Abstract
Ovarian steroid cell tumors NOS are rare sex cord-stromal tumors. They account for less than 0.1% of ovarian tumors. We present a case of a 17-year-old girl with the complaint of amenorrhea. The serum testosterone level was 11.55 nmol/L (reference value, 0.35-2.6 nmol/L) and the serum value of dehydroepiandrosterone-sulfate (DHEA-S) was 5.9 μmol/L (reference value, 0.49-8.71 μmol/L). A computed tomography (CT) pelvic scan identified a solid, right ovarian tumor and detected no adrenal gland enlargement or additional tumors. We took a surgical excision and a wedge resection of the normal contralateral ovary. The histopathologic examination on microscopy confirmed it was a benign ovarian steroid cell tumor NOS. Four days postoperative, her sex hormones were declined to normal levels and her serum testosterone level was 2.37 nmol/L (reference value, 0.35-2.6 nmol/L) a month after surgery. Her serum testosterone level was in the normal range and there was no evidence of recurrence 6 months after surgery.
Keywords: Ovarian steroid cell tumor, NOS, virilization, pathology, therapy
Introduction
Ovarian steroid cell tumors account for less than 0.1% of ovarian tumors and are rare sex cord-stromal tumors [1]. They have been classified into three subtypes: Stromal luteoma, Leydig cell tumors and not otherwise specified (NOS) [2,3]. NOS tumors usually develop in reproductive-aged women with an average age of 47 years and are rare in adolescent girls [4]. The major symptoms of NOS tumors are hirsutism and virilization that caused by the high level of sex hormones [5]. The mainstay of NOS tumors treatment is surgery. This report describes a case of NOS tumor in an adolescent girl of 17 years.
Case report
A 17-year-old girl (virgin) visited our hospital with the complaint of amenorrhea for one and half years. The girl had menarche at 14 years old and experienced oligomenorrhea for about one year. She suffered amenorrhea since one and a half years ago which could be relieved by oral contraceptives (OCs).
She had a low-pitched, deepened male voice, thin and greasy skin and severe facial hair growth that required daily chin and lip shaving. Her BMI was 32.27 kg/m2 (height: 167 cm, weight: 90 kg). A pelvic examination revealed an 8*7*6 cm, enlarged clitoris, and right adnexal fullness.
The laboratory analysis showed that renal and liver parameters were within normal limits and tumor markers, including carcinoembryonic antigen (CEA), a-fetoprotein (AFP), human epididymis protein 4 (HE-4), CA19-9, CA125 were found to be normal. The hemoglobin levels were 157 g/L (reference value, 115-150 g/L). The serum testosterone level was 14.28 nmol/L (reference value, 0.35-2.6 nmol/L) and the serum value of dehydroepiandrosterone-sulfate (DHEA-S) was 5.9 μmol/L (reference value, 0.49-8.71 μmol/L) (Table 1).
Table 1.
Laboratory analysis before and after surgery
| Before surgery | 4 days after surgery | 1 month after surgery | Normal values | |
|---|---|---|---|---|
| Hemoglobin | 157 | 132 | 125 | 115-150 g/L |
| Testosterone | 14.28 | 0.97 | 2.37 | 0.35-2.6 nmol/L |
| CA125 | 16.5 | - | 19 | < 35 U/ml |
| CA199 | 17.37 | - | 15.19 | < 39 U/ml |
| HE-4 | 46.06 | - | 39.76 | < 140 pmol/L |
| CEA | 0.68 | - | 0.50 | < 4.7 ng/ml |
| AFP | 1.24 | - | 1.25 | < 20 ng/ml |
| CA724 | 1.51 | - | 1.32 | < 6.90 U/ml |
| Premenopausal ROMA | 6.24 | - | 4.52 | < 11.4% |
Transvaginal ultrasound identified an 8.3*6.2*7.2 cm, solid, right ovarian tumor. A computed tomography (CT) pelvic scan confirmed the ultrasound findings and showed no adrenal gland enlargement or additional tumors. There were no enlarged lymph nodes around the mass as well. Magnetic resonance imaging (MRI) showed (red arrow) a 78*63*72 mm solid mass on the upper right of the uterus with heterogeneous hyperintense on T2-weighted imaging (T2WI). No diffusion restriction of water was noted on either diffusion-weighted images (DWIs) or apparent diffusion coefficient (ADC) maps. The mass showed obvious heterogeneous enhancement on T1-weighted images after intravenous gadolinium administration (Figure 1). The chromosome screening confirmed that she was clinically female.
Figure 1.

A. MRI identified (red arrow) a solid mass on the upper right of the uterus with heterogeneous hyperintensity on T2WI. B, C. No diffusion restriction of water was noted on either DWIs or ADC maps. D. The mass showed obvious heterogeneous enhancement on T1-weighted images after intravenous gadolinium administration.
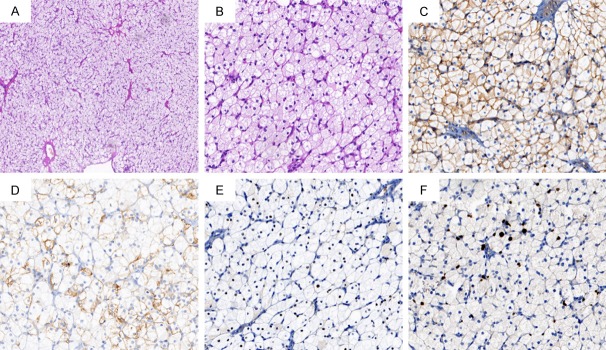
The patient underwent exploratory laparotomy. During the laparotomy, 50 ml washings of the pelvis were collected first. The enlarged right ovary was found to be smooth and had no external excrescences and measured 8*6*6 cm. The left ovary and all the surfaces of peritoneum, omentum majus and bowels were normal. We performed surgical excision and a wedge resection of the normal right ovary. Microscopically, the tumor consisted of irregular nests of cells containing abundant eosinophilic cytoplasm and they had distinct cell borders. There was sporadic mitotic activity and no significant necrosis, or nuclear atypia. Immunohistochemistry showed diffuse positive staining for CD99 and was partially positive for CK-pan, ER, and Ki-67 (Figure 2). Calretinin, Inhibin, CD10, RCC, WT-1, PR, EMA, S-100 and Syn were negative on microscopic examination. All these features confirmed it was a benign ovarian steroid cell tumor NOS.
Figure 2.
(A, B) Microscopic appearance: cells with small round nuclei, sporadic mitosis, and no significant necrosis or atypia are arranged in a diffuse pattern of columns or nests (A: H&E, 100×; B: H&E, 400×). (C-F) Immunohistochemistry showed diffuse positive staining for CD99 (C) and partial positive for CK-pan (D), ER (E), and Ki-67 (F) (C-F: 400×).
Four days postoperative, the hemoglobin declined to normal levels (135 g/L) (reference value, 115-150 g/L) and her serum testosterone level declined to 0.97 nmol/L (reference value, 0.35-2.6 nmol/L). The patient was discharged from the hospital seven days after surgery with no complications. At the first visit to the outpatient clinic a month after surgery, her serum testosterone level was 2.37 nmol/L (reference value, 0.35-2.6 nmol/L) (Table 1). The patient has been advised to get regular follow up with the department of gynecology. 6 months after surgery, her serum testosterone level was in normal range and there was no evidence of recurrence.
Discussion
Ovarian steroid cell tumors are rare sex cord-stromal tumors and account for less than 0.1% of all ovarian tumors [1]. Peking Union Medical College Hospital treated 7301 patients of ovarian tumor from 1986 to 2008, of which only 14 cases were ovarian steroid cell tumor, which accounted for about 0.19% (14/7301) [6]. Ovarian steroid cell tumors have been classified into three subtypes: Stromal luteoma, Leydig cell tumors and NOS [2,3]. NOS tumors are the most common subtype and comprise a larger portion for about 60% while stromal luteoma and Leydig cell tumors each account for about 20% [1].
Ovarian steroid cell tumors NOS can occur at any age, the youngest age is 2.5 years and the oldest age is 93 years as we found [7,8]. It is the most common subtype of ovarian steroid cell tumor and usually develops in reproductive-aged women with an average age of 47 years [9]. Most of them are unilateral [8] and characterized by the proliferation of steroid cells. The major symptoms are caused by high levels of sex hormones. More than half of the NOS patients shows hyperandrogenic symptoms and virilization signs [8], such as hirsutism, acne, deepened voice, clitoromegaly, amenorrhea, and infertility. Estradiol secretion has been detected in about 6-23% patients [10]. In these patients, irregular bleeding and endometrial polyp were noted. Some patients also developed endometrial cancer [11]. Ovarian steroid cell tumors can also secrete other kinds of steroid hormones, such as progesterone, cortisol and aldosterone, which will cause corresponding clinical symptoms [5]. About 25% of these patients were detected during a physical examination without any increase in the level of hormones [8]. Additional symptoms in some patients included abdominal distension and palpable abdominal mass. Kim et al. reported a NOS steroid cell tumor case with virilization and massive ascites [11].
Serum laboratory analysis typically shows elevated levels of testosterone and androstenedione, indicating the ovarian origin of androgen release and normal DHEA-S levels, and ruling out adrenal causes of hyperandrogenism [13]. Tumor markers such as CA125, CA199, CEA, AFP, HE4 and RISK of Ovarian Malignancy Algorithm (ROMA) are not helpful in the diagnosis of this disease. Compared with CT, the features of MRI possess practical value for analysis of the tumors. No diffusion restriction of water noted on either DWIs or ADC maps showed insufficient evidence of malignancy in our case.
Ovarian steroid cell tumors are mostly considered benign and a few show a low degree of malignancy. However, almost all the malignant cases reported in the literature are steroid cell tumors NOS [6]. In Hayes and Scully’s series, steroid cell tumors NOS are clinically malignant in up to 43% of the tumors. Hayes and Scully reported the most predictive malignant features of ovarian steroid cell tumors, NOS [6]. In their point of view, the presence of two or more mitotic figures per 10 high-power fields and grade 2-3 nuclear atypia, necrosis, hemorrhage are the predictors of malignant behavior of ovarian steroid cell tumors. In addition, a tumor diameter greater than 7 cm is also demonstrated by most of the malignant tumors [8]. In our case, the pathologic features of the accessory ovarian tumor suggested its benign behavior, although the size was larger than 7 cm.
The mainstay of all ovarian steroid cell tumor treatment is surgery. Operative method should be individualized based on surgical stage, tumor pathology, age, and the desire for preserving fertility. Ovarian steroid cell tumors are generally considered benign because they are often detected in an early stage and metastases are rare when they are diagnosed. So, for benign tumors, unilateral salpingo-oophorectomy or tumor removal is acceptable in all reproductive-aged women. A mandatory follow-up evaluation including the measurement of the sex hormone levels is required after surgery. Surgical treatments using total abdominal hysterectomy and bilateral salpingo-oophorectomy are an appropriate management option for postmenopausal women and for those who have completed childbearing. Endometrial sampling should be performed when fertility-sparing surgery is planned since many of these patients may have coexisting hyperplasia or adenocarcinoma that may affect the decision for hysterectomy. Surgical treatments using total abdominal hysterectomy, bilateral salpingo-oophorectomy, or complete surgical staging for malignant ovarian steroid cell tumor have both been reported. Unfortunately, there are few data to support a preferred approach.
Too few cases exist to have good information as to the role of adjuvant therapy. The 5-day bleomycin, etoposide, and cisplatin (BEP) regimen is the most widely used first-line chemotherapy combination for steroid cell tumors. However, there were few reports about the chemotherapy of steroid cell tumors and no evidence showed the efficiency of chemotherapy. Brewer reported a steroid cell tumor NOS case with demonstrated progressive disease after surgical debulking. Treatment with multiagent chemotherapy failed, but the patient subsequently had a robust response to GnRH agonist therapy [8]. Therefore, although typically treated solely with surgery, GnRH-agonist may be required when persistent abnormal serum hormone levels, the doubt of residual tumor, recurrences or metastases are noted. GnRH analogue treatment may be considered prior to cytotoxic chemotherapy in cases of steroid cell tumors NOS [14]. Following final pathologic diagnosis, further adjuvant chemotherapy or GnRH-a therapy was not given, and hormonal levels have been monitored as part of the patient’s postoperative follow-up.
Conclusion
Ovarian steroid cell tumor NOS accounts for less than 0.1% of ovarian tumors and yet due to its rarity, only a limited number of cases have been reported in literature. The major symptoms are hirsutism and virilization. There are no standardized treatment programs because of the lower rates of this disease. Surgical removal is the mainstay of steroid cell tumor NOS treatment. GnRH-a therapy or cytotoxic chemotherapy may be considered in cases of malignant steroid cell tumors NOS.
Disclosure of conflict of interest
None.
References
- 1.Young RH, Scully RE. Steroid cell tumors of the ovary. In: Fox H, Wells M, editors. Obstetric & Gynecological Pathology. Spain: Churchill Livingstone; 2003. pp. 845–856. [Google Scholar]
- 2.Berek JS. Berek & Novak’s Gynaecology. 15th edition. Lippincott Williams & Wilkins Publishers; 2012. p. 1098. [Google Scholar]
- 3.Schorg JO, Schaffer JI, Halvorson LM, Hoffman BL, Bradshaw KD, Cunningham FG. Williams Gynaecology. 2nd edition. McGraw-Hill Companies; 2008. p. 750. [Google Scholar]
- 4.Chen VW, Ruiz B, Killeen JL, Cot TR, Wu XC, Correa CN. Pathology and classification of ovarian tumors. Cancer. 2003;97:2631–2642. doi: 10.1002/cncr.11345. [DOI] [PubMed] [Google Scholar]
- 5.Young RH, Scully RE. Ovarian sex cord-stromal tumours: recent advances and current status. Clin Obstet Gynaecol. 1984;11:93–134. [PubMed] [Google Scholar]
- 6.Wang Z, Sun A, Lang J. Clinical analysis of 14 cases of ovarian steroid cell tumors. J Reprod Med. 2010;19:6–12. [Google Scholar]
- 7.Powell JL, Dulaney DP, Shiro BC. Androgen-secreting steroid cell tumor of the ovary. South Med J. 2000;93:1201–1204. [PubMed] [Google Scholar]
- 8.Hayes MC, Scully RE. Ovarian steroid cell tumors (not otherwise specified). A clinicopathological analysis of 63 cases. Am J Surg Pathol. 1987;11:835–845. doi: 10.1097/00000478-198711000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Lin CJ, Jorge AA, Latronico AC, Marui S, Fragoso MC, Martin RM, Carvalho FM, Arnhold IJ, Mendonca BB. Origin of an ovarian steroid cell tumor causing isosexual pseudoprecocious puberty demonstrated by the expression of adrenal steroidogenic enzymes and adrenocorticotropin receptor. J Clin Endocrinol Metab. 2000;85:1211–1214. doi: 10.1210/jcem.85.3.6454. [DOI] [PubMed] [Google Scholar]
- 10.Chung DH, Lee SH, Lee KB. A case of ovarian steroid cell tumor, not otherwise specified, treated with surgery and gonadotropin releasing hormone agonist. J Menopausal Med. 2014;20:39–42. doi: 10.6118/jmm.2014.20.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luk WT, Lee N, Chang TC, Chu KK. Lipid cell tumor of the ovary associated with endometrial adenocarcinoma--a case report. Changgeng Yi Xue Za Zhi. 1989;12:244–248. [PubMed] [Google Scholar]
- 12.Kim YT, Kim SW, Yoon BS, Kim SH, Kim JH, Kim JW, Cho NH. An ovarian steroid cell tumor causing virilization and massive ascites. Yonsei Med J. 2007;48:142–146. doi: 10.3349/ymj.2007.48.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reedy MB, Richards WE, Ueland F, Uy K, Lee EY, Bryant C, van Nagell JR Jr. Ovarian steroid cell tumors, not otherwise specified: a case report and literature review. Gynecol Oncol. 1999;75:293–7. doi: 10.1006/gyno.1999.5549. [DOI] [PubMed] [Google Scholar]
- 14.Brewer CA, Shevlin D. Encouraging response of an advanced steroid-cell tumor to GnRH agonist therapy. Obstet Gynecol. 1998;92:661–663. doi: 10.1016/s0029-7844(98)00166-5. [DOI] [PubMed] [Google Scholar]