Abstract
Neuraminidase is the second major surface antigen on influenza virus. We investigated the immunogenicity and cross protective efficacy of virus-like particle containing neuraminidase derived from 2009 pandemic H1N1 influenza virus (N1 VLP) in comparison with inactivated split influenza vaccine. Immunization of mice with N1 VLP induced antibody responses specific for virus and cross-reactive neuraminidase inhibition activity whereas an inactivated split vaccine induced strain-specific hemagglutination inhibition activity. N1 VLP-immunized mice developed cross protective immunity against antigenically different influenza viruses, as determined by body weight changes, lung viral titers, infiltrating innate immune cells, and cytokines, and antibody secreting cells, and germinal center B cells. Also, N1 VLP-immune sera provided cross-protection in naïve mice. Immunity by N1 VLP vaccination was not compromised in Fc receptor γ-chain deficient mice. These results suggest that neuraminidase-presenting VLP can be developed as an effective cross-protective vaccine candidate along with current influenza vaccination.
Keywords: Influenza virus, Virus-like particle, Neuraminidase vaccine, Cross protection
1. Introduction
Annual vaccination is recommended to protect seasonal influenza viruses worldwide although its efficacy is highly variable year to year. Hemagglutinin (HA) is a major target for inducing immunity by currently licensed influenza vaccines since antibodies to HA can block the ability of the receptor binding site of HA and thus have neutralizing activity, a protective correlate. However, the antigenicity of HA is highly variable and HA immunity is specific for virus strains with matching HA.
Neuraminidase (NA) is the second major surface protein, playing key roles in the life cycle of influenza virus. NA has an enzymatic function that cleaves terminal sialic acids from glycans, facilitating virus release on the host cell surface (Wohlbold and Krammer, 2014). NA is also suggested to play a role in promoting virus entry into the target host cells (Sakai et al., 2017; Su et al., 2009). Antiviral drugs (oseltamivir, zanamivir) target NA enzymatic activity. Therefore, NA can provide an important vaccine target antigen. Antibodies specific for NA antigens could provide an independent protective correlate alleviating disease in humans (Couch et al., 2013; Memoli et al., 2016; Monto et al., 2015; Murphy et al., 1972). In addition, NA undergoes much slower antigenic changes than HA, suggesting NA as a desirable vaccine target (Kilbourne et al., 1990; Krammer et al., 2018).
Currently, the only HA is required to be quantified in inactivated influenza vaccines while the amount of NA has been found to vary drastically from different manufacturers even if the same vaccine strains were used to make the vaccines (Gravel et al., 2010). HA is known to be immune dominant and has suppressive effects on inducing NA specific immune responses when both HA and NA antigens are present in the same influenza virus particles as in the inactivated influenza vaccines (Johansson et al., 1987). Co-vaccination with separate HA and NA vaccines was shown to avoid intravirionic antigenic competition, inducing balanced immune responses to both HA and NA antigens (Johansson and Kilbourne, 1993; Johansson et al., 2002).
Different approaches were taken to study the role of NA in protecting against influenza virus, which include purified NA proteins (Brett and Johansson, 2005; Johansson, 1999; Wohlbold et al., 2015), DNA plasmids (Qiu et al., 2006; Sandbulte et al., 2007), and live virus-vectored vaccines expressing NA (Mooney et al., 2017). Vaccination inducing antibodies against NA could suppress viral replication in the lungs and reduce disease severity upon subsequent challenge in animals (Job et al., 2018a; Job et al., 2018b; Liu et al., 2015; Smith et al., 2017; Walz et al., 2018; Wohlbold et al., 2015). However, some limitations might be associated with previous approaches in developing NA vaccines. Specifically, it is laborious and costly to prepare recombinant protein vaccines which often require adjuvants to be included in the vaccination. DNA vaccines are poor in immunogenicity and thus need multiple immunizations, while live vectored vaccines may induce immune responses against the vectors and have safety concerns in humans.
Influenza virus-like particles (VLPs) have distinct advantages over the aforementioned strategies in that they mimic viral structure and morphology, representing one of the novel platforms to develop efficacious vaccines against influenza (Bright et al., 2008). Influenza VLP vaccines (2009 H1N1, H5N1, H7N9) were safe and efficacious in clinical trials (Fries et al., 2013; Khurana et al., 2011; Lopez-Macias et al., 2011). Previous studies demonstrated that vaccination of animals with VLPs containing NA induced high titers of anti-NA antibodies and protection against viruses with the same subtype NA (Easterbrook et al., 2012; Quan et al., 2012; Smith et al., 2017). However, the roles of NA immunity in cross protection and Fc receptors (FcR) in NA antibody-mediated protection have not been well studied yet. In this study, we constructed influenza VLPs containing NA derived from A/California/2009 (H1N1) virus (N1 VLP) and investigated immunogenicity and efficacy of homologous and cross protection in comparison with inactivated split virus vaccine. N1 VLP was found to induce cross-reactive neuraminidase inhibition activity correlating with cross protection whereas an inactivated split vaccine induced strain-specific hemagglutination inhibition activity. FcR was not required for protection by N1 VLP vaccination or anti-NA immune sera.
2. Materials and Methods
2.1. Animals, viruses, and reagents
BALB/c and Fc receptor (FcR) γ-chain gene knockout (FcRγ KO on the BALB/c genetic background) mice were purchased from Taconic Farms (Hudson, NY) and maintained in the animal facility at Georgia State University (GSU). Animal experiments were performed under the guidelines of the approved IACUC protocol (A18001). Influenza A viruses, A/California/04/09 (A/Cal, H1N1), A/Philippines/2/82 (A/Phil, H3N2), and A/Puerto Rico/8/34 (A/PR8, H1N1) were grown in 10 days old chicken eggs (Hy-Line North America, Mansfield, GA) at 37 °C for 2 days. The reassortant rgH5N1 virus contains HA and NA derived from A/Vietnam/1203/2004 (H5N1) and the remaining backbone genes from A/PR8 (Song et al., 2010). The viruses grown in embryonated eggs were inactivated using formalin at a concentration of 1:4000 (v/v) (Quan et al., 2008). Influenza split vaccine (sCal) derived from the 2009 H1N1 pandemic strain A/Cal/07/2009 was kindly provided by the WHO-certified vaccine manufacturing company (Green Cross, South Korea). The split vaccine of A/PR8 (sPR8) was prepared with inactivated PR8 virus by treating with 1% triton X-100 to disrupt virus particles and dialyzing with phosphate-buffered saline (PBS) as described (Ko et al., 2018). The total protein concentrations of sPR8 and N1 VLP were determined by DC protein assay kit (Bio-rad, Hercules, CA). HCA-2 monoclonal antibody (mAbs) specific for pan NA proteins (NA220–230, LRTQESEC) has been well described in previous studies (Doyle et al., 2013; Gravel et al., 2010).
2.2. Preparation of influenza N1 NA VLP
Virus-like particle expressing NA (N1 VLP) derived from A/Cal (H1N1) was produced in insect cells as previously described (Kim et al., 2018b; Quan et al., 2012; Quan et al., 2010). Briefly, the plasmid pCI with cDNA encoding N1 NA derived from A/Cal (H1N1) virus kindly provided by Dr. Ruben Donis (CDC, Atlanta, GA) and cloned into pFastBac shuttle vector using primers synthesized with forward primer (5’-AAAGAATCCGCCGCC ACCATGA ATCCAAACCAAAAGATAATAACC −3’) and reverse primer (5’-AAAAAGCTTTT ACTTGTCAATGGTAAATFF-3’). The cloned pFastBac-NA plasmid was subsequently used to generate recombinant Bacmid DNA containing NA in transformed DH10Bac cells (Invitrogen). Recombinant baculovirus (rBV) expressing N1 NA was generated in Sf9 insect cells transfected with recombinant Bacmid containing NA. N1 VLP was prepared from the culture supernatants from Sf9 cells co-infected with rBVs expressing N1 NA and matrix protein M1 (Quan et al., 2012) by removing cells via centrifugation (3000 rpm, 20 min) and then purified by ultracentrifugation (30,000 rpm, 60 min) (Quan et al., 2012; Quan et al., 2010). Expression and incorporation of N1 NA into VLP were confirmed by ELISA and western blot using anti-rabbit NA222–230 mAb, HCA2 (Doyle et al., 2013; Gravel et al., 2010). The amount of NA was estimated to be in a low range of 1% to 3% of total N1 VLP proteins, implicating that the NA amount in 10μg N1 VLP would be approximately 0.2 μg NA. Nanoparticle size distribution of N1 VLP was determined by dynamic light scattering (DLS) with a Malvern Zetasizer Nano ZS (Malvern Instruments, Westborough, MA). Functional NA activity of N1 VLP was estimated by enzyme linked lectin assay (ELLA) as described (Doyle et al., 2013).
2.3. Immunization and viral infection
BALB/c mice (6- to 8-week old) and FcRγ KO mice (6- to 8 week old, n=8–10 per group) were intramuscularly prime-boost immunized with N1 VLP (10 μg total protein per mouse), split vaccines (1 μg total protein per mouse) from A/PR8 (sPR8) or A/Cal (sCal) at weeks 0 and 3. Blood samples were collected at 2 weeks after prime and boost immunization. Immunized mice were challenged with a lethal dose of 3× LD50 A/Cal (H1N1), 2.5× LD50 rgH5N1, or 1.5× LD50 A/Phil (H3N2) at 4 weeks after boost.
2.4. Enzyme-linked immunosorbent assay (ELISA)
IgG antibody responses specific for virus antigens were determined by ELISA in immune sera collected after boost, in bronchoalveolar lavage fluids (BALF) and lung lysates harvested on 7 days post infection (dpi). ELISA coating antigens include inactivated A/Cal (H1N1), A/PR8 (H1N1), and rgH5N1 virus. Serially diluted samples (sera, BALF, lung lysates) were added into a 96-well plate coated with inactivated virus as previously described (Kim et al., 2018a). To determine antibody production in vitro, the cells from mediastinal lymph node (MLN) collected at 7 dpi with rgH5N1 virus infection were cultured in the presence of inactivated virus antigens and incubated for 2 days at 37 °C. Antigen-specific antibodies in culture supernatants were determined by ELISA. The levels of cytokines and chemokine were measured from BALF and lung lysates using ELISA kits (eBioscience, San Diego, CA) according to the manufacturer’s instructions.
2.5. Neuraminidase inhibition (NAI) analysis by enzyme-linked lectin assay (ELLA)
NA inhibition (NAI) activity against virus in sera was determined by ELLA using a fetuin-based procedure as described (Doyle et al., 2013). Briefly, 96 well plates were coated at 25 ng per each well with fetuin (Sigma-Aldrich) and incubated overnight at 4 °C. After blocking the plates with PBS containing 1% bovine serum albumin (BSA), a mixture of 2-fold serially diluted immune sera and virus with 90% maximum activity was incubated and then added to the fetuin-coated plates, then incubated for 2 hours at 37 °C. Peroxidase-labeled peanut agglutinin (1 μg/ml) was added to the plates for 2 hours at room temperature. NAI activity was measured using tetramethylbenzidine substrate (eBioscience, San Diego, CA) and OD values were read at 490nm. The inhibition percent was calculated using the formula: 100 × (ODvirus only control - ODtest sample)/ODvirus only control.
2.6. Hemagglutination inhibition (HAI) assay
To determine HAI titers, immune sera were treated with receptor destroying enzymes (RDE, Sigma Aldrich, St. Louis, MO) at 1:3 ratio (sera: RDE) and then incubated for 16 hours at 37 °C as previously described (Ko et al., 2017). The RDE-treated serum samples were inactivated at 56 °C for 30 min, serially 2 fold diluted, and incubated with 4 HA units of A/PR8, A/Cal, or rgH5N1 virus for 30 min, and then admixed with 0.5% chicken red blood cells (RBC) (Lampire Biological Laboratories, Pipersville, PA). The highest serum dilutions interfering with the red spot formation were determined for HAI titers.
2.7. Lung viral titration
Lung viral loads were determined in embryonated chicken eggs. Lung homogenates were serially diluted and injected into the egg allantoic sacs per each dilution of samples and then incubated at 37 °C for 3 days. Highest dilutions displaying hemagglutination activity were measured using RBC to determine viral titers as described (Kim et al., 2018a).
2.8. Flow cytometry
Bronchoalveolar lavage fluids (BALF) and lung tissues were collected at 7 dpi with rgH5N1 virus. BALF cells were harvested by infusing 1 ml of PBS into the trachea using a catheter (Exelint International Co., Los Angeles, CA) to harvest non-adherent cells in the airways as described (Kim et al., 2015). The lung tissues without perfusion were homogenized and spun on 44%/67% Percoll gradients at 2800rpm for 15 min. The lung cells were collected from the layer between 44% and 67%. To determine cell phenotypes in the airways, BAL and lung cells were stained with surface marker antibodies specific for anti-mouse CD11b, CD11c, Ly6c, CD45, F4/80, Siglec F, MHC II (eBioscience or BD Pharmingen, San Diego, CA). MLN cells were collected at 7dpi to determine germinal center (GC) B cells and plasma cells. The MLN cells were stained with specific anti-mouse phenotypic marker antibodies for CD3, CD19, IgD, B220, GL-7, CD138 (eBioscience or BD Pharmingen, San Diego, CA). All samples were analyzed on a Becton-Dickinson LSR-II/Fortessa flow cytometer (BD, San Diego, CA) and analyzed using the Flowjo software (FlowJo V10, Tree Star, Inc., Ashland, OR).
2.9. In vivo protective assay of N1 VLP immune sera
To investigate the roles of N1 VLP immune sera in contributing to cross protection, N1 VLP immune or unvaccinated naïve sera were collected from wild type (WT) BALB/c and FcRγ KO mice, inactivated at 56 °C 30 min, diluted 2-fold with PBS, and mixed with a lethal dose (10× LD50) of rgH5N1 at same volume (25 μl + 25 μl) as previously described (Song et al., 2011). Naïve WT BALB/c and FcRγ KO mice were intranasally infected with a mixture (50 μl) of sera and virus and monitored for the survival rates and body weight changes for 14 days.
2.10. Statistical analysis
All results are presented as the mean ± the standard errors of the mean (SEM). The statistical significance for all experiments was performed by one- or two-way analysis of variance (ANOVA). Prism software (GraphPad Software, Inc., San Diego, CA) was used for all data analysis. The comparison used to generate a P value is indicated by horizontal lines (*; p<0.05, **; p<0.01, ***; p<0.001).
3. Results
3.1. Characterization of influenza VLP expressing neuraminidase (N1 VLP)
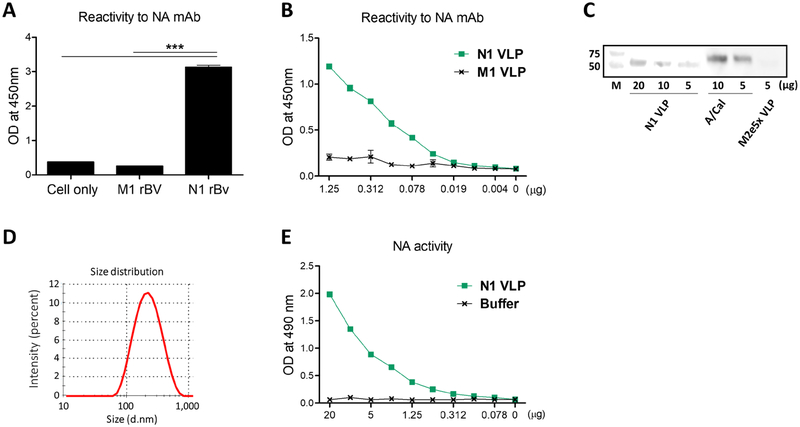
The rBV expressing N1 NA protein derived from A/California/04/2009 (A/Cal, H1N1) was confirmed as evidenced by high reactivity of N1 rBV-infected insect cells against HCA-2 mAb specific for NA by cell-surface ELISA (Fig. 1A). N1 NA VLP purified from insect cell culture supernatants also displayed strong reactivity to HCA-2 mAb (Fig. 1B). The NA protein presented on VLPs was also confirmed by western blot at different loading amounts using HCA-2 mAb and estimated to be present at 2–3 folds less than A/Cal virus (Fig. 1C). The particle sizes of NI VLP were an average diameter of 220nm as measured by dynamic light scattering (DLS) using Malvern Zetasizer Nano ZS (Fig. 1D). The functional enzyme activity of NA expressed on the VLP was found to be dependent on the concentrations of N1 NA VLP (Fig. 1E). These results showed that NA displaying on the surface of VLP exhibits NA enzyme activity, implicating native NA conformation.
Figure 1. Characterization of VLP containing N1 NA (N1 VLP).
(A) The ELISA reactivity to NA mAb (HCA-2) in the M1 (from A/PR8 virus) or N1 NA (from A/Cal, H1N1 virus) rBV-infected insect cells. NA expression on the insect cells infected with M1 or N1 rBVs was determined with anti-NA mAb (HCA-2) by ELISA. (B) The reactivity of N1 VLP to NA mAb (HCA-2) by ELISA. (C) The expression of NA protein on VLPs was determined by western blot probed with rabbit HCA-2 mAb. Influenza N1 VLP (20, 10, 5 μg), inactivated A/Cal (H1N1) virus (10, 5 μg), M2e5x VLP (5 μg) were loaded. (D) Size distribution of N1 VLP as measured by Malvern Zetasizer with dynamic light scattering (DLS). (E) Neuraminidase activity by an enzyme-linked lectin assay. Statistical significance was determined by using one-way ANOVA. Data are representative of individual animal out of two independent experiments. Error bars indicate the means ± SEM. ***, p<0.001.
3.2. N1 VLP vaccine induces Th1-biased IgG2a antibody responses
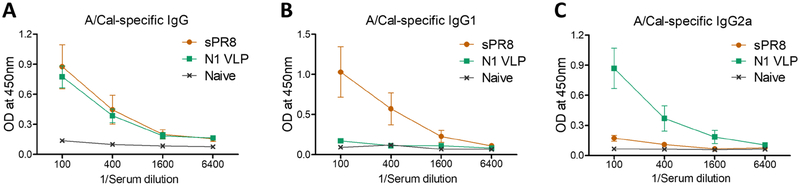
Groups of mice were intramuscularly immunized with N1 VLP by a prime boost regimen in comparison with influenza split vaccine (sPR8, H1N1). Serum IgG antibodies binding to A/Cal virus were induced after boost immunization at comparable levels in the sPR8 and N1 VLP groups (Fig. 2A). The split sPR8 vaccine group induced T helper type 2 (Th2) IgG1 isotype antibody predominantly (64 folds compared to N1 VLP) whereas the N1 VLP group raised Th1 type IgG2a isotype antibody dominantly (16 folds compared to sPR8) (Fig. 2B, 2C). We further found that split sPR8 vaccine was highly effective in inducing IgG and IgG1 antibodies specific for the homologous A/PR8 virus antigen (Supplementary Fig. S1). These results suggest that N1 VLP vaccine effectively induce Th1 type IgG2a antibodies compared to split influenza vaccine.
Figure 2. N1 VLP immunization induces Th1-biased IgG2a isotype antibody responses.
BALB/c mice (n=8) were immunized with N1 (A/Cal, H1N1) VLP (10 μg) and split vaccine from A/PR8 (H1N1) virus (sPR8, 1 μg total protein) intramuscularly with an interval of 2 weeks and blood samples were collected at week 2 after boost. (A-C) A/Cal (H1N1) virus antigen-specific IgG antibodies were determined by ELISA.
3.3. N1 VLP immunization induces NA-inhibiting cross-reactive antibodies
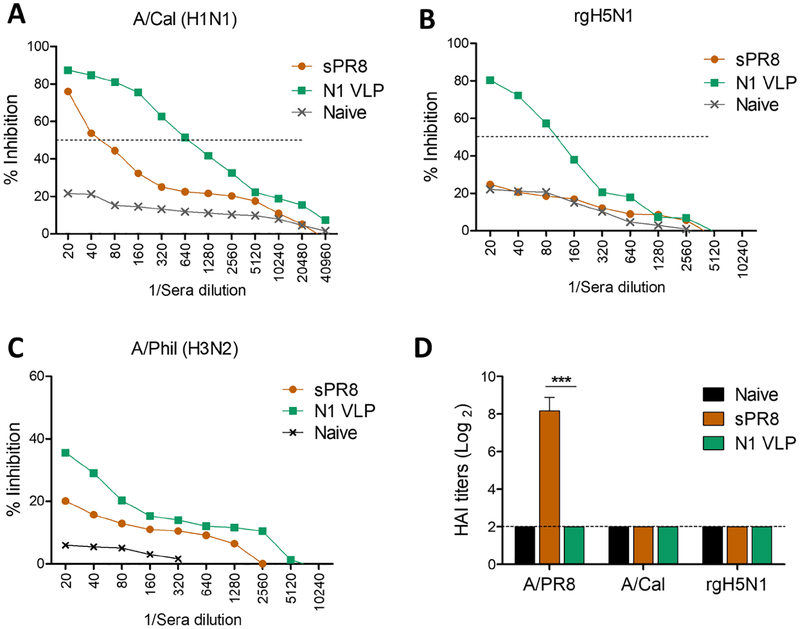
NA inhibiting (NAI) IgG antibodies prevent release and spread of virus. N1 VLP immune sera exhibited significantly higher levels of NI activity by 11 folds and 16 folds or 4 folds than those of the sPR8 group against A/Cal (H1N1) (Fig. 3A), heterologous rgH5N1 virus (Fig. 3B), and heterosubtypic A/Phil (H3N2) (Fig. 3C) respectively. Split vaccine sPR8 raised high hemagglutination inhibition (HAI) titers against homologous A/PR8 (H1N1) while N1 VLP group did not display HAI activity (Fig. 3D). These results support that N1 VLP is effective in inducing cross reactive NAI antibody responses.
Figure 3. NA inhibition activity is induced in sera from N1 VLP immunized mice.
NA inhibition (NAI) activity in boost immune sera was determined against different stains of influenza viruses by an enzyme-linked lectin assay. (A) NAI activity against homologous A/Cal (H1N1) virus. (B) NAI activity against heterologous rgH5N1 virus. Dotted lines indicate the concentration inhibiting 50% of NA enzyme activity. (C) NAI activity against heterosubtypic A/Phil (H3N2) virus. (D) HAI titers against influenza viruses. Statistical significance was determined by using one-way ANOVA. Data (n=4) are representative of individual animal out of two independent experiments. Error bars indicate the means ± SEM. ***, p<0.001.
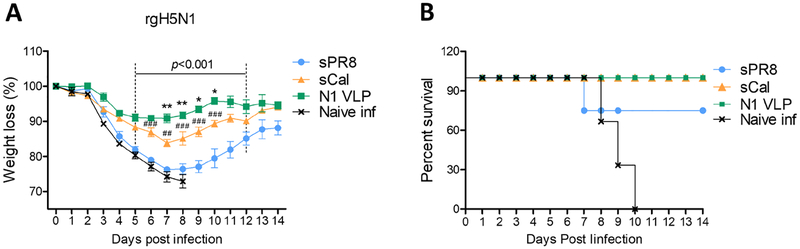
3.4. N1 VLP provides cross protection against HA variant viruses
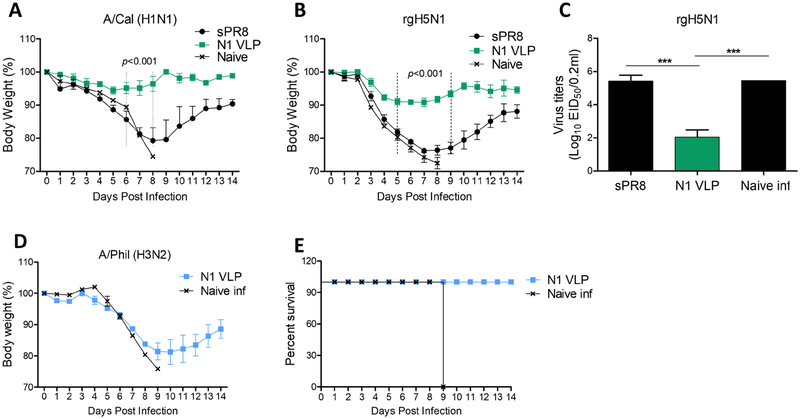
To investigate cross protective efficacy, the groups of mice immunized with sPR8 or N1 VLP were intranasally challenged with A/Cal (H1N1), heterologous rgH5N1, or heterosubtypic A/Phil (H3N2) (Fig 4). The sPR8 group showed severe weight loss of approximately 20% and 24% against A/Cal (H1N1) and rgH5N1 infections respectively (Fig. 4A–B). In contrast, N1 VLP-immunized mice displayed slight to moderate weight loss of approximately 5% and 10%, and were 100% protected against homologous A/Cal (H1N1) and heterologous rgH5N1 virus lethal challenge respectively (Fig. 4A–B). The mice with sPR8 vaccination displayed high lung viral titers similar to those in unvaccinated naïve mice as determined at 7dpi with rgH5N1 whereas the N1 VLP group showed over 1,000 folds lower lung viral titers (Fig. 4C). Moreover, when we tested cross-protection efficacy against heterosubtypic A/Phil (H3N2), N1 VLP-immunized mice all survived the virus challenge although the recovery was delayed (Fig. 4D–E). Therefore, these results suggest that N1 VLP platform could confer cross protection against HA variant influenza virus strains.
Figure 4. Cross protection induced by mice immunized with N1 VLP.
The groups of mice with sPR8 or N1 VLP vaccination (n=4 or 8) were challenged with 3× LD50 A/Cal (H1N1), 2.5× LD50 rgH5N1, and 1.5× LD50 A/Phil (H3N2) intranasally at 4 weeks after boost immunization. Body weight changes were monitored for 14 days. (A) Body weight changes against A/Cal (H1N1) virus. (B) Weight changes after rgH5N1 virus infection. (C) Lung viral titers at 7 dpi with rgH5N1. (D) The mortality against A/Phil (H3N2) virus in N1 VLP immunized mice. Data are representative of individual animal out of two independent experiments. Error bars indicate the means ± SEM. ***, p<0.001.
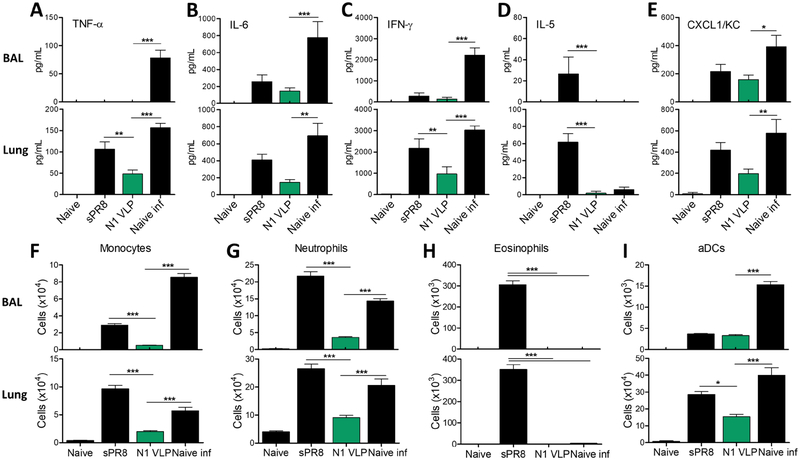
3.5. N1 VLP vaccination prevents heightened lung inflammation due to heterologous virus infection
To better understand the protective efficacy, we determined whether N1 VLP vaccination would prevent excessive inflammations in the lungs following influenza virus infection. Naïve mice infected with lethal rgH5N1 exhibited highest levels of cytokines (TNF-α, IL-6, IFN-γ) and chemokine (CXCL1/KC) indicating severe inflammation except IL-5 in BALF and lungs (Fig. 5A–E). In comparison, the sPR8-immunized group induced moderate levels of proinflammatory cytokines (TNF-α, IL-6, IFN-γ) and chemokine (CXCL1/KC) (Fig. 5A–C, E), and highest levels of IL-5 cytokine in BALF and lungs (Fig. 5D). In contrast, N1 VLP-immunized mice showed lowest levels of cytokines and chemokines among the virus infection groups (Fig. 5A–E).
Figure 5. N1 VLP vaccination prevents lung inflammation due to heterologous rgH5N1 virus infection.
Inflammatory cytokines, chemokines, and cellular phenotypes were determined in BALF and lung samples collected at 7 dpi with rgH5N1 virus. (A-E) ELISA of cytokines and chemokines in BALF and lungs. (A) TNF-α. (B) IL-6. (C) IFN-γ. (D) IL-5. (E) chemokine CXCL/KC. (F-I) Phenotypes of cellular infiltrates as determined by flow cytometry. (F) Monocytes (CD11b+Ly6chiF4/80+). (G) Neutrophils (CD11b+Ly6c+F4/80−). (H) Eosinophils (CD11b+CD11c+SiglecF+). (I) Activated dendritic cells (aDCs, CD45+CD11b+MHCII+). Naïve: unvaccinated mice without virus infection. sPR8: split sPR8 vaccinated mice with rgH5N1 virus infection. N1 VLP: N1 VLP vaccinated mice with rgH5N1 virus infection. Naïve inf: unvaccinated mice with rgH5N1 virus infection. Statistical significance was determined by using one-way and dunnett’s multiple comparison test ANOVA. Data (n=4) are representative of individual animal out of two independent experiments. Error bars indicate the means ± SEM. *, p<0.05, **, p<0.01, ***, p<0.001.
Since inflammatory cytokines and chemokines recruit cellular infiltrates during influenza virus infection, innate immune cells were analyzed in the BALF and lungs 7 dpi with rgH5N1 virus (Fig. 5F–H). N1 VLP group showed significantly lower cell numbers of inflammatory monocytes (CD11b+Ly6chiF4/80+), neutrophils (CD11b+Ly6c+F4/80−), eosinophils (CD11b+CD11c+SiglecF+), and activated dendritic cells (aDCs, CD45+CD11b+MHCII+) in the BALF and lungs compared to those in the sPR8 and naïve groups after infection (Fig. 5F–I). In comparison with sPR8 group, N1 VLP-immunized mice exhibited reductions of 5 or 17 folds in monocytes, 6 or 3 folds in neutrophils, 300 or 350 folds in eosinophils in the BALF and lungs respectively post rgH5N1 infection (Fig. 5F–H). Furthermore, histological analysis displayed diminished immune cell infiltration in the lungs from mice with N1 VLP immunization and heterosubtypic infection whereas the sPR8 and naïve infections groups showed severe infiltrations of immune cells (data not shown). Taken together, these results suggest that N1 VLP confers effective protection in preventing pulmonary inflammation and innate immune cell infiltration after heterologous influenza virus infection.
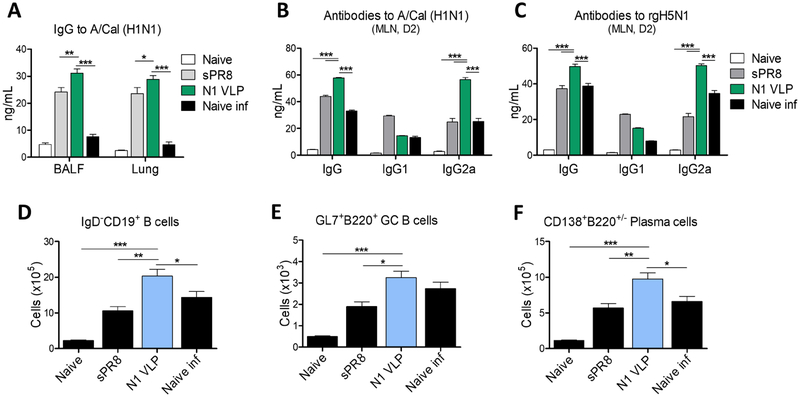
3.6. N1 VLP immunization induces mucosal IgG and antibody-secreting plasma cells in draining lymph nodes
To investigate the effect of N1 VLP immunization on enhancing humoral immunity, we determined antigen-specific antibody responses from BALF and lung lysates collected at 7dpi with rgH5N1. IgG antibodies specific for A/Cal (H1N1) virus were induced at higher levels in mucosal BALF and lung samples from mice immunized with N1 VLP than those in sPR8 vaccine and naïve mice after infection (Fig. 6A). Furthermore, we determined the secretion of IgG antibodies specific for homologous or heterologous viruses from in vitro cultures of MLN cells collected at 7dpi with rgH5N1. Consistent with serum IgG antibody responses, N1 VLP immunized group showed higher levels of IgG and IgG2a, but not IgG1, specific for A/Cal (H1N1) and rgH5N1 viruses than those of sPR8 vaccine and naïve mice (Fig. 6B–C). To better understand effector B cell responses induced by N1 VLP immunization, germinal center (GC) B cells and antibody-secreting plasma cells were determined from MLN cells collected day 7 post infection by flow cytometry. B cells (IgD−CD19+), GC B cells (GL7+B220+IgD−CD19+), and plasma cells (CD138+B220+/−IgD−CD19+) were detected with higher numbers in the MLN from the N1 VLP immunized group when compared with the sPR8 and naïve groups (Fig. 6D–F). Therefore, these results suggest that N1 VLP immunization induces cross-reactive IgG antibody responses and B cell differentiation into plasma cells.
Figure 6. Mucosal IgG and antibody-secreting plasma cells in draining lymph nodes after N1 VLP vaccination and rgAH5N1 virus challenge.
(A) IgG antibodies specific for A/Cal (H1N1) virus in mucosal BALF and lung samples collected at 7 dpi with rgH5N1 challenge. (B-C) IgG antibody production from in vitro cultures with MLN cells collected at 7 dpi. MLN day 2 culture in vitro-secreted IgG antibodies specific for A/Cal (H1N1) virus (B) and for rgH5N1 virus (C). (D) Total mature B cells (IgD−CD19+ B cells) in MLN cells collected at 7dpi. (E and F) Germinal center B cells (GL7+B220+ B cells) and plasma cells (CD138+B220+/− B cells) in MLN cells collected at 7dpi were determined from gated IgD−CD19+B220+ B cells by flow cytometry. Data (n=4) are representative of individual animal out of two independent experiments. Error bars indicate the means ± SEM. *, p<0.05, **, p<0.01, ***, p<0.001.
3.7. N1 VLP is effective in conferring cross protection
We next compared cross protective efficacy from mice immunized with N1 VLP and split vaccine platforms derived from A/Cal (H1N1, sCal) and A/PR8 (H1N1, sPR8) against heterologous influenza virus. The sPR8- and sCal-immunized groups displayed severe and moderate weight loss of approximately 24% and 16%, and survival rates of 75% and 100%, respectively, after rgH5N1 virus infection (Fig. 7A, B). In contrast, N1 VLP-immunized mice induced effective cross protection against heterologous influenza virus infection as evidenced by significantly less body weight loss (~10%) (Fig. 7A–B). These results provide evidence that NA VLP can be relatively effective in conferring cross protection against influenza A viruses with HA antigenic variants.
Figure 7. N1 VLP provides effective cross protection against rgH5N1 virus compared to HA-based split vaccines.
Mice (n=4) immunized with split vaccines from A/Cal (H1N1, sCal) or A/PR8 (sPR) in 0.3 μg HA and 10 μg N1 VLP derived from A/Cal (H1N1) were intranasally infected with rgH5N1. (A) Body weigh changes. (B) Survival rates. Statistical significance was determined by using two-way ANOVA. Data are representative of individual animal out of two independent experiments. Error bars indicate the means ± SEM. *, p<0.05, **, p<0.01 in comparison of sCal and N1 VLP; ##, p<0.01, ### p<0.001 in comparison of sPR8 and sCal; p<0.001 in comparison of N1 VLP and sPR8 group.
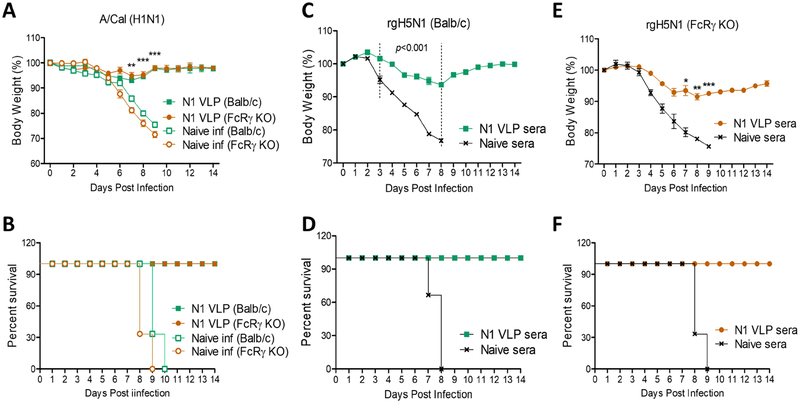
3.8. N1 VLP vaccination and N1 VLP immune sera confer protection in Fc receptor deficient mice
Next, we determined whether Fc receptors (FcR) would be required for N1 VLP immune serum-mediated protection. Wild type (WT) and FcRγ KO mice were immunized with N1 VLP by a prime boost regimen, then intranasally challenged with A/Cal (H1N1) virus, and daily monitored for 14 days (Fig. 8A–B). FcRγ KO mice with N1 VLP vaccination were well protected, displaying only a slight weight loss in a similar pattern as observed in WT mice with N1 VLP (Fig. 8A–B). Thus, N1 VLP vaccination induces protection in FcRγ KO mice.
Figure 8. Roles of FcRγ in providing protection by N1 VLP vaccination or N1 VLP immune sera.
(A and B) Protection (weight changes and survival rates) against A/Cal virus in BALB/c and FcRγ KO after N1 VLP vaccination. WT BALB/c and FcRγ KO mice (n=5) that were immunized with N1 VLP were challenged with A/Cal (H1N1) at 4 weeks after boost immunization. (C and D) Protection (weight changes and survival rates) against rgH5N1 virus by N1 VLP immune sera in naive BALB/c mice (n=3). Immune sera collected from N1 VLP-immunized BALB/c mice were incubated with a lethal dose (10× LD50) of rgH5N1 virus. Naïve BALB/c mice were intranasally infected with influenza virus mixed with immune sera or naïve sera. (E and F) Protection (weight changes and survival rates) against rgH5N1 virus by N1 VLP immune sera in naive FcRγ KO mice (n=3). Naïve FcRγ KO mice were intranasally infected with influenza virus mixed with immune sera or naïve sera. Statistical significance was determined by using tow-way ANOVA. Data are representative of individual animal out of two independent experiments. Error bars indicate the means ± SEM. *, p<0.05, **, p<0.01, ***, p<0.001 in comparison between N1 VLP immunization (or immune sera) and unvaccinated naïve infection (or naïve sera) in BALB/c or FcRγ KO mice.
To determine the roles of N1 VLP immune sera in conferring cross protection, naïve mice were intranasally inoculated with a mixture of influenza virus and immune sera collected from N1 VLP-immunized or unvaccinated naïve mice and then monitored for weight changes and survival rates for 14 days (Fig. 8 C–D). The naïve mice with unvaccinated sera and virus were not protected against rgH5N1 virus as evidenced by severe weight loss (>24%) and 0% survival rates (Fig. 8C–D). In contrast, naïve mice with N1 VLP immune sera and rgH5N1 virus were protected against rgH5N1 virus with slight weight loss of 5–6% (Fig. 8C–D).
To further investigate the roles of FcR, naïve FcRγ KO mice were inoculated with heterologous rgH5N1 virus and N1 VLP immune sera (Fig. 8E–F). Naïve FcRγ KO mice that received N1 VLP immune sera and rgH5N1 virus were protected, as evidenced by a moderate body weight loss of 7–9% and survival rates of 100% (Fig 8E–F). The cross protection against rgH5N1 mediated by N1 (A/Cal) VLP immune sera was similarly observed in WT (Fig. 8A, B) and FcRγ KO mice despite a delay in recovery in FcRγ KO mice. Taken together, these results suggest that FcRγ is not required for N1 VLP immune sera-mediated protection or protection by N1 VLP vaccination.
4. Discussion
NA is the second major surface antigen on influenza viruses and NA immunity was indicated to be an independent correlate of protection as evidenced by the analysis of human samples (Memoli et al., 2016; Monto et al., 2015). Inactivated split influenza vaccines contain variable contents of NA (Gravel et al., 2010). Vaccination with split vaccines was not effective in inducing antibody responses to NA since HA outcompetes NA in the priming of B and T cell responses when both antigens are close each other on the same virus particles as shown in a mouse model (Johansson et al., 1987). A separate entity of NA vaccines would be desirable. Here we explored the roles of NA VLP immunity in conferring protection against antigenically different influenza viruses in comparison with inactivated influenza split virus, the most common vaccine platform. Influenza virus is estimated to contain approximately 25% – 30% HA and to 8% NA out of total virus proteins (Gravel et al., 2010; Hutchinson et al., 2014; Ko et al., 2018; Tumpey et al., 2001), suggesting 0.25 to 0.3 μg HA in 1 μg split virus total protein used to immunize the mice. Although the NA amount in 10μg N1 VLP was estimated to be approximately 0.2 μg NA, N1 NA VLP vaccine was immunogenic, preferentially inducing Th1 type humoral and cellular immune responses in mice with a prime boost regimen. Notably, N1 VLP vaccine was found to be relatively effective in conferring cross protection against influenza viruses with antigenically different HA antigens, compared to HA-based split vaccine inducing strain-specific HAI activity. Protection by N1 VLP vaccination or by immune sera of N1 VLP vaccination was also observed in FcR deficient mice.
NA is known to be less variable and to undergo lower antigenic mutation rates than those of HA (Kilbourne et al., 1990; Sandbulte et al., 2011). The mice that received N1 (A/Cal, H1N1) NA VLP vaccination were well protected against homologous A/Cal (H1N1) virus and heterologous rgH5N1 within the same N1 subtype, resulting in over 1000 folds lower lung viral loads and minimal weight loss. In contrast, inactivated split (sPR8, H1N1) virus vaccination induced either survival protection against A/Cal (H1N1) virus or no protection against rgH5N1. The levels of IgG antibodies specific for A/Cal virus antigens were observed in sPR8 vaccine immune mice at similar to or higher than those in NA VLP immune mice. This data indicates that virus binding IgG antibody levels induced by split vaccination would not be correlated with cross protection. Transfer of virus-mixed immune sera of NA VLP to naïve mice could confer protection against rgH5N1 virus, without displaying substantial weight loss. The homo and heterologous NA immune-mediated protection observed in this study is consistent with previous studies that anti-NA antibodies can provide cross protection against different antigenic HA viruses within the same NA subtype (Easterbrook et al., 2012; Halbherr et al., 2015; Wan et al., 2013; Wohlbold et al., 2015). The survival protection against heterosubtypic A/Phil (H3N2) virus was observed with N1 VLP immune sera, despite of accompanying significant weight loss.
The underlying mechanisms for homo, heterologous, and heterosubtypic cross protection by NA immunity need to be further studied. Protective NA antibodies can inhibit NA enzymatic function, interfering with virus egress on the infected cells. We found that N1 VLP induces cross-reactive NAI antibodies. Also, the levels of NAI activities against A/Cal (H1N1), rgH5N1, and A/Phil (H3N2) induced by N1 VLP appear to be correlated with the efficacy of homo and cross protection as measured by body weight changes and survival rates. Previous studies reported that plaque sizes are significantly reduced in the presence of NA antibodies at the stages of virus spreading even at low concentrations (Jiang et al., 2016; Wan et al., 2013; Wohlbold et al., 2016; Wohlbold et al., 2017). Most NA inhibiting antibodies are considered to mediate non-neutralizing immunity, thus resulting in infection-permissive protection but significantly mitigating the severity of disease (Easterbrook et al., 2012; Quan et al., 2011; Wohlbold et al., 2015), consistent with the results in this study. NA was also reported to promote HA-mediated cell fusion and viral infection into the target cells, suggesting a role of NA in viral entry (Sakai et al., 2017; Su et al., 2009). Thus, induction of NAI antibodies would be effective in conferring protection against influenza viruses with homo or closely related heterologous NA as shown in A/Cal (H1N1) and rgH5N1 viruses.
HA stalk-specific antibodies were demonstrated to mediate broad cross protection in vivo via Fc receptor interactions (DiLillo et al., 2016; DiLillo et al., 2014) and promoting immune complexes’ phagocytosis by neutrophils (Mullarkey et al., 2016). Similarly, in vivo cross protection by antibodies to conserved influenza virus M2 extracellular domains (M2e) also requires the engagement of Fc receptors (Kim et al., 2014; Lee et al., 2014; Van den Hoecke et al., 2017) and possibly alveolar macrophages (Song et al., 2011). The requirement of Fc receptors and macrophages for NA antibody-mediated in vivo protection remains less well known. It was shown that clodronate-mediated depletion of dendritic and macrophage cells prior to transfer of NA immune sera partially reduced the protective efficacy but resulted in no effects on protective efficacy of neutralizing HA antibodies (Quan et al., 2012). Similarly, in vivo protection by immune sera of NA protein vaccination was partially dependent on the Fc receptors (Kim et al., 2017). In this study, similar levels of protection against NA homo A/Cal (H1N1) virus were observed in WT and FcRγ KO mice with NA VLP immune sera. Also, N1 VLP immune sera provided substantial protection in FcRγ KO mice although a moderate delay in recovering weight loss was displayed. It is likely that protection by NA antibodies is less dependent on or does not require the involvement of Fc receptors, in contrast to a critical role of FcR-mediated protection by M2e or HA stalk antibodies (DiLillo et al., 2016; DiLillo et al., 2014; Kim et al., 2014; Lee et al., 2014; Van den Hoecke et al., 2017).
HA-based vaccines inducing neutralizing antibodies provide the most effective homologous protection, which is superior to M2e- or NA-based vaccines inducing non-neutralizing antibodies (Kim et al., 2017; Lee et al., 2016; Mullarkey et al., 2016). However, when challenged with antigenically different HA viruses as expected, sPR8 vaccine (A/PR8, H1N1) could not confer protection against heterologous (A/Cal, H1N1) and heterosubtypic virus (rgH5N1). In contrast, N1 VLP vaccine was found to be effective in conferring cross protection against these viruses, inducing Th1 type immune responses, and rapidly generating virus specific antibody secreting plasma cells upon challenge. Therefore, developing supplemental vaccines inducing NA immunity independent of HA would provide substantial benefits in conferring cross protection. Priming or supplementing split vaccines with N1 and N2 NA VLP vaccines would provide a promising vaccination strategy conferring cross protection. Mice provide a preferred small animal model for testing preclinical experimental influenza vaccines and adjuvants at early developmental stages. However, mice are not a natural host for influenza virus and immune responses and pathogenesis in BALB/c mice might be different from what are expected in humans. Ferrets would be a better animal model for testing advanced preclinical influenza vaccines, which should be a future direction.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) grants AI1093772 (S.M.K), AI105170 (S.M.K), and AI134132 (S.M.K). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There is no conflict of interest in this study presentation
References:
- Brett IC, Johansson BE, 2005. Immunization against influenza A virus: comparison of conventional inactivated, live-attenuated and recombinant baculovirus produced purified hemagglutinin and neuraminidase vaccines in a murine model system. Virology 339, 273–280. [DOI] [PubMed] [Google Scholar]
- Bright RA, Carter DM, Crevar CJ, Toapanta FR, Steckbeck JD, Cole KS, Kumar NM, Pushko P, Smith G, Tumpey TM, Ross TM, 2008. Cross-Clade Protective Immune Responses to Influenza Viruses with H5N1 HA and NA Elicited by an Influenza Virus-Like Particle. PLoS ONE 3, e1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch RB, Atmar RL, Franco LM, Quarles JM, Wells J, Arden N, Nino D, Belmont JW, 2013. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J Infect Dis 207, 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLillo DJ, Palese P, Wilson PC, Ravetch JV, 2016. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. The Journal of clinical investigation 126, 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLillo DJ, Tan GS, Palese P, Ravetch JV, 2014. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nature medicine 20, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle TM, Hashem AM, Li C, Van Domselaar G, Larocque L, Wang J, Smith D, Cyr T, Farnsworth A, He R, Hurt AC, Brown EG, Li X, 2013. Universal anti-neuraminidase antibody inhibiting all influenza A subtypes. Antiviral research 100, 567–574. [DOI] [PubMed] [Google Scholar]
- Easterbrook JD, Schwartzman LM, Gao J, Kash JC, Morens DM, Couzens L, Wan H, Eichelberger MC, Taubenberger JK, 2012. Protection against a lethal H5N1 influenza challenge by intranasal immunization with virus-like particles containing 2009 pandemic H1N1 neuraminidase in mice. Virology 432, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries LF, Smith GE, Glenn GM, 2013. A recombinant viruslike particle influenza A (H7N9) vaccine. The New England journal of medicine 369, 2564–2566. [DOI] [PubMed] [Google Scholar]
- Gravel C, Li C, Wang J, Hashem AM, Jaentschke B, Xu KW, Lorbetskie B, Gingras G, Aubin Y, Van Domselaar G, Girard M, He R, Li X, 2010. Qualitative and quantitative analyses of virtually all subtypes of influenza A and B viral neuraminidases using antibodies targeting the universally conserved sequences. Vaccine 28, 5774–5784. [DOI] [PubMed] [Google Scholar]
- Halbherr SJ, Ludersdorfer TH, Ricklin M, Locher S, Berger Rentsch M, Summerfield A, Zimmer G, 2015. Biological and protective properties of immune sera directed to the influenza virus neuraminidase. Journal of virology 89, 1550–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson EC, Charles PD, Hester SS, Thomas B, Trudgian D, Martinez-Alonso M, Fodor E, 2014. Conserved and host-specific features of influenza virion architecture. Nat Commun 5, 4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Fantoni G, Couzens L, Gao J, Plant E, Ye Z, Eichelberger MC, Wan H, 2016. Comparative Efficacy of Monoclonal Antibodies That Bind to Different Epitopes of the 2009 Pandemic H1N1 Influenza Virus Neuraminidase. J Virol 90, 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job ER, Schotsaert M, Ibanez LI, Smet A, Ysenbaert T, Roose K, Dai M, de Haan CAM, Kleanthous H, Vogel TU, Saelens X, 2018a. Antibodies Directed toward Neuraminidase N1 Control Disease in a Mouse Model of Influenza. J Virol 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job ER, Ysenbaert T, Smet A, Christopoulou I, Strugnell T, Oloo EO, Oomen RP, Kleanthous H, Vogel TU, Saelens X, 2018b. Broadened immunity against influenza by vaccination with computationally designed influenza virus N1 neuraminidase constructs. NPJ Vaccines 3, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BE, 1999. Immunization with influenza A virus hemagglutinin and neuraminidase produced in recombinant baculovirus results in a balanced and broadened immune response superior to conventional vaccine. Vaccine 17, 2073–2080. [DOI] [PubMed] [Google Scholar]
- Johansson BE, Kilbourne ED, 1993. Dissociation of influenza virus hemagglutinin and neuraminidase eliminates their intravirionic antigenic competition. J Virol 67, 5721–5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BE, Moran TM, Kilbourne ED, 1987. Antigen-presenting B cells and helper T cells cooperatively mediate intravirionic antigenic competition between influenza A virus surface glycoproteins. Proc Natl Acad Sci U S A 84, 6869–6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BE, Pokorny BA, Tiso VA, 2002. Supplementation of conventional trivalent influenza vaccine with purified viral N1 and N2 neuraminidases induces a balanced immune response without antigenic competition. Vaccine 20, 1670–1674. [DOI] [PubMed] [Google Scholar]
- Khurana S, Wu J, Verma N, Verma S, Raghunandan R, Manischewitz J, King LR, Kpamegan E, Pincus S, Smith G, Glenn G, Golding H, 2011. H5N1 virus-like particle vaccine elicits cross-reactive neutralizing antibodies that preferentially bind to the oligomeric form of influenza virus hemagglutinin in humans. Journal of virology 85, 10945–10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourne ED, Johansson BE, Grajower B, 1990. Independent and disparate evolution in nature of influenza A virus hemagglutinin and neuraminidase glycoproteins. Proc Natl Acad Sci U S A 87, 786–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Kwon YM, Lee YT, Hwang HS, Kim MC, Ko EJ, Wang BZ, Quan FS, Kang SM, 2018a. Virus-like particles presenting flagellin exhibit unique adjuvant effects on eliciting T helper type 1 humoral and cellular immune responses to poor immunogenic influenza virus M2e protein vaccine. Virology 524, 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Kwon YM, Lee YT, Kim MC, Hwang HS, Ko EJ, Lee Y, Choi HJ, Kang SM, 2018b. Virus-Like Particles Are a Superior Platform for Presenting M2e Epitopes to Prime Humoral and Cellular Immunity against Influenza Virus. Vaccines (Basel) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Lee YT, Hwang HS, Kwon YM, Kim MC, Ko EJ, Lee JS, Lee Y, Kang SM, 2015. Virus-like particle vaccine containing the F protein of respiratory syncytial virus confers protection without pulmonary disease by modulating specific subsets of dendritic cells and effector T cells. Journal of virology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MC, Lee YN, Ko EJ, Lee JS, Kwon YM, Hwang HS, Song JM, Song BM, Lee YJ, Choi JG, Kang HM, Quan FS, Compans RW, Kang SM, 2014. Supplementation of influenza split vaccines with conserved M2 ectodomains overcomes strain specificity and provides long-term cross protection. Molecular therapy: the journal of the American Society of Gene Therapy 22, 1364–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Ko EJ, Kim MC, Lee YN, Kim KH, Jung YJ, Kang SM, 2017. Roles of antibodies to influenza A virus hemagglutinin, neuraminidase, and M2e in conferring cross protection. Biochem Biophys Res Commun 493, 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko EJ, Lee Y, Lee YT, Kim YJ, Kim KH, Kang SM, 2018. MPL and CpG combination adjuvants promote homologous and heterosubtypic cross protection of inactivated split influenza virus vaccine. Antiviral Res 156, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko EJ, Lee YT, Kim KH, Lee Y, Jung YJ, Kim MC, Lee YN, Kang T, Kang SM, 2017. Roles of Aluminum Hydroxide and Monophosphoryl Lipid A Adjuvants in Overcoming CD4+ T Cell Deficiency To Induce Isotype-Switched IgG Antibody Responses and Protection by T-Dependent Influenza Vaccine. Journal of immunology 198, 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Fouchier RAM, Eichelberger MC, Webby RJ, Shaw-Saliba K, Wan H, Wilson PC, Compans RW, Skountzou I, Monto AS, 2018. NAction! How Can Neuraminidase-Based Immunity Contribute to Better Influenza Virus Vaccines? MBio 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YN, Lee YT, Kim MC, Gewirtz AT, Kang SM, 2016. A Novel Vaccination Strategy Mediating the Induction of Lung-Resident Memory CD8 T Cells Confers Heterosubtypic Immunity against Future Pandemic Influenza Virus. Journal of immunology 196, 2637–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YN, Lee YT, Kim MC, Hwang HS, Lee JS, Kim KH, Kang SM, 2014. Fc receptor is not required for inducing antibodies but plays a critical role in conferring protection after influenza M2 vaccination. Immunology 143, 300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WC, Lin CY, Tsou YT, Jan JT, Wu SC, 2015. Cross-Reactive Neuraminidase-Inhibiting Antibodies Elicited by Immunization with Recombinant Neuraminidase Proteins of H5N1 and Pandemic H1N1 Influenza A Viruses. Journal of virology 89, 7224–7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Macias C, Ferat-Osorio E, Tenorio-Calvo A, Isibasi A, Talavera J, Arteaga-Ruiz O, Arriaga-Pizano L, Hickman SP, Allende M, Lenhard K, Pincus S, Connolly K, Raghunandan R, Smith G, Glenn G, 2011. Safety and immunogenicity of a virus-like particle pandemic influenza A (H1N1) 2009 vaccine in a blinded, randomized, placebo-controlled trial of adults in Mexico. Vaccine 29, 7826–7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memoli MJ, Shaw PA, Han A, Czajkowski L, Reed S, Athota R, Bristol T, Fargis S, Risos K, Powers JH, Davey RT Jr., Taubenberger JK, 2016. Evaluation of Antihemagglutinin and Antineuraminidase Antibodies as Correlates of Protection in an Influenza A/H1N1 Virus Healthy Human Challenge Model. MBio 7, e00417–00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto AS, Petrie JG, Cross RT, Johnson E, Liu M, Zhong W, Levine M, Katz JM, Ohmit SE, 2015. Antibody to Influenza Virus Neuraminidase: An Independent Correlate of Protection. The Journal of infectious diseases 212, 1191–1199. [DOI] [PubMed] [Google Scholar]
- Mooney AJ, Gabbard JD, Li Z, Dlugolenski DA, Johnson SK, Tripp RA, He B, Tompkins SM, 2017. Vaccination with Recombinant Parainfluenza Virus 5 Expressing Neuraminidase Protects against Homologous and Heterologous Influenza Virus Challenge. J Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullarkey CE, Bailey MJ, Golubeva DA, Tan GS, Nachbagauer R, He W, Novakowski KE, Bowdish DM, Miller MS, Palese P, 2016. Broadly Neutralizing Hemagglutinin Stalk-Specific Antibodies Induce Potent Phagocytosis of Immune Complexes by Neutrophils in an Fc-Dependent Manner. MBio 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BR, Kasel JA, Chanock RM, 1972. Association of serum anti-neuraminidase antibody with resistance to influenza in man. N Engl J Med 286, 1329–1332. [DOI] [PubMed] [Google Scholar]
- Qiu M, Fang F, Chen Y, Wang H, Chen Q, Chang H, Wang F, Wang H, Zhang R, Chen Z, 2006. Protection against avian influenza H9N2 virus challenge by immunization with hemagglutinin- or neuraminidase-expressing DNA in BALB/c mice. Biochem Biophys Res Commun 343, 1124–1131. [DOI] [PubMed] [Google Scholar]
- Quan FS, Compans RW, Nguyen HH, Kang SM, 2008. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J Virol 82, 1350–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Kim MC, Lee BJ, Song JM, Compans RW, Kang SM, 2012. Influenza M1 VLPs containing neuraminidase induce heterosubtypic cross-protection. Virology 430, 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Li ZN, Kim MC, Yang D, Compans RW, Steinhauer DA, Kang SM, 2011. Immunogenicity of low-pH treated whole viral influenza vaccine. Virology 417, 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Vunnava A, Compans RW, Kang SM, 2010. Virus-Like Particle Vaccine Protects against 2009 H1N1 Pandemic Influenza Virus in Mice. PLoS One 5, e9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Nishimura SI, Naito T, Saito M, 2017. Influenza A virus hemagglutinin and neuraminidase act as novel motile machinery. Scientific reports 7, 45043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ, 2007. Cross-Reactive Neuraminidase Antibodies Afford Partial Protection against H5N1 in Mice and Are Present in Unexposed Humans. PLoS Med 4, e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbulte MR, Westgeest KB, Gao J, Xu X, Klimov AI, Russell CA, Burke DF, Smith DJ, Fouchier RA, Eichelberger MC, 2011. Discordant antigenic drift of neuraminidase and hemagglutinin in H1N1 and H3N2 influenza viruses. Proc Natl Acad Sci U S A 108, 20748–20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GE, Sun X, Bai Y, Liu YV, Massare MJ, Pearce MB, Belser JA, Maines TR, Creager HM, Glenn GM, Flyer D, Pushko P, Levine MZ, Tumpey TM, 2017. Neuraminidase-based recombinant virus-like particles protect against lethal avian influenza A(H5N1) virus infection in ferrets. Virology 509, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JM, Kim YC, Barlow PG, Hossain MJ, Park KM, Donis RO, Prausnitz MR, Compans RW, Kang SM, 2010. Improved protection against avian influenza H5N1 virus by a single vaccination with virus-like particles in skin using microneedles. Antiviral Res 88, 244–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JM, Van Rooijen N, Bozja J, Compans RW, Kang SM, 2011. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc Natl Acad Sci U S A 108, 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B, Wurtzer S, Rameix-Welti MA, Dwyer D, van der Werf S, Naffakh N, Clavel F, Labrosse B, 2009. Enhancement of the influenza A hemagglutinin (HA)-mediated cell-cell fusion and virus entry by the viral neuraminidase (NA). PLoS One 4, e8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumpey TM, Renshaw M, Clements JD, Katz JM, 2001. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol 75, 5141–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hoecke S, Ehrhardt K, Kolpe A, El Bakkouri K, Deng L, Grootaert H, Schoonooghe S, Smet A, Bentahir M, Roose K, Schotsaert M, Schepens B, Callewaert N, Nimmerjahn F, Staeheli P, Hengel H, Saelens X, 2017. Hierarchical and Redundant Roles of Activating FcgammaRs in Protection against Influenza Disease by M2e-Specific IgG1 and IgG2a Antibodies. J Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz L, Kays SK, Zimmer G, von Messling V, 2018. Neuraminidase-Inhibiting Antibody Titers Correlate with Protection from Heterologous Influenza Virus Strains of the Same Neuraminidase Subtype. J Virol 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Gao J, Xu K, Chen H, Couzens LK, Rivers KH, Easterbrook JD, Yang K, Zhong L, Rajabi M, Ye J, Sultana I, Wan XF, Liu X, Perez DR, Taubenberger JK, Eichelberger MC, 2013. Molecular basis for broad neuraminidase immunity: conserved epitopes in seasonal and pandemic H1N1 as well as H5N1 influenza viruses. J Virol 87, 9290–9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlbold TJ, Chromikova V, Tan GS, Meade P, Amanat F, Comella P, Hirsh A, Krammer F, 2016. Hemagglutinin Stalk- and Neuraminidase-Specific Monoclonal Antibodies Protect against Lethal H10N8 Influenza Virus Infection in Mice. J Virol 90, 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlbold TJ, Krammer F, 2014. In the shadow of hemagglutinin: a growing interest in influenza viral neuraminidase and its role as a vaccine antigen. Viruses 6, 2465–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlbold TJ, Nachbagauer R, Xu H, Tan GS, Hirsh A, Brokstad KA, Cox RJ, Palese P, Krammer F, 2015. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. MBio 6, e02556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlbold TJ, Podolsky KA, Chromikova V, Kirkpatrick E, Falconieri V, Meade P, Amanat F, Tan J, tenOever BR, Tan GS, Subramaniam S, Palese P, Krammer F, 2017. Broadly protective murine monoclonal antibodies against influenza B virus target highly conserved neuraminidase epitopes. Nat Microbiol 2, 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.