinically significant.
Table 2:
Clinical Factors Associated with a Pre-Radiograph Plan to Prescribe Antibiotics
| Overall (n=195) |
Plan to Prescribe Antibiotics (n=68) |
Do Not Plan to Prescribe Antibiotics (n=127) |
p-value | |
|---|---|---|---|---|
| Historical Features (n, (%)) | ||||
| Fever | 176 (90.3) | 65 (95.6) | 111 (87.4) | 0.07 |
| Difficulty Breathing | 158 (92.7) | 63 (92.7) | 95 (74.8) | <0.01 |
| Wheezing | 122 (62.6) | 45 (66.2) | 77 (60.6) | 0.45 |
| Physical Exam Findings | ||||
| Nasal Flaring | 16 (8.2) | 8 (11.8) | 8 (6.3) | 0.13 |
| Retractions | 65 (33.3) | 28 (41.2) | 37 (29.1) | 0.09 |
| Wheeze | 54 (27.7) | 16 (23.5) | 38 (29.9) | 0.4 |
| Crackles | 55 (28.2) | 40 (58.8) | 15 (11.8) | <0.01 |
| Rhonchi | 63 (32.5) | 19 (27.9) | 44(34.9) | 0.32 |
| Decreased Breath Sounds | 62 (31.8) | 35 (51.5) | 27 (21.3) | <0.01 |
| Oxygen Saturation (%), (median, IQR) | 97 (95, 98) | 98 (95, 99) | 96 (93, 98) | <0.01 |
| Tachypnea | 92 (47.2) | 44 (64.7) | 48 (37.8) | <0.01 |
| Clinician Impressions before Seeing CXR | ||||
| Overall Clinical Impression | <0.01 | |||
| Mild | 116 (59.8) | 26 (38.2) | 90 (71.4) | |
| Moderate | 75 (38.7) | 40 (58.8) | 35 (27.8) | |
| Severe | 3 (1.6) | 2 (2.9) | 1 (0.8) | |
| Probability of Pneumonia | <0.01 | |||
| <1% | 45 (23.2) | 1 (1.5) | 44 (34.9) | |
| 1–5% | 67 (34.5) | 10 (14.7) | 57 (45.2) | |
| 5–10% | 22 (11.3) | 12 (17.7) | 10 (7.9) | |
| 10–25% | 23 (11.9) | 14(20.9) | 9 (7.1) | |
| 25–50% | 15 (7.7) | 11 (16.2) | 4 (3.2) | |
| 50–75% | 16 (8.3) | 15 (22.1) | 1 (0.8) | |
| 75–100% | 6 (3.1) | 5 (7.4) | 1 (0.8) | |
Clinical Impressions
Children who were thought to have moderate or severe disease were more likely to have a plan to receive an antibiotic prescription. Similarly, clinicians were more likely to have a pre-CXR plan for antibiotics for children for whom they deemed to have a higher probability of pneumonia prior to CXR results being known (Table 2).
Clinical Outcomes
Antibiotics were administered or prescribed in the ED to 34.9% (n=68) of children, including 57.4% of children with a pre-CXR plan for an antibiotic prescription (n=39) and 22.8% of children with a pre-CXR plan for no antibiotic prescription (n=29) (p<0.01). The prevalence of radiographic pneumonia was 14.2% overall (n=27), with radiographic pneumonia in 19.4% of cases with a pre-CXR plan for an antibiotic prescription (n=13) and 11.4% in cases without a plan for an antibiotic prescription (n=14) (p=0.13) (Table 3). The rates of radiographic pneumonia increased with increasing suspicion for pneumonia (Supplemental Table 1).
Table 3:
Outcomes Stratified by Pre-Radiograph Plan for Antibiotic prescription
| Overall (n=195) | Plan to Prescribe Antibiotics (n=68) | Do Not Plan to Prescribe Antibiotics (n=127) | p-value | |
|---|---|---|---|---|
| Radiographic Pneumonia (n, %) | 27 (14.2) | 13 (19.4) | 14 (11.4) | 0.13 |
| Disposition (n, %) | ||||
| Admit | 88 (45.13) | 45 (66.2) | 43 (33.9) | <0.01 |
| Discharge | 107 (54.87) | 23 (33.8) | 84 (66.14) | |
| Receipt of Antibiotics/Prescription in ED (n, %) | 68 (34.9) | 39 (57.35) | 29 (22.83) | <0.01 |
| Return to Medical Care within 7–15 Days (n, %) * | 60 (44.4) | 23(46.0) | 37 (43.5) | 0.86 |
| Reason for Return to Medical Care | ||||
| Unscheduled follow-up visit for the same illness | 5 (8.3) | 1 (4.35) | 4 (10.8) | 0.56 |
| A scheduled follow-up visit for the same illness | 46 (76.7) | 20 (86.9) | 26 (70.3) | |
| A scheduled follow-up illness for the same illness but the child is getting worse | 8 (13.3) | 2 (8.7) | 6 (16.2) | |
| Well-child/routine visit | 1 (1.7) | 0 | 1 (2.7) |
60 observations lost to follow up
In the logistic regression model, a pre-CXR plan for an antibiotic prescription was the strongest predictor for ultimate receipt of an antibiotic prescription, controlling for the presence of radiographic pneumonia (OR 6.39, 95% CI 3.7–11.0). Radiographic pneumonia was also a significant predictor (OR 3.49, 95% CI 1.98–6.14) in the model, although to a lesser degree than the pre-CXR plan for antibiotic prescription.
Of the 195 children in the analysis, 60 were lost to follow up (30.8%). Analysis of secondary outcomes was performed on the remaining 135 children. Medical care was sought in the 7–15 days following ED discharge in 44% (n=60) of children. There was no statistical difference in follow-up or reasons for follow up between children with a pre-CXR plan for an antibiotic prescription (46%, n=23) and children with a pre-CXR plan for no antibiotic prescription (44%, n=37) (p=0.78) (Table 3). In a separate logistic regression model, which included receipt of antibiotic prescription, pre-CXR plan for an antibiotic prescription, and radiographic pneumonia, the strongest predictor of medical care sought after discharge from the ED was receipt of antibiotics or an antibiotic prescription (OR 1.85, 95% CI 1.13–3.05), while children with radiographic pneumonia were less likely to seek care after discharge (OR 0.18, 95% CI 0.09–0.39) (Table 4). There was no statistical difference in rates of unscheduled visits in children treated and not treated with antibiotics (18 vs. 24%, p=0.75). Of the 60 participants who are missing follow-up data, 7 had radiographic pneumonia and 21 received antibiotics from the ED.
Table 4:
Multivariable logistic regression models examining the association of the intention to prescribe antibiotics in the ED and radiographic PNA with actual antibiotic prescription and medical care sought after discharge.
| Odds Ratio | 95% Confidence Limits | |
|---|---|---|
| Model 1: Prescription or receipt of antibiotics in the ED * | ||
| Plan to receive antibiotics | 6.39 | (3.70, 11.04) |
| Radiographic pneumonia | 3.49 | (1.98, 6.14) |
| Model 2: Medical care sought after discharge * | ||
| Plan to receive antibiotics | 0.9 | (0.46, 1.77) |
| Radiographic pneumonia | 0.18 | (0.09, 0.39) |
| Prescription or Receipt of antibiotics in the ED | 1.85 | (1.13, 3.05) |
Only variables listed were included in model
DISCUSSION:
In this prospective study of children evaluated for CAP, the pre-CXR plan to prescribe antibiotics was a stronger predictor for antibiotic prescription than the presence of radiographic pneumonia. Specific features of the clinical history and exam, clinician overall impression, and physician assessment of the probability of pneumonia were associated with the plan to prescribe antibiotics prior to the results of the chest radiograph being known. In addition, receipt of antibiotic prescription was the strongest predictor of return to medical care within 7–15 days, after controlling for radiographic pneumonia.
The usefulness of chest radiographs to change provider diagnosis and prescribing behavior has been debated since the 1980s.10 The 2011 PIDS/IDSA pediatric pneumonia guideline recommends against routine chest radiographs in children well enough to be treated as outpatients. The cited rationale is a randomized controlled study of chest radiographs in outpatient pediatric pneumonia conducted in South Africa, which showed no difference in time to complete clinical recovery, return visits, or subsequent admissions.16 A similar study to ours performed by Nelson et al. prior to the publication of the PIDS/IDSA guidelines similarly demonstrated that provider behavior is more influenced by the history and physical (i.e., pre-test probability) than actual chest radiograph results.11 Both the Nelson study and ours suggest that clinicians may still rely on CXR to avoid antibiotics in patients with negative CXRs in whom they had a low suspicion for pneumonia. Our study was not designed to determine the effect of chest radiography and antibiotics on overall care or outcomes, however, the relatively low prevalence of detectable bacteria compared to viruses in hospitalized children with pneumonia in the recent EPIC cohort raises further questions about the indications for antibiotic use.17
Several clinical factors were more prevalent in children with a pre-CXR plan to prescribe antibiotics in our study, including difficulty breathing, retractions, crackles, decreased breath sounds, and tachypnea. Although we cannot comment on the statistical effect of interactions between these clinical variables on prescribing antibiotics, it is notable that none of the clinical factors associated with the plan to give antibiotics are reliably associated with the diagnosis of pneumonia.5–7 Additionally, of the physical exam findings associated with the plan to prescribe antibiotics, only tachypnea had an acceptable level of interrater reliability between clinicians.8 Given the challenges inherent in diagnosing pneumonia based on clinical history and exam, antibiotics are commonly prescribed empirically.2
Antibiotic overuse is a major concern worldwide, given the spread of antimicrobial resistance, in addition to antibiotic-associated adverse effects and cost. Respiratory illness is the most common source of potentially unnecessary antibiotic use in children in the United States.18 A study conducted in Israel suggested that reliance on clinical symptoms alone without radiologic confirmation in an urgent care setting could lead to overtreatment in more than half of cases.19 However, even when confirmatory chest radiographs are used, as in our cohort, the plan to give antibiotics remained the strongest predictor of antibiotic use, surpassing the presence of radiographic pneumonia. Despite the presence of radiographic pneumonia in only 14% of patients in our study, 35% received antibiotics. Recently, Lipsett et al. found that the negative predictive value for pneumonia of a negative CXR is likely greater than 97%, suggesting that many outpatients with negative CXRs may have been overtreated.20 However, children who were treated with antibiotics despite negative CXRs or without CXRs were excluded from the analysis, suggesting potentially greater overtreatment. Furthermore, not all children with pneumonia require antibiotics, as viruses are the most likely pathogen in many children, particularly those younger than 5 years.21 While the 2011 PIDS/IDSA guideline strongly discourages the use of routine antibiotics in preschool aged children, it is unclear how closely this recommendation is followed in practice.4 Further studies are needed to assist clinicians to determine which children are more likely to be helped by antibiotics, including better pathogen-specific diagnostic methods.
Our study noted an association between antibiotic prescription in the ED and return to medical care after discharge. While the loss of 31% of our cohort to follow up limits the potential validity of this finding, it highlights the potential downsides of excess antibiotic use. The cause of this association is unclear but one explanation may be that return visits are due to common adverse effects of antibiotics, such as diarrhea, especially in younger children.22, 23
This study has several limitations. The study was conducted at a single tertiary care academic medical center and conclusions about clinician behavior may not be generalizable to other settings. In addition, in CARPE DIEM, CXRs from 81% of children enrolled were seen by the clinician prior to answering the provider survey. However, all children in the cohort received a CXR and only children in whom a CXR was not seen prior to answering the survey were included in the analysis for this study. There were no systematic reasons why different clinicians were approached at different times (i.e., before or after seeing the CXR) to complete the survey. Despite this, there may have been selection bias against children with more severe illness, as providers may have been quicker to review the CXRs in in these cases. However, the clinician perception of severity for patients in this analysis was similar to the overall cohort. A relatively high proportion of the cohort was lost to follow up, limiting our ability to definitively address our secondary outcomes. In addition, as this study was part of a larger cohort in which every participant received a CXR, we are unable to determine whether are findings are applicable to a real world setting in which children at very high or very low suspicion for pneumonia likely do not receive CXRs. We also do not know the rates of antibiotic prescription and how they related to CXR results in our ED outside of our cohort. Finally, it is difficult to measure the decision process that a provider goes through when deciding to prescribe antibiotics for a child with pneumonia. This study attempted to account for this process by not only including clinical characteristics of the patient, but also by asking the provider their predicted probability of radiographic pneumonia prior to receiving results of any diagnostic tests. In addition, the prospective nature of this study allows for stronger causal inferences regarding the decision to prescribe antibiotics and the act of prescribing antibiotics. We are unable to fully assess whether antibiotics were prescribed for indications other than CAP, but the clinicians were asked about their plans to treat CAP explicitly.
We found that the intention to prescribe antibiotics based on clinical presentation and physical exam was the strongest predictor for actual antibiotic prescription in the ED in our study. Antibiotic prescription was associated with return to medical care after controlling for the presence of radiographic pneumonia and the plan to prescribe antibiotics based on clinical presentation and physical exam. While our findings support the current PIDS/IDSA guidelines’ recommendation on CXR use, the surprising association between antibiotic prescription and return to medical care highlights the need for more precise methods of identifying children who are at low risk of having bacterial pneumonia and who thus are unlikely to be helped by antibiotics. The precise association between antibiotic prescription and clinical outcomes, such as return visits, hospitalizations, and antibiotic related adverse events in children suspected of CAP remains unclear.
Supplementary Material
Funding:
This work was supported by a pediatric research grant from the National Institute for Allergy and Infectious Diseases and the National Institutes of Health (grant 1K23 AI121325–01 to TAF and K01 AI125413–01A1 to LA) and a Pediatric Research Grant from The Gerber Foundation (to TAF). The funders had no role in the design and conduct of the study, collection, management, analysis and interpretation of the data.
Footnotes
This work was presented as a poster presentation at the Pediatric Academic Societies meeting in Toronto, ON in May 2018.
REFERENCES
- [1].Alpern ER, Stanley RM, Gorelick MH, et al. Epidemiology of a Pediatric Emergency Medicine Research Network. Pediatr Emerg Care. 2006;22:11. [DOI] [PubMed] [Google Scholar]
- [2].Handy LK, Bryan M, Gerber JS, et al. Variability in Antibiotic Prescribing for Community-Acquired Pneumonia. Pediatrics. 2017;139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Launay E, Levieux K, Levy C, et al. Compliance with the current recommendations for prescribing antibiotics for paediatric community-acquired pneumonia is improving: data from a prospective study in a French network. BMC Pediatr. 2016;16:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rambaud-Althaus C, Althaus F, Genton B, et al. Clinical features for diagnosis of pneumonia in children younger than 5 years: a systematic review and meta-analysis. Lancet Infect Dis. 2015;15:439–50. [DOI] [PubMed] [Google Scholar]
- [6].Shah SN, Bachur RG, Kim D, et al. Lack of predictive value of tachypnea in the diagnosis of pneumonia in children. Pediatr Infect Dis J. 2010;29:406–9. [DOI] [PubMed] [Google Scholar]
- [7].Shah SN, Bachur RG, Simel DL, et al. Does This Child Have Pneumonia?: The Rational Clinical Examination Systematic Review. JAMA. 2017;318:462–71. [DOI] [PubMed] [Google Scholar]
- [8].Florin TA, Ambroggio L, Brokamp C, et al. Reliability of Examination Findings in Suspected Community-Acquired Pneumonia. Pediatrics. 2017;140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Grossman LK, Caplan SE. Clinical, Laboratory, and Radiologic Information in the Diagnosis of Pneumonia in Children. Annals of Emergency Medicine. 1988;17:4. [DOI] [PubMed] [Google Scholar]
- [10].Alario AJ, McCarthy PL, Markowitz R, et al. Usefulness of chest radiographs in children with acute lower respiratory tract disease. J Pediatr. 1987;111:187–93. [DOI] [PubMed] [Google Scholar]
- [11].Nelson KA, Morrow C, Wingerter SL, et al. Impact of Chest Radiography on Antibiotic Treatment for Children With Suspected Pneumonia. Pediatr Emerg Care. 2016;32:6. [DOI] [PubMed] [Google Scholar]
- [12].Jain S, Self WH, Wunderink RG, et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N Engl J Med. 2015;373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Feudtner C, Feinstein JA, Zhong W, et al. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rosenbaum PR, Rubin DB. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika. 1983;70:41–55. [Google Scholar]
- [15].Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Swingler GH, Hussey GD, Zwarenstein M. Randomised controlled trial of clinical outcome after chest radiograph in ambulatory acute lower-respiratory infection in children. Lancet. 1998;351:404–8. [DOI] [PubMed] [Google Scholar]
- [17].Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011. JAMA. 2016;315:1864–73. [DOI] [PubMed] [Google Scholar]
- [19].Zimmerman DR, Kovalski N, Fields S, et al. Diagnosis of childhood pneumonia: clinical assessment without radiological confirmation may lead to overtreatment. Pediatr Emerg Care. 2012;28:646–9. [DOI] [PubMed] [Google Scholar]
- [20].Lipsett SC, Monuteaux MC, Bachur RG, et al. Negative Chest Radiography and Risk of Pneumonia. Pediatrics. 2018;142. [DOI] [PubMed] [Google Scholar]
- [21].Hamano-Hasegawa K, Morozumi M, Nakayama E, et al. Comprehensive detection of causative pathogens using real-time PCR to diagnose pediatric community-acquired pneumonia. J Infect Chemother. 2008;14:424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Turck D, Bernet J, Marx M, et al. Incidence and Risk Factors of Oral Antibiotic-Associated Diarrhea in an Outpatient Pediatric Population. Journal of Pediatric Gastroenterology and Nutrition. 2003;37:5. [DOI] [PubMed] [Google Scholar]
- [23].Lovegrove MC, Geller AI, Fleming-Dutra KE, et al. US Emergency Department Visits for Adverse Drug Events From Antibiotics in Children, 2011–2015. J Pediatric Infect Dis Soc. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.