Abstract
Emerging studies have shown that microRNAs (miRNAs) play key roles in regulating progression of pancreatic cancer (PaCa). miR-103 has been reported to serve as an oncomiR in hepatocellular carcinoma and colorectal cancer. However, litter is known regarding the function and molecular mechanism of miR-103 in PaCa. Here, we observed that miR-103 was markedly highly expressed in PaCa tissues and cell lines. Up-regulation of miR-103 expression was associated with advanced TNM stage, positive lymph node metastasis, and poor prognosis. Furthermore, knock-down of miR-103 by its inhibitor resulted in inhibited cell invasion and migration. Using a dual-luciferase reporter assay, we identified that USP10 (Ubiquitin specific peptidase 10) was a directly target of miR-103. In addition, by using qRT-PCR assay and western blotting analysis, we found that miR-103 down-regulated the expression of USP10 in PaCa tissues and cell lines. Taken together, the present study demonstrates that up-regulation of miR-103 is associated with tumor metastasis and poor prognosis in PaCa patients. Our data further indicates that miR-103 is an upregulated oncomiR and promotes cell metastasis by targeting USP10, suggesting miR-103 may be a potential prognosis and treatment target for PaCa.
Keywords: PaCa, miR-103, metastasis, prognosis, USP10
Introduction
Pancreatic cancer (PaCa) is the fourth leading cause of cancer-associated death for both men and women all over the world, which is characterized by early metastasis, rapid invasion and resistance to standard treatments [1,2]. PaCa is one of the most aggressive tumors, with a 5-year survival rate of less than 5% [3,4]. During past decades, though several risk factors have been illustrated to play important roles in PaCa development, little progress has made on the molecular mechanisms underlying the progression of PaCa [5-7]. Therefore, identifying novel molecular mechanisms involved in PaCa progression is necessary to provide early diagnosis and develop effective therapeutic options.
MicroRNAs (miRNAs) are a subset of endogenous, small, non-coding RNA of consisting of 18-24 nucleotides that inhibit target genes expression by directly binding with the 3’-untranslated regions (UTRs) of mRNA [8-10]. Accumulating studies suggest that miRNAs are involved in various of cell processes, including proliferation, apoptosis, differentiation, metastasis and endocrine homeostasis [11]. Recently, miRNAs have been shown to be involved in the regulation of a variety of diseases in eukaryote [12,13]. Dysregulation of miRNAs have been identified in many human cancers, which are closely associated with tumor initiation, progression and prognosis [14,15]. Some miRNAs act as oncomiRs to promote tumor development and progression, while others serve as tumor-suppressive miRNAs [16]. For instance, miR-615-5p functions as a tumor-suppressive miRNA in pancreatic ductal adenocarcinoma [17,18]. In contrast, miR-3646 and miR-1228 are two oncomiRs in breast cancer [19,20].
MiR-103 is identified as an oncomiR or tumor-suppressive miRNA, which is aberrant expression in several types of human cancers, including prostate cancer, gastric carcinoma and colorectal cancer [21-23]. Microarray expression profiles study has showed that miR-103 is up-regulated in PaCa [24]. However, the biologic function and molecular mechanism of miR-103 in PaCa remain to be elucidated. In this study, we confirmed that miR-103 was up-regulated in PaCa tissues and cell lines. Up-regulation of miR-103 was associated with tumor metastasis and poor prognosis in PaCa patients. Notably, we identified that USP10 (ubiquitin specific peptidase 10) was a target gene of miR-103 in PaCa. Knockdown of miR-103 suppressed PaCa cell migration and invasion through reducing USP10 expression.
Materials and methods
PaCa samples and cell lines
Thirty-four paired PaCa tissues and their adjacent matched non-tumorous pancreatic epithelial tissues were obtained from Department of Hepatobiliary Surgery, the First Affiliated Hospital of Jiaxing University between March 2008 to March 2013. All samples were flash-frozen in liquid nitrogen immediately and stored at -80°C until further use. The patients included 22 males and 12 females, with average age of 51.7 ± 5.9 years. All tissues were histologically confirmed by hemotoxylin-eosin (H&E) staining and pathological evidences. Consent forms was signed from all patients and conducted in accordance with the regulations of Declaration of Helsinki. This study was also approved by the Ethic Committees at the First Affiliated Hospital of Jiaxing University. Follow-up information was available for all participants. Overall survival (OS) was defined as the time from the day of operation to death or the date of last follow-up.
The human pancreatic cancer cell lines (Capan-2 and PANC-1) and a pancreatic ductal cell line (HPDE6-C7) were obtained from were obtained from the Chinese Academy of Sciences (Shanghai, China). All cell lines were cultured in RPMI-1640 (Invitrogen, Carlsbad, CA, USA), supplemented with 10% FBS (Invitrogen, Carlsbad, CA, USA) containing streptomycin (100 mg/ml) and penicillin (100 U/ml), and maintained in an incubator at 37°C with 5% CO2.
RNA oligonucleotides and cell transfections
The miR-103 inhibitor and inhibitor control were bought from Shanghai GenePharma, Co. Ltd., (Shanghai, China). Cells were cultured in 6-well plates until 75-85% confluence. Transfections were performed by using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocols. For each well, 20 µl of miR-103 inhibitor and inhibitor control were added to 500 µl DMEM with 5 µl Lipofectamine 3000. The mixture was added to each well and incubated for 48 h at 37°C with 5% CO2. Total RNA and/or protein were extracted for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and/or western blot.
Cell migration assay
Cell migration was assessed by a wound-healing assay. Briefly, Capan-2 and PANC-1 cells were seeded to 6-well plates. At 24 h after transient transfection, an artificial wound was created onto the monolayer by using a sterile 100-μl tip. After scratching, cells were washed with PBS for three times and incubated at 37°C with 5% CO2. Images of cell migration were captured at time points of 0 and 24 h under a TE2000-U microscope (Nikon Corporation, Tokyo, Japan).
Cell invasion assay
Cell invasion assay was detected by using the Transwell chambers coated Matrigel (BD Biosciences, MA, USA). Capan-2 and PANC-1 cells were seeded in Matrigel pre-coated upper chambers (8 × 104 cells) in RPMI-1640 medium. The lower chambers were filled with 500 μl RPMI-1640 medium with 10% FBS. After 24 hours of incubation, the cell in upper chambers was removed, while cell invaded into lower chambers was fixed by 4% formaldehyde (Sigma-Aldrich, St. Louis, MO, USA) for 20 min, and stained with 0.5% crystal violet (Sigma-Aldrich, St. Louis, MO, USA) for 15 min, and finally observed at × 200 magnification under a LSM710 inverted light microscope (Zeiss, Germany).
Dual luciferase reporter assay
MiRcode 11 (http://www.mircode.org) was used to illustrate complementary sequences between the miR-103 and USP10 gene. Human USP10 3’-UTR, including the complimentary binding site of miR-103, was cloned into downstream of the firefly cassette of pmirGLO dual-luciferase reporter plasmid (Promega, WI, USA) to construct USP10-3’-UTR-wild type (WT) luciferase reporter plasmid. The mutated USP10 3’-UTR was also inserted into pmirGLO plasmid to produce USP10-3’-UTR-mutant (MUT) luciferase reporter plasmid. In Capan-2 and PANC-1 cells, the USP10-3’-UTR-WT or MUT luciferase reporter plasmid, and miR-103 inhibitor or inhibitor control was co-transfected by using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocols. 48 h after transfection, a Dual-Luciferase Reporter Assay system (Promega, WI, USA) was applied to detected Firefly and Renilla activities. Firefly luciferase activity was normalized to Renilla luciferase activity.
RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA of PaCa cells and tissues was isolated by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. qPCR reaction was performed on an ABI Prism 7100 Sequence Detector System (Applied Biosystems, CA, USA) by using a miScript SYBR® Green PCR Kit (Qiagen, Germany) and a SYBR Premix Ex Taq™ kit (Takara, Japan) to determine the expression levels of miR-103 and USP10, respectively. The qPCR conditions consisted of 20 min of DNA polymerase activation at 95°C, followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec. U6 small nuclear RNA (U6) and (Glyceraldehyde-3-phosphate dehydrogenase) GAPDH were served as two internal normalized references. The primers used for the amplification were as follows: miR-103: 5’-AGCAGCATTGTACAGGGCTATCA-3’ (forward), universal primer (reverse); U6: 5’-CTCGCTTCGGCAGCACATA-3’ (forward), 5’-CGAATTTGCGTGTCATCCT-3’ (reverse); USP10:5’-TTATGAGAAGACTGGTGGGT-3’ (sense), 5’-TGTTGCCGTGATGGTAGA-3’ (antisense); GAPDH: 5’-TGCACCACCAACTGCTTAGC-3’ (sense), 5’-AGCTCAGGGATGACCTTGCC-3’ (antisense). The relative mRNA and miRNA expression levels were calculated by using the delta-delta Ct method [25].
Western blot analysis
Cells were collected and extracted by RIPA buffer on ice. Total proteins were separated by 8% SDS-PAGE and then blotted onto PVDF membranes. The membranes were then blocked with 5% non-fat milk for 2 h at 37°C and followed incubated with anti-USP10 antibody (1:1000; CST Technologies, Inc., Chicago, IL, USA) and anti-GAPDH antibody (1:2000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C. After washing with TBST buffer for 3 times, the membranes were incubated with a horseradish peroxidase-conjugated (HRP) goat anti-mouse IgG (1:5,000; Abcam, Inc., CA, USA) for 1 h at 37°C. Positive bands were detected by using a ECL western blotting Detection kit (GE Healthcare, Pittsburgh, PA, USA), according to the manufacturer’s protocols.
Statistical analysis
Data are presented as the means ± standard deviation (SD). The SPSS software version 19.0 (SPSS, Chicago, IL, USA) was applied for statistical analysis. One-way ANOVA (One-way analysis of variance) or Student’s t-test was used to analyze the differences between groups. Survival curves were evaluated by the Kaplan-Meier survival analysis. P < 0.05 was considered a significant difference.
Results
MiR-103 was up-regulated in PaCa tissues and cell lines
We performed RT-qPCR assay to examine the miR-103 expression levels in both PaCa samples and cell lines. Paired PaCa tissues and their adjacent matched non-tumorous pancreatic epithelial tissues were obtained from 34 patients diagnosed with PaCa. The results showed that miR-103 was significantly up-regulated in PaCa tissues than in non-tumorous pancreatic epithelial tissues (Figure 1A, P < 0.05).
Figure 1.
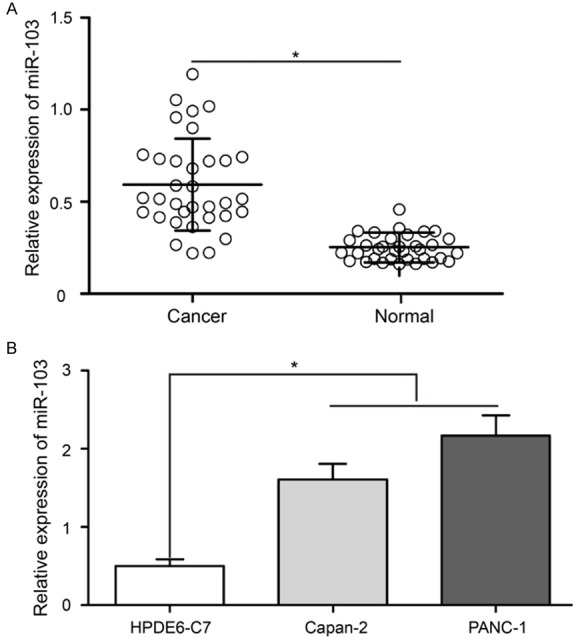
miR-103 was significantly up-regulated in PaCa tissues and cell lines. A. RT-qPCR analysis of miR-103 expression levels in PaCa tissues and adjacent matched non-tumorous pancreatic epithelial tissues. U6 was used as an internal control. B. miR-103 was markedly up-regulated in the Capan-2 and PANC-1 cells compared with HPDE6-C7 cell. RT-qPCR: reverse transcription-quantitative polymerase chain reaction; Pancreatic cancer: PaCa; miR: microRNA. *P < 0.05.
In addition, miR-103 expression level was also compared between a pancreatic ductal cell line (HPDE6-C7) and two well-defined PaCa cell lines (Capan-2 and PANC-1). Analysis of RT-qPCR revealed that, similar to the expression pattern in PaCa tissues, miR-103 was also markedly up-regulated in the two PaCa cell lines (Figure 1B, P < 0.05).
Up-regulation of miR-103 was associated with tumor metastasis and poor prognosis in PaCa patients
Using the median miR-103 expression in 34 PaCa samples as the threshold, the patients were grouped into two subgroups, including high miR-103 expression and low miR-103 expression. Relationships between miR-103 expression and clinicopathologic features were evaluated. The data showed that high miR-103 expression was significantly associated with high TNM stage and positive lymph node metastasis (Figure 2A and 2B, P < 0.05). Furthermore, Kaplan-Meier survival analysis revealed that PaCa patients with high expression of miR-103 had a significantly shorter overall survival than those patients with low expression of miR-103 (Figure 2C, P < 0.05).
Figure 2.
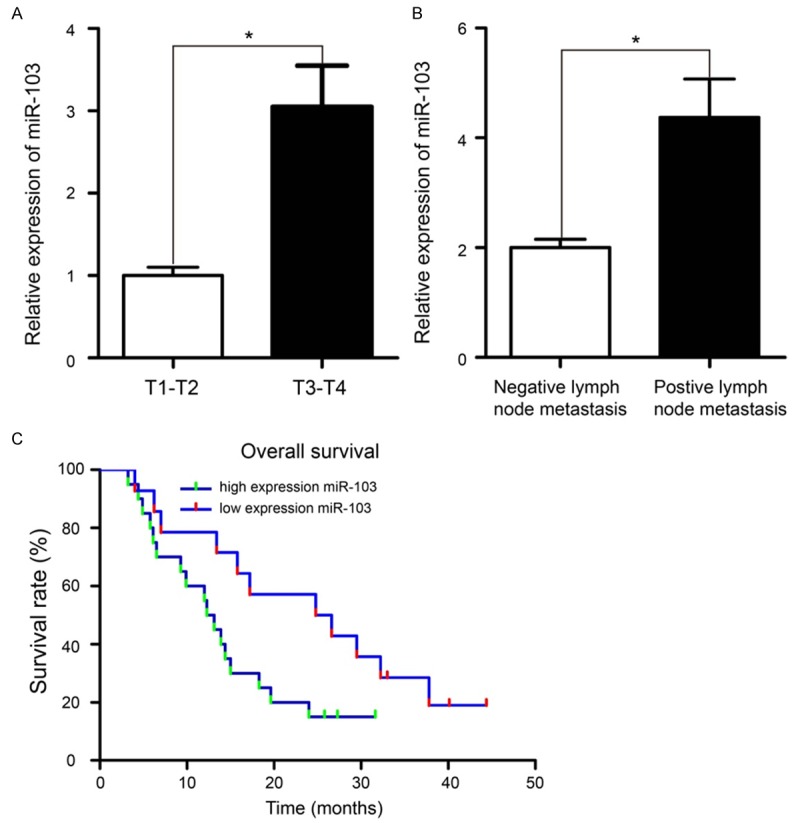
Up-regulation of miR-103 was associated with tumor metastasis and poor prognosis in PaCa patients. A. Patients with T3-T4 stage have higher miR-103 expression than those patients with T1-T2 stage. B. Patients with positive lymph node metastasis have higher miR-103 expression that those patients with negative lymph node metastasis. C. Overall survival curves for two groups of patients with PaCa. The high expression miR-103 group had a significantly shorter overall survival than the low expression miR-103 group. *P < 0.05.
MiR-103 down-regulation suppressed PaCa cell migration and invasion
In order to evaluate the potential roles of miR-103 in PaCa cells, we transfected miR-103 inhibitor into Capan-2 and PANC-1 cell lines to produce PaCa cell with stable miR-103 inhibition. The data of RT-qPCR assay confirmed that, Capan-2 and PANC-1 cells treated with miR-103 inhibitor had significant lower miR-103 expression than that transduced with inhibitor control (Figure 3A, P < 0.05). Then, we explored the cancer-related effects of knockdown of miR-103 expression in Capan-2 and PANC-1 cells. A wound-healing assay showed that miR-103 downregulation significantly reduced Capan-2 and PANC-1 cells migration in vitro (Figure 3B, P < 0.05). Second, a transwell assay was performed to detect the invasion ability of Capan-2 and PANC-1 cells. The results revealed that significantly less cells invaded into lower chambers after transfected with miR-103 inhibitor in Capan-2 and PANC-1 cells (Figure 3C, P < 0.05). These results demonstrated that anti-miR-103 inhibited the metastasis capabilities of PaCa cells.
Figure 3.
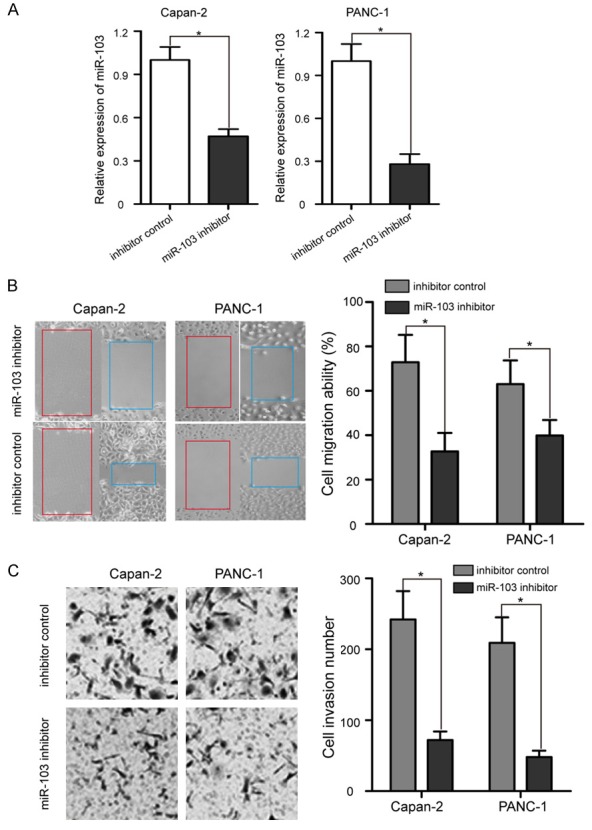
miR-103 down-regulation suppressed PaCa cell migration and invasion. A. Expression level of miR-103 was detected in Capan-2 and PANC-1 cells by RT-qPCR after transfection with miR-103 inhibitor or inhibitor control. B. Cell migration ability was determined at 0 h and 24 h in Capan-2 and PANC-1 cells by wound healing assay after treatment with miR-103 inhibitor or inhibitor control. C. Capan-2 and PANC-1 cells were transfected with miR-103 inhibitor or inhibitor control for 24 h; cell invasion ability was measured by Matrigel invasion assay. After transfection with miR-103 inhibitor, the invasion abilities of Capan-2 and PANC-1 cells were lower compared with those of inhibitor control treated cells. *P < 0.05.
USP10 was a direct target gene of miR-103
To elucidate the potential molecular mechanism underlying miR-103 mediated regulation of invasion and migration, bioinformatics analysis based on the computer-aided algorithm (MiRcode 11) was performed to predicted target genes. The most promising candidate gene was USP10, which was confirmed by algorithm. As shown in Figure 4A, there was a putative 7-mer-binding sites for miR-103 in the 3’-UTRs of the USP10 mRNA. In addition, USP10 was a well-established invasion and migration inhibition gene, which was down-regulated in several types of human cancers [26-28].
Figure 4.
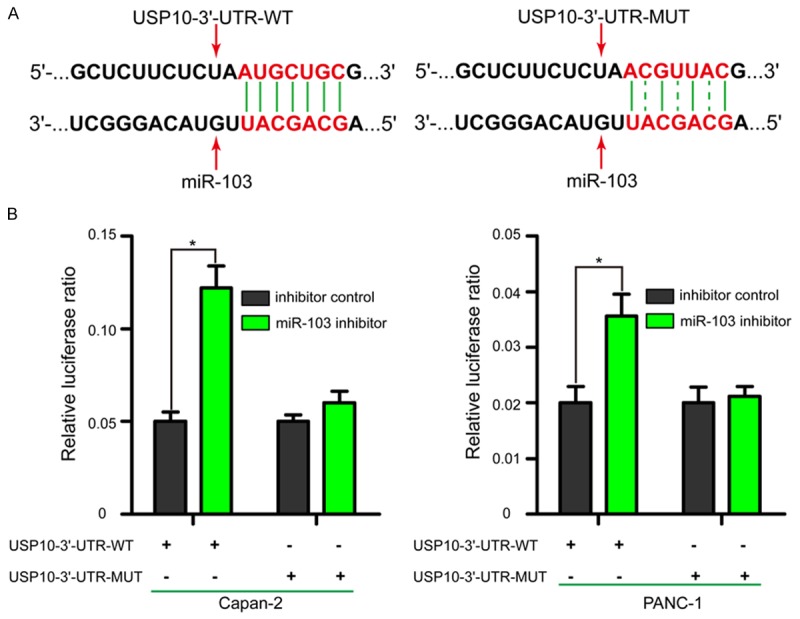
USP10 is a direct target gene of miR-103. A. USP10-3’-UTR wild type (WT) or mutant (MUT) sequence was cloned into pmirGLO dual-luciferase reporter plasmid. Binding sites are indicated by solid lines and mutant binding sites by dotted line. B. Relative luciferase ratio in different groups after co-transfected with different factors. Transfection with miR-103 inhibitor in Capan-2 and PANC-1 cells significantly increased relative luciferase ratio in the USP10-3’-UTR-WT luciferase reporter plasmid, while relative luciferase ratio of the USP10-3’-UTR-MUT luciferase reporter plasmid remained unaffected. USP10: ubiquitin specific peptidase 10; UTR: untranslated region. *P < 0.05.
To identify whether miR-103 could directly regulate USP10, a dual-luciferase reporter assay was performed. Luciferase reporter plasmid containing the USP10-3’-UTR-WT or MUT was constructed to verify the binding region between miR-103 and USP10. As shown in Figure 4B, transfected with miR-103 inhibitor in Capan-2 and PANC-1 cells significantly increased relative luciferase ratio in the USP10-3’-UTR-WT luciferase reporter plasmid (P < 0.05); however, relative luciferase ratio of the USP10-3’-UTR-MUT luciferase reporter plasmid remained unaffected. These observations indicated that miR-103 directly targeted USP10 through binding with the complementary region in its 3’-UTR.
MiR-103 down-regulated the expression of USP10 in PaCa tissues and cell lines
The USP10 mRNA level was also measured by RT-qPCR assay in PaCa tissues and their adjacent matched non-tumorous pancreatic epithelial tissues. As shown in Figure 5A, the level of USP10 mRNA decreased when miR-103 expression increased. The USP10 mRNA expression negatively correlated with miR-103 in 34 PaCa samples. In addition, transfection of Capan-2 and PANC-1 cells with miR-103 inhibitor significantly increased the mRNA and protein expression levels of USP10 compared with levels in the inhibitor control treated cells (Figure 5B and 5C, P < 0.05). These data suggested that USP10 was indeed regulated by miR-103 in PaCa tissues and cell lines.
Figure 5.
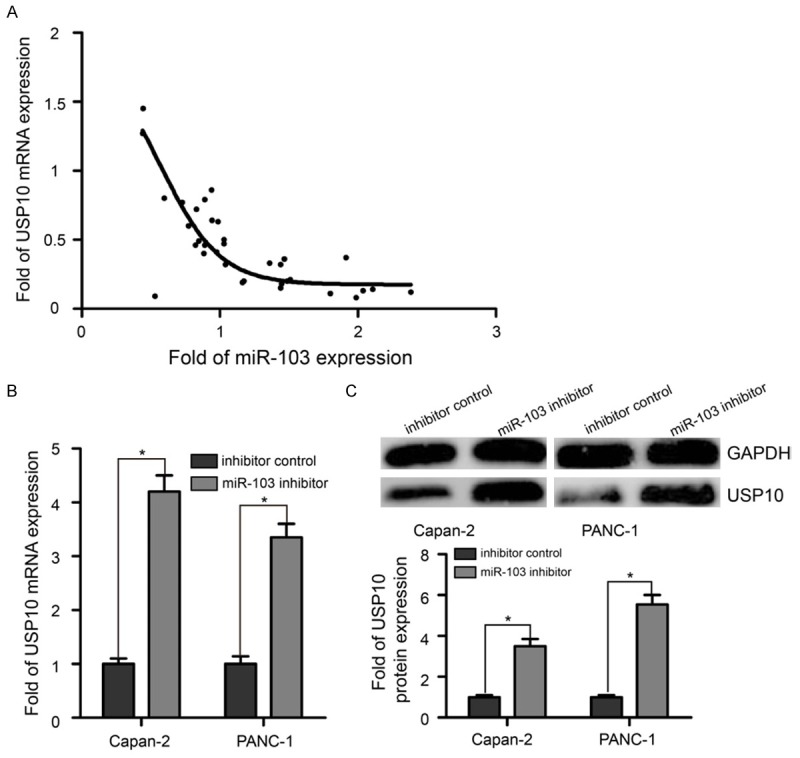
miR-103 down-regulated the expression of USP10 in PaCa tissues and cell lines. A. The level of USP10 mRNA decreased when miR-103 expression increased. USP10 mRNA expression negatively correlated with miR-103 in 34 PaCa samples by RT-qPCR. B. miR-103 negatively regulated the expression of USP10 mRNA in Capan-2 and PANC-1 cells. C. Transfection of Capan-2 and PANC-1 cells with miR-103 inhibitor significantly increased protein expression level of USP10 compared with levels in the inhibitor control treated cells. GAPDH: glyceraldehyde-3-phosphate dehydrogenase. *P < 0.05.
Discussion
Identification of aberrant regulatory molecular mechanisms involved in the progression of PaCa is utmost importance [29]. Dysregulation of miRNAs has been shown to be associated with the tumorigenesis and progression of various of human cancers [14,15]; however, elucidation of their potential functions in PaCa remains poorly unclear. In this study, we found that up-regulation of miR-103 was associated with tumor metastasis and poor prognosis in PaCa patients. We also discovered that USP10 was directly targeted by miR-103. Thus, our data supported a potential mechanism by which miR-103 promoted cell metastasis in PaCa.
Previous studies reported that many miRNAs have been dysregulated in PaCa tissues, such as miR-10a [30,31], miR-20a [32], miR-21 [33], miR-145 [34], miR-196a [35], miR-211 [36], and miR-320a [37]. Some of them were associated with the metastatic abilities of PaCa cells. For example, miR-10a was up-regulated in PaCa and involved in its invasiveness partially through suppressing HOXA1 expression [30]. miR-20a overexpression inhibited metastasis of PaCa cells [32]. miR-211 modulates gemcitabine activity via downregulation of ribonucleotide reductase and suppressed the invasive behavior of PaCa cells [36]. miR-103 was found to be aberrantly expressed in many human cancers, and to act as an oncogenic or tumor suppressive miRNA by regulating tumor cell proliferation and metastasis [21-23]. Here, we observed that miR-103 expression in pancreatic cancer was much similar with other cancers. The analysis of RT-qPCR on clinical samples from 34-pancreatic cancer patients and two PaCa cell lines demonstrated miR-103 was significantly up-regulated in PaCa. Increased miR-103 expression significantly correlated with advanced TNM stage and positive lymph node metastasis, and predicted poor survival. These results were in line with a previous microarray expression profiles study [24], not only confirming the up-regulation pattern of miR-103, but also suggesting an important role of miR-103 in regulating PaCa metastasis.
Subsequently, the biological functions of miR-103 were examined in two pancreatic cancer cell lines (Capan-2 and PANC-1). The data on cell transwell and wound healing assays in vitro demonstrated that miR-103 down-regulation significantly suppressed PaCa cell migration and invasion, confirming previous studies suggesting an oncogenic role for miR-103. These results provided substantial evidences that miR-103 was involved in the metastatic progression of PaCa. It is clear that miRNAs execute their tumor suppressor or oncogenic role by regulating the expression of target genes [16]. By using bioinformatics analysis, we found potential target genes of miR-103 and selected USP10 as potential candidate. USP10 is an important regulator of tumor progression in many human cancers [26-28]. Previous data showed an interesting correlation between miR-103 and aggressive clinicopathologic characteristics of PaCa patients. Following knockdown of miR-103 in Capan-2 and PANC-1 cells, USP10 expression was significantly increased. miR-103 and USP10 expression were inversely correlated in PaCa tissues and adjacent non-tumorous pancreatic epithelial tissues, suggesting that increased expression of miR-103 and subsequent down-regulation of USP10 may be an important driver of PaCa progression. Furthermore, a dual luciferase reporter assay also provided the directly evidence that USP10 was a direct target gene of miR-103.
In conclusion, the present study observes that up-regulation of miR-103 is associated with tumor metastasis and poor prognosis in PaCa patients. MiR-103 promotes PaCa cell migration and invasion by mediating USP10 suppression. Our research identifies, for the first time, the miR-103/USP10 axis in PaCa that is responsible for tumor metastasis, suggesting miR-103 may be useful as a new promising therapeutic target for the treatment of PaCa.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muniraj T, Jamidar PA, Aslanian HR. Pancreatic cancer: a comprehensive review and update. Dis Mon. 2013;59:368–402. doi: 10.1016/j.disamonth.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol. 2009;6:699–708. doi: 10.1038/nrgastro.2009.177. [DOI] [PubMed] [Google Scholar]
- 5.Maitra A, Kern SE, Hruban RH. Molecular pathogenesis of pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:211–226. doi: 10.1016/j.bpg.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 7.Rossi ML, Rehman AA, Gondi CS. Therapeutic options for the management of pancreatic cancer. World J Gastroenterol. 2014;20:11142–11159. doi: 10.3748/wjg.v20.i32.11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 9.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 12.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruan K, Fang X, Ouyang G. MicroRNAs: novel regulators in the hallmarks of human cancer. Cancer Lett. 2009;285:116–126. doi: 10.1016/j.canlet.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 14.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z. Advances with microRNAs in tumorigenesis and cancer therapy. Curr Pharm Des. 2014;20:5245. doi: 10.2174/1381612820666140128223856. [DOI] [PubMed] [Google Scholar]
- 16.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Zhang T, Wang C, Jin X, Jia C, Yu S, Chen J. MiRNA-615-5p functions as a tumor suppressor in pancreatic ductal adenocarcinoma by targeting AKT2. PLoS One. 2015;10:e0119783. doi: 10.1371/journal.pone.0119783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao W, Gu Y, Li Z, Cai H, Peng Q, Tu M, Kondo Y, Shinjo K, Zhu Y, Zhang J, Sekido Y, Han B, Qian Z, Miao Y. miR-615-5p is epigenetically inactivated and functions as a tumor suppressor in pancreatic ductal adenocarcinoma. Oncogene. 2015;34:1629–1640. doi: 10.1038/onc.2014.101. [DOI] [PubMed] [Google Scholar]
- 19.Tao S, Liu YB, Zhou ZW, Lian B, Li H, Li JP, Zhou SF. miR-3646 promotes cell proliferation, migration, and invasion via regulating G2/M transition in human breast cancer cells. Am J Transl Res. 2016;8:1659–1677. [PMC free article] [PubMed] [Google Scholar]
- 20.Lin L, Liu D, Liang H, Xue L, Su C, Liu M. MiR-1228 promotes breast cancer cell growth and metastasis through targeting SCAI protein. Int J Clin Exp Pathol. 2015;8:6646–6655. [PMC free article] [PubMed] [Google Scholar]
- 21.Fu X, Zhang W, Su Y, Lu L, Wang D, Wang H. MicroRNA-103 suppresses tumor cell proliferation by targeting PDCD10 in prostate cancer. Prostate. 2016;76:543–551. doi: 10.1002/pros.23143. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Qu X, Li C, Fan Y, Che X, Wang X, Cai Y, Hu X, Liu Y. miR-103/107 modulates multidrug resistance in human gastric carcinoma by downregulating Cav-1. Tumour Biol. 2015;36:2277–2285. doi: 10.1007/s13277-014-2835-7. [DOI] [PubMed] [Google Scholar]
- 23.Geng L, Sun B, Gao B, Wang Z, Quan C, Wei F, Fang XD. MicroRNA-103 promotes colorectal cancer by targeting tumor suppressor DICER and PTEN. Int J Mol Sci. 2014;15:8458–8472. doi: 10.3390/ijms15058458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piepoli A, Tavano F, Copetti M, Mazza T, Palumbo O, Panza A, di Mola FF, Pazienza V, Mazzoccoli G, Biscaglia G, Gentile A, Mastrodonato N, Carella M, Pellegrini F, di Sebastiano P, Andriulli A. Mirna expression profiles identify drivers in colorectal and pancreatic cancers. PLoS One. 2012;7:e33663. doi: 10.1371/journal.pone.0033663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Xu XF, Zhao Y, Tang MC, Zhou YQ, Lu J, Gao FH. MicroRNA-191 promotes pancreatic cancer progression by targeting USP10. Tumour Biol. 2014;35:12157–12163. doi: 10.1007/s13277-014-2521-9. [DOI] [PubMed] [Google Scholar]
- 27.Lin Z, Yang H, Tan C, Li J, Liu Z, Quan Q, Kong S, Ye J, Gao B, Fang D. USP10 antagonizes c-Myc transcriptional activation through SIRT6 stabilization to suppress tumor formation. Cell Rep. 2013;5:1639–1649. doi: 10.1016/j.celrep.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reece KM, Figg WD. A novel regulator (USP10) of p53: Implications for tumor suppression and therapeutic targeting. Cancer Biol Ther. 2010;9:583–584. doi: 10.4161/cbt.9.8.11690. [DOI] [PubMed] [Google Scholar]
- 29.Li D, Abbruzzese JL. New strategies in pancreatic cancer: emerging epidemiologic and therapeutic concepts. Clin Cancer Res. 2010;16:4313–4318. doi: 10.1158/1078-0432.CCR-09-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohuchida K, Mizumoto K, Lin C, Yamaguchi H, Ohtsuka T, Sato N, Toma H, Nakamura M, Nagai E, Hashizume M, Tanaka M. MicroRNA-10a is overexpressed in human pancreatic cancer and involved in its invasiveness partially via suppression of the HOXA1 gene. Ann Surg Oncol. 2012;19:2394–2402. doi: 10.1245/s10434-012-2252-3. [DOI] [PubMed] [Google Scholar]
- 31.Nakata K, Ohuchida K, Mizumoto K, Kayashima T, Ikenaga N, Sakai H, Lin C, Fujita H, Otsuka T, Aishima S, Nagai E, Oda Y, Tanaka M. MicroRNA-10b is overexpressed in pancreatic cancer, promotes its invasiveness, and correlates with a poor prognosis. Surgery. 2011;150:916–922. doi: 10.1016/j.surg.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Yan H, Wu J, Liu W, Zuo Y, Chen S, Zhang S, Zeng M, Huang W. MicroRNA-20a overexpression inhibited proliferation and metastasis of pancreatic carcinoma cells. Hum Gene Ther. 2010;21:1723–1734. doi: 10.1089/hum.2010.061. [DOI] [PubMed] [Google Scholar]
- 33.Wei X, Wang W, Wang L, Zhang Y, Zhang X, Chen M, Wang F, Yu J, Ma Y, Sun G. MicroRNA-21 induces 5-fluorouracil resistance in human pancreatic cancer cells by regulating PTEN and PDCD4. Cancer Med. 2016;5:693–702. doi: 10.1002/cam4.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han T, Yi XP, Liu B, Ke MJ, Li YX. MicroRNA-145 suppresses cell proliferation, invasion and migration in pancreatic cancer cells by targeting NEDD9. Mol Med Rep. 2015;11:4115–4120. doi: 10.3892/mmr.2015.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang F, Tang J, Zhuang X, Zhuang Y, Cheng W, Chen W, Yao H, Zhang S. MiR-196a promotes pancreatic cancer progression by targeting nuclear factor kappa-B-inhibitor alpha. PLoS One. 2014;9:e87897. doi: 10.1371/journal.pone.0087897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maftouh M, Avan A, Funel N, Frampton AE, Fiuji H, Pelliccioni S, Castellano L, Galla V, Peters GJ, Giovannetti E. miR-211 modulates gemcitabine activity through downregulation of ribonucleotide reductase and inhibits the invasive behavior of pancreatic cancer cells. Nucleosides Nucleotides Nucleic Acids. 2014;33:384–393. doi: 10.1080/15257770.2014.891741. [DOI] [PubMed] [Google Scholar]
- 37.Wang W, Zhao L, Wei X, Wang L, Liu S, Yang Y, Wang F, Sun G, Zhang J, Ma Y, Zhao Y, Yu J. MicroRNA-320a promotes 5-FU resistance in human pancreatic cancer cells. Sci Rep. 2016;6:27641. doi: 10.1038/srep27641. [DOI] [PMC free article] [PubMed] [Google Scholar]


