Abstract
Aims
The aim of this study was to systematically review the literature for evidence of the effect of a high-fat diet (HFD) on the onset or progression of osteoarthritis (OA) in mice.
Methods
A literature search was performed in PubMed, Embase, Web of Science, and Scopus to find all studies on mice investigating the effects of HFD or Western-type diet on OA when compared with a control diet (CD). The primary outcome was the determination of cartilage loss and alteration. Secondary outcomes regarding local and systemic levels of proteins involved in inflammatory processes or cartilage metabolism were also examined when reported.
Results
In total, 14 publications met our inclusion criteria and were included in our review. Our meta-analysis showed that, when measured by the modified Mankin Histological-Histochemical Grading System, there was a significantly higher rate of OA in mice fed a HFD than in mice on a CD (standardized mean difference (SMD) 1.27, 95% confidence interval (CI) 0.63 to 1.91). Using the Osteoarthritis Research Society International (OARSI) score, there was a trend towards HFD causing OA (SMD 0.78, 95% CI -0.04 to 1.61). In terms of OA progression, a HFD consistently worsened the progression of surgically induced OA when compared with a CD. Finally, numerous inflammatory cytokines such as tumour necrosis factor alpha (TNF-α), interleukin (IL)-1β, and leptin, among others, were found to be altered by a HFD.
Conclusion
A HFD seems to induce or exacerbate the progression of OA in mice. The metabolic changes and systemic inflammation brought about by a HFD appear to be key players in the onset and progression of OA.
Cite this article: Bone Joint Res 2019;8:582–592.
Keywords: Osteoarthritis, Cartilage degeneration, High-fat diet, Metabolic syndrome, Obesity
Article focus
Is a high-fat diet (HFD) related to the onset of osteoarthritis (OA)?
Is a HFD related to the progression of OA?
Which inflammatory factors are seen in the HFD mouse model that might be linked to the development and progression of OA?
Key messages
A HFD accelerates the progression of surgically induced OA.
A HFD proved capable of inducing OA in the mouse model; however, this effect was manifested inconsistently among the papers included in this systematic review. Honing this model to make it more reproducible could help elucidate mechanisms that link diets high in fat to OA.
Inflammatory cytokines (tumour necrosis factor alpha (TNF-α), interleukin (IL)-1β, IL-13, IL-6, IL-8), adipokine (leptin), and cartilage metabolism proteins (vascular endothelial growth factor-a (VEGF-a), transforming growth factor-beta 1 (TGF-β1), and matrix metallopeptidase (MMP)-13) increased in mice fed a HFD compared with mice fed a control diet.
Strengths and limitations
This review compiles the latest information regarding the effect of a HFD on OA in the mouse model.
There was substantial heterogeneity in the OA scoring methods used in the included studies.
Introduction
Despite the prevalence of osteoarthritis (OA), especially in the adult and older adult populations,1 the aetiology of this disease remains incompletely understood, although a complex, multifactorial origin is now well established. However, the mechanisms by which the mechanical, genetic, environmental, and metabolic risk factors interact in inducing progressive degradation of the involved joints is still open to question. Obesity is an increasingly frequent condition that is widely accepted as a risk factor in both the incidence and progression of OA.1,2 A twofold higher risk of knee arthroplasty has been reported in obese patients when compared with normal-weight subjects.3 Beyond the obvious mechanical overload that can cause excessive stress on large weight-bearing joints, inducing articular degradation, recent research has revealed an enlarging role of metabolic factors independent of mechanical loading.4-6 Furthermore, several epidemiological studies found that, aside from knee and hip joints, obese people are also frequently affected by OA in non-weight-bearing joints such as those of the hands.7-10 Some experimental studies have also shown that morbidly obese mice do not develop OA when fed standard chow.11 These findings suggest that factors other than adiposity or body weight, such as dietary content or systemic and local levels of inflammation, may contribute to OA.6,12
In addition, many epidemiological and experimental studies in humans and animals have shown an association of OA with cardiovascular-related conditions, such as hypertension, hypercholesterolaemia, abdominal obesity, dyslipidaemia, and type 2 diabetes.13
Supported by numerous in vivo and in vitro studies, a common biochemical and pathophysiological milieu shared by OA and metabolic syndrome has hence been hypothesized, in which several biochemical, cellular, and molecular mediators, such as glucose, fatty acids, hormones, growth factors, transcription factors, nitric oxide, cytokines, and oxygen radicals may also participate in the chondrocyte damage.14
Among the aforementioned metabolic conditions, much attention has been dedicated to the altered lipid metabolism. Recent studies found that high-density lipoprotein cholesterol (HDL-C) is lower in the serum of OA patients when compared with individuals with no signs of OA, suggesting a potential relationship between HDL and OA aetiopathogenesis.15-17
Furthermore, in the literature there is an emerging body of evidence suggesting a potential role of a high-fat diet (HFD) or western-type diet (WTD) in the occurrence of OA. However, although the first observations that mice develop early-onset OA when fed a HFD date from more than 50 years ago,18 the link remains ambiguous.
To our knowledge, no prior systematic review has examined the evidence for the relationship between a HFD and the development of OA in mice. Thus, we aim to systematically evaluate the effects of a HFD on the onset and progression of OA in mice.
Materials and Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines19 for reporting, and used the PICOS (population, intervention, comparator, outcome, setting) criteria reported in Supplementary Table i to perform this systematic review.
Eligibility criteria
We included studies matching the following criteria: 1) randomized controlled trials or controlled observational studies; 2) enrolled mice strains to study OA; 3) compared a HFD, defined as at least 30% of caloric intake from fat, to a control diet (CD) consisting of a low-fat diet or standard chow; 4) measured cartilage damage or alteration; 5) published in English, Spanish or Italian; and 6) full-text articles. We excluded studies with cointerventions (e.g. high-fat–high-sucrose diet), studies that included transgenic mice only, and studies without a control group.
Search strategy
In order to identify all primary studies, we searched the following electronic databases: PubMed, Embase, Web of Science, and Scopus. The search strategy was developed using the keywords “high-fat diet”, “osteoarthritis”, and “mice”, and was similar across all databases. The complete search strategies are shown in Supplementary Table ii. We also examined the reference list of potentially eligible studies. The last search was run in April 2018.
Outcomes
Cartilage damage and alterations and progression of OA were the primary outcomes, measured in mice knee joints primarily according to the Osteoarthritis Research Society International (OARSI) score20 or the modified Mankin Histological-Histochemical Grading System (HHGS).21 In order to evaluate the link between a HFD and the onset and progression of OA, as a secondary outcome, data reported on cytokines and growth factors related to OA were extracted and an analysis of the principle cytokines was conducted.
In detail, interleukin (IL)-1β, IL-6, IL-8, IL-13, interferon gamma (IFNγ), and tumour necrosis factor alpha (TNF-α), representing the principally involved inflammatory cytokines in OA, were evaluated. IL-4, IL-10, and IL-1 receptor antagonist (IL-1Ra), representing the anti-inflammatory cytokines able to counteract the inflammatory status of cartilage, were also considered. Transforming growth factor-beta 1 (TGF-β1), vascular endothelial growth factor-a (VEGF-a), collagen X (ColX), SRY-Box Transcription Factor 9 (SOX9), matrix metallopeptidase 13 (MMP)-13, and TIMP metallopeptidase inhibitor 3 (TIMP-3) were selected for their direct involvement in cartilage metabolism. Finally, the three main adipokines involved in obesity and inflammatory processes – leptin, adiponectin, and resistin – were analyzed.22
Study selection and data collection
Two researchers (VS, RCA) independently reviewed title and abstract for inclusion according to eligibility criteria. Disagreements were resolved by consensus.
Two authors (RCA, VP) independently extracted the data in an extraction form. The following information were recorded: 1) characteristics of animals (age, sex, strain); 2) characteristics of the studies (study design); 3) data about cartilage damage or alteration (OARSI score or modified Mankin HHGS score); and 4) indications of systemic and local inflammation or changes in cartilage metabolism. When the data were not directly available, we contacted the authors to request it.
Risk of bias assessment
Two reviewers (VP, RCA) independently evaluated the studies’ quality using the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) risk of bias tool for animal studies.23 This tool contains nine domains (sequence generation, baseline mice characteristics, allocation concealment, housing, blinding researchers, random outcome assessment, blinding outcome assessors, incomplete outcome data, and selective outcome reporting) relating to five types of bias: selection, performance, detection, attrition, and reporting bias. Furthermore, it contains another domain to state any important concerns bias not covered by the other domains. Thus, we evaluated other possible methodological flow considering the four items from the Animals in Research: Reporting In Vivo Experiments (ARRIVE) guidelines for reporting animal experiments.24 The quality items were as follows: 1) ethical statement (ethical review permissions and national or institutional guidelines for the care and use of animals); 2) experimental procedures description (precise details of all procedures performed); 3) experimental animals’ details (including number of mice, strain, and age); and 4) financial conflicts of interest. Every domain was classified as having either “low”, “high”, or “unclear” risk of bias based on the information reported in each study.
Statistical analysis
To quantify the pooled effects of the primary outcome, we used standardized mean difference (SMD) with a 95% confidence interval (CI) according to the two principal scales that were used to measure the onset of OA. As there was a great degree of heterogeneity regarding the methods used for scoring among the papers included in the meta-analysis, data were meta-analyzed using the random effect models.
If the meta-analysis was not appropriate due to a high statistical heterogeneity (I2 > 80%)25 or an insufficient number of studies, we planned to present only a narrative summary of studies. All analyses were conducted with Review Manager (RevMan5) software version 5.3 (RevMan; Cochrane, London, UK).
Results
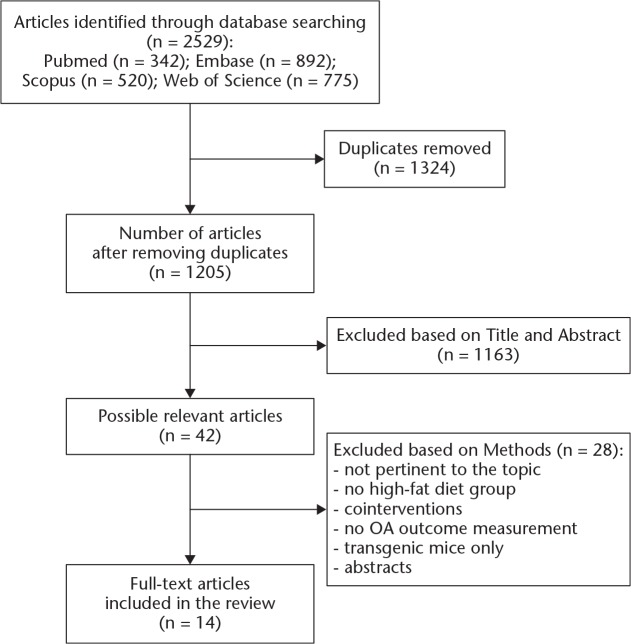
The search strategy identified a total of 2529 articles (Figure 1), of which 1324 were duplicates. Of the 1205 papers remaining after removing duplicates, 1163 papers were excluded on the basis of title and abstract. The remaining 42 publications were retrieved for full-text evaluation, of which 14 papers met the inclusion criteria. Of the 14 selected papers, only three aimed to evaluate the primary research question of determining whether a HFD correlates to the onset or progression of OA.12,26,27 In total, four of the 14 papers reported multiple intervention groups that met our inclusion criteria, allowing our review to assess data from 24 unique intervention groups (compared with 20 control groups).4,6,12,26 Details of the 14 included papers are outlined in Tables I to III.4-6,12,17,26-34
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram of the study selection process for this systematic review. OA, osteoarthritis.
Table I.
Study characteristics; all studies were conducted in male C57BL/6 mice, except for Griffin et al,34 who used female C57BL/6 mice, and Wu et al,6 who did not specify the strain of mice used
| Study | Experimental model | % of kcal from fat in HFD groups, % (n) | % of kcal from fat in CD groups, % (n) | Starting age, wks | Duration of diet, wks |
|---|---|---|---|---|---|
| Barboza et al27 (2017) | Diet | 60 (13) | 10.0 (12) | 6 | 46 |
| Datta et al4 (2017) | Diet | 60.3 (10) | LFD: 10.2 (10); chow: 18.0 (5) | 9 | 18* |
| Diet + surgery | 60.3 (10) | 10.2 (10) | 9 | 18* | |
| Kozijn et al28 (2018) | Diet (study 1) | 45.0 (12) | 16.0 (12) | 12 | 0, 6, 12, 24 |
| Diet (study 2) | 45.0 (15) | 16.0 (10) | 12 | 24 | |
| Diet (study 3) | 45.0 (10 to 15) | 10.0 (10 to 15) | 12 | 52 | |
| Aibibula et al29 (2016) | Diet | 56.7 (10) | 12.2 (10) | 7 | 12 |
| Asou et al5 (2016) | Diet | 56.7 (5†) | 12.2 (5†) | 7 | 12 |
| Kc et al30 (2015) | Diet | 35.0 (5) | 18.0 (5) | 6 to 8 | 10 |
| Wu et al6 (2015) | Diet (SFA) ± surgery | 60.0 (12‡) | 10.0 (11‡) | 4 | 20 |
| Diet (⍵-6 FA) ± surgery | 60.0 (14‡) | 10.0 (11‡) | 4 | 20 | |
| Diet (⍵-3 FA) ± surgery | 60.0 (12‡) | 10.0 (11‡) | 4 | 20 | |
| Iwata et al31 (2013) | Diet | 56.7 (5) | 12.2 (5) | 7 | 12 |
| O’Conor et al32 (2013) | Diet | 60.0 (9) | 10.0 (9) | 10 | 22 |
| Triantaphyllidou et al17 (2013) | Diet | 42.0 (6) | 10.6 (6) | 10 to 12 | 24 |
| Griffin et al33 (2012) | Diet | 60.0 (5) | 13.5 (5) | 12 | 12 |
| Louer et al12 (2012) | Diet | 60.0 (6) | 13.5 (8) | 4 | 20 to 23 |
| Diet + surgery | 60.0 (6) | 13.5 (9) | 4 | 20 | |
| Mooney et al26 (2011) | Diet ± surgery | 60.0 (4 to 9‡) | 10.0 (4 to 9‡) | 5 | 28 |
| Griffin et al34 (2010) | Diet | 45.0 (9) | 10.0 (9) | 9 | 45 |
The intervention diets (HFD and LFD) were provided for only 18 weeks; mice were then returned to normal chow. Evaluation timepoints were at 18, 36, and 52 weeks for the non-surgery groups and at 18, 28, and 38 weeks for the surgically induced OA groups
Missing samples were explained in personal communication with authors
In each animal, one hind limb was operated and one hind limb was left intact, providing a non-operated control in the same mouse
HFD, high-fat diet; CD, control diet; LFD, low-fat diet; SFA, saturated fatty acids; ⍵-3 FA, omega-3 fatty acids; ⍵-6 FA, omega-6 fatty acids; OA, osteoarthritis
Table III.
Summary of results on the progression of OA via HFD interventions in mice with surgically induced OA; all studies were conducted in male C57BL/6 mice, except for Griffin et al,34 who used female C57BL/6 mice, and Wu et al,6 who did not specify the strain of mice used
| Study | OA scoring system | Significantly higher OA score in HFD versus CD? | Osteophyte summary | Local inflammation | Systemic inflammation (serum/visceral fat) |
|---|---|---|---|---|---|
| Datta et al4 (2017) | OARSI | Yes (p < 0.05) | N/A | ↑ Leptin in the articular cartilage | No increase in leptin |
| Wu et al6 (2015) | Modified Mankin | SFA: Yes (p < 0.05); ⍵-6 FA: Yes (p < 0.05); ⍵-3 FA: No | SFA and ω-6 FA mice trended towards greater osteophyte severity than control and ω-3 FA mice | Increased frequency of macrophages in the synovium of ω-6 FA mice | SFA and ω-6 FA mice had elevated leptin concentrations versus control and ω-3 FA mice, while ω-3 FA mice had higher adiponectin and PGE2 levels and lower resistin levels. Leptin and resistin had a positive association with OA |
| Louer et al12 (2012) | Modified Mankin | Yes (p < 0.05) | N/A | Increased synovial inflammation | ↑ IL-6, KC, IL-12p70; ↓ adiponectin. No significant effects of IL-1β, IL-10, or IFNγ |
| Mooney et al26 (2011) | OARSI | Yes (p < 0.01) | Osteophytes present in HFD | N/A | N/A |
OA, osteoarthritis; HFD, high-fat diet; CD, control diet; OARSI, Osteoarthritis Research Society International; N/A, not applicable in article nor in supplement; SFA, saturated fatty acids; ⍵-6 FA, omega-6 fatty acids; ⍵-3 FA, omega-3 fatty acids; PGE2, prostaglandin E2; IL, interleukin; KC, keratinocyte-derived chemokine; IFNγ, interferon gamma
Characteristics of mice and diets
Overall, 12 of the 14 papers were conducted in male C57BL/6 mice.4,5,12,17,26-33 One paper used female C57BL/6 mice,34 and one paper did not specify the strain nor sex of the mice.6 At baseline, the age of the mice varied from four to 12 weeks with a mean age of eight weeks. The number of mice per diet group varied from five to 15; however, two studies32,34 did not report the number of mice per diet group. The maximum duration of the diets varied between ten and 52 weeks, and the mean percentage of calories derived from fat was 54.1% (35% to 60.3%) for the HFD and 11.6% (10% to 18%) for CD (Table I). More extensive diet content details can be found in Supplementary Table iii.
Risk of bias assessment
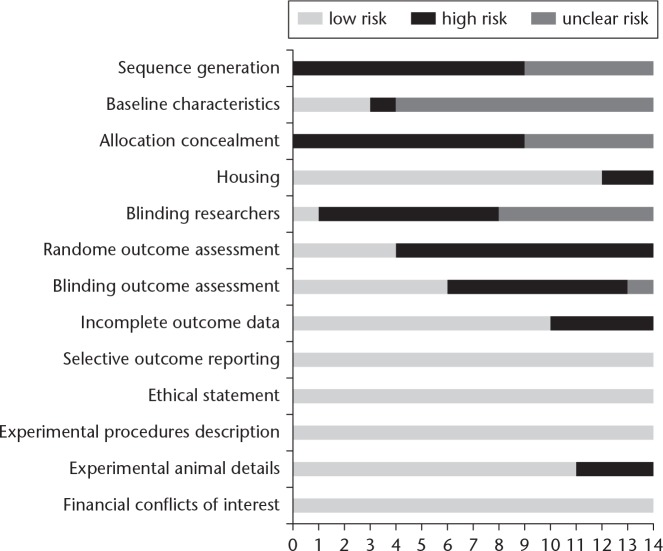
The majority of studies were judged at low risk of bias (Figure 2). Four studies were randomized and were judged at low risk of detection bias, but none described the methods used to generate and conceal the allocation sequence. They were therefore classified as having an unclear risk of selection bias. Only three studies reported the baseline characteristics of mice included. In total, 12 of the 14 studies reported measures used to house the animals within the animal room. Researchers were blinded in one study, and were unclear in six studies; seven studies were at high risk of performance bias. However, outcome assessors were blinded in six studies, so they were judged as low risk of detection bias. Ten studies describe the details of each main outcome, so they were at low risk of attrition bias. A total of 11 studies reported animal details, two did not report the number of mice included, and one neglected to mention the strain and number of mice used. All studies described details of experimental procedures, reported financial conflict of interest and ethical statement, and were at low risk of reporting bias.
Fig. 2.
Risk of bias assessment.
The effects of HFD on the onset of osteoarthritis
Overall, seven of the 14 papers used OARSI to measure the onset of OA induced by the HFD;4,5,17,26,28-30 six used a modified Mankin grading system;6,12,27,32-34 and one used a cartilage destruction score and terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay (Table II).31
Table II.
Summary of results of interventions that induced OA via HFD alone; all studies were conducted in male C57BL/6 mice, except for Griffin et al,34 who used female C57BL/6 mice, and Wu et al,6 who did not specify the strain of mice used
| Study | OA scoring system | Significantly higher OA score in HFD versus CD? | Osteophyte summary |
|---|---|---|---|
| Barboza et al27 (2017) | Modified Mankin | Yes (p < 0.01) at 20 wks and 46 wks | Significantly higher osteophyte score at 20 wks but not at 46 wks |
| Datta et al4 (2017) | OARSI | Yes (p < 0.05) | N/A |
| Kozijn et al28 (2018), study 1 | OARSI | No | Osteophyte score significantly greater at 12 wks and 24 wks |
| Kozijn et al28 (2018), study 2 | OARSI | No | No significant difference in HFD and CD |
| Kozijn et al28 (2018), study 3 | OARSI | No | No significant difference in HFD and CD |
| Aibibula et al29 (2016) | OARSI | Yes (p < 0.05) | Osteophyte area was significantly greater in HFD (p < 0.05) |
| Asou et al5 (2016) | OARSI | Yes (p < 0.05) | HFD was associated with enhanced osteophyte formation from 8 wks |
| Kc et al30 (2015) | OARSI | No | Osteophytes were present primarily in the operated joints. SFA and ω-6 FA mice trended towards greater osteophyte severity |
| Wu et al6 (2015) | Modified Mankin | No | Osteophytes were present primarily in the operated joints. Osteophyte score correlated positively with OA |
| Iwata et al31 (2013) | Cartilage destruction score | Yes (p < 0.05) | Osteophyte volume was significantly increased in HFD mice beginning at 8 wks (p < 0.05) |
| O'Conor et al32 (2013) | Modified Mankin | No | N/A |
| Triantaphyllidou et al17 (2013) | OARSI | No | N/A |
| Griffin et al33 (2012) | Modified Mankin | Yes (p < 0.05) | N/A |
| Louer et al12 (2012) | Modified Mankin | No | N/A |
| Mooney et al26 (2011) | OARSI | No | In HFD groups, osteophytes were present in both surgery and sham conditions |
| Griffin et al34 (2010) | Modified Mankin | Non-significant trend (p = 0.10) | N/A |
OA, osteoarthritis; HFD, high-fat diet; CD, control diet; OARSI, Osteoarthritis Research Society International; SFA, saturated fatty acids; ⍵-6 FA, omega-6 fatty acids; N/A, not applicable in article nor in supplement
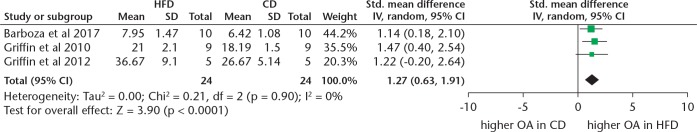
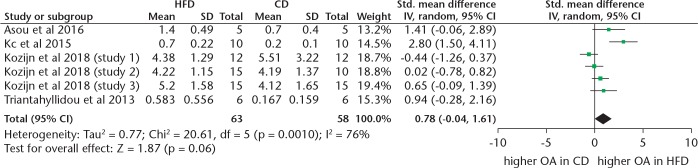
Our meta-analysis showed that when measured via the modified Mankin score, mice fed a HFD are more likely to develop OA when compared with controls (SMD 1.27, 95% CI 0.63 to 1.91; p < 0.001, Z-test) (Figure 3). No heterogeneity was found in the modified Mankin data (I2 = 0%). However, when OA was measured via the OARSI score, only a trend towards HFD mice developing more OA than controls was found (SMD 0.78, 95% CI -0.04 to 1.61; p = 0.06, Z test) (Figure 4). Substantial heterogeneity was also found (I2 = 76%).
Fig. 3.
Forest plot of studies reporting data from modified Mankin scores. IV, inverse variance; HFD, high-fat diet; CD, control diet; CI, confidence interval; OA, osteoarthritis.
Fig. 4.
Forest plot of studies reporting data from Osteoarthritis Research Society International (OARSI) scores. IV, inverse variance; HFD, high-fat diet; CD, control diet; CI, confidence interval; OA, osteoarthritis.
All studies measured OA at the medial femoral condyles and medial tibial plateau; some studies also measured OA of the lateral tibia and femur. One study specified that the measurements were made in the coronal plane;12 two other studies specified using the sagittal plane for its sections.31,34
In six studies, HFD groups showed significantly higher OA scores when compared with controls (p < 0.05).4,5,27,29,31,33 Osteoarthritis was detectable after eight,31 12,5,29,31,33 18,4 and 4627 weeks of the intervention diet.
The effects of HFD on the progression of OA
Two of the four studies investigating the effect of a HFD on the progression of OA via a surgically induced model used OARSI to measure the progression of OA4,26 and two studies used modified Mankin (Table III).6,12 Datta et al4 and Wu et al6 surgically induced OA by destabilizing the medial meniscus; Louer et al12 made moderate articular fractures of the left tibial plateau; and Mooney et al26 induced OA by transecting the medial collateral ligament and excising a segment of the medial meniscus of the knee. In five of six intervention pairs, a HFD combined with surgically induced OA showed significantly higher OA scores when compared with CD surgically induced OA groups. Of note, the sole HFD intervention that did not find a significant correlation between a HFD and the progression of OA was part of a paper that included three diverse interventions with varying fat profiles. Unlike two interventions by Wu et al6 containing saturated fatty acids (SFAs) and omega-6 fatty acids (ω-6 FAs), which did show significant progression of OA when compared with controls, the HFD intervention with omega-3 fatty acids (ω-3 FAs) – a deliberate attempt to mitigate the effects of the HFD – showed no difference when compared with the controls. Osteoarthritis progression was measured at 18,4 20,6,12 and 2826 weeks of diet.
Secondary outcomes
Overall, 11 of the 14 papers included in this review reported data for cytokines in C57BL/6 (wild type) mice. All of the data can be found in Table IV. Below, the results are described for the most common cytokines and growth factors related to OA. Results are divided by the source; proteins from the serum and adipose tissue reflect levels of systemic inflammation, whereas proteins or genes analyzed from articular knee tissues reflect levels of local inflammation. The proteins were quantified via various methods, including gene expression, enzyme-linked immunosorbent assay (ELISA), and immunohistochemistry (IHC). All increases and decreases mentioned below were a result of the HFD intervention and were reported to have been statistically significant (p < 0.05) when compared with the CD.
Table IV.
Table reporting the response of genes or proteins involved in OA for all studies in which data were reported, the source tissue for the analysis, and the detection methods
| Gene/protein | Source tissue | Method | Increase in HFD versus CD (p < 0.05) | No statistically significant difference between HFD and CD | Decrease in HFD versus CD (p < 0.05) |
|---|---|---|---|---|---|
| Proinflammatory cytokines | |||||
| IL-1α | Serum | ELISA | N/A | 2 studies33,34 | N/A |
| IL-1β | Knee joint | GE | 2 studies27,29 | N/A | N/A |
| IL-1β | Serum | ELISA | N/A | 2 studies12,33 | N/A |
| IL-6 | Knee joint;5 visceral adipose6 | GE | 1 study5 | 1 study6 | N/A |
| IL-6 | Serum | ELISA | 1 study12 | N/A | N/A |
| IL-8 or KC | Serum | ELISA | N/A | 1 study12 | N/A |
| IL-13 | Knee joint | GE | 1 study27 | N/A | N/A |
| IFNγ | Serum | ELISA | N/A | 3 studies12,33,34 | N/A |
| TNF-α | Knee joint | GE | 3 studies5,27,31 | 1 study6 | N/A |
| TNF-α | Serum | ELISA | Excluded | N/A | N/A |
| NLRP3 | Knee joint | GE | 1 study29 | N/A | N/A |
| CCL2 or MCP-1 | Knee joint;27 visceral adipose6 | GE | 1 study27 | 1 study6 | N/A |
| CCL3 or MIP1α | Knee joint | GE | 1 study27 | 1 study34 | N/A |
| CCL7 or MCP-3 | Knee joint | GE | 1 study27 | N/A | N/A |
| CD11c | Visceral adipose | GE | N/A | 1 study6 | N/A |
| Casp1 | Knee joint | GE | 1 study27 | N/A | N/A |
| CCR1 or CD191 | Knee joint | GE | N/A | 1 study27 | N/A |
| CCR3 | Knee joint | GE | N/A | 1 study27 | N/A |
| CCR5 | Knee joint | GE | N/A | 1 study27 | N/A |
| MIG or CXCL9 | Serum | ELISA | 1 study33 | N/A | N/A |
| IL-12 | Serum | ELISA | N/A | 1 study34 | N/A |
| IL-12p70 | Serum | ELISA | 1 study12 | N/A | N/A |
| F4/80 | Visceral adipose | GE | N/A | 1 study6 | N/A |
| Anti-inflammatory cytokines | |||||
| IL-4 | Knee joint | GE | N/A | 1 study27 | N/A |
| IL-4 | Serum | ELISA | N/A | N/A | 1 study33 |
| IL-10 | Knee joint | GE | N/A | 1 study27 | N/A |
| IL-10 or CXCL10 | Serum | ELISA | N/A | 2 studies12,33 | N/A |
| IL-1Ra | Serum | ELISA | 1 study33 | 1 study33 | N/A |
| Proteins directly involved with cartilage metabolism | |||||
| TGF-β1 | Knee joint | GE | 1 study5 | N/A | N/A |
| TGF-β1 | Serum | ELISA | N/A | 1 study6 | N/A |
| VEGF-a | Knee joint | GE | 2 studies5,31 | N/A | N/A |
| ColX | Knee articular cartilage | IHC | N/A | 1 study30 | N/A |
| SOX9 | Knee articular cartilage | IHC | N/A | 1 study30 | N/A |
| MMP13 | Knee articular cartilage | IHC | 1 study29 | N/A | N/A |
| MMP13 | Knee articular cartilage | IHC | N/A | 1 study30 | N/A |
| MMP3 | Knee joint | GE | 1 study27 | N/A | N/A |
| TIMP-3 | Knee joint | GE | 1 study27 | N/A | N/A |
| TIMP-3 | Knee articular cartilage | IHC | N/A | 1 study30 | N/A |
| Lipocalin2 | Knee joint | GE | 1 study31 | N/A | N/A |
| Nampt | Knee joint | GE | 2 studies5,31 | N/A | N/A |
| PGE2 | Serum | ELISA | N/A | N/A | N/A |
| p-PKCδ | Knee articular cartilage | IHC | N/A | 1 study30 | N/A |
| p-ERK1/2 | Knee articular cartilage | IHC | N/A | 1 study30 | N/A |
| RUNX2 | Knee articular cartilage | IHC | N/A | 1 study30 | N/A |
| bFGF | Serum | ELISA | N/A | 1 study33 | N/A |
| ADAMTS5 | Knee articular cartilage | IHC | N/A | 1 study30 | N/A |
| iNOS | Knee joint | GE | 1 study29 | N/A | N/A |
| p-NF-κB | Knee articular cartilage | IHC | N/A | 1 study30 | N/A |
| Adipokines involved in obesity and inflammatory processes | |||||
| Leptin | Knee joint | GE | 2 studies5,31 | N/A | N/A |
| Leptin | Serum | ELISA | 3 studies4,6,33 | 1 study34 | N/A |
| Adiponectin | Knee joint | GE | N/A | 1 study12 | N/A |
| Adiponectin | Serum | ELISA | 1 study33 | N/A | 1 study12 |
| Resistin | Serum | ELISA | N/A | Undetected12 | N/A |
| Chemerin | Knee joint | GE | 1 study31 | N/A | N/A |
| Insulin | Serum | ELISA | 2 studies4,6 | N/A | N/A |
OA, osteoarthritis; HFD, high-fat diet; CD, control diet; IL, interleukin; ELISA, enzyme-linked immunosorbent assay; N/A, not applicable; GE, gene expression; KC, keratinocyte-derived chemokine; IFNγ, interferon gamma; TNF, tumor necrosis factor; NRLP3, NOD-like receptor family pyrin domain-containing 3; CCL, chemokine ligand; MCP, monocyte-chemotactic protein; MIP, macrophage inflammatory protein; CD11c, integrin alpha X; Casp1, caspase-1; CCR, C-C chemokine receptor; MIG, monokine induced by gamma; CXCL, chemokine (C-X-C motif) ligand; F4/80, adhesion G protein-coupled receptor E1; IL-1RA, interleukin-1 receptor antagonist; TGF-β1, transforming growth factor beta 1; VEGF-a, vascular endothelial growth factor-a; IHC, immunohistochemistry; ColX, collagen X; SOX9, SRY-Box Transcription Factor 9; MMP, matrix metallopeptidase; TIMP-3, TIMP metallopeptidase inhibitor 3; Nampt, nicotinamide phosphoribosyltransferase; PGE2, prostaglandin E2; p-PKCδ, phospho-protein kinase C delta; p-ERK1/2, phospho-extracellular signal-regulated protein kinases 1 and 2; RUNX2, Runt-related transcription factor 2; bFGF, basic fibroblast growth factor; ADAMTS5, a disintegrin-like and metalloproteinase with thrombospondin-1 motifs, member 5; iNOS, inducible nitric oxide synthase; p-NF-κB; phospho-nuclear factor kappa-light-chain-enhancer of activated B cells
Proteins originating from serum or adipose tissue
Regarding the principal proinflammatory cytokines, ELISA analysis of serum samples showed that a HFD, in comparison with a CD, showed no statistically significant difference in proinflammatory IL-1β in two studies.12,33 For IL-6, an increase was noted in one study.12 Neither IL-812 nor IFNγ12,33,34 showed any statistically significant differences. Finally, TNF-α was below the detectable limit and was excluded from the authors’ analysis.12
Regarding the most prevalently studied anti-inflammatory cytokines, when serum samples were analyzed by ELISA, IL-4 decreased,33 while IL-10 showed no differences.12,33 Last, IL1-Ra showed an increase in one study33 and no differences in another study.34
For the predominant proteins involved in cartilage metabolism, only TGF-β1 was measured via serum sample, and it showed no significant differences.6
As for the ELISA serum sample quantification of the main adipokines involved in obesity and inflammatory processes, leptin increased in three studies,4,6,34 and adiponectin increased in one study34 but decreased in another study.12 Finally, resistin was undetected.12
Gene or protein analysis from knee articular tissues
Gene expression of the infrapatellar fat pad (IPFP) showed that a HFD, compared with a CD, induced an increase of the proinflammatory gene IL-1β in two studies.27,29
IL-6 increased in one study5 and showed no statistically significant differences in another study.6 IL-8 also showed no statistically significant differences.12 Last, TNF-α increased in three studies,5,27,31 and in one study no statistically significant differences were found.6
When analyzed via gene expression of the local fat pads, the anti-inflammatory cytokines IL-4 and IL-10 showed no statistical differences between HFD mice and CD mice; however, IL-13 increased in one study.27
Regarding the main proteins involved in the metabolic processes of knee cartilage, when comparing a HFD with a CD, TGF-β1 increased in one study,5 the angiogenic VEGF-a increased in two studies,5,31 MMP-13 increased in one study,29 and MMP-3 and TIMP-3 increased in one study.27 Collagen X, SOX9, and TIMP-3 were analyzed via IHC staining of knee cartilage; none showed any statistically significant differences between a HFD and CD.30 In MMP-13, IHC showed no difference in one study30 and an increase in another study.29
For the adipokines detected in the local articular tissue, leptin increased in two studies,5,31 and adiponectin showed no statistically significant differences in one study.31
Change in other indicators of inflammation
A HFD was associated with a significant macrophage infiltration6,27,31. Additionally, an HFD seems to induce chondrocyte apoptosis, which is known to be involved in OA development,4,5,31 and it is linked to proteoglycan depletion.5,31
Discussion
HFD and OA outcome
To our knowledge, this is the first systematic review to examine the evidence for the relationship between a HFD or WTD and OA in mice. The pooled effects of the data showed that when compared with a CD, HFD-fed mice showed significantly higher OA scores when measured by modified Mankin (SMD 1.27; p < 0.001, Z test) and a trend towards higher scores when measured by OARSI (SMD 0.78; p = 0.06, Z test). Overall, 11 of the 20 interventions (six using HFD alone and five using HFD plus surgery) included in this review found that a HFD induced the onset, or significantly worsened the progression of, OA when compared with a CD. Eight papers did not find the HFD intervention alone to be sufficient in giving rise to significantly greater OA scores when compared with CD mice. Finally, one paper did not find an ω-3 FA-rich HFD capable of worsening the progression of OA.6 This study by Wu et al6 underlines an important concept that not all fats are made equal. Indeed, their results proved that the inclusion of 5.2% of ω-3 FA within a very HFD (60%) reduced the deleterious effects of the HFD. Meanwhile, their SFA and ω-6 FA interventions caused significantly greater advancement of OA when compared with CD groups.
Although the results were discordant among the intervention groups that studied the effects of a HFD alone, the results of the six progression intervention pairs were nearly unanimous in finding a significant degree of OA progression in the HFD groups (with the justifiable exception of ω-3 FA-rich HFD from the study by Wu et al,6 as mentioned above). Regarding the inconsistency of the results among the interventions that employed HFD alone, it is impossible to pinpoint a singular rationale behind why this discrepancy exists; instead, we find it likely that many variables are implicated.
There was a great disparity in high-fat content among the studies. This systematic review included both a HFD, which we defined as a dietary HF content of 30% kcal to 40% kcal, and a very HFD (> 40% kcal from fat). The lowest percentage HFD used 35% kcal from fat, and the highest percentage HFD (used in seven of the 14 papers) contained 60% kcal from fat (Supplementary Table iii). The mean high-fat content used in the studies that did find a significant correlation between a HFD alone and OA was higher than in studies that did not find a significant correlation (58.4% kcal fat (sd 1.7; n = 6) vs 50.9% kcal fat (sd 9.57; n = 8), respectively). Among the control groups, the high-fat content was similar.
Although the present review is focused on the influence of fat, it would be remiss to neglect to consider the protein and carbohydrate content of the diets. Five of the interventions maintained protein levels at 20% kcal for the HFD and CD.26-28,32 Therefore, to compensate for the higher fat levels in the HFDs, the levels of carbohydrates were necessarily much higher in the CD than in the HFD, and in some cases, the carbohydrates were composed of very high levels of sucrose. A recent paper that sought to evaluate the independent effects of dietary fat and sucrose content on chondrocyte metabolism and OA pathology35 hypothesized that studies using a CD comprised of exceedingly high levels of sucrose could inadvertently induce OA. If this were true, the so-called ‘control group’ would actually become a competing experimental group, obscuring the results and making it difficult to distinguish a significant difference in OA outcomes between the two groups. The study by Donovan et al35 showed that low-fat diets higher in sucrose caused significant changes in serum metabolites and joint pathology without showing differences in body mass. These results indicate that the formulation of the CD must be carefully considered. We therefore examined the dietary content of both the experimental and control groups (Supplementary Table iii); however, we were not able to identify any OA trends related to variations in dietary content. Nonetheless, it is recommended that future experiments in mice models carefully select the fat and carbohydrate content and composition for both the experimental and CDs.
Another variable that must also be considered is the inherent biological variation of the mice. Although all of the mice in this review were seemingly homogeneous, non-transgenic C57BL/6 mice, and all were male except in a study by Griffin et al,34 considerable biological variation could have existed among the mice. In a study by Choi et al,36 in male C57BL/6 mice on a HFD, the authors identified two very distinct subgroups of mice; one group of mice was described as being obesity-resistant, while the other was described as being obesity-prone.36 The obesity-resistant mice were distinguishable from the obesity-prone mice by divergent transcriptomes, phenotypes, and metabolic processes. Furthermore, in one study of female C57BL/6 mice on a HFD, a clear stratification emerged of some mice gaining more weight than others in response to the imposed HFD, allowing the researchers to divide the mice into high gainer and low gainer phenotypes by weight. Bivariate and multivariate analysis of the high and low gainer mice demonstrated that variation in susceptibility to diet-induced obesity determined the progression of OA.34
These results may help explain the tendency towards very high SDs of the OA scores reported in the papers included in this review. Moreover, they may explain why some authors were not able to find evidence of significantly greater OA in HFD groups when compared with CD groups.
Aside from the variation in the intervention methods and the mice, several different methods were used to quantify OA. The OARSI and modified Mankin grading systems were often measured in different ways, summing the scores in some instances, and calculating the mean scores in other papers. It is not clear which method is the most effective. However, a more recent method used by Iwata et al,31 called the TUNEL assay, proved to be a very effective way of identifying the earliest signs of OA.31 After just eight weeks, the authors were able to see a measurable difference in the presence of OA in the HFD groups compared with the CD groups. They reported significantly greater TUNEL-positive cells in the articular cartilage, indicating higher rates of chondrocyte apoptosis (i.e. cartilage degradation). In addition, they observed an abundance of proliferating cell nuclear antigen (PCNA) positive cells at the site of osteophyte formation.31 We find the methods of Iwata et al31 to be the most sensitive in identifying the first signs of OA, and would therefore recommend that future studies consider implementing their methods in the assessment of OA.
Another recommendation for future studies would be to use sufficiently large sample sizes. Not a single paper reported calculating the sample size, and several papers may not have been able to show a significant difference between experimental and control group outcomes as a result of being underpowered. According to the calculation by Donovan et al,35 as well as our own calculation, the sample size should be at least ten mice per group. Furthermore, several of the papers reported unexpected mice death or damaged, unusable histological samples; consequently, ten mice should really be the absolute minimum starting sample size per intervention group.
H2HFD and early signs of OA via inflammation
It is well recognized that proinflammatory cytokines play an important role in the development of OA and that a HFD induces an increase in the organism’s inflammatory status.28 Therefore, the main cytokines involved in articular cartilage inflammation and destruction were analyzed as well as the adipokines known to be correlated with diet. Several papers did not find any statistically significant difference in the proteins that were measured. However, as expected, an increase of several proinflammatory cytokines was observed in the HFD mice when compared with CD mice. IL-1β, IL-6, and TNF-α increased at the knee joint level, and IL-8 increased at the systemic level. Interestingly, at a systemic level the anti-inflammatory cytokine IL-1Ra, the direct antagonist of IL-1β, also increased in HFD mice. On the other hand, the data were contradictory with regard to two cytokines that share the IL-4 type 1 receptor and would therefore have been expected to respond similarly. Instead, IL-4 decreased33 while IL-13 increased.27 This discrepancy may have resulted from variations in the methodology used in the two papers (for example, the different tissue origin, serum for IL-4 and epididymal fat tissue for IL-13).
A HFD induced an increase in the proinflammatory status. An increase was found at the knee joint level of the catabolic cartilage markers MMP-13 and MMP-3, complimented by an increase in its inhibitor TIMP-3. Moreover, an increase in markers of hypertrophic cartilage, such as TGF-β1 and ColX, was observed. In accordance with OA progression, VEGF-a, which is associated with catabolic processes in chondrocytes, also increased.
Finally, the adipocytokine evaluation demonstrated a clear increase of leptin, which is directly associated with obesity and OA,37 at both local and systemic levels, as protein released or gene expressed. Conversely, the anti-inflammatory adiponectin, inversely correlated with OA, showed conflicting data at the systemic level.
Future studies are needed to investigate the impact of inflammation on joint disease, to define the molecular pathways mediating the inflammation in OA, and to discover new potential therapeutic targets. Although this review was conducted in mice, Panchal and Brown38 concluded that a HFD mimics most of the symptoms of metabolic syndrome in humans. Therefore, these findings may provide new insights into the pathogenesis of human OA.
In conclusion, many of the studies included in this review carried out experiments designed to reveal the mechanisms of the presumed correlation between a HFD and OA in the mice model, regardless of whether or not the correlation existed. In the surgically induced model of OA, a HFD unequivocally exacerbated the progression of OA when compared with a CD. However, this systematic review and meta-analysis revealed a more ambiguous result with regard to the effects of a HFD on the induction of OA in mice. The lack of reproducibility in the use of a high-fat dietary intervention to produce OA in mice merits consideration. If the HFD model in mice can be established to reliably induce OA, it would facilitate further exploration of the identification of the key inflammatory mediators involved, potentially leading to the discovery of dietary and pharmacological preventions and treatments for this debilitating disease.
Acknowledgments
The authors would like to thank Massimo Del Fabbro for his support with regards to the technicalities of reporting in systematic reviews.
Footnotes
Author contributions: V. Sansone: Designed and performed the systematic review, Wrote the manuscript.
R. C. Applefield: Designed and performed the systematic review, Analyzed the data, Wrote the manuscript.
P. De Luca: Analyzed the data.
V. Pecoraro: Designed and performed the systematic review, Analyzed the data, Wrote the manuscript.
S. Gianola: Designed and performed the systematic review, Analyzed the data, Wrote the manuscript.
W. Pascale: Wrote the manuscript.
V. Pascale: Wrote the manuscript.
Supplementary Material
Tables showing: the population, intervention, comparator, outcome, and setting (PICOS) criteria used in performing the systematic review; the electronic databases and search strategies used to identify all primary studies; and dietary information for high-fat diets and control diets for the mice models.
Funding statement
The publication of this paper was supported by the Italian Ministry of Health “Ricerca Corrente”.
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Ostir GV, Carlson JE, Black SA, et al. Disability in older adults. 1: Prevalence, causes, and consequences. Behav Med 1999;24:147-156. [DOI] [PubMed] [Google Scholar]
- 2. Lementowski PW, Zelicof SB. Obesity and osteoarthritis. Am J Orthop (Belle Mead NJ) 2008;37:148-151. [PubMed] [Google Scholar]
- 3. Munugoda IP, Wills K, Cicuttini F, et al. The association between ambulatory activity, body composition and hip or knee joint replacement due to osteoarthritis: a prospective cohort study. Osteoarthritis Cartilage 2018;26:671-679. [DOI] [PubMed] [Google Scholar]
- 4. Datta P, Zhang Y, Parousis A, et al. High-fat diet-induced acceleration of osteoarthritis is associated with a distinct and sustained plasma metabolite signature. Sci Rep 2017;7:8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Asou Y, Iwata M, Ochi H, et al. Pleiotropic functions of high fat diet in the etiology of osteoarthritis. PLoS One 2016;11:e0162794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu CL, Jain D, McNeill JN, et al. Dietary fatty acid content regulates wound repair and the pathogenesis of osteoarthritis following joint injury. Ann Rheum Dis 2015;74:2076-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carman WJ, Sowers M, Hawthorne VM, Weissfeld LA. Obesity as a risk factor for osteoarthritis of the hand and wrist: a prospective study. Am J Epidemiol 1994;139:119-129. [DOI] [PubMed] [Google Scholar]
- 8. Felson DT, Chaisson CE. Understanding the relationship between body weight and osteoarthritis. Baillieres Clin Rheumatol 1997;11:671-681. [DOI] [PubMed] [Google Scholar]
- 9. Dahaghin S, Bierma-Zeinstra SM, Reijman M, et al. Does hand osteoarthritis predict future hip or knee osteoarthritis? Arthritis Rheum 2005;52:3520-3527. [DOI] [PubMed] [Google Scholar]
- 10. Dahaghin S, Bierma-Zeinstra SM, Koes BW, Hazes JM, Pols HA. Do metabolic factors add to the effect of overweight on hand osteoarthritis? The Rotterdam Study. Ann Rheum Dis 2007;66:916-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Griffin TM, Huebner JL, Kraus VB, Guilak F. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum 2009;60:2935-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Louer CR, Furman BD, Huebner JL, et al. Diet-induced obesity significantly increases the severity of posttraumatic arthritis in mice. Arthritis Rheum 2012;64:3220-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rahman MM, Kopec JA, Anis AH, Cibere J, Goldsmith CH. Risk of cardiovascular disease in patients with osteoarthritis: a prospective longitudinal study. Arthritis Care Res (Hoboken) 2013;65:1951-1958. [DOI] [PubMed] [Google Scholar]
- 14. Velasquez MT, Katz JD. Osteoarthritis: another component of metabolic syndrome? Metab Syndr Relat Disord 2010;8:295-305. [DOI] [PubMed] [Google Scholar]
- 15. Karvonen-Gutierrez CA, Sowers MR, Heeringa SG. Sex dimorphism in the association of cardiometabolic characteristics and osteophytes-defined radiographic knee osteoarthritis among obese and non-obese adults: NHANES III. Osteoarthritis Cartilage 2012;20:614-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soran N, Altindag O, Cakir H, et al. Assessment of paraoxonase activities in patients with knee osteoarthritis. Redox Rep 2008;13:194-198. [DOI] [PubMed] [Google Scholar]
- 17. Triantaphyllidou IE, Kalyvioti E, Karavia E, et al. Perturbations in the HDL metabolic pathway predispose to the development of osteoarthritis in mice following long-term exposure to western-type diet. Osteoarthritis Cartilage 2013;21:322-330. [DOI] [PubMed] [Google Scholar]
- 18. Silberberg M, Silberberg R. Effects of a high fat diet on the joints of aging mice. AMA Arch Pathol 1950;50:828-846. [PubMed] [Google Scholar]
- 19. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage 2010;18(Suppl 3):S17-S23. [DOI] [PubMed] [Google Scholar]
- 21. Cai A, Hutchison E, Hudson J, et al. Metabolic enrichment of omega-3 polyunsaturated fatty acids does not reduce the onset of idiopathic knee osteoarthritis in mice. Osteoarthritis Cartilage 2014;22:1301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nguyen LT, Sharma AR, Chakraborty C, et al. Review of prospects of biological fluid biomarkers in osteoarthritis. Int J Mol Sci 2017;18:E601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hooijmans CR, Rovers MM, de Vries RB, et al. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010;8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mooney RA, Sampson ER, Lerea J, Rosier RN, Zuscik MJ. High-fat diet accelerates progression of osteoarthritis after meniscal/ligamentous injury. Arthritis Res Ther 2011;13:R198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barboza E, Hudson J, Chang WP, et al. Profibrotic infrapatellar fat pad remodeling without m1 macrophage polarization precedes knee osteoarthritis in mice with diet-induced obesity. Arthritis Rheumatol 2017;69:1221-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kozijn AE, Gierman LM, van der Ham F, et al. Variable cartilage degradation in mice with diet-induced metabolic dysfunction: food for thought. Osteoarthritis Cartilage 2018;26:95-107. [DOI] [PubMed] [Google Scholar]
- 29. Aibibula Z, Ailixiding M, Iwata M, et al. Xanthine oxidoreductase activation is implicated in the onset of metabolic arthritis. Biochem Biophys Res Commun 2016;472:26-32. [DOI] [PubMed] [Google Scholar]
- 30. Kc R, Li X, Forsyth CB, et al. Osteoarthritis-like pathologic changes in the knee joint induced by environmental disruption of circadian rhythms is potentiated by a high-fat diet. Sci Rep 2015;5:16896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iwata M, Ochi H, Hara Y, et al. Initial responses of articular tissues in a murine high-fat diet-induced osteoarthritis model: pivotal role of the IPFP as a cytokine fountain. PLoS One 2013;8:e60706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O’Conor CJ, Griffin TM, Liedtke W, Guilak F. Increased susceptibility of Trpv4-deficient mice to obesity and obesity-induced osteoarthritis with very high-fat diet. Ann Rheum Dis 2013;72:300-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Griffin TM, Huebner JL, Kraus VB, Yan Z, Guilak F. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: effects of short-term exercise. Arthritis Rheum 2012;64:443-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Griffin TM, Fermor B, Huebner JL, et al. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis Res Ther 2010;12:R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Donovan EL, Lopes EBP, Batushansky A, Kinter M, Griffin TM. Independent effects of dietary fat and sucrose content on chondrocyte metabolism and osteoarthritis pathology in mice. Dis Model Mech 2018;11:dmm034827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Choi JY, McGregor RA, Kwon EY, et al. The metabolic response to a high-fat diet reveals obesity-prone and -resistant phenotypes in mice with distinct mRNA-seq transcriptome profiles. Int J Obes 2016;40:1452-1460. [DOI] [PubMed] [Google Scholar]
- 37. MacDonald IJ, Liu SC, Huang CC, et al. Associations between adipokines in arthritic disease and implications for obesity. Int J Mol Sci 2019;20:E1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Panchal SK, Brown L. Rodent models for metabolic syndrome research. J Biomed Biotechnol 2011;2011:351982. [DOI] [PMC free article] [PubMed] [Google Scholar]