Abstract
Cholesteatoma is characterized by the presence of a squamous epithelium invading the middle ear altering its growth properties. Wnt/β-catenin signaling controls cell proliferation and differentiation by regulating expressions of target genes. Elevated levels of β-catenin are related to tissue pathogenesis and tumor progression. Nevertheless, the mechanisms through which β-catenin contributes to middle ear cholesteatoma development remain to be elucidated. We used proliferation assay, qRT-PCR assay, and western blotting to measure levels of the Wnt/β-catenin signaling pathway. β-Catenin expression evidently increased in middle ear cholesteatoma cells when compared with normal epithelial cells. Next, we found that treatment of Wnt inhibitor dickkopf1 (Dkk1) decreased β-catenin expression, as well as the expression levels of cytokeratin 16 (CK16), CK18, Ki67 and PCNA. Overexpression of Wnt3a or β-catenin induced the expression levels of CK16, CK18, Ki67 and PCNA. Furthermore, Dkk1 treatment significantly inhibited proliferation activity of middle ear cholesteatoma cells, whereas forced expression of Wnt3a or β-catenin promoted proliferation activity of middle ear cholesteatoma cells. Wnt/β-catenin signaling induced cell proliferation and up-regulated expressions of targeted genes in human middle ear cholesteatoma.
Keywords: Beta-catenin, cell proliferation, cholesteatoma, middle ear, Wnt signaling pathway
Introduction
Cholesteatoma is a destructive squamous epithelial lesion of the temporal bone which gradually expands and leads to serious complications by destruction of nearby bony structures [1,2]. Human middle ear cholesteatoma is a progressive disease. Clinical appearances vary from well-localized pathology with normal hearing to advanced involvement with serious complications such as brain abscess. Therefore, the difficulties of operation, management, and outcomes are different according to the severity. Several attempts have been made in cholesteatoma staging [3-5], but to date, they have not met the global consensus like the TNM classification of cancer. Thus, the pathogenesis of human middle ear cholesteatoma has not been well investigated.
As a signaling pathway existing in all species, Wnt was first identified as a product of Wingless gene (wg) in Drosophila melanogaster (fruit fly), and its mutations resulted in frequent wing loss [6]. Nusse and Varmus [7] reported that inserting mouse mammary tumor virus into the int1 locus activated proto-oncogene Wnt in the genome and formed tumor, giving Wnt-1 as the first member of the Wnt signaling pathway. Wnt genes encode a large family of secreted glycoproteins that act as extracellular signaling molecules and activate β-catenin. β-catenin is generally identified in three distinct loci: at cellular adherent junctions, where it directly interacts with E-cadherin; in the cytosolic space; and in the nucleus. In most normal unstimulated adult cells, the Wnt/β-catenin signaling pathway is inactive, and this is ensured by the absence of Wnt protein and the degradation of β-catenin. When Wnt/β-catenin signaling is not activated, cytosolic β-catenin is phosphorylated by core proteins adenomatous polyposis coli, axin, glycogen synthase kinase 3, and casein kinase 1 [8]. The β-catenin protein is encoded by CTNNB1. Wnt/β-catenin signaling pathway regulates various biologic events in cells, such as gene expression, cell growth, metabolism, apoptosis and metastasis [9-11].
Herein we showed that expression of β-catenin efficiently increased in middle ear cholesteatoma cells when compared with normal epithelial cells. Next, we found that treatment of Wnt inhibitor Dkk1 decreased cell proliferation and β-catenin expression, as well as the expression levels of CK16, CK18, Ki67 and PCNA. Overexpression of Wnt3a or β-catenin induced Wnt/β-catenin signaling and cell proliferation. In this study, we reveal a new mechanism by which Wnt/β-catenin signaling participates in cell growth and pathogenesis of human middle ear cholesteatoma, benefiting the development of novel prognostic biomarkers and the treatment of human middle ear cholesteatoma.
Materials and methods
Clinical sample collection
Sixty middle ear cholesteatoma tissue samples surgically resected from patients enrolled in our hospital from July 2015 to September 2017 were collected as a group. The patients consisted of 24 males and 36 females aged 18~64 years old, with an average of (38.92±10.21). Middle ear cholesteatoma was confirmed by preoperative clinical diagnosis and postoperative pathologic diagnosis. The external ear canal skin samples of 40 patients were collected as a control group. All collected samples were immediately fixed and embedded in paraffin. This study has been approved by the ethics committee of our hospital, and written informed consent has been obtained from all patients.
Detection of β-catenin expression by immunohistochemical assay
Sample sections were deparaffinized, fully hydrated, and washed with PBS for 5 min three times. After 10 min of antigen retrieval with citric acid, the sections were naturally cooled down to room temperature and immersed in PBS for 5 min three times. After endogenous peroxidase was blocked by 3% H2O2, the samples were incubated at room temperature for 20 min, immersed in PBS for 5 min three times, received 50 μL of goat serum, and were blocked with endogenous biotin at room temperature for 20 min, incubated with 50 μL of primary antibody at 4°C overnight, immersed in PBS for 5 min three times and incubated with 50 μL of secondary antibody at 37°C for 30 min. Subsequently, the sections were immersed in PBS for 5 min three times, incubated with 50 μL of HRP-labeled streptomycin at 37°C for 30 min, immersed in PBS again for 5 min three times, and color-developed with DAB, which was terminated by adding distilled water. Then the sections were counterstained with hematoxylin, dehydrated by using gradient concentrations of ethanol solutions, transparentized by xylene mounted with neutral resin and observed by microscopy. A known positive section was used as positive control, and PBS instead of primary antibody was employed as negative control.
Determination of results: The experimental results were analyzed in a double-blinded manner by two experienced pathologists. For each section, five visual fields (×400) were randomly selected. Under each field, 100 cells were counted to calculate the proportion of positive cells and the positive expression rate. Negative (-): Positive rate <5%; mildly positive (+): 6%~25%; moderately positive (++): 26%~50%; strongly positive (+++): >50%.
Cell culture
Human primary cells from human middle ear cholesteatoma tissue and adjacent normal epithelium tissue were maintained in DMEM supplemented with 100 units/ml penicillin, 10% fetal bovine serum and 100 µg/ml streptomycin, and cultured in a humidified incubator with 5% CO2 at 37°C.
Cell proliferation assay
Cells were inoculated into 96-well plates at the density of 2000/well. According to manufacturer’s instructions, the cell proliferation capacity was detected by a CCK8 kit (Dojindo Laboratories, Kumamoto, Japan) at different time points. Data were reported based on three independent experiments, with six replicates each time.
Separation of cell nucleus, cytosol and membrane
Cell nucleus, cytosol, and membrane were separated according to the instructions of Nuc-Cyto-Mem Preparation Kit P120 (Beijing Applygen Gene Technology Co., Ltd., China).
Quantitative real-time PCR (qPCR)
According to manufacturer’s instructions, total RNA was extracted from cells by TRIzol reagent (Invitrogen, CA, USA). To detect the mRNA expression levels of these genes, the extracted total RNA was reverse-transcribed with oligodT primer into cDNA by a RT reagent kit (Takara, China). Housekeeping gene GAPDH was employed as internal reference. Afterwards, cDNA was amplified with SYBR Green Master Mix (Takara, China) by qPCR using a 7900HT system. Relative fold changes in the expressions of target genes were measured by the 2-ΔΔCt method.
Western blotting
Ground tissues or cells were lysed for 30 on ice min in RIPA buffer (prepared by 100 mM pH 8.0 Tris, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 5 mM EDTA, 1% sodium deoxycholate and 10 mM NaF) that was supplemented by 2 mM leupeptin, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 2 mM aprotinin, 2 mM pepstatin A and 1 mM DTT. Then the lysate was centrifugated at 4°C and 12,000 rpm for 15 min, and the resulting supernatant was collected. Subsequently, the protein concentration was measured with the BCA assay (Beyotime Institute of Biotechnology, China). After separation with SDS-PAGE, the protein extracts were transferred onto a nitrocellulose membrane in transfer buffer (150 mM glycine, 20 mM Tris and 20% methanol (v/v)). Then the membrane was blocked by 5% skimmed milk in 0.05% Tween-20-containing 1× PBS and incubated by using antibodies against β-catenin, p-β-catenin, Cyclin D1, c-myc (Cell Signaling Technology, Beverly, MA, USA), CK16, CK18 (Proteintech Group, Rosemont, USA), Ki67, PCNA and GAPDH (Bioworlde, USA). The protein bands were thereafter probed by horseradish peroxidase-conjugated secondary antibody IgG, and visualized using SuperSignal West Pico Chemiluminescent Substrate Kit (Thermo Scientific, MA, USA).
Statistical analysis
All experimental data were analyzed by GraphPad Prism 5 software (La Jolla, CA, USA) using Student’s t-test. P<0.05 was considered significant.
Results
β-catenin expression in middle ear cholesteatoma tissue exceeded that in normal ear skin
β-Catenin was strongly positively expressed (brownish yellow) in the epithelial cell nucleus and cytosol of middle ear cholesteatoma tissue (Figure 1). Of 60 middle ear cholesteatoma tissue samples, 46 had positive expression, with a positive rate of 76.67% which was significantly higher than that of normal external ear canal skin (10/40, 25.00%) (χ2=26.001, P=0.000).
Figure 1.
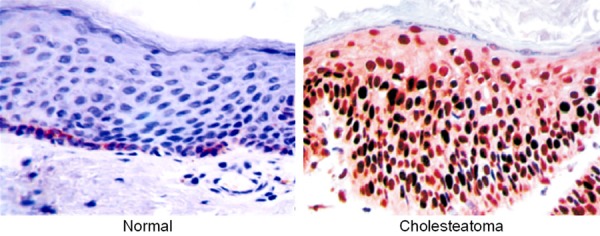
β-Catenin expression in normal external ear canal skin and middle ear cholesteatoma tissue detected by immunohistochemical assay. Magnification: ×400.
Cholesteatoma cells showed high activity of cell growth and induced Wnt/β-catenin signaling
Cholesteatoma cells showed higher activity of growth than normal cells (Figure 2A). Moreover, qRT-PCR and western blotting showed that cholesteatoma cells induced Wnt/β-catenin signaling. The expressions of β-catenin, Cyclin D1 and c-myc were up-regulated in cholesteatoma cells, but that of p-β-catenin was down-regulated (Figure 2B and 2C). Our results indicated that cholesteatoma cells showed high activity of cell growth and induced Wnt/β-catenin signaling.
Figure 2.
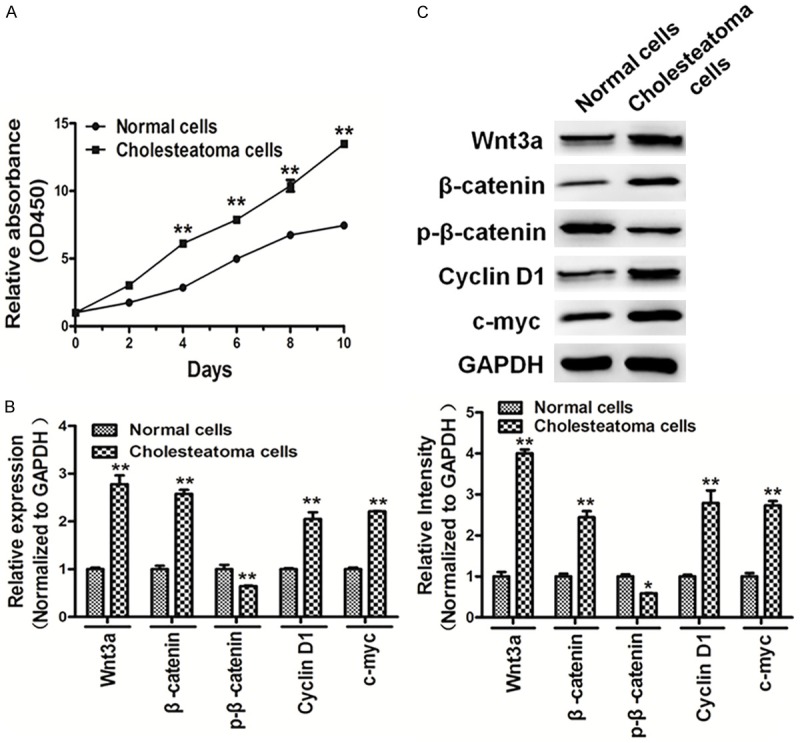
Cholesteatoma cells showed high cell growth activity and induced Wnt/β-catenin signaling. A. Cells were seeded and cultured in 96-well plates. Cell viability was assayed using a CCK8 kit according to the manufacturer’s instruction at indicated time points. The results showed that cholesteatoma cells showed high cell growth activity compared with normal cells. B, C. qRT-PCR and western blotting showed that cholesteatoma cells induced Wnt/β-catenin signaling, the expression levels of β-catenin, Cyclin D1 and c-myc were up-regulated in cholesteatoma cells, and the expression levels of p-β-catenin was down-regulated in cholesteatoma cells. The density of protein levels of above was quantified by ImageJ software and normalized to the level of GAPDH. Data represent mean ± SD of three replicates. *Indicates significant difference at P<0.05. **Indicates significant difference at P<0.01.
Also, we used a Nuc-Cyto-Mem Preparation Kit to separate cell nucleus, cytosol and membrane, and then to detect the subcellular location of β-catenin. Both qRT-PCR (Figure 3A) and western blotting (Figure 3B) exhibited that the expressions of β-catenin in cholesteatoma cell nucleus and cytosol were significantly higher than those of normal cells.
Figure 3.
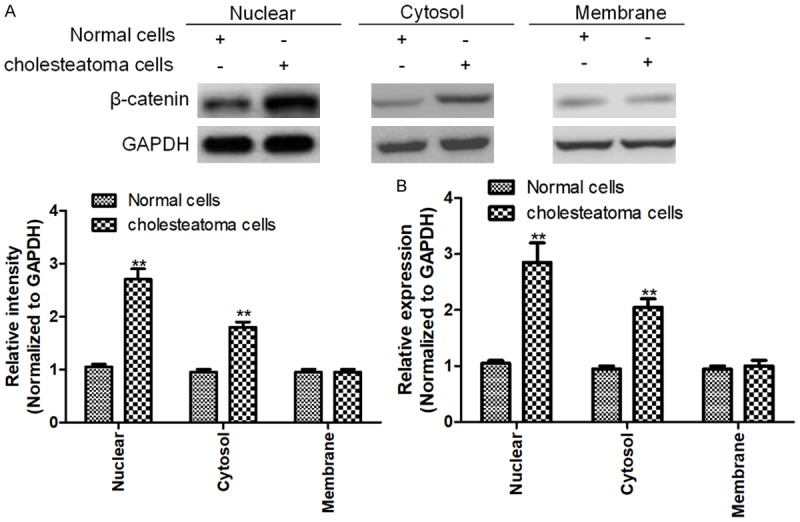
Subcellular location of β-catenin in cholesteatoma and normal cells. A, B. qRT-PCR and western blotting showed that the expression of β-catenin in cholesteatoma cell nucleus and cytosol were significantly higher than those of normal cells. Data represent mean ± SD of three replicates. *Indicates significant difference at P<0.05. **Indicates significant difference at P<0.01.
Wnt/β-catenin signaling was significantly activated in cell culture after 6 days
Next, we cultured cholesteatoma cells for indicated times. In our study, qRT-PCR and western blotting showed that cholesteatoma cells induced Wnt/β-catenin signaling. The expressions of β-catenin, CK16, CK18, Ki67 and PCNA were up-regulated in cholesteatoma cells, and significantly activated in cell culture after 6 days. The protein levels were quantified by ImageJ software and normalized to that of GAPDH (Figure 4A and 4B). Given the important role of CK16, CK18, Ki67 and PCNA in proliferation, Wnt/β-catenin signaling was significantly activated in cell culture after 6 days.
Figure 4.
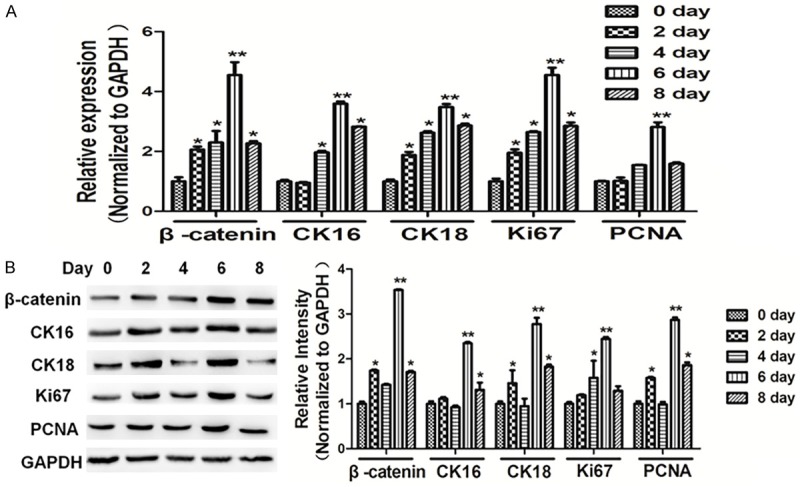
Wnt/β-catenin signaling was significantly activated in cell culture for 6 days. Cells were seeded and cultured in 6-well plates. Protein and RNA were harvested at the indicated time point, as 0 day, 2 day, 4 day, 6 day, 8 day. A, B. The mRNA and protein levels of β-catenin, CK16, CK18, Ki67 and PCNA were analyzed using RT-qPCR and western blotting respectively, and normalized by GAPDH expression. Data represent mean ± SD of three replicates. *indicates significant difference at P<0.05. **Indicates significant difference at P<0.01.
Dkk1 suppressed proliferation and Wnt/β-catenin signaling in cells
Wnt/β-catenin signaling is predominantly regulated by several inhibitors, among which dickkopf-related protein 1 (Dkk1) and sclerostin have been most comprehensively studied. We found that overexpression of Dkk1 suppressed cell proliferation (Figure 5A). Moreover, qRT-PCR and western blotting showed that overexpression of Dkk1 in cholesteatoma cells repressed Wnt/β-catenin signaling, and the expressions of β-catenin, CK16, CK18, Ki67, and PCNA were down-regulated in cholesteatoma cells in the Dkk1 overexpression group (Figure 5B and 5C).
Figure 5.
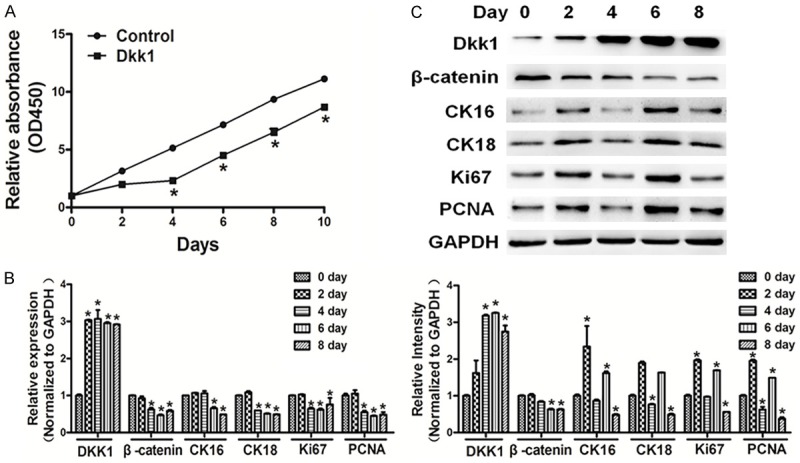
Dkk1 suppressed cell proliferation and Wnt/β-catenin signaling in cells. A. Cells were seeded and cultured in 96-well plates. Cell viability was assayed using a CCK8 kit according to the manufacturer’s instruction at indicated time points. Overexpression of Dkk1 suppressed cell proliferation. B, C. qRT-PCR and western blotting showed that overexpression of Dkk1 in cholesteatoma cells repressed Wnt/β-catenin signaling, the expression levels of β-catenin, CK16, CK18, Ki67 and PCNA were down-regulated in cholesteatoma cells. The density of protein levels of above was quantified by ImageJ software and normalized to the level of GAPDH. Data represent mean ± SD of three replicates. *Indicates significant difference at P<0.05.
Overexpression of Wnt3a induced proliferation and Wnt/β-catenin signaling in cells
The Wnt family consists of a number of small, cysteine-rich, secreted glycoproteins involved in regulation of a variety of cellular activities with critical roles during development [13-15]. Wnt proteins trigger signaling pathways within cells that proceed through several protein complexes including β-catenin. We found that overexpression of Wnt3a promoted cell proliferation (Figure 6A). Moreover, qRT-PCR and western blotting showed that overexpression of Wnt3a in cholesteatoma cells induced Wnt/β-catenin signaling, and the expressions of β-catenin, CK16, CK18, Ki67, and PCNA were up-regulated in cholesteatoma cells in the Wnt3a overexpression group (Figure 6B and 6C).
Figure 6.
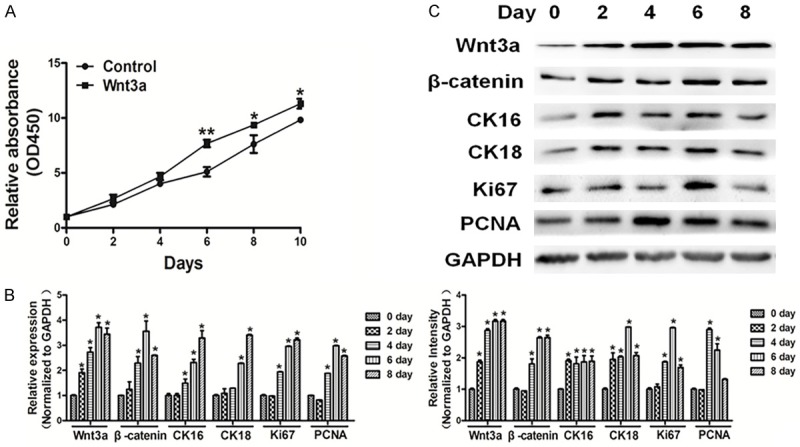
Overexpression of Wnt3a induced cell proliferation and Wnt/β-catenin signaling in cells. A. Cells were seeded and cultured in 96-well plates. Cell viability was assayed using a CCK8 kit according to the manufacturer’s instruction at indicated time point. Overexpression of Wnt3a promoted activity of cell proliferation. B, C. qRT-PCR and western blotting showed that overexpression of Wnt3a in cholesteatoma cells promoted Wnt/β-catenin signaling, the expression levels of β-catenin, CK16, CK18, Ki67 and PCNA were down-regulated in cholesteatoma cells. The densities of protein levels of the above were quantified by ImageJ software and normalized to the level of GAPDH. Data represent mean ± SD of three replicates. *Indicates significant difference at P<0.05. **Indicates significant difference at P<0.01.
Overexpression of β-catenin induces proliferation and Wnt/β-catenin signaling in cells
Afterwards, we found that overexpression of β-catenin promoted cell proliferation (Figure 7A). Moreover, qRT-PCR and western blotting showed that overexpression of β-catenin in cholesteatoma cells induced Wnt/β-catenin signaling, and the expressions of CK16, CK18, Ki67 and PCNA were up-regulated in cholesteatoma cells in β-catenin overexpression group (Figure 7B and 7C).
Figure 7.
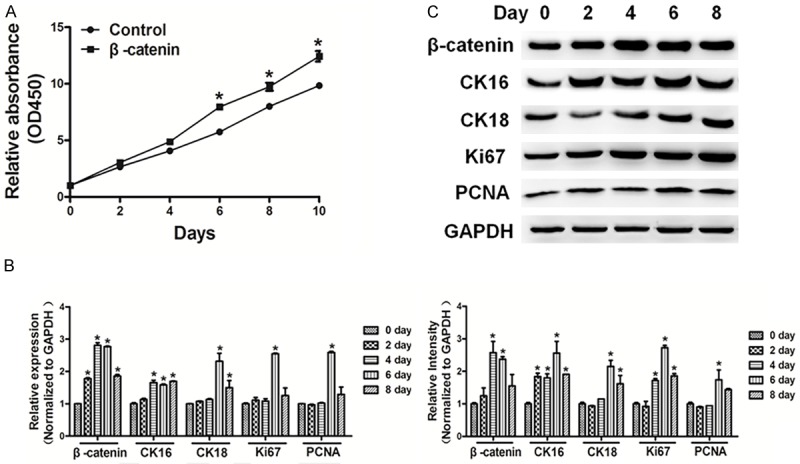
Overexpression of β-catenin induced cell proliferation and Wnt/β-catenin signaling in cells. A. Cells were seeded and cultured in 96-well plates. Cell viability was assayed using a CCK8 kit according to the manufacturer’s instruction at indicated time point. Overexpression of β-catenin promoted cell proliferation. B, C. qRT-PCR and western blotting showed that overexpression of β-catenin in cholesteatoma cells promoted Wnt/β-catenin signaling. The expression levels of CK16, CK18, Ki67 and PCNA were down-regulated in cholesteatoma cells. The density of protein levels of above was quantified by ImageJ software and normalized to the level of GAPDH. Data represent mean ± SD of three replicates. *Indicates significant difference at P<0.05.
Discussion
Although cholesteatoma is not a malignant disease, it can invade neighboring bones and exhibit invasive biological behaviors due to its severe destructive effect and high potential for recurrence [16,17]. The common feature of cholesteatoma is the migration of keratinized hyperproliferative squamous epithelium, from the fibrous stroma into the mastoid cavity and middle ear [18,19]. A previous study has demonstrated that cholesteatoma keratinocyte proliferation and migration is regulated by growth factors and receptors [20]. The up-regulation of keratinocyte growth factor and its receptor, as well as the epidermal growth factor and its receptor, have been reported in cholesteatoma [21-23]. Herein we showed that expression of β-catenin efficiently increased in middle ear cholesteatoma cells when compared with normal epithelial cells.
Wnt/β-catenin is one of the classic signal pathways [24,25]. The canonical Wnt signaling pathway regulates the amount of transcriptional co-activator β-catenin, and thereby controls the crucial developmental gene expression programs [25]. In addition, the Wnt/β-catenin signaling pathway has widely been linked with several pathologic processes, including tumor metastasis, epithelial-mesenchymal transition (EMT), stem cell renewal, and other diseases [24-27]. During the fusion of embryonic stem cells with somatic cells, periodic activation of the Wnt/β-catenin signaling pathway could significantly enhance fusion-mediated cell reprogramming [26]. Recently, Matsuura has reported that β-catenin/BCL9L/TCF4 signal pathway directly targeted the GCM1/syncytin pathway and thereby regulated the fusion of human choriocarcinoma cells [28]. However, the precise molecular activities of the Wnt/β-catenin signaling pathway in human middle ear cholesteatoma need further study.
Conclusion
Herein, β-catenin expression significantly increased in middle ear cholesteatoma cells when compared with normal epithelial cells. Next, we found that treatment by Wnt inhibitor Dkk1, Wnt3a, or β-catenin influenced the Wnt/β-catenin pathway. Forced expression of Dkk1 decreased the expressions of β-catenin, CK16, CK18, Ki67 and PCNA. Overexpression of Wnt3a or β-catenin induced the expression levels of CK16, CK18, Ki67 and PCNA. Furthermore, Dkk1 treatment significantly inhibited proliferative activity of middle ear cholesteatoma cells, whereas forced expression of Wnt3a or β-catenin promoted proliferation activity of middle ear cholesteatoma cells. The Wnt/β-catenin pathway was enhanced in middle ear cholesteatoma cells compared with that in normal epithelial cells, indicating that this signaling influenced the pathogenesis of cholesteatoma. In summary, Wnt/β-catenin signaling induced cell proliferation and regulated the pathogenesis of human middle ear cholesteatoma, suggesting potential applicability of some inhibitors of this pathway.
Acknowledgements
This work is supported by grant from the Key Clinical Specialist Program, Natural Science Foundation of Fujian Province (2014J01286).
Disclosure of conflict of interest
None.
References
- 1.Tos M. Incidence, etiology and pathogenesis of cholesteatoma in children. Adv Otorhinolaryngol. 1988;40:110–117. doi: 10.1159/000415679. [DOI] [PubMed] [Google Scholar]
- 2.Black B, Gutteridge I. Acquired cholesteatoma: classification and outcomes. Otol Neurotol. 2011;32:992–995. doi: 10.1097/MAO.0b013e3182255874. [DOI] [PubMed] [Google Scholar]
- 3.Austin DF. Reporting results in tympanoplasty. Am J Otol. 1985;6:85–88. [PubMed] [Google Scholar]
- 4.Meyerhoff WL, Truelson J. Cholesteatoma staging. Laryngoscope. 1986;96:935–939. [PubMed] [Google Scholar]
- 5.Saleh HA, Mills RP. Classification and staging of cholesteatoma. Clin Otolaryngol Allied Sci. 1999;24:355–359. doi: 10.1046/j.1365-2273.1999.00272.x. [DOI] [PubMed] [Google Scholar]
- 6.Sharma RP, Chopra VL. Effect of the Wingless (wg1) mutation on wing and haltere development in drosophila melanogaster. Dev Biol. 1976;48:461–465. doi: 10.1016/0012-1606(76)90108-1. [DOI] [PubMed] [Google Scholar]
- 7.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 8.Kimelman D, Xu W. Beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- 9.Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13:4042–4045. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- 10.Duchartre Y, Kim YM, Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol. 2016;99:141–149. doi: 10.1016/j.critrevonc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 12.Wallingford JB, Habas R. The developmental biology of dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- 13.Huelsken J, Birchmeier W. New aspects of wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev. 2001;11:547–553. doi: 10.1016/s0959-437x(00)00231-8. [DOI] [PubMed] [Google Scholar]
- 14.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of wnt signaling through β-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 15.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 16.James AL, Chadha NK, Papsin BC, Stockley TL. Pediatric cholesteatoma and variants in the gene encoding connexin 26. Laryngoscope. 2010;120:183–187. doi: 10.1002/lary.20649. [DOI] [PubMed] [Google Scholar]
- 17.Juhász A, Sziklai I, Rákosy Z, Ecsedi S, Adány R, Balázs M. Elevated level of tenascin and matrix metalloproteinase 9 correlates with the bone destruction capacity of cholesteatomas. Otol Neurotol. 2009;30:559–565. doi: 10.1097/MAO.0b013e31819fe6ed. [DOI] [PubMed] [Google Scholar]
- 18.Preciado DA. Biology of cholesteatoma: special considerations in pediatric patients. Int J Pediatr Otorhinolaryngol. 2012;76:319–321. doi: 10.1016/j.ijporl.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Chen X, Qin Z. MicroRNA let-7a suppresses the growth and invasion of cholesteatoma keratinocytes. Mol Med Rep. 2015;11:2097–2103. doi: 10.3892/mmr.2014.2971. [DOI] [PubMed] [Google Scholar]
- 20.Louw L. Acquired cholesteatoma: summary of the cascade of molecular events. J Laryngol Otol. 2013;127:542–549. doi: 10.1017/S0022215113000601. [DOI] [PubMed] [Google Scholar]
- 21.Alves AL, Pereira CS, Carvalho Mde F, Fregnani JH, Ribeiro FQ. EGFR expression in acquired middle ear cholesteatoma in children and adults. Eur J Pediatr. 2012;171:307–310. doi: 10.1007/s00431-011-1526-2. [DOI] [PubMed] [Google Scholar]
- 22.Barbara M, Raffa S, Murè C, Manni V, Ronchetti F, Monini S, Torrisi MR. Keratinocyte growth factor receptor (KGF-R) in cholesteatoma tissue. Acta Otolaryngol. 2008;128:360–364. doi: 10.1080/00016480701785004. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto-Fukuda T, Takahashi H, Koji T. Expression of keratinocyte growth factor (KGF) and its receptor in a middle-ear cavity problem. Int J Pediatr Otorhinolaryngol. 2012;76:76–81. doi: 10.1016/j.ijporl.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miki T, Yasuda SY, Kahn M. Wnt/beta-catenin signaling in embryonic stem cell self-renewal and somatic cell reprogramming. Stem Cell Rev. 2011;7:836–846. doi: 10.1007/s12015-011-9275-1. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, Bai X, Chen W, Ma T, Hu Q, Liang C, Xie S, Chen C, Hu L, Xu S, Liang T. Wnt/beta-catenin signaling enhances hypoxia-induced epithelial-mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1alpha signaling. Carcinogenesis. 2013;34:962–973. doi: 10.1093/carcin/bgt027. [DOI] [PubMed] [Google Scholar]
- 28.Matsuura K, Jigami T, Taniue K, Morishita Y, Adachi S, Senda T, Nonaka A, Aburatani H, Nakamura T, Akiyama T. Identification of a link between wnt/beta-catenin signalling and the cell fusion pathway. Nat Commun. 2011;2:548. doi: 10.1038/ncomms1551. [DOI] [PubMed] [Google Scholar]


