Abstract
Purpose: Metastasis is the hallmark of gastric cancer (GC) and is the most widely recognized reason for GC-related deaths. However, the underlying mechanism of GC metastasis remains unknown. Herein we sought to investigate the biologic function of Gpx3 in gastric tumor metastasis and the underlying mechanism. Methods: Cell migration and invasion was determined with Transwell chamber assay. Western blotting was used to determine protein expression levels of Gpx3, EMT markers and Wnt signaling related molecules. In vivo metastasis was determined with experiment lung metastasis model in tumor xenografts. Results: Gpx3 expression was lower in GC patients and GC cell lines when compared with normal tissues and cells. Further studies showed that overexpression of Gpx3 was able to inhibit GC cell migration and invasion whereas Gpx3 knockdown promoted cell migration and invasion. Furthermore, AGS cells overexpressing Gpx3 showed lower metastatic potential when compared with the parental cells. Gpx3 was also found to regulate the expression of EMT markers. Mechanistic study showed that Gpx3 selectively inhibited Wnt/JNK signaling pathway over canonical Wnt/β-catenin pathway. The data revealed that blockade of NFкB and JNK signaling pathway abolished siGpx3-induced cell migration and invasion. Conclusions: Taken together, we identify Gpx3 as a suppressor of GC metastasis. Above results provide the rationale that regulation of Gpx3 serves as a potential therapeutic option for GC.
Keywords: GC, Gpx3, metastasis, Wnt
Introduction
Gastric cancer (GC), a significant cause of cancer death, is subtyped into diffuse and intestinal types with different prognostic and epidemiological characteristics as per the Lauren classification [1]. It is a multifactorial disease with the risk factors including geneticand environmental factors [2]. The large majority of the patients are diagnosed at very late stages when the therapeutics is substantially less effective [3]. Currently, surgery, radiotherapy, chemotherapy, and phototherapy are the therapeutics for GC treatment [4]. However, chemotherapy remains the most favorable treatment for patients with gastric cancer [5]. Despite recent advances in understanding of the biology of gastric cancer, treatment of patients with advanced gastric cancer remains a major problem.
Glutathione peroxidase (GPx), a selenium-dependent enzyme, is a group of proteins which are utilized by cells to confront free-radical-mediated attacks [6]. There are seven isoforms with diverse substrate profiles and different tissue distribution pattern [7]. Glutathione peroxidase 3 (GPx3), which is located in 5q23, is an extracellular enzyme that plays an important role in the detoxification of hydrogen peroxide and other oxygen free radicals [8]. Gpx3 mRNA is expressed in multiple tissues including gastrointestinal tract, kidney, brain, breast, liver, heart, lung and adipose tissue [9]. Gpx3 promoter hypermethylation in various tumor types including gastric cancer has been reported [10]. Furthermore, several types of tumors exhibited reduced Gpx3 levels compared with normal tissues, including hepatocellular carcinoma (HCC) [11], lung cancer [9], gastric cancer [12], thyroid cancer [13] and colorectal cancer [14]. Gpx3 is reported to be involved in the metastatic progression of various cancers [13,15]. However, the role of Gpx3 in GC metastasis has not yet been elucidated.
Wnt signaling regulates a diversity of processes fundamental to normal development, including cell proliferation, differentiation, polarity, adhesion and motility [16]. It diversifies into three branches including the β-catenin pathway which is the canonical Wnt pathway, the planar cell polarity pathway, which involves jun N-terminal kinase (JNK) and the Wnt/Ca2+ pathway [16]. The aberrant activation of the Wnt/β-catenin signaling pathway is involved in the development and progression of a significant proportion of gastric cancer cases [17]. Wnt5a, which is upregulated in gastric cancer regardless of their histological phenotype, has an increased expression in advanced stages of gastric cancer and it is correlated with poor prognosis [18].
The epithelial-mesenchymal transition (EMT) is a developmental process in which epithelial cells acquire mesenchymal characteristics that change their morphology and enhance their migratory capacity [19]. In cancer, EMT is thought to facilitate the detachment of cancer cells from the primary tumor to enable their infiltration into surrounding tissues [20]. Triggering EMT in normal development and in cancer is associated with activation of several signaling pathways, including TGF-β, Wnt, EGF and FGF [20]. While it is well-established that canonical Wnt/β-catenin signaling can be an activator of EMT, it was recently found that non-canonical Wnt5a signaling components such as PKC and JNK are also upregulated during EMT [21].
In the present study, we confirmed that Gpx3 expression was lower in GC patients and GC cell lines when compared with normal tissues and cells. Overexpression of Gpx3 caused significant metastatic suppression of GC cells in vitro and in vivo. A mechanistic study revealed that Gpx3 was involved in the metastasis of GC through the NFкB/Wnt5a/JNK signaling pathway.
Materials and methods
Patients and samples
Totally 13 pairs of GC tissues and matched normal tissues were collected from the patients with GC, who were enrolled in Qilu Hospital of Shandong University according to the protocols approved by the Ethics Review Board. All patients provided signed, informed consent for their tissues to be used for scientific research.
Cell culture
Human GC cell lines (AGS, BGC-823, SGC-7901, MGC-803) were obtained respectively from Cell Library, China Academy of Science (Shanghai, China) and the cells were cultured in RPMI 1640 (Sigma-Aldrich, St. Louis, MO, USA), with 10% fetal bovine serum (Gibco-BRL, Grand Island, NY, USA). Human normal gastric epithelium cell line GES-1 was purchased from Cell Library, China Academy of Science and cultured in DMEM (Sigma-Aldrich, St. Louis, MO, USA) with 10% fetal bovine serum at 37°C in a 5% CO2 incubator.
Vector construction and cell transfection
Human full-length Gpx3 was cloned into the pcDNA™3.1 vector (Thermo Fisher). Plasmid transfections were carried out using Fugene HD (Promega) according to the manufacturer’s protocol. The cells were seeded onto 6-well culture plates at a density of 2×105 cells/well and transfected with the specific siRNAs for Gpx3 or controls (Genepharma, Shanghai, China) at a concentration of 50 nM by using Lipofectamine TM 2000 (Invitrogen, Shanghai, China) according to the manufacturer’s protocol.
Cell invasion and migration assay
Cell invasion and migration activity were analyzed by using 24-well trans-well chambers coated with or without Matrigel (BD Biosciences) on the upper surface of the membrane with a pore size of 8 μm (Sigma). Briefly, cells (1×104 cells/well) were suspended in culture media and added into the upper trans-well chamber. The lower chamber was soaked with 500 μL DMEM medium containing 10% FBS. After incubation for 16 h, the chambers were washed three times and fixed with 4% paraformaldehyde for 10 min. Finally, the chambers were stained with crystal violet and the cells which passed through the membrane were counted visually under a microscope (OLYMPUS).
Western blot analysis and antibodies
The proteins were first subjected to SDS polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred onto polyvinylidene difluoride (PVDF) filter membranes (Millipore, Bedford, MA, USA). After blocking with PBS containing 5% non-fat milk and 0.1% Tween-20 for 2 hours, the membranes were then incubated with the primary antibodies overnight at 4°C. Then the membranes were washed three times with PBST for 5 minutes each time and incubated with horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. Finally, the membranes were scanned by the ECL detection system. The band density was quantified using the Image J analysis system (Wayne Rasband, National Institutes of Health, USA). The primary antibodies included: anti-Gpx3 antibody (ab104448; 1:1000; Abcam), anti-Ecad antibody (14472; 1:1000; Cell Signaling Technology), anti-Ncad antibody (D4R1H; 1:1000; Cell Signaling Technology), anti-pβ-catinin antibody (D10A8; 1:1000; Cell Signaling Technology), anti-β-catinin antibody (D2F1; 1:1000; Cell Signaling Technology), anti-pJNK antibody (9255; 1:1000; Cell Signaling Technology), anti-JNK antibody (9258; 1:1000; Cell Signaling Technology), anti-GSK-3β antibody (D5C5Z; 1:1000; Cell Signaling Technology), anti-pGSK-3β antibody (D85E12; 1:1000; Cell Signaling Technology), anti-β-actin antibody (8H10D10; 1:1000; Cell Signaling Technology). The experiments were carried out on three separate occasions.
EC lung metastasis mouse model
GC lung metastases mouse model were established by tail vein injections of AGC cells overexpressing Gpx3 (3×105 in 0.1 ml PBS) into nude mice. The mice were anesthetized and euthanized 25 days later. The lungs were excised, fixed and paraffin embedded for counting of macroscopic lung metastases. All experimental procedures involving animals were performed according to the Principles of Laboratory Animal Care (NIH publication no. 85-23, revised 1985) and/or the Declaration of Helsinki promulgated in 1964 as amended in 1996.
Statistical analysis
Each experiment was repeated at least three times. All values are reported as means ± SEM. The calculations were analyzed with the Statistical Package for the Social Science SPSS 20.0 software (SPSS Inc., An IBM Company, Chicago, IL, USA). The probability of 0.05 or less was considered statistically significant.
Results
Gpx3 is downregulated in GC tissues and GC cell lines
Expression of Gpx3 was confirmed in 13 gastric cancer and paired adjacent normal tissues by western blot analysis. Gpx3 was downregulated in gastric cancer tissues compared with paired adjacent normal tissues (Figure 1A and 1B). We then assayed the expression level of Gpx3 in GC cell lines versus normal gastric epithelium cell line GES-1. The results showed that Gpx3 was downregulated in GC cell lines when compared with normal gastric epithelium cell line GES-1 (Figure 1C and 1D).
Figure 1.
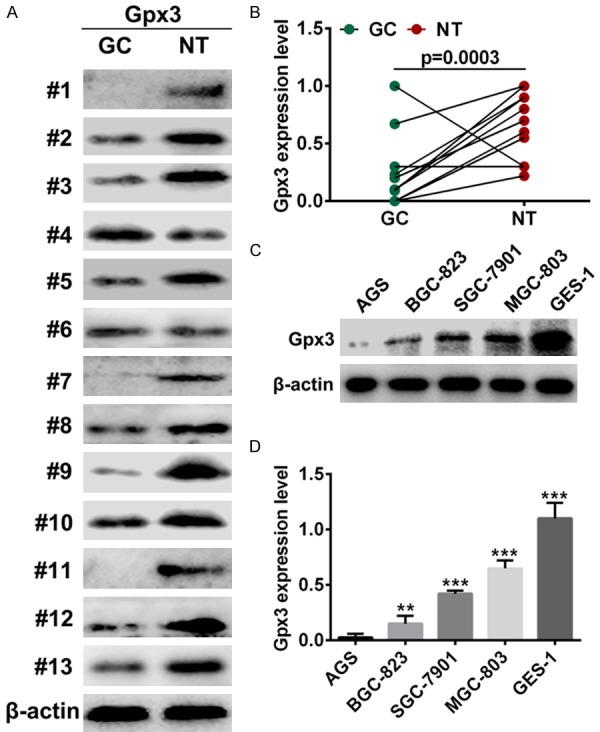
Decreased Gpx3 expression in GC patients and cell lines. A, B. Western blot assays revealed the protein level of Gpx3 in 13 pairs of GC cases and normal tissues. C, D. The expression of Gpx3 in GC cell lines and normal cells “**” denotes P < 0.01. “***” denotes P < 0.001. Data are expressed as the mean ± SD.
Gpx3 regulates GC cell migration and invasion in vitro and in vivo
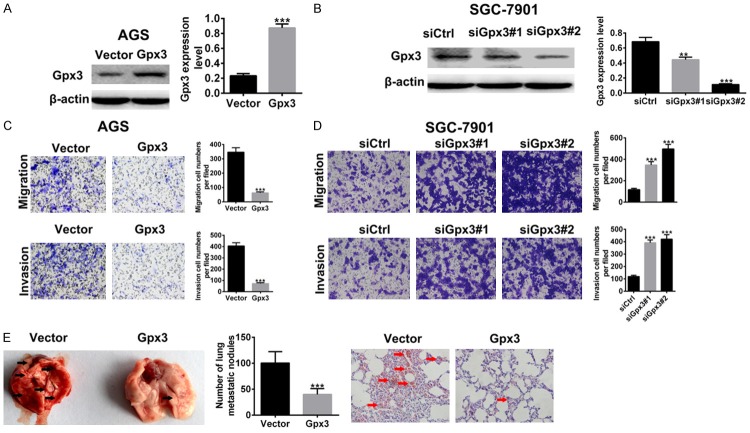
To determine the role of Gpx3 in GC migration and invasion in vitro, cell migration and invasion assay was conducted in AGS cells overexpessing Gpx3 and SGC-7901 cells with Gpx3 knockdown. The efficiency of the Gpx3 silencing and overexpression was confirmed by western blot (Figure 2A and 2B). As shown in Figure 2C, overexpression of Gpx3 caused inhibition of cell migration and invasion in AGS cells, while silencing of Gpx3 let to increase in cell migration and invasion in SGC-7901 cells (Figure 2D). Furthermore, we tested the effect of Gpx3 on GC metastasis using a xenograft model. The AGS cells overexpressing Gpx3 were implanted via tail vein of NCG mice and the number of lung metastatic foci were evaluated 25 days later. A significantly lower number of lung metastatic foci could be observed for Gpx3-overexpressing AGS cells compared with control cells. The lung metastases were also validated in H&E stained sections of lung (Figure 2E). The results above demonstrated that Gpx3 acted as metastasis suppressor in GC.
Figure 2.
Gpx3 suppressed GC cell migration, invasion, and metastasis. A. Protein expression of Gpx3 was detected in AGS cells when overexpressed with Gpx3 by using western blot. B. The protein expression of Gpx3 was detected in SGC-7901 cells when transfected with the specific siRNA of Gpx3 by using western blot. C. Cell migration and invasion of AGS cells was analyzed after overexpression of Gpx3 for 72 h by using transwell chamber assay, respectively. D. The cell migration and invasion of SGC-7901 cells after transfection with the specific siRNA of Gpx3 for 72 h by using transwell chamber assay, respectively. E. AGS cells overexpressing Gpx3 were i.v. injected into NCG animals (n=6 per group). The lung metastatic loci were counted and lung sections were subjected to H&E staining. “**” denotes P < 0.01. “***” denotes P < 0.001. Data are expressed as the mean ± SD.
Gpx3 regulates EMT in GC cells
Due to the pivotal role of EMT in tumor metastasis, EMT features including changes in the epithelial marker such as Ecadherin and mesenchymal markers including N-cadherin and vimentin was assessed upon changes of Gpx3 in GC cells. Overexpression of Gpx3 dramatically induced increase in E-cadherin expression but decrease in expression of N-cadherin and vimentin in AGS cells (Figure 3A). Conversely, downregulation of Gpx3 resulted in decrease of E-cadherin and increase of N-cadherin and vimentin in SGC-7901 cells (Figure 3B). Taken together, Gpx3 is involved in the regulation of EMT in GC cells.
Figure 3.
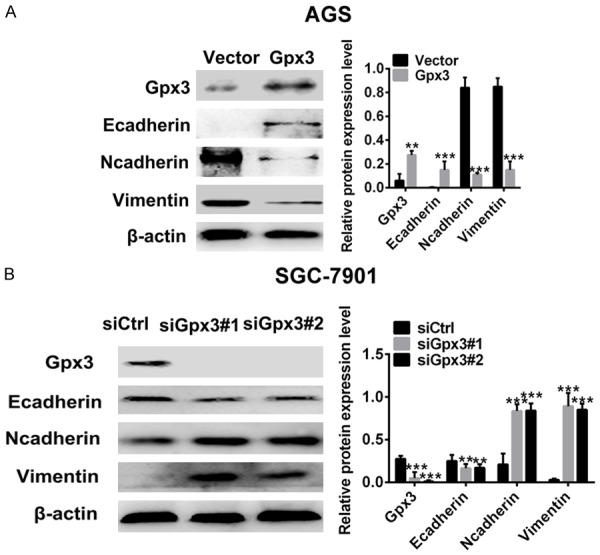
Gpx3 regulated EMT in GC cells. A. Protein expression of Gpx3 and EMT markers was detected in AGS cells when overexpressed with Gpx3 by using western blot. B. The protein expression of Gpx3 and EMT markers was detected in SGC-7901 cells when transfected with the specific siRNA of Gpx3 by using western blot. “**” denotes P < 0.01 and “***” denotes P < 0.001 Data are expressed as the mean ± SD.
Gpx3 regulates WNT/JNK signaling in GC cells
To further explore the underlying mechanism of Gpx3 in cell migration and invasion, the effect of Gpx3 on Wnt signaling pathway was evaluated by measuring the changes of canonical and non-canonical signaling pathways. The results showed that overexpression of Gpx3 inhibited JNK/c-Jun signaling in AGS cells, whereas silencing of Gpx3 enhanced JNK/c-Jun signaling in SGC-7901 cells (Figure 4A and 4B). However, Gpx3 was found to have no obvious effect on β-catenin/GSK-3β signaling (Figure 4A and 4B). Collectively, the data above indicated that Gpx3 selectively regulated the non-canonical Wnt/JNK signaling pathway over the β-catenin/GSK-3β canonical signaling pathway.
Figure 4.
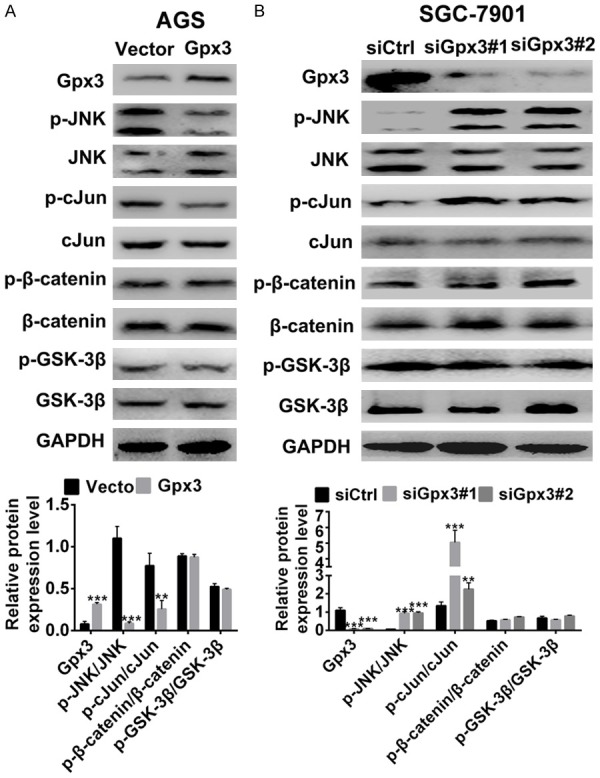
Gpx3 regulated Wnt/JNK signaling in GC cells. A. Protein expression of Gpx3, β-catenin and JNK signaling were detected in AGS cells when overexpressed with Gpx3 by using western blot. B. Protein expression of Gpx3, β-catenin, and JNK signaling were detected in SGC-7901 cells when transfected with the specific siRNA of Gpx3 by using western blot. “**” denotes P < 0.01 and “***” denotes P < 0.001. Data are expressed as the mean ± SD.
Gpx3 regulates WNT5A/JNK signaling via NFкB in GC cells
To understand the regulation of Wnt/JNK/c-Jun signaling by Gpx3, we hypothesized that Gpx3 regulates the Wnt5a expression mediated by NFκB. As shown in Figure 5A, knocking down of Gpx3 was able to upregulate the expression of Wnt5a. However, silencing p65 resulted in abolishment of Wnt5a upregulation by Gpx3. Moreover, treatment with NFкB inhibitor PDTC reversed upregulation of phosphorylation level of JNK by Gpx3 as well. In summary, the data above indicated that Gpx3 regulated the non-canonical Wnt/JNK signaling pathway by NFкB.
Figure 5.
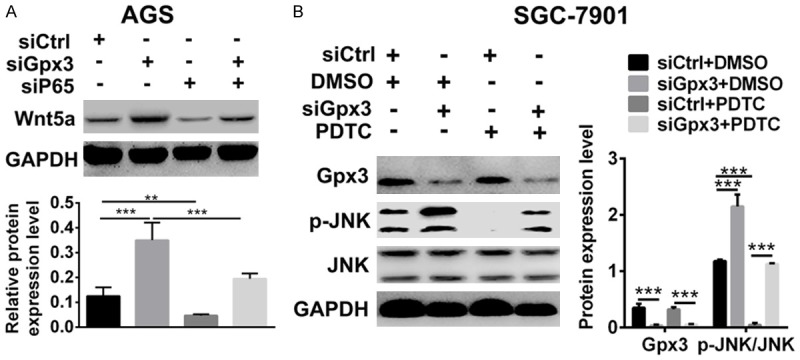
Gpx3 regulated Wnt/JNK signaling via NFкB in GC cells. A, B. Protein expression of Wnt5a was detected in SGC-7901 cells when transfected with the specific siRNA of Gpx3 or control siRNA in the absence or presence of transfection of specific siRNA of p65 or control siRNA by using western blot. B. Protein expression of JNK and p-JNK were detected in SGC-7901 cells when transfected with the specific siRNA of Gpx3 or control siRNA in the absence or presence of PDTC (2 μM) by using western blot. “**” denotes P < 0.01 and “***” denotes P < 0.001. Data are expressed as the mean ± SD.
Gpx3 regulates cell migration and invasion through NFкB/Wnt5a/JNK signaling in GC cells
To further investigate whether Gpx3 regulates cell migration and invasion by NFкB/WNT5a/JNK signaling in GC cells, blockade of NFкB and JNK were utilized for cell treatment. The results showed that PDTC treatment abolished the Gpx3 silencing-induced increase in cell migration and invasion in SGC-7901 cells (Figure 6A). Similar to the results with PDTC, JNK inhibitor SP600125 could also abolish the Gpx3 silencing-induced increase in cell migration and invasion (Figure 6C). Moreover, Gpx3 overexpression was able to reduce cell migration and invasion. However, Wnt5a treatment reversed Gpx3 induced cell migration and invasion (Figure 6C).
Figure 6.
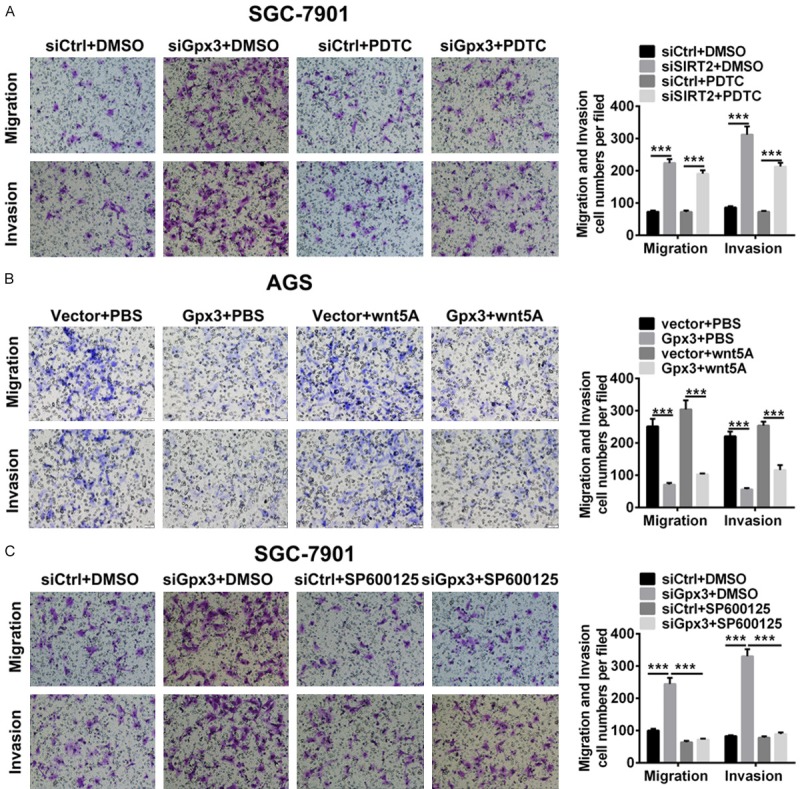
Gpx3 regulated cell migration by NFкB/ wnt/JNK signaling in GC cells. A. Cell migration and invasion were detected in SGC-7901 cells when transfected with the specific siRNA of Gpx3 or control siRNA in the absence or presence of transfection of specific siRNA of p65 or control siRNA for 72 h by transwell chamber assay. B. Cell migration and invasion were detected in SGC-7901 cells when transfected with the specific siRNA of Gpx3 or control siRNA in the absence or presence of PDTC (2 μM) by transwell chamber assay. C. Cell migration and invasion were detected in AGC cells when overexpressed Gpx3 or control vector in the absence or presence of treatment of Wnt5a (100 ng/ml) by transwell chamber assay. “***” denotes P < 0.001. Data are expressed as the mean ± SD.
Discussion
Gastric carcinoma (GC) is the second leading cause of cancer-related death worldwide and fourth most common malignant tumor [4]. In China, GC has an estimated 380000 new cases annually. Metastasis, the hallmark of GC, results in a high mortality rate and a poor prognosis [2]. GC metastasis is composed of multiple biologic processes, which is a complex progression [5]. Therefore, the understanding of comprehensive mechanisms of GC metastasis could assist early diagnosis and therapy.
Gpx3 showed highly selective expression in normal human tissues but dramatic reduction in multiple tumor types, including gastric cancer [10]. The epigenetic mechanism regulating Gpx3 expression has been extensively studied. DNA copy number loss and promoter hypermethylation as possible mechanisms mediating silencing of Gpx3 in gastric cancers has been reported [12]. These data corroborate a role for Gpx3 in cancer and pinpoint it as a bona fide tumor suppressor in gastric cancer. Consistent with previous reports, in this study Gpx3 was confirmed to be downregulated in gastric tumor tissues and cells while expressed at comparatively reduced levels in normal samples and cells.
It has been reported that down-regulation of Gpx3 may be related to tumor metastasis [22]. For example, Gpx3 was found to suppress tumor migration and invasion by the FAK/AKT pathway in esophageal squamous cell carcinoma [23]. Over-expression of GPx3 or administration of rGPx3 significantly inhibited proliferation and invasiveness of HCC cells in vitro and in vivo [11]. Thus, we are interested in the effect of Gpx3 on GC metastasis. The data showed that Gpx3 inhibited cell migration and invasion capability in vitro as well as suppressed the lung metastatic loci in metastasis experimental animal model. The role of EMT in gastric carcinogenesis has been demonstrated extensively [24]. Consequently, a systematic exploration of the role of EMT in GC could deepen our understanding of GC tumorigenesis and progression. Aberrant activation of Wnt signaling pathways including canonical signaling and non-canonical signaling are implicated in EMT during tumor initiation and progression [16]. Interestingly, we found that Gpx3 inhibited EMT and metastasis via regulation of Wnt non-canonical signaling pathway but sparing canonical wnt/β-catenin signaling in GC cells.
Qi et al. demonstrated that NFκB nuclear translocation was significantly attenuated upon GPx3 administration, indicating that activation of NFκB could be inhibited by GPx3 treatment [11]. Wnt5a, a non-transforming Wnt family member, is an activator of the non-canonical Wnt signaling pathway and it is commonly linked with EMT in tumor metastasis [25]. Upregulation of Wnt5a were frequently reported and correlated with aggressiveness of gastric tumors [26]. Wnt5a transcription is regulated by many proteins, included NFκB signaling pathway [27]. Wnt5a expression induced GC metastasis by several mechanisms [28]. Overexpression of Wnt5a expression into MKN-7 cells induced high expression of genes related to EMT and cancer stem cells (CSCs) [26]. In this study we found that Gpx3 regulated Wnt5a expression by NFκB signaling in GC cells. Furthermore, we also found that Wnt5a induced GC cell migration by the JNK singling pathway.
Collectively, in this study we demonstrated that Gpx3 was downregulated when compared with normal tissues and cells. The in vitro and in vivo results provide evidence that Gpx3 participated in the cell migration, invasion, and metastasis of GC. Gpx3 functions as an GC metastasis inhibitor by blocking the NFκB/Wnt/JNK pathway. Gpx3 may be a candidate for the development of a targeted gastric cancer therapy.
Acknowledgements
This work was supported by Shandong Provincial Natural Science Foundation, China (Grant No. BS2015YY017), Applied Basic Research Priorities Program of Qingdao (Grant No. 15-9-140-jch), Key Research and Development Project in Shandong Province (Grant No. 2017GSF218108), National Natural Science Fund (No. 81602271).
Disclosure of conflict of interest
None.
References
- 1.Pasechnikov V, Chukov S, Fedorov E, Kikuste I, Leja M. Gastric cancer: prevention, screening and early diagnosis. World J Gastroenterol. 2014;20:13842–13862. doi: 10.3748/wjg.v20.i38.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barati N, Momtazi-Borojeni AA, Majeed M, Sahebkar A. Potential therapeutic effects of curcumin in gastric cancer. J Cell Physiol. 2019;234:2317–2328. doi: 10.1002/jcp.27229. [DOI] [PubMed] [Google Scholar]
- 3.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singapore Med J. 2014;55:621–628. doi: 10.11622/smedj.2014174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon H, Kim N. Diagnosis and management of high risk group for gastric cancer. Gut Liver. 2015;9:5–17. doi: 10.5009/gnl14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou JD, Yao DM, Zhang YY, Ma JC, Wen XM, Yang J, Guo H, Chen Q, Lin J, Qian J. GPX hypermethylation serves as an independent prognostic biomarker in non-M3 acute myeloid leukemia. Am J Cancer Res. 2015;5:1786–1794. [PMC free article] [PubMed] [Google Scholar]
- 7.Herault O, Hope KJ, Deneault E, Mayotte N, Chagraoui J, Wilhelm BT, Cellot S, Sauvageau M, Andrade-Navarro MA, Hébert J, Sauvageau G. A role for GPx3 in activity of normal and leukemia stem cells. J Exp Med. 2012;209:895–901. doi: 10.1084/jem.20102386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi X, Ng KT, Shao Y, Li CX, Geng W, Ling CC, Ma YY, Liu XB, Liu H, Liu J, Yeung WH, Lo CM, Man K. The clinical significance and potential therapeutic role of GPx3 in tumor recurrence after liver transplantation. Theranostics. 2016;6:1934–1946. doi: 10.7150/thno.16023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.An BC, Choi YD, Oh IJ, Kim JH, Park JI, Lee SW. GPx3-mediated redox signaling arrests the cell cycle and acts as a tumor suppressor in lung cancer cell lines. PLoS One. 2018;13:e0204170. doi: 10.1371/journal.pone.0204170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Yang JJ, Kim YS, Kim KY, Ahn WS, Yang S. An 8-gene signature, including methylated and down-regulated glutathione peroxidase 3, of gastric cancer. Int J Oncol. 2010;36:405–414. [PubMed] [Google Scholar]
- 11.Qi X, Ng KT, Lian QZ, Liu XB, Li CX, Geng W, Ling CC, Ma YY, Yeung WH, Tu WW, Fan ST, Lo CM, Man K. Clinical significance and therapeutic value of glutathione peroxidase 3 (GPx3) in hepatocellular carcinoma. Oncotarget. 2014;5:11103–11120. doi: 10.18632/oncotarget.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng DF, Hu TL, Schneider BG, Chen Z, Xu ZK, El-Rifai W. Silencing of glutathione peroxidase 3 through DNA hypermethylation is associated with lymph node metastasis in gastric carcinomas. PLoS One. 2012;7:e46214. doi: 10.1371/journal.pone.0046214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao H, Li J, Li X, Han C, Zhang Y, Zheng L, Guo M. Silencing GPX3 expression promotes tumor metastasis in human thyroid cancer. Curr Protein Pept Sci. 2015;16:316–321. doi: 10.2174/138920371604150429154840. [DOI] [PubMed] [Google Scholar]
- 14.Pelosof L, Yerram S, Armstrong T, Chu N, Danilova L, Yanagisawa B, Hidalgo M, Azad N, Herman JG. GPX3 promoter methylation predicts platinum sensitivity in colorectal cancer. Epigenetics. 2017;12:540–550. doi: 10.1080/15592294.2016.1265711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Zheng Z, Yingji S, Kim H, Jin R, Renshu L, Lee DY, Roh MR, Yang S. Downregulation of glutathione peroxidase 3 is associated with lymph node metastasis and prognosis in cervical cancer. Oncol Rep. 2014;31:2587–2592. doi: 10.3892/or.2014.3152. [DOI] [PubMed] [Google Scholar]
- 16.Lerner UH, Ohlsson C. The WNT system: background and its role in bone. J Intern Med. 2015;277:630–649. doi: 10.1111/joim.12368. [DOI] [PubMed] [Google Scholar]
- 17.Astudillo P, Larrain J. Wnt signaling and cell-matrix adhesion. Curr Mol Med. 2014;14:209–220. doi: 10.2174/1566524014666140128105352. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Wang W, Zhang N, Ma T, Zhao C. IL-1beta mediates MCP-1 induction by Wnt5a in gastric cancer cells. BMC Cancer. 2014;14:480. doi: 10.1186/1471-2407-14-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng Z, Wang CX, Fang EH, Wang GB, Tong Q. Role of epithelial-mesenchymal transition in gastric cancer initiation and progression. World J Gastroenterol. 2014;20:5403–5410. doi: 10.3748/wjg.v20.i18.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L, Wu RL, Xu AM. Epithelial-mesenchymal transition in gastric cancer. Am J Transl Res. 2015;7:2141–2158. [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Lin L, Jin Y, Lin Y, Cao Y, Zheng C. Overexpression of WNT5B promotes COLO 205 cell migration and invasion through the JNK signaling pathway. Oncol Rep. 2016;36:23–30. doi: 10.3892/or.2016.4772. [DOI] [PubMed] [Google Scholar]
- 22.Yang ZL, Yang L, Zou Q, Yuan Y, Li J, Liang L, Zeng G, Chen S. Positive ALDH1A3 and negative GPX3 expressions are biomarkers for poor prognosis of gallbladder cancer. Dis Markers. 2013;35:163–172. doi: 10.1155/2013/187043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang LL, Zhan L, Jin YD, Min ZL, Wei C, Wang Q, Chen YJ, Wu QM, Hu XM, Yuan Q. SIRT2 mediated antitumor effects of shikonin on metastatic colorectal cancer. Eur J Pharmacol. 2017;797:1–8. doi: 10.1016/j.ejphar.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Davis FM, Stewart TA, Thompson EW, Monteith GR. Targeting EMT in cancer: opportunities for pharmacological intervention. Trends Pharmacol Sci. 2014;35:479–488. doi: 10.1016/j.tips.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Saitoh T, Mine T, Katoh M. Frequent up-regulation of WNT5A mRNA in primary gastric cancer. Int J Mol Med. 2002;9:515–519. [PubMed] [Google Scholar]
- 26.Kanzawa M, Semba S, Hara S, Itoh T, Yokozaki H. WNT5A is a key regulator of the epithelial-mesenchymal transition and cancer stem cell properties in human gastric carcinoma cells. Pathobiology. 2013;80:235–244. doi: 10.1159/000346843. [DOI] [PubMed] [Google Scholar]
- 27.Rothgiesser KM, Erener S, Waibel S, Luscher B, Hottiger MO. SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci. 2010;123:4251–4258. doi: 10.1242/jcs.073783. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto H, Kitadai Y, Yamamoto H, Oue N, Ohdan H, Yasui W, Kikuchi A. Laminin gamma2 mediates Wnt5a-induced invasion of gastric cancer cells. Gastroenterology. 2009;137:242–252. doi: 10.1053/j.gastro.2009.02.003. [DOI] [PubMed] [Google Scholar]