Abstract
Background: The formation and rupture of aneurysms is a reversible process involving the destruction and repair of smooth muscle cells, and the proliferation of vascular smooth muscle cells (VSMC) and inflammation play an important role. In our study, we investigated whether Interleukin-10 (IL-10) treatment delays and prevents the development of aneurysms, and the molecular mechanism whereby IL-10 could inhibit proliferation of VSMC by inhibiting inflammatory responses in abdominal aortic aneurysms. Methods: Models of rabbit abdominal aortic aneurysm (AAA) were established by elastin pressurization and perfusion, and recombinant IL-10 was used as a drug to intervene in treatment of the AAA model by rabbit ear vein injection. 1 week, 2 weeks and 4 weeks after establishing the AAA model, color Doppler ultrasound and H&E staining was used to observe the development of AAA. Western blotting and RT-qPCR were used to detect the gene expression of PCNA, OPN and α-SMA, Th1/Th2 cytokines were detected by RT-qPCR, Nf-kB and MCP-1 protein was analyzed by immunochemistry. Activation of Macrophage was analyzed by immunofluorescence. Results: Compared with the model group without any intervention, after treatment with IL-10, a decreased cell number was recorded and number of layers of smooth muscle cells in rabbit abdominal aortic aneurysms were significantly reduced, as was elastin breakage and smooth muscle cell degradation. The gene expression of PCNA and OPN, the mRNA expression of IFN-γ and TNF-α, and the protein expression of NF-kB and MCP-1 were elevated (P < 0.05), but α-SMA, IFN-γ, TNF-α, IL-4 and IL-13 were decreased (P < 0.05) in abdominal aortic aneurysm. The M2/M1 macrophage ratio increased significantly. Conclusion: With treatment by IL-10, the development of rabbit abdominal aortic aneurysm was delayed. The molecular mechanism may have been that IL-10 treatment inhibits inflammation in aneurysm tissue by promoting the activation of M2 macrophages and altering Th1/Th2 cytokine production.Tthe inhibited inflammatory response promoted the proliferation and phenotypic transformation of VSMC.
Keywords: Interleukin-10, abdominal aortic aneurysm, inflammation, vascular smooth muscle cells
Introduction
Aneurysm damages the normal structure of the aortic wall, especially the the elastic fiber layer of the arterial wall, which is caused by congenital or acquired diseases. Itleads to the gradual expansion or enlargement of the aorta. In general, an aneurysm is defined as an artery with a diameter greater than 50% of normal [1]. Abdominal aortic aneurysm (AAA) is a high-risk disease that refers to the abnormal expansion or limited expansion of a segment of the abdominal aorta that ultimately renders the wall unable to withstand the impact of blood flow. In China, the incidence of AAA is 2%, and has a yearly rising trend. Because of the limitations in the understanding of the molecular mechanisms underlying the development and rupture of AAA, we have no effective drug to delay or cure AAA without surgical treatment [2,3].
The formation and rupture of aneurysm is a progressive process from the inner to middle and outer layers, and the degeneration of smooth muscle cells plays an important role during the formation of the entire aneurysm. In other words, the formation and rupture of aneurysms is a reversible process with the destruction and repair of smooth muscle cells, and the rupture of aneurysm is the result of damage caused by blood flow shear stress. Inflammation is then much greater than the repair of the smooth muscle cell layer [4,5]. Vascular smooth muscle cells (VSMC) are a highly specialized cell with the functions of constricting blood vessels, regulating vascular tone, and maintaining blood pressure [6]. VSMC play an important role during the formation and rupture of AAA. When the rate of apoptosis of VSMC exceeds proliferation, the number of smooth muscle layers gradually decreases. When the number of smooth muscle layers decreases to a certain extent, the wall of the aneurysm ruptures, endangering life [7,8]. Therefore, the proliferation of VSMC is very important.
Until now, the pathogenesis of AAA was not clear. However, it is certain that AAA is a chronic inflammatory disease, and many inflammatory related factors are closely related to the incidence of abdominal aortic aneurysm [9,10]. IL-10 is a cytokine that suppresses and terminates inflammatory responses, and it can prevent the secretion of inflammatory cytokines and regulate the differentiation and proliferation of T cells, B cells, and NK cells [11]. IL-10 had been widely used to treat inflammatory bowel diseases such as chronic ulcerative colitis [12,13] and Crohn’s disease [14,15].
In our study, we used IL-10 to treat a rabbit AAA model, and investigated whether Interleukin-10 (IL-10) treatment delays and prevents the development of aneurysms. The molecular mechanism is that IL-10 can inhibit proliferation of VSMC by inhibiting inflammatory responses in abdominal aortic aneurysms. Collectively, our study offers a new therapeutic strategy for treating or preventing AAA by inhibiting inflammation.
Materials and methods
Experimental animals and groups
There were 70 New Zealand male rabbits (SCXK2006-0006) with weight (2.5 ± 0.20) kg chosen as research subjects. After adaptive feeding (room temperature 20-24°C, half day and night, air humidity 60%) for one week, they were randomly divided into A, B, C, D, E, F, and H groups. Group A was the sham group, and groups B, C, D, E, F, and H were AAA model groups. In groups E, F, and H, 5 μl of recombinant IL-10 (CYT-500, Amyjet Scientific, China) (dissolved in 5 mol/L NaP) was injected into rabbit ear vein immediately after the model was completed, and the same dose was injected weekly until rabbits were killed. Groups A, B, C, and D were injected with 5 mol/L NaP solvent. One week after the completion of the rabbit AAA model, the rabbits in B and E groups were sacrificed. 2 weeks later we killed C and F groups, and D and H groups rabbits were sacrificed after 4 weeks. Group A was defined as the Sham group, groups B, C, and D were defined as the Model group, and groups E, F, and H were defined as the IL-10 treatment group. The study was approved by Ethics committee of Yantai Yuhuangding Hospital.
Rabbit abdominal aorta aneurysm model
The rabbit AAA model was established by elastase pressurization and perfusion, and the details of methods was as follows: all experimental animals fasted and were denied water for 4 h before surgery. 10% chloral hydrate (Sinopharm Chemical Reagent Co.,Ltd, China) 3.5 ml/kg was used for intraperitoneal injection, strictly aseptically. To free the femoral artery: The common femoral artery was isolated and dissociated 0.5-1 cm, and was threaded on the proximal end and distal end, respectively. Laparotomy: We did a midline abdominal incision and exposes the abdominal aorta. We selected a 1.5-2.0 abdominal aortic segment with less branches and dissociated the abdominal aorta. We placed rubber filters on the proximal and distal ends. Femoral artery was punctured and we placed the catheter (4F Forgaty, Beijing Life Oasis Technology Co., Ltd., China): the catheter was gently delivered to the aortic segment of the patient through the femoral artery incision, and the proximal end of the abdominal aorta was blocked with an arterial clamp and a rubber filter. The distal end was blocked by a rubber filter, forming a closed chamber that communicates with the catheter, and the blood in the chamber was drained and flushed with physiological saline 3 times. According to the group design, 10 μl saline (Sham group) or 100 U/mL porcine trypsin solution (Model group and IL-10 treatment group) (45124, Sigma-aldrich, USA) was injected into the arterial cavity through the low pressure of the double-lumen catheter. After 7 minutes of perfusion, the solution was withdrawn and the heparin saline (H32021978, Suzhou Xinbao Pharmaceutical Co., Ltd., China) was injected from the auxiliary tube. The tube was aspirated from the main tube and washed repeatedly in the lumen of the artery. We removed the filter, removed the microcatheter and closed the abdomen.
Color doppler ultrasound
Experimental animals were subjected to color ultrasonography of the abdominal aorta before surgery, 7 days after surgery, and before sacrifice (ClearVue 580, Philips, Holland). The longitudinal and transverse sections were examined separately, and the maximum lumen diameter was measured by the ultrasound instrument post-processing software. We tookthe average value as the diameter of the experimental animal tumor; abdominal aortic expansion rate = (AAA diameter-average abdominal aortic diameter)/average abdominal aortic diameter; abdominal aortic mean diameter = (upper end of tumor + lower end of tumor)/2.
H&E staining
The experimental rabbits were anesthetized, and then we cut the abdomen to get the perfusion arterial segments and collected aneurysm specimens. A portion of the specimen was dehydrated, transparently and paraffin-embedded, and the specimen was cut into 5 μm slices by a serial slicer (SLEE, CUT4062, Germany). Paraffin sections were routinely dewaxed and washed with staining kits for H&E staining (G1120, Solarbio, USA) as follows: staining with hematoxylin for 5 to 10 minutes, washing for 3 to 5 minutes, placing in 0.5 to 1% hydrochloric acid in alcoholic solution for several seconds, washing for 3 to 5 minutes, coloring with alkaline aqueous solution for 1 min, washing with water for about 10 min. We then examined the extent of staining under the microscope. After suitable coloring, it was washed 1 or 2 times, stained with 0.5% Yihong alcohol for 1 to 2 minutes, dehydrated with alcohol, transparent with xylene, and neutral resin was used for sealing. Optical microscope (LSM800, Olympus, Japan) was used to observe the pathologic changes after H&E staining of each slice.
Immunofluorescence
We removed the prepared tissue section and placed it at room temperature for 20 minutes. After drying, we marked the area of immunohistochemical tissue, washed 3 times for 5 minutes at room temperature with PBS (135 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8 mM K2HPO4, adjusted pH to 7.2 with HCl and NaOH), and then sealed with goat serum (Hyclone, MA, USA) at 37°C for 1 hour. The goat serum was removed and it wa incubated with anti-Galectin 3 (ab76245, 1:1000) and anti-iNOS antibody (ab178847, 1:100) at 4°C overnight. The PBS was rinsed 3 times for 5 minutes at room temperature in the dark. The goat anti-rabbit secondary antibody was incubated at 37°C for 1 h and washed 3 times with PBS for 5 mins at room temperature. The slides were sealed and observed by fluorescence microscopy (Carl Zeiss, Weimar, German). Unless otherwise specified, all antibodies were purchased from Abcam.
Western blotting
The experimental rabbits were anesthetized, and then we cut the abdomen to get the perfusion arterial segments and collected aneurysm specimens. AAA tissue and RIPA lysate (1 mM PSMF was added in advance, Beyotime, China) were added into a glass homogenizer (Shanghai Broadcom Chemical Technology Co., Ltd., China), and then placed on ice for 10 min, centrifuged at 12000 rpm for 10 min to collect the supernatant, and we measured total tissue protein.
20% SDS buffer solution was added to the supernatant until the final concentration of SDS was 1%, and the mixture was boiled at 100°C for 5 min. The concentration of protein was determined by BCA protein concentration assay kit (Beyotime, China). 75 μg of total protein was loaded into each lane and separated by 15% SDS-PAGE (90 V, 0.5 h; 120 V, 1 h) and transferred (400 mA, 1.5 h) to polyvinylidene fluoride film (Amersham Biosciences, UK), fixed with methanol for 1 min, washed three times (5 min each) with TBST (10 mM Tris-HCl, 150 mM NaCl, 0.1% tween 20, pH = 7.6) and blocked with blocking buffer (5% skim milk in TBST) for 1 h. anti-PCNA (ab29, 1:10000), anti-OPN (ab8448, 1:200) or anti-α-SMA [EPR5368] (ab124964, 1:1000), or anti-GAPDH antibody (ab8227, 1:2000) or anti-NF-kB p65 (ab16502, 1:2000) or anti-MCP-1 (ab124964, 1:1000) diluted by 5% skim milk was added and incubated at 4°C overnight. We washed with TBST for 3 times (10 min each). Sheep anti-rabbit secondary antibody (ab205718, 1:2000) or Sheep anti-mouse secondary antibody (ab6808, 1:2000) was added and incubated for 1 h at room temperature, and then ECL solution was added for detection. The expression of the target protein was analyzed by Image J software, and the relative expression level of target protein was characterized by the gray value of the target protein/gray value of β-actin or lamin A protein. Unless otherwise specified, all antibodies were purchased from Abcam.
Real-time fluorescence quantitative PCR
The experimental rabbits were anesthetized, and then we cut the abdomen to get the perfusion arterial segments and collected aneurysm specimens. AAA tissue and Trizol (Takara Biomedical Technology (Beijing) Co., Ltd., China) were added into a glass homogenizer. Total RNA was extracted by the Trizol method. DEPC (Takara, China) water was added to dissolve the RNA according to RNA concentration, and RNA concentration was measured by NanoDrop 2000 Ultramicro Spectrophotometer (Thermo Scientific, USA). The extracted RNA was reverse transcribed into cDNA by using PrimeScript™RT Master Mix reverse transcription kit (RR036B, Takara, China). PCR parameters set: 37°C/60 minutes, 85°C/5 seconds.
RT-qPCR: 20 μl RT-qPCR system was prepared according to the SYBR Green qPCR Master Mix kit instructions (638320, TakaRa, China) and amplified using ABI 7500 fluorescence quantitative PCR instrument (Applied Biosystems, USAA). PCR parameters set: 95°C/30 s, [90°C/5 s, 65°C/30 s] -40 cycles. β-actin was used as an internal control. The relative expression level of the target gene was calculated by 2-ΔtΔt method. RT-qPCR specific primer sequences are shown in Table 1.
Table 1.
RT-qPR specific primer sequences
| Gene | Primer sequences (5’-3’) |
|---|---|
| PCNA | F: GTTTAAGTATCTTCACGTTC |
| R: CGGGTTCCTTCGACATTCCT | |
| OPN | F: CGTGATTTGCTTTTGTCTCTTG |
| R: TGGGGTACACAGTGACTTCATC | |
| α-SMA | F: TGCTGTCCTTCTGGCTGAACATT |
| R: GAATCAGACAGCTTTCGGAAGTTGG | |
| TNF-α | F: ACCCTCACACTCACAAACCA |
| R: ATAGCAAATCGGCTGACGGT | |
| IFN-γ | F: CCTCATGGCTGTTTCTGGCT |
| R: TCCTTTTGCCAGTTCCTCCA | |
| IL-13 | F: TGCCATCTACAGGACCCAGA |
| R: CTCATTAGAAGGGGCCGTGG | |
| IL-4 | F: GGATGACAACTAGCTGGGGG |
| R: ATGGATGTGCCAAACGTCCT | |
| GAPDH | F: GATGAACCTAAGCTGGGACCC |
| R: TGTGAACGGATTTGGCCGTA |
Statistical analysis
The data were analyzed by SPSS 20.0 software package for statistical analysis and the data were expressed as (mean ± standard deviation). Student’s t test, χ2 test and Fisher’s exact test were used to compare the differences between groups. P < 0.05 indicated a significant difference.
Results
IL-10 delays the development of elastase-induced AAA
Color Doppler ultrasonography was used to detect and measure abdominal aortic diameter. The results showed that the maximum diameter of rabbit abdominal aorta increases with time after elastin perfusion induction, but IL-10 treatment could significantly reduce the expansion of the abdominal aorta (Figure 1A, 1B). The results of H&E staining showed that the rabbit abdominal aortic aneurysm wall (Figure 1D) was damaged and gradually disappeared compared with the normal blood vessel wall (Figure 1C). The elastic fibers ruptured, the smooth muscle cells atrophied, and the smooth muscle number and number of layers decreased. During the development of the aneurysm, the destruction of the elastic layer of the tumor wall increased. The number of smooth muscle cells became less. The wall of the blood vessel became thinner. At the 4th week, the aneurysm wall was the thinnest (Figure 1D). This may be related to extracellular matrix degradation, proteolytic enzymes, smooth muscle inflammation, apoptosis, and the like. After IL-10 treatment, atrophy of smooth muscle cells, breakage of elastic fibers, decrease in the number and number of smooth muscle cells, and thinning of the wall of the aneurysm still occurred. However, compared with the Model group, the degree of decrease in the number and number of layers of smooth muscle cells was significantly reduced, and the extent of breakage of elastic fibers and degradation of smooth muscle cells was also reduced.
Figure 1.
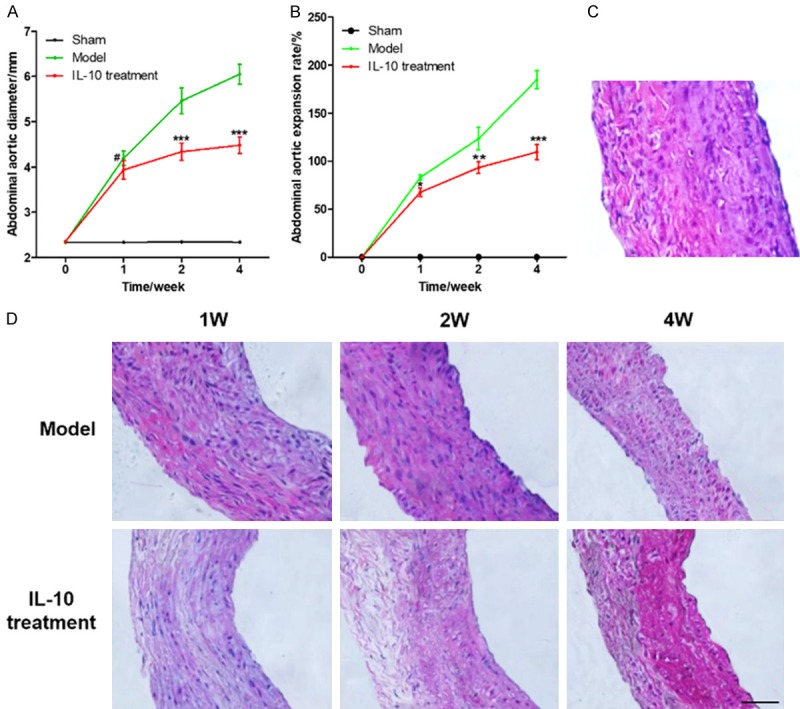
IL-10 delays the development of abdominal aortic aneurysm. A. Diameter of rabbit abdominal aortic aneurysm in different groups which were measured by color Doppler ultrasonography; B. The expansion rate of rabbit AAA model in different groups; C. The H&E staining of normal rabbit abdominal aorta (400×); D. H&E staining of AAA model and IL-10 treatment group (400×); Compared with the model group, # was P > 0.05, * was P < 0.05, ** was P < 0.01, and *** was P < 0.001.
IL-10 promotes proliferation of VSMC in rabbit AAA
α-SMA is a marker of cells derived from smooth muscle, but the content of cells in different mature states is different. In the less-differentiated VSMC, the content is minimal, but opposite in differentiated and matured VSMC. The expression of α-SMA would decrease and cell replication activity would also decrease during the development of organisms, which was a sign of cell contraction [16,17]. It was found that the degree of OPN expression was positively correlated with the degree of arteriosclerosis, but negatively correlated with the expression of α-SMA. In the study, OPN was commonly used as a marker of VSMC’s synthetic phenotype, i.e., the higher the expression of VSMC in aneurysm, the stronger the proliferation activity of VSMC [18,19]. Proliferating cell nuclear antigen (PCNA) existed only in normal proliferating cells, played an important role in the initiation of cell proliferation, and was a good indicator of cell proliferation status [20].
In order to study the effect of IL-10 treatment on the proliferation of rabbit AAA wall smooth muscle cells, the expression of PCNA, α-SMA, and OPN genes was detected by western blotting and RT-qPCR in this study. The results showed that the PCNA and OPN genes in rabbit AAA that were treated with IL-10 were significantly higher than those in the untreated Model group (P < 0.05), while α-SMA was significantly decreased (Figure 2), This suggests that IL-10 in the treatment of abdominal aortic aneurysms in rabbits can promote a proliferation of smooth muscle cells in the abdominal aortic aneurysm and promote the conversion of VSMCs from contractile to synthetic.
Figure 2.
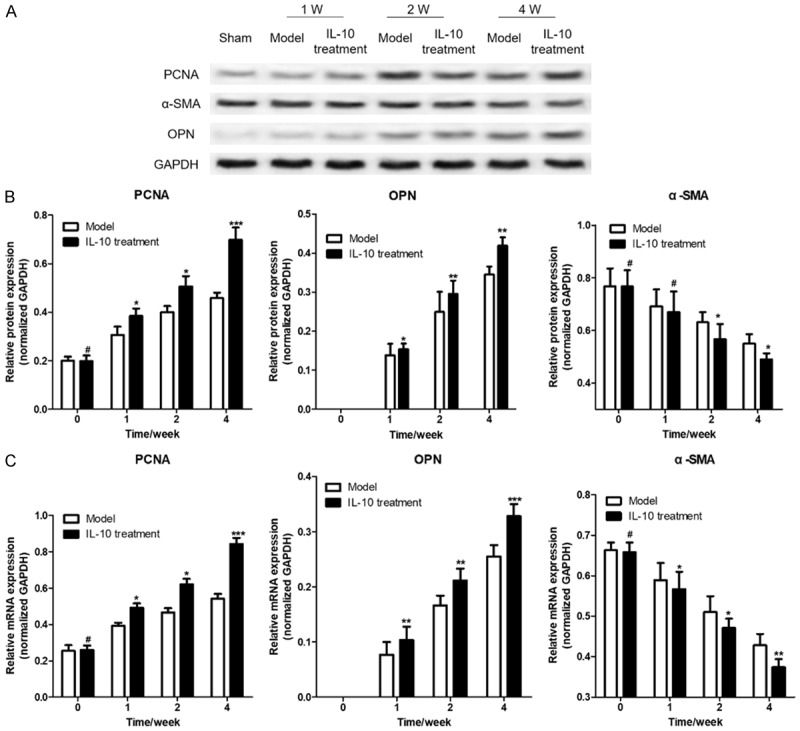
IL-10 promotes the expression of PCNA, α-SMA and OPN gene in rabbit AAA. A. Western blotting was used to detect the protein expression of PCNA, α-SMA and OPN in different rabbit AAA; B. The statistics of PCNA, α-SMA and OPN protein expression gray value; C. RT-qPCR was used to detect the protein expression of PCNA, α-SMA and OPN in different rabbit AAA. Compared with the model group, # was P > 0.05, * was P < 0.05, ** was P < 0.01, and *** was P < 0.001.
IL-10 inhibits inflammation in rabbit AAA
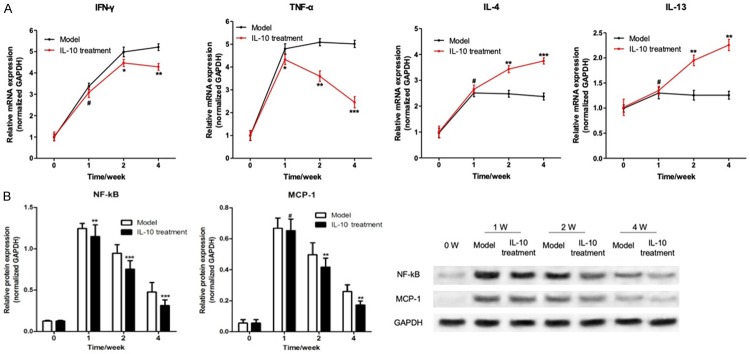
The formation of AAA is closely related to the inflammatory response. At present, AAA is considered to be a chronic inflammatory disease. A variety of inflammatory related factors are closely related to the incidence of AAA. This study found that the expression of IFN-γ and TNF-α mRNA in the tumor tissue of rabbit AAAs that were treated with IL-10 was significantly higher than that of the Model group without any treatment (P < 0.05). The expression of IL-4 and IL-13 was significantly decreased (P < 0.05), as shown in Figure 3A. It intimated that IL-10 could inhibit the expression of inflammatory factors in abdominal active tissue.
Figure 3.
IL-10 inhibits the expression of inflammatory factors in rabbit AAA. A. RT-qPCR was used to detect the mRNA expression of IFN-γ, TNF-α, IL-4, and IL-13 in the tissue of rabbit AAA; B. Western blot was used to detect the expression of NF-kB and MCP-1 protein in abdominal aortic aneurysms in the tissue of rabbit AAA; Western blot was used to detect the expression of MCP-1 protein in abdominal aortic aneurysms; Compared with the model group, # was P > 0.05, * was P < 0.05, ** was P < 0.01, and *** was P < 0.001.
NF-κB is one of the hubs of pro-inflammatory gene expression. Recent studies have shown that an NF-κB transcriptional regulatory binding site is located in many pro-inflammatory cytokines, which could initiate the expression of some pro-inflammatory genes at the transcriptional level. It may play an important role in the formation and development of experimental abdominal active tumors [21,22]. MCP-1 is a downstream pro-inflammatory protein that regulates the transcriptional level of NF-kB, and it plays an important role in the regulation of macrophage infiltration [23]. This study had found that after one week of induction with elastase, the expression of NF-κB and MCP-1 protein was significantly increased in AAA model rabbit abdominal active lesions, and then decreased with time. However, at the fourth week after the completion of the AAA model it was still at a relatively high level. In the AAA rabbit model treated with IL-10, the expression of NF-κB and MCP-1 protein were significantly lower than that of the untreated Model group (Figure 3B), which suggested that IL-10 treatment in the AAA model may inhibit the inflammatory response of abdominal aortic aneurysm tissue by inhibiting the expression of NF-κB protein.
IL-10 promotes activation of M2 macrophages in rabbit AAA
In the research of the development of AAA disease, the view widely accepted by scholars at home and abroad is that the infiltration of inflammatory cells promotes the secretion of matrix metalloproteinases and causes damage to the elastic fibers and collagen fibers of the arterial wall and the development of AAA. Macrophages can be polarized into two major subpopulations of M1 and M2 cells, namely the inflamed “classically activated” macrophages and the “alternatively activated” macrophages that inhibit inflammation [24]. In general, activated M1 macrophages produce high levels of inflammatory cytokines (IL-12, IL-23, IL-1β, TNF-α) and cytotoxicity factor (iNOS) expression [25]. Therefore, M1 is often described as a pro-inflammatory cell, while M2 macrophage is the opposite type of cell that exerts an inhibitory effect on inflammation [26].
When the body is in a normal physiological state, M1 and M2 macrophages are in homeostasis [24].
In this study, we found there was no infiltrate of macrophages in normal rabbit abdominal aortic tissue, but macrophages began to infiltrate aortic aneurysm tissue after being induced by elastase for one week, and there were more M1 macrophages than M2 macrophages (Figure 4). Over time, the proportion of M2/M1 macrophages gradually increased, but the M2 macrophages in the Model group were still lower than those in M1 macrophages. From the second week of IL-10 treatment, M2 macrophages in rabbit abdominal aortic aneurysms began to be more than M1 macrophages. These results suggested that IL-10 treatment can promote the preferential differentiation of macrophages infiltrating the wall of active tumors into M2 macrophages and reduce the number of the M1 macrophage population to make the M1/M2 ratio of macrophages in an imbalanced state.
Figure 4.
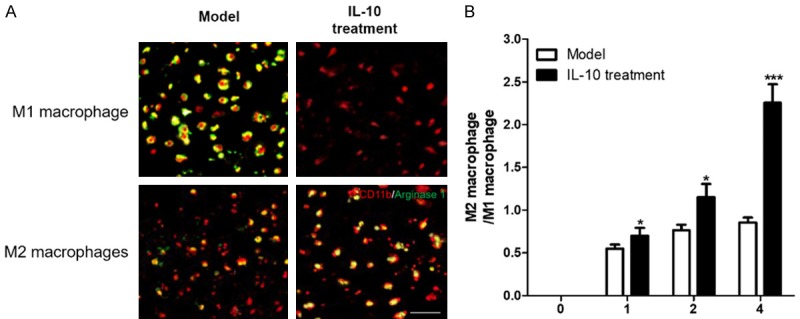
IL-10 promotes activation of M2 macrophages in rabbit AAA. A. 4 weeks after IL-10 treatment, immunofluorescence was used to detect markers of M1 and M2 macrophages in rabbit AAA. iNOS for M1 macrophages, arginase for M2 macrophages; B. The ratio of M2/M1 macrophage in rabbit AAA; Compared with the model group * was P < 0.05 and *** was P < 0.001.
Discussion
The formation and rupture of aneurysm is a progressive process from the inner to middle and outer layers, and the degeneration of smooth muscle cells plays an important role during the formation of the entire aneurysm. In other words, the formation and rupture of aneurysms is a reversible process with the destruction and repair of smooth muscle cells, and the rupture of aneurysm is due to damage caused by blood flow shear stress, where inflammation is much greater than the repair of smooth muscle cell layer [4,5]. The proliferation of VSMC and inflammation plays an important role during the process of formation and rupture of an aneurysm. When the rate of apoptosis of VSMC increases to more than the rate of proliferation, the number of smooth muscle layers gradually decreases. When the number of smooth muscle layers decreases to a certain extent, the wall of the aneurysm ruptures [7,8]. The impact of inflammation on aneurysms is mainly reflected in two aspects. First, infiltration of inflammatory cells in the aneurysm would secrete MMPS protein, which promotes the degradation of elastin and collagen in the arterial wall, causes the expansion of the arterial wall, destroys the extracellular matrix and affectes the occurrence and development of aneurysms [9,10]. On the other hand, a sustained inflammatory response inhibits VSMC proliferation, accelerates VSMC apoptosis, and promotes rupture of the aneurysm [27]. Therefore, inhibiting the inflammatory response of aneurysm tissue is of great significance for delaying and preventing rupture of the aneurysm.
IL-10 is a secreted single-chain glycoprotein, consisting of 178 amino acids, and its main biologic function is to suppress and stop inflammation, prevent the secretion of inflammatory cytokines, and regulate the proliferation and differentiation of T cells, B cells, NK cells, etc. [11]. Previous research has confirmed that IL-10 can be used to treat chronic ulcerative colitis [12,13] and Crohn’s disease [14,15] and other inflammatory bowel diseases, rheumatoid arthritis, systemic lupus erythematosus, and acute pancreatitis [28]. In this study, we used IL-10 to treat rabbit AAA models and found that IL-10 could promote the proliferation and phenotypic transformation of smooth muscle cells of abdominal aortic aneurysm, inhibit the degradation of smooth muscle cells and breakage of elastic fibers in rabbit abdominal aortic aneurysm, reduce the speed of the decrease of smooth muscle number and number of layers, and delay the development of aneurysms.
There are many different functional T cells clustering on AAA tissue, and animal experiments had confirmed that the loss of natural regulatory T lymphocytes (CD80-/-, CD86-/- or CD28-/-) would promote the development of AAA. This may be related to the decreased expression of interleukin-10 (IL-10) caused by the loss of natural regulatory T lymphocytes, as studies had demonstrated that deletion of IL-10 significantly increases AngII-induced AAA formation [29]. Zhou et al. [30] had found that intraperitoneal injection of recombinant Treg cells protected AngII-induced formation of AAA in ApoE-deficient mice, but the recombinant IL-10 deletion type Treg has no protective effect. It suggests that the function of multiple T cells to regulate AAA may be related to the secretion of IL-10. In this study, we found that with the treatment of IL-10, the expression of IFN-γ and TNF-α mRNA increased, and IL-4 and IL-13 decreased in in AAA rabbit abdominal active tissue. That meant that IL-10 suppresses the inflammatory response by changing the production of Th1/Th2 cytokines in the rabbit’s abdominal active tissue. On the other hand, IL-10 treatment significantly reduced the expression of NF-κB and MCP-1 proteins in AAA rabbit abdominal aneurysms.
The occurrence, development, and rupture of aneurysms are essentially the process of vascular remodeling. Various factors control the inflammatory infiltrate in the lesions. Crompton and Kosierkiewicz had confirmed that the inflammatory cells infiltrated in the wall of unruptured and ruptured aneurysms, and the inflammatory reaction exists before the rupture of the aneurysm [31,32]. Of all the infiltrating inflammatory cells, macrophages played the most important role, while MCP-1 played an important role in regulating the infiltration of macrophages, and NF-κB regulated the expression of pro-inflammatory genes such as MCP-1 at the transcriptional level [23]. Macrophages are innate immune cells that exist in all tissues of the body and can engulf and kill intracellular parasites, bacteria, tumor cells, and cells that are aging and dead. They also exert their immune defenses and immune self-stability, immune surveillance, and other functions [33]Different phenotypes of macrophages get activated in different microenvironments [34]. M1 and M2 macrophages are the two most common forms of macrophage activation, and both mediate the inflammatory response in the body’s diseased area. M1 is the “classically activated” macrophage that causes inflammation and M2 acts as an anti-inflammatory agent, that is, “alternative Activation” Macrophages [35,36]. This study found that IL-10 treatment could significantly increase the ratio of M2/M1 macrophages, and promote the activation of M2 cells, and thereby inhibit the inflammatory response.
Conclusion
In this study, we found that IL-10 could delay the development of aneurysms by promoting the proliferation of VSMC, inhibiting the degradation of smooth muscle cells of the abdominal aortic aneurysm in rabbits and breaking the elastic fibers, and reducing the number of smooth muscle cells and the decrease in the number of layers. These positive effects were at least partly attributable to the fact that IL-10 treatment could inhibit the inflammation of abdominal aortic aneurysms by altering the secretion of Th1/Th2 cytokines and promoting the activation of M2 macrophages.
Disclosure of conflict of interest
None.
References
- 1.Sidloff D, Stather P, Dattani N, Bown M, Thompson J, Sayers R, Choke E. Aneurysm global epidemiology study: public health measures can further reduce abdominal aortic aneurysm mortality. Circulation. 2014;129:747–753. doi: 10.1161/CIRCULATIONAHA.113.005457. [DOI] [PubMed] [Google Scholar]
- 2.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8:92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 3.George J, Rapsomaniki E, Pujades-Rodriguez M, Shah AD, Denaxas S, Herrett E, Smeeth L, Timmis A, Hemingway H. How does cardiovascular disease first present in women and men? Incidence of 12 cardiovascular diseases in a contemporary cohort of 1,937,360 people. Circulation. 2015;132:1320–1328. doi: 10.1161/CIRCULATIONAHA.114.013797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature. 2011;473:308–16. doi: 10.1038/nature10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonobe M, Yamazaki T, Yonekura M, Kikuchi H. Small unruptured intracranial aneurysm verification study: SUAVe study, Japan. Stroke. 2010;41:1969–1977. doi: 10.1161/STROKEAHA.110.585059. [DOI] [PubMed] [Google Scholar]
- 6.Bailey MA, Rode B, Simpson CM, Green B. Functional significance of the novel CRAC channel inhibitor, JPIII in abdominal aortic aneurysm vascular smooth muscle cells. British Journal of Surgery. 2015;102:10–10. [Google Scholar]
- 7.López-Candales A, Holmes DR, Liao S, Scott MJ, Wickline SA, Thompson RW. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol. 1997;150:993–1007. [PMC free article] [PubMed] [Google Scholar]
- 8.Nataatmadja M, West M, West J, Summers K, Walker P, Nagata M, Watanabe T. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation. 2003;108(Suppl 1):II329–34. doi: 10.1161/01.cir.0000087660.82721.15. [DOI] [PubMed] [Google Scholar]
- 9.Brophy CM, Reilly JM, Smith GJ, Tilson MD. The role of inflammation in nonspecific abdominal aortic aneurysm disease. Ann Vasc Surg. 1991;5:229–33. doi: 10.1007/BF02329378. [DOI] [PubMed] [Google Scholar]
- 10.Hellenthal FA, Buurman WA, Wodzig WK, Schurink GW. Biomarkers of abdominal aortic aneurysm progression. Part 2: inflammation. Nat Rev Cardiol. 2009;6:543–52. doi: 10.1038/nrcardio.2009.102. [DOI] [PubMed] [Google Scholar]
- 11.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 12.Tedde A, Laura Putignano A, Bagnoli S, Congregati C, Milla M, Sorbi S, Genuardi M, Papi L. Interleukin-10 promoter polymorphisms influence susceptibility to ulcerative colitis in a gender-specific manner. Scand J Gastroenterol. 2008;43:712–8. doi: 10.1080/00365520701885507. [DOI] [PubMed] [Google Scholar]
- 13.Lackeyram D, Young D, Kim CJ, Yang C, Archbold TL, Mine Y, Fan MZ. Interleukin-10 is differentially expressed in the small intestine and the colon experiencing chronic inflammation and ulcerative colitis induced by dextran sodium sulfate in young pigs. Physiol Res. 2017;66:147–162. doi: 10.33549/physiolres.933259. [DOI] [PubMed] [Google Scholar]
- 14.Schreiber S, Fedorak RN, Nielsen OH, Wild G, Williams CN, Nikolaus S, Jacyna M, Lashner BA, Gangl A, Rutgeerts P, Isaacs K, van Deventer SJ, Koningsberger JC, Cohard M, LeBeaut A, Hanauer SB. Safety and efficacy of recombinant human interleukin 10 in chronic active crohn’s disease. Crohn’s disease IL-10 cooperative study group. Gastroenterology. 2000;119:1461–1472. doi: 10.1053/gast.2000.20196. [DOI] [PubMed] [Google Scholar]
- 15.Lindsay JO, Hodgson HJ. The immunoregulatory cytokine interleukin-10-a therapy for crohn’s disease? Aliment Pharmacol Ther. 2001;15:1709–16. doi: 10.1046/j.1365-2036.2001.01093.x. [DOI] [PubMed] [Google Scholar]
- 16.Chappell J, Harman JL, Narasimhan VM, Yu H, Foote K, Simons BD, Bennett MR, Jørgensen HF. Extensive proliferation of a subset of differentiated, yet plastic, medial vascular smooth muscle cells contribute to neointimal formation in mouse injury and atherosclerosis models. Circ Res. 2016;119:1313–1323. doi: 10.1161/CIRCRESAHA.116.309799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li YH, Choi DH, Lee EH, Seo SR, Lee S, Cho EH. Sirtuin 3 (SIRT3) regulates α-smooth muscle actin (α-SMA) production through the succinate dehydrogenase-G protein-coupled receptor 91 (GPR91) pathway in hepatic stellate cells. J Biol Chem. 2016;291:10277–92. doi: 10.1074/jbc.M115.692244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Zhang Y, Yang P, Enkhjargal B, Manaenko A, Tang J, Pearce WJ, Hartman R, Obenaus A, Chen G, Zhang JH. Recombinant osteopontin stabilizes smooth muscle cell phenotype via integrin receptor/integrin-linked kinase/Rac-1 pathway after subarachnoid hemorrhage in rats. Stroke. 2016;47:1319–27. doi: 10.1161/STROKEAHA.115.011552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SJ, Baek SE, Jang MA, Kim CD. Osteopontin plays a key role in vascular smooth muscle cell proliferation via EGFR-mediated activation of AP-1 and C/EBPβ pathways. Pharmacol Res. 2016;108:1–8. doi: 10.1016/j.phrs.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 20.Vidal AE, Kannouche P, Podust VN, Yang W, Lehmann AR, Woodgate R. Proliferating cell nuclear antigen. J Biol Chem. 2004;279:48360–8. doi: 10.1074/jbc.M406511200. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Lenardo MJ, Baltimore D. Baltimore, 30 years of NF-κB: a blossoming of relevance to human pathobiology. Cell. 2017;168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aurora AB, Biyashev D, Mirochnik Y, Zaichuk TA, Sánchez-Martinez C, Renault MA, Losordo D, Volpert OV. NF-kappaB balances vascular regression and angiogenesis via chromatin remodeling and NFAT displacement. Blood. 2016;116:475–84. doi: 10.1182/blood-2009-07-232132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eardley KS, Zehnder D, Quinkler M, Lepenies J, Bates RL, Savage CO, Howie AJ, Adu D, Cockwell P. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int. 2006;69:1189–1197. doi: 10.1038/sj.ki.5000212. [DOI] [PubMed] [Google Scholar]
- 24.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Standiford TJ, Strieter RM, Chensue SW, Westwick J, Kasahara K, Kunkel SL. IL-4 inhibits the expression of IL-8 from stimulated human monocytes. J Immunol. 1990;145:1435–9. [PubMed] [Google Scholar]
- 26.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–61. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 27.Zhang LL, Gao CY, Fang CQ, Wang YJ, Gao D, Yao GE, Xiang J, Wang JZ, Li JC. PPARγ attenuates intimal hyperplasia by inhibiting TLR4-mediated inflammation in vascular smooth muscle cells. Cardiovasc Res. 2011;92:484–493. doi: 10.1093/cvr/cvr238. [DOI] [PubMed] [Google Scholar]
- 28.Geginat J, Larghi P, Paroni M, Nizzoli G, Penatti A, Pagani M, Gagliani N, Meroni P, Abrignani S, Flavell RA. The light and the dark sides of Interleukin-10 in immune-mediated diseases and cancer. Cytokine Growth Factor Rev. 2016;30:87–93. doi: 10.1016/j.cytogfr.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Ait-Oufella H, Wang Y, Herbin O, Bourcier S, Potteaux S, Joffre J, Loyer X, Ponnuswamy P, Esposito B, Dalloz M, Laurans L, Tedgui A, Mallat Z. Natural regulatory T cells limit angiotensin II-induced aneurysm formation and rupture in mice. Arterioscler Thromb Vasc Biol. 2013;33:2374–9. doi: 10.1161/ATVBAHA.113.301280. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Wu W, Lindholt JS, Sukhova GK, Libby P, Yu X, Shi GP. Regulatory T cells in human and angiotensin II-induced mouse abdominal aortic aneurysms. Cardiovasc Res. 2015;107:98–107. doi: 10.1093/cvr/cvv119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crompton MR. The comparative pathology of cerebral aneurysms. Brain. 1966;89:789–796. doi: 10.1093/brain/89.4.789. [DOI] [PubMed] [Google Scholar]
- 32.Kosierkiewicz TA, Factor SM, Dickson DW. Immunocytochemical studies of atherosclerotic lesions of cerebral berry aneurysms. J Neuropathol Exp Neurol. 1994;53:399–406. doi: 10.1097/00005072-199407000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 34.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–61. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 36.Busch SA, Hamilton JA, Horn KP, Cuascut FX, Cutrone R, Lehman N, Deans RJ, Ting AE, Mays RW, Silver J. Multipotent adult progenitor cells prevent macrophage-mediated axonal dieback and promote regrowth after spinal cord injury. J Neurosci. 2011;31:944–53. doi: 10.1523/JNEUROSCI.3566-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]