Abstract
Objective: Oxygen free radicals (ROS) are considered to be one of the important factors involved in the pathophysiology of aged renal ischemia-reperfusion (I/R) injury. Hydrogen gas has been reported to alleviate I/R injury by scavenging free radicals. The aim of this study was to evaluate the effect of hydrogen-rich saline (HRS) on renal I/R injury in aged rats. Materials and methods: A rat model of renal I/R injury was induced by 45-min occlusion of the bilateral renal pedicles and 24-h reperfusion. Physiological saline or HRS (8 ml/kg) was administered intraperitoneally 5 min before reperfusion. Parameters indicating renal function (blood urea nitrogen (BUN) and serum creatinine (SCr)) and those indicating oxidative stress (tissue levels of malondialdehyde (MDA) and 8-hydroxy-deoxyguanosine (8-OHdG), tissue activities of superoxide dismutase (SOD), and tissue expression of heme oxygenase-1 (HO-1)) were measured. Results: After I/R injury, BUN, SCr, tissue levels of MDA and 8-OHdG, and gene expression of HO-1 were all significantly increased while tissue activities of SOD were significantly decreased. HRS reversed these changes, with the exception of HO-1 expression, which was increased further, and improved renal morphology. Conclusions: HRS improves the renal response to I/R in aged rats, possibly by reducing oxidative stress and upregulating HO-1 gene expression.
Keywords: Ischemia-reperfusion (I/R) injury, hydrogen-rich saline (HRS), heme oxygenase-1 (HO-1), reactive oxygen species (ROS), malondialdehyde (MDA), superoxide dismutase (SOD)
Introduction
The human lifespan has increased through medical advancements and improved healthcare. However, as the elderly population has increased worldwide, the incidence of acute kidney injury (AKI) has also steadily risen [1]. Cardiovascular problems caused by age-related conditions, such as hypertension, diabetes, and ischemia-reperfusion (I/R), contribute to AKI susceptibility [2,3]. In addition, the severity of AKI also increases with age and functional renal recovery is lower. A recent study has estimated that patients older than 65 years of age have an approximately 30% greater risk for non-recovery of complete renal function after AKI than those younger than 65 years old [4]. Thus, it is very important to find an effective treatment to alleviate renal I/R injury in the aging population.
Kidney aging has been recognized as a chronic process of compromised renal function and structural changes in the tubulointerstitium and glomerulus [5]. There is considerable evidence that aging occurs as a consequence of oxidative stress, and reactive oxygen species (ROS) produced during cellular metabolism lead to an age-dependent increase in oxidatively modified proteins, lipids, and nucleic acids in tissue. Oxygen free radicals are considered to be one of the important factors involved in the pathophysiology of I/R.
Recently, it was demonstrated that molecular hydrogen could selectively reduce cytotoxic ROS and reactive nitrogen species (RNS), such as hydroxyl radicals and peroxynitrite, in vitro and exert a therapeutic antioxidant activity in a rat middle cerebral artery occlusion model [6]. Hydrogen-rich saline (HRS) can attenuate renal I/R injury by reducing oxidative stress and inflammation in adult rats [7,8]. However, the potential effect of hydrogen on renal I/R injury in aged rats has not been examined. Therefore, the present study investigated the possible therapeutic effects of HRS on renal I/R injury in aged rats.
Materials and methods
Animals
A total of 30 male Sprague-Dawley rats weighing 225-250 g and 24 months of age (aged model) were used in the present study, which was approved by the Institutional Animal Care and Use Committee at Soochow University. All experiments were performed in accordance with the National Institutes of Health guidelines (NIH Publ. No. 86-23, revised 1985). Before the experiments, the animals were fed a standard rat chow, drank water ad libitum, and were housed in metabolic cages under controlled temperature in 12-h light/dark cycles for at least one week.
Surgical procedure
Animals were divided into three groups consisting of 10 rats each: (1) sham-operated plus physiological saline treatment; (2) renal I/R plus physiological saline treatment; and (3) renal I/R plus HRS treatment. Sham-operated animals underwent the same surgical procedures except that the bilateral renal pedicles were not clamped. Physiological saline or HRS (8 ml/kg) was injected intraperitoneally 5 min before reperfusion. Twenty-four hours after the initiation of renal ischemia, the rats were killed under anesthesia, blood was drawn, and the left kidneys were harvested and frozen in liquid nitrogen.
HRS production
Hydrogen was dissolved in physiological saline for 6 h under high pressure (0.4 MPa) to a supersaturated level using a hydrogen-rich, water-producing apparatus produced by our department. The saturated hydrogenated saline was stored under atmospheric pressure at 48°C in an aluminum bag with no dead volume. HRS was sterilized by gamma irradiation and was freshly prepared each week, which ensured that a concentration of 0.6 mM was maintained. Gas chromatography was used to confirm the content of hydrogen in the saline by the method described by Ohsawa et al [9]. We referenced a paper to intraperitoneal injection [8].
Analysis of renal function
Twenty-four hours after renal ischemia, blood was used to assess renal function by measuring blood urea nitrogen (BUN) and serum creatinine (SCr). The samples were analyzed on a COBAS Mira chemical analyzer (Roche; Basel, Switzerland) with commercial kits from Sigma Chemical Co. (St. Louis, MO, USA).
Histopathology evaluation
Kidney tissue samples from 12-week post-ischemic kidneys were stained with hematoxylin and eosin, and the staining was semi-quantitatively graded for tubulointerstitial damage (tubular dilation or atrophy and interstitial expansion with edema, inflammatory infiltrate, or fibrosis) based on a scale of 0 to 3 as follows: A score of 0 was given for a normal cortical tubulointerstitium; a score of 1 was given for mild tubulointerstitial damage affecting up to 25% of an objective field at 200× magnification; a score of 2 was given for moderate tubulointerstitial damage affecting 25-50% of the field; and a score of 3 was given for severe tubulointerstitial damage affecting more than 50% of the field. The examiners were blinded to the identity of each group, and ten randomly selected cortical fields were used to score each animal, with the mean score being attributed to that animal [10].
Immunohistochemistry
Kidney tissue sections (4-µm thick) were subjected to immunohistochemical analyses. Sections were dewaxed in xylene, rehydrated through graded ethanol solutions, rinsed in phosphate-buffered saline (PBS) for 5 min, and immersed in 3% hydrogen peroxide in methanol for 15 min to block endogenous peroxidase activity. The slides were rinsed in PBS for 5 min, blocked with 5% bovine serum albumin at room temperature for 15 min, and then incubated with primary monoclonal antibody against heme oxygenase-1 (HO-1; 1:100 dilution; Boster Biotechnology Ltd.; Wuhan, China) at 4°C overnight. Rabbit IgG isotype was used as a negative control. Slides were then incubated with biotinylated mouse anti-rabbit IgG secondary antibody (Maixin Biotechnology Ltd.; Fuzhou, China). Finally, slides were incubated with hydrogen peroxide-diaminobenzidine for 1 min. Sections were counterstained with hematoxylin. Estimates of staining intensity were performed by a blinded observer on coded sections (3-4 sections per kidney and 10-12 fields per section). The observer performed light microscopy and semiquantitatively scored the intensity of HO-1 staining in the whole section (0 = none, 1 = weak, 2 = moderate, and 3 = strong).
Measurement of antioxidant enzyme activity and malondialdehyde (MDA) content
Kidney MDA content and superoxide dismutase (SOD) activity were determined according to the technical manuals of the detection kits (Nanjing Jiancheng Biochemistry Co.; Nanjing, China). Kidney tissue was homogenized in phosphate buffer (pH 7.4). After centrifugation at 12,000 g for 20 min, MDA content and SOD activity in the supernatant of each sample were measured using the corresponding kits. MDA content was measured by the thiobarbituric acid (TBA) assay. The method obtained measurements of the color produced during the reaction of TBA with MDA at 532 nm in a spectrophotometer; estimated MDA levels were expressed as nmol/mg protein. SOD activity was measured using the nitroblue tetrazolium (NBT) reduction assay, by following the reduction of nitrite by a xanthine-xanthine oxidase system, which is a superoxide generator. One unit of SOD activity is defined as the amount that shows 50% inhibition of NBT reduction.
Real-time reverse transcription polymerase chain reaction (RT-PCR)
mRNA levels were determined by real-time RT-PCR. Primers and probes are listed in Table 1. Total RNA was extracted from tissues using a total RNA purification kit (Sheneregy Biocolor BioScience and Technology Co.; Shanghai, China), according to the manufacturer’s instructions. The purity and concentration of total RNA was measured by the 280 nm: 260 nm absorbance ratio. Total RNA (2 µg) was reverse-transcribed to cDNA using a first-strand cDNA synthesis kit (Fermantas; Vilnius, Lithuania), according to the manufacturer’s instructions. All PCRs were performed in a Roche Light Cycler (Roche; Basel, Switzerland) with a final volume of 25 µl. Optimum conditions were obtained with 2.5 µl buffer, 1.5 µl MgCl2, 0.5 µl dNTPs, 0.25 µl Taq DNA polymerase, 0.1 µl specific sense primer (s), 0.1 µl specific antisense primer(s), 0.1 µl specific probe(s), and 2 µl template cDNA. Finally, 17.95 µl doubly-distilled water was added to the reaction mixture. PCR cycling conditions were as follows: initial denaturation at 95°C for 3 min, 40 cycles at 95°C for 5 s, and 60°C for 12 s, with measurement of the fluorescence signal at 40°C. Standards for target genes were simultaneously analyzed using PCR with the appropriate templates. From each amplification plot, a threshold cycle (Ct) value (defined as the number of PCR cycles required for the fluorescence signal of the sample to exceed the threshold value) was calculated and then used to determine the number of molecules of the particular gene. All samples were amplified in duplicate, and the average values were then exported to Microsoft Excel for further analysis.
Table 1.
Primers and probes for rat HO-1 and β-actin
| Gene | Primers and probes | Sequence (5’ to 3’) |
|---|---|---|
| HO-1 | Forward primer | GCCTGCTAGCCTGGTTCAAGATACTAC |
| Reverse primer | AGGAAACTGAGTGTGAGGACCCATC | |
| Probe | FAM-AGAGACGCCCCGAGGAAAATCCCAGA-TAMRA | |
| β-Actin | Forward primer | GCCACTGCCGCATCCTCT |
| Reverse primer | CTGGAAGAGAGCCTCGGGG | |
| Probe | FAM-AGCTGCCTGACGGTCAGGTCATCACTATC-TAMRA |
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 4.0 software package (GraphPad Software, Inc.; San Diego, CA, USA). All data were expressed as mean ± SD. Statistical analyses were done using one-way ANOVA followed by the Student-Newman-Keuls (SNK) t-test for multiple comparisons. A P-value less than 0.05 was considered as statistically significant.
Results
Renal function
Renal function of rats was assessed by measuring the BUN and SCr levels in plasma (Table 2). In the aged I/R group, BUN and SCr levels were significantly (P < 0.05) greater than those in the aged group by nearly 2-fold, indicating the development of renal failure. In the aged + I/R + HRS group, BUN and SCr levels were significantly (P < 0.01) less than those in the aged + I/R group. In contrast, the levels of BUN and SCr in the aged + I/R + HRS and the aged groups did not significantly differ from one another (P > 0.05).
Table 2.
Effect of HRS treatment on renal function
| Aged | Aged + I/R | Aged + I/R + HRS | |
|---|---|---|---|
| BUN (mM) | 11.3 ± 7.1 | 19.9 ± 5.4# | 12.3 ± 5.5* |
| SCr (µM) | 129 ± 41 | 227 ± 64# | 147 ± 52* |
Data are expressed as mean ± SD, n = 10. Both BUN and SCr levels were greater in the aged + I/R group as compared to those of the aged group. HRS treatment significantly decreased both BUN and SCr levels.
P < 0.01 vs. the aged + I/R group;
P < 0.01 vs. the aged group.
Renal oxidative stress
MDA content was significantly greater in the aged + I/R group than in the aged group (P < 0.05; Figure 1A). HRS treatment significantly decreased MDA levels compared with the aged + I/R group. Similarly, the level of 8-OHdG in aged + I/R rats was significantly greater than that in rats not exposed to I/R (P < 0.05; Figure 1B). Additionally, the level of 8-OHdG in the aged + I/R + HRS group was significantly less than that in the aged + I/R group (P < 0.01; Figure 1B).
Figure 1.
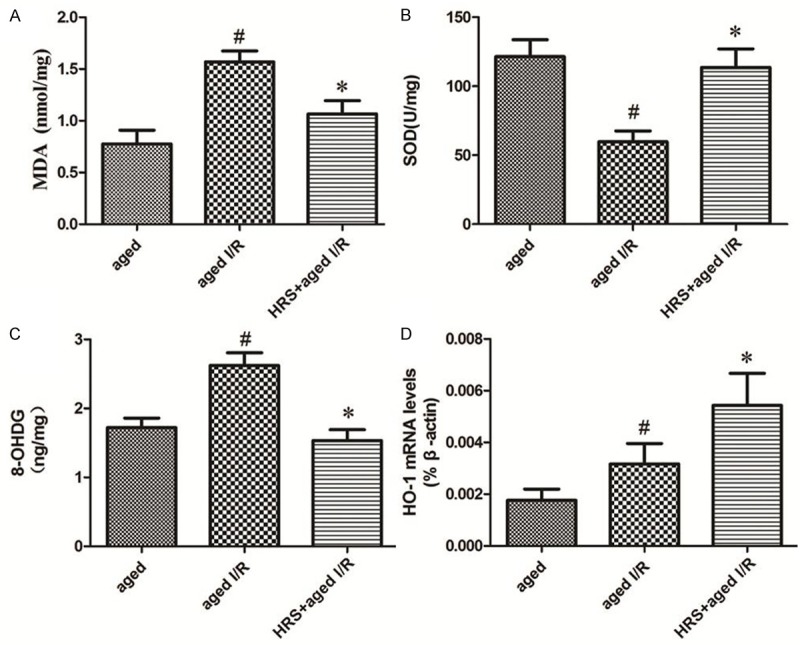
Markers of oxidative stress in the different treatment groups. A. MDA level in the kidneys of different groups (n = 10). Data are expressed as mean ± SD. *P < 0.05 vs. the aged I/R group; #P < 0.05 vs. the aged group. B. 8-OHdG level in the kidney in different groups (n = 10). Data are expressed as mean ± SD. *P < 0.05 vs. the aged I/R group; #P < 0.05 vs. the aged group. C. SOD activity in the kidney of different groups (n = 10). Data are expressed as mean ± SD. *P < 0.05 vs. the aged I/R group; #P < 0.05 vs. the aged group. D. mRNA level of HO-1 in the kidney of different groups (n = 10). Data are expressed as mean ± SD. *P < 0.05 vs. the aged I/R group; #P < 0.05 vs. the aged group.
Renal antioxidant enzymatic activities
The activity of SOD in renal tissue was significantly less in the aged + I/R group than in the aged group (P < 0.05; Figure 1C). HRS treatment significantly increased the SOD activity as compared to that in the aged I/R group (P < 0.05; Figure 1C).
Histopathology findings
Histopathology analysis showed that tubulointerstitial damage presented as tubular dilation, interstitial inflammatory infiltrate, and fibrosis in the majority of animals (Figure 2). The tubulointerstitial damage was greater in the aged + I/R group than in the aged group (0.80 ± 0.38 vs. 0.39 ± 0.25; P < 0.05; Figure 2). However, HRS treatment significantly decreased the score for tubulointerstitial damage (0.48 ± 0.24 vs. 0.80 ± 0.38 for the aged + I/R + HRS group vs. the aged + I/R group; P < 0.05; Figure 2).
Figure 2.
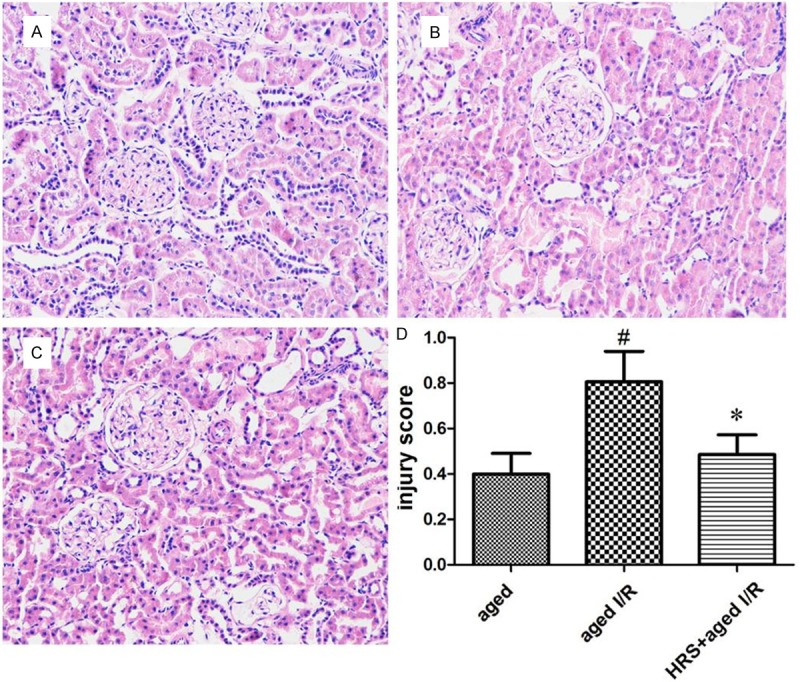
Histology changes in the kidney of different treatment groups. Kidney tissues from rats of all groups were fixed, embedded, sectioned, and stained with hematoxylin and eosin. A-C. Representative histology specimens from the young group, aged group, young I/R group, and aged I/R group. Original magnification: 200×. Histology changes were observed 24 h after renal I/R injury and included tubular dilation or atrophy and interstitial expansion with edema, inflammatory infiltrate, or fibrosis. D. Injury scores in different groups (n = 10). Data are expressed as mean ± SD. *P < 0.05 vs. the aged I/R group; #P < 0.05 vs. the aged group.
Immunohistochemical staining for HO-1
HO-1 immunostaining was localized to the proximal and distal tubules in the cortex and was prominent in the outer stripe region of the outer medulla (Figure 3). HO-1 immunostaining intensity in the aged + I/R + HRS group was significantly greater than that in the aged + I/R group (4.71 ± 2.54 vs. 2.51 ± 1.30, P < 0.01). HO-1 staining in the aged + I/R group did not differ significantly from that in the aged group (2.51 ± 1.30 vs. 1.53 ± 0.89, P > 0.05; Figure 3).
Figure 3.
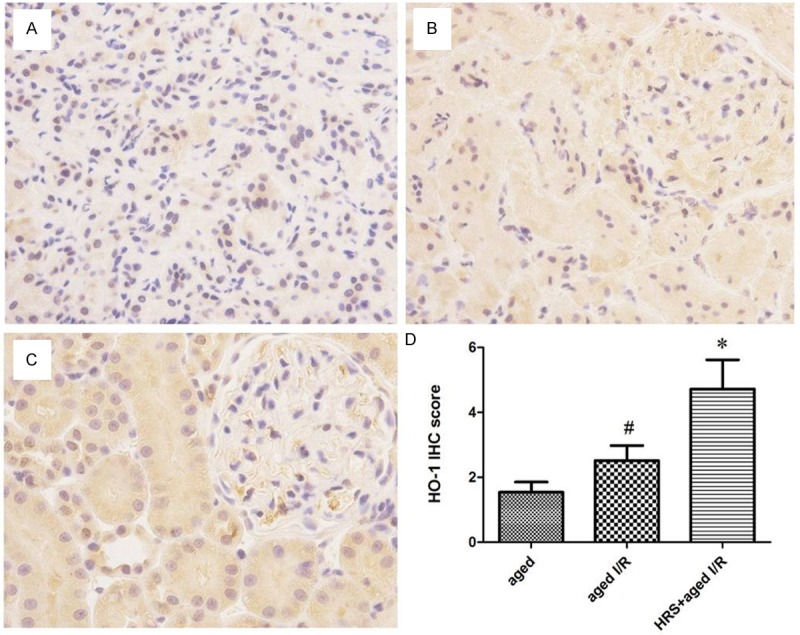
HO-1 expression in the kidneys from different treatment groups. A-C. Representative HO-1 staining of specimens from the aged group, aged I/R group, and aged I/R + HRS group. Original magnification: 400×. D. Immunohistochemistry score for HO-1 staining in different groups (n = 10). Data are expressed as mean ± SD. *P < 0.01 vs. the aged I/R group; #P < 0.01 vs. the aged group.
Renal tissue HO-1 gene expression
HO-1 mRNA levels in the aged, aged + I/R, and aged + I/R + HRS groups were determined by real-time RT-PCR using β-actin as the reference gene (Figure 1D). The HO-1 mRNA level in the aged + I/R + HRS group was significantly greater than that of the aged + I/R group (0.00543 ± 0.0035 vs. 0.00317 ± 0.00223, P < 0.05). The levels of HO-1 mRNA in the aged + I/R and aged groups were not significantly different from one another (0.00317 ± 0.00223 vs. 0.00176 ± 0.00124, P < 0.05).
Discussion
The present study demonstrated that intraperitoneal injection of HRS significantly attenuated I/R-induced renal dysfunction and tissue injury, as observed by the reversal of increases in the levels of MDA and 8-OHdG and decreases in SOD activity that resulted from I/R. This improved renal function was accompanied by an increase in HO-1 expression. Because HO-1 is an inducible antioxidant enzyme, its induction may be part of the mechanism underlying the protective effects afforded by HRS therapy. This finding is consistent with results from a recent study showing that HRS was able to attenuate renal I/R injury in adult rats. Compared to normal adult rats, aged rats are more susceptible to I/R-induced renal failure, which is associated with increased oxidative stress, as shown in our previous study [11].
Persistent oxidative stress is one of the underlying causes or components of the aging process [6]. Antioxidant treatment has been proposed to prevent aging-related general disturbances [12]. In the kidneys, the local synthesis of ROS, at least in I/R-injured animals and cultured cells, seems to increase with age [13]. Antioxidant treatment may also prevent the morphological and functional aging-related changes in the kidneys. Our data demonstrate that aging aggravates ischemic acute renal failure in the rat. Compared with same-age control animals, rats that underwent I/R had greater levels of MDA in renal tissue. However, renal MDA content was significantly less in those groups that received HRS as compared to those groups that received physiological saline. These results suggest that HRS is more effective in aging animals than in young adult animals.
The decreased SOD activity seemed to be linked to the increased MDA levels in the aged I/R rats. SOD is an antioxidant enzyme that is important in aging, and a decrease in this parameter is correlated with advanced age. Here, we found that HRS significantly increased SOD activity compared to that in the control animals. 8-OHdG is another marker of oxidative damage that arises from the reaction of ROS with cellular DNA and may accumulate with advancing age. The present study showed that HRS treatment significantly alleviated oxidative stress following I/R injury by reducing levels of both renal MDA and 8-OHdG.
HO-1 induction is one of the most sensitive indicators of cellular stress [14]. Induction of HO-1 in kidney, heart, and liver grafts prior to transplantation provided these grafts protection after reperfusion and was associated with decreased levels of inflammation [15,16]. Recent studies have indicated that kidneys from older animals have an impaired ability to upregulate HO-1 in response to I/R injury and exhibit a worse injury phenotype compared to young animals. Administration of the heme precursor hemin to one-year-old mice robustly induced HO-1 protein expression and provided aged mice with both structural and functional protection from I/R injury [17]. Similarly, administration of hydrogen gas had a protective effect on injured lung tissue by promoting the levels of HO-1 mRNA and protein [18]. Consistent with these previous studies, we found that HRS increased the levels of HO-1 mRNA and HO-1 protein in aged rat tissue, suggesting that the protective effects afforded by hydrogen gas may be mediated by HO-1 induction. The protection due to the increased expression of HO-1 may in turn be linked to the generation of carbon monoxide by the action of HO-1 on biliverdin and bilirubin. Carbon monoxide has potent vasodilating effects by activating guanylate cyclase and has been shown to prevent I/R injury during organ transplantation [19]. Bilirubin and biliverdin, two metabolites of heme degradation, act as scavengers of toxic oxygen radicals [20]. Moreover, HO-1 induction in grafts prior to reperfusion may eliminate free heme, which is released from the kidneys during the I/R process [21].
It is well known that mitochondria are not only the source of energy but also the major source of ROS [19]. Mitochondrial function and morphology are impaired in aging, as shown by declines in the membrane potential and SOD activity [22]. These changes enable accumulation of oxidatively damaged macromolecules with aging and render the mitochondria of aged animals more susceptible to oxidative injury [23]. Qiao et al. [24] demonstrated an aging-related increase of tubular cell apoptosis in renal I/R injury, which may be due to enhanced activation of the mitochondrial apoptosis pathway that results from the increase in oxidative stress. Although not directly tested in the present study, the findings of decreased SOD activity and increased MDA levels in aged animals after renal I/R injury are consistent with mitochondrial dysfunction occurring during the injury. Furthermore, the exaggerated response in older animals as compared to that in young animals suggests that mitochondrial dysfunction is also more extensive in the older animals. Hydrogen gas may protect mitochondria by scavenging ROS [13], although the exact molecular mechanism by which this occurs is unknown.
In conclusion, HRS has a protective effect on age-dependent renal I/R injury by reducing free radical peroxidation of lipid membranes and increasing the activity of antioxidant enzymes. The upregulation of HO-1 may play an essential role in the protective effects of HRS.
Acknowledgements
We are also grateful to patients for their important contribution to this study. This project was financially support in part by the Medical Science and Technique Foundation of Jiangsu Province, P. R. China (No. BL2012045).
Disclosure of conflict of interest
None.
References
- 1.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992-2001. J Am Soc Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 2.Esposito C, Plati A, Mazzullo T, Fasoli G, De Mauri A, Grosjean F, Mangione F, Castoldi F, Serpieri N, Cornacchia F, Dal Canton A. Renal function and functional reserve in healthy elderly individuals. J Nephrol. 2007;20:617–625. [PubMed] [Google Scholar]
- 3.De Luca L, Fonarow GC, Adams KF Jr, Mebazaa A, Tavazzi L, Swedberg K, Gheorghiade M. Acute heart failure syndromes: Clinical scenarios and pathophysiologic targets for therapy. Heart Fail Rev. 2007;12:97–104. doi: 10.1007/s10741-007-9011-8. [DOI] [PubMed] [Google Scholar]
- 4.Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR. Recovery of kidney function after acute kidney injury in the elderly: A systematic review and meta-analysis. Am J Kidney Dis. 2008;52:262–271. doi: 10.1053/j.ajkd.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Ding G, Franki N, Kapasi AA, Reddy K, Gibbons N, Singhal PC. Tubular cell senescence and expression of TGF-1 and p21 (WAF1/C1P1) in tubulointerstitial fibrosis of aging rats. Exp Mol Pathol. 2001;70:43–53. doi: 10.1006/exmp.2000.2346. [DOI] [PubMed] [Google Scholar]
- 6.Yu BP. Aging and oxidative stress: modulation by dietary restriction. Free Radic Biol Med. 1996;21:651–668. doi: 10.1016/0891-5849(96)00162-1. [DOI] [PubMed] [Google Scholar]
- 7.Shingu C, Koga H, Hagiwara S, Matsumoto S, Goto K, Yokoi I, Noguchi T. Hydrogen-rich saline solution attenuates renal ischemia-reperfusion injury. J Anesth. 2010;24:569–574. doi: 10.1007/s00540-010-0942-1. [DOI] [PubMed] [Google Scholar]
- 8.Wang F, Yu G, Liu S-Y. Hydrogen-rich saline protects against renal ischemia/reperfusion injury in rats. J Surg Res. 2011;167:e339–e344. doi: 10.1016/j.jss.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 10.Thomas GL, Yang B, Wagner BE, Savill J, El Nahas AM. Cellular apoptosis and proliferation in experimental renal fibrosis. Nephrol Dial Transpl. 1998;13:2216–2226. doi: 10.1093/ndt/13.9.2216. [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Fan M, He X, Liu J, Qin J, Ye J. Aging aggravates long-term renal ischemia-reperfusion injury in rats. J Surg Res. 2014;187:289–296. doi: 10.1016/j.jss.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1981;116:53–64. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-Torres MP, Gonzalez-Rubio M, Lucio-Cazan FJ, Ruiz-Villaesesa M, Rodriguez-Puyol M, Rodriguez-Puyol D. Reactive oxygen species and platelet activating factor synthesis in age-related glomerulosclerosis. J Lab Clin Med. 1994;124:489–495. [PubMed] [Google Scholar]
- 14.Maines MD. The heme oxygenase system: A regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–524. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 15.Tullius SG, Nieminen-Kelha M, Buelow P, Reutzel-Selke A, Martins PN, Pratschke J, Bachmann U, Lehmann M, Southard D, Iyer S, Schmidbauer G, Sawitzki B, Reinke P, Neuhaus P, Volk HD. Inhibition of ischemia/reperfusion injury and chronic graft deterioration by a single-donor treatment with cobalt-protoporphyrin for the induction of heme oxygenase-1. Transplantation. 2002;74:591–598. doi: 10.1097/00007890-200209150-00001. [DOI] [PubMed] [Google Scholar]
- 16.Katori M, Buelow R, Ke B, Ma J, Coito AJ, Iyer S, Southard D, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 overexpression protects rat hearts from cold ischemia/reperfusion injury via an antiapoptotic pathway. Transplantation. 2002;73:287–292. doi: 10.1097/00007890-200201270-00023. [DOI] [PubMed] [Google Scholar]
- 17.Holzen JP, August C, Bahde R, Minin E, Lang D, Heidenreich S, Dietl KH, Spiegel HU. Influence of heme oxygenase-1 on microcirculation after kidney transplantation. J Surg Res. 2008;148:126–135. doi: 10.1016/j.jss.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Huang CS, Kawamura T, Toyoda Y, Nakao A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic Res. 2010;44:971–982. doi: 10.3109/10715762.2010.500328. [DOI] [PubMed] [Google Scholar]
- 19.Wei YH, Lee HC. Oxidative stress, mitochondrial DNA mutation and impairment of antioxidant enzymes in aging. Exp Biol Med (Maywood) 2002;227:671–682. doi: 10.1177/153537020222700901. [DOI] [PubMed] [Google Scholar]
- 20.Stocker R. Antioxidant activities of bile pigments. Antiox Redox Signal. 2004;6:841–849. doi: 10.1089/ars.2004.6.841. [DOI] [PubMed] [Google Scholar]
- 21.Blydt-Hansen TD, Katori M, Lassman C, Ke B, Coito AJ, Iyer S, Buelow R, Ettenger R, Busuttil RW, Kupiec-Weglinski JW. Gene transfer-induced local heme oxygenase-1 overexpression protects rat kidney transplants from ischemia/reperfusion injury. J Am Soc Nerphrol. 2003;14:745–754. doi: 10.1097/01.asn.0000050760.87113.25. [DOI] [PubMed] [Google Scholar]
- 22.Bak MI, Wei JY, Ingwall JS. Interaction of hypoxia and aging in the heart: analysis of high energy phosphate content. J Mol Cell Cardiol. 1998;30:661–672. doi: 10.1006/jmcc.1997.0633. [DOI] [PubMed] [Google Scholar]
- 23.Mather M, Rottenberg H. Aging enhances the activation of the permeability transition pore in mitochondria. Biochem Biophys Res Commun. 2000;273:603–608. doi: 10.1006/bbrc.2000.2994. [DOI] [PubMed] [Google Scholar]
- 24.Qiao X, Chen X, Wu D, Ding R, Wang J. Mitochondrial pathway is responsible for aging-related increase of tubular cell apoptosis in renal ischemia/reperfusion injury. J Gerontol A Biol Sci Med Sci. 2005;60:830–839. doi: 10.1093/gerona/60.7.830. [DOI] [PubMed] [Google Scholar]


