Abstract
Indolent T-lymphoblastic proliferation (IT-LBP) is referred to as extrathymic immature TDT+T-cell hyperplasia, non-neoplastic lesion, often misdiagnosed as T-lymphoblastic lymphoma and overtreated. We report a case of a 20-year-old male patient with a right adrenal gland mass, diagnosed as indolent T-lymphoblastic proliferation (IT-LBP) associated with hyaline vascular Castleman disease (HV-CD) and low grade follicular dendric cell sarcoma (LG-FDCS). The case is a rare combination of finding, and it is the first case occurring in adrenal gland. IT-LBP is a clinically indolent disease, requiring no treatment, often associated with other tumors. Because of the high ki67 index, IT-LBP is easily misdiagnosed as T-lymphoblastic lymphoma, causing overtreatment. Understanding the biological behavior, treatment, prognosis and the associated diseases of IT-LBP is important.
Keywords: Indolent T-lymphoblastic proliferation, Castleman disease, low grade follicular dendric cell sarcoma, associated with
Introduction
Indolent T-lymphoblastic proliferation (IT-LBP) is the immature TDT+T cell proliferation from the extrathymic lymphoid tissues. The pathological change is of a primitive small lymphocyte nonclonal proliferation, consistent with the cortical thymocyte immunophenotype (TDT+CD3+CD99+CD4+CD8+CD1a+CD34-), not thymic epithelium. Because of the high proliferation index (Ki67 index of 40%-90%), it is often misdiagnosed as T lymphoblastic lymphoma [1]. In the literature, IT-LBP has been reported to be associated with Castleman disease (CD), follicular dendric cell sarcoma (FDCS), angioimmunoblastic T-cell lymphoma (AITL), myasthenia gravis (MG), hepatocellular carcinoma (HCC), and acinic cell carcinoma (ACC). To the best of our knowledge, there are only 15 cases [1-14] reported, and the pathogenesis of this rare disease remains unclear. Some researchers hypothesized that the autoimmune dysregulation and cytokine homeostasis may play a part. Up to now, there have been reports about IT-LBP in the upper aerodigestive tract, oropharynx, cervical lymph nodes, retroperitoneum, neck and inguinal lymph nodes, and parotid gland. However, there is no report occurring in the adrenal gland. We report a case of IT-LBP associated with CD and LG-FDCS in the adrenal gland, along with literature review, to deepen understanding.
Case report
A 20-year-old male patient was detected with a right adrenal gland mass for 4 days. CT and MRI showed the border-clear mass in the right adrenal gland, about 52 mm × 50 mm × 57 mm in size. CT showed the right adrenal region mass shadow (Figure 1A) which was considered to be a pheochromocytoma originating from the adrenal. There was no other occupying lesion and enlarged lymph nodes. MRI demonstrated the mass in the right adrenal gland, showing slightly longer T1 and T2 signal, and obviously inhomogeneous in the arterial phase and markedly evenly enhanced in the venous phase on enhancement scanning (Figure 1B). Subsequently, the mass was removed and sent for pathological examination.
Figure 1.
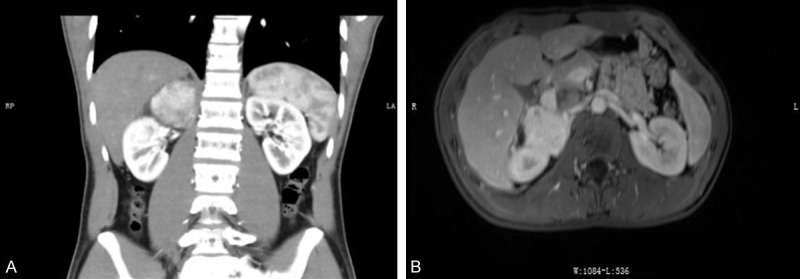
CT and MRI scan of the right adrenal gland. A. The CT scan; B. The MRI scan.
Materials and methods
The specimens were fixed with 4% neutral formaldehyde, routinely taken, embedded in paraffin, sliced with 4 μm thickness, stained with H&E, and observed under light microscope and photographed by BX53F Olympus. Immunohistochemical staining was performed by EnVision two-step method, antibodies TDT, CD3, CD99, CD4, CD8, CD1a, CD34, CD20, CD23 and CD21 were purchased from Maixin. PAS staining was performed according to the operation instructions. The TCR gene rearrangement studies were as follows: DNA isolated from formalin-fixed paraffin-embedded tissues was digested overnight using HPA II restriction enzyme, then used as template for PCR reaction; The polymerase chain reaction products were analyzed by an ABI7500 with date analysis using a Peak scanner 2.
Pathological findings
The gross appearance had circumscribed borders and medium-sized rounded shape, 50 mm × 50 mm × 47 mm in size.
Microscopically, the H&E-stained sections showed the following characteristics: 1. The remaining follicular germinal center showed hyaline vessels and the mantle zone had onion-like hyperplasia (Figure 2A); 2. In the interfollicular region, focal slight atypia spindle cells arranged in a swirling pattern (Figure 2B); 3. A large number of immature and deeply stained small lymphocytes infiltrated between the interfollicular areas and spindle cells (Figure 2C).
Figure 2.
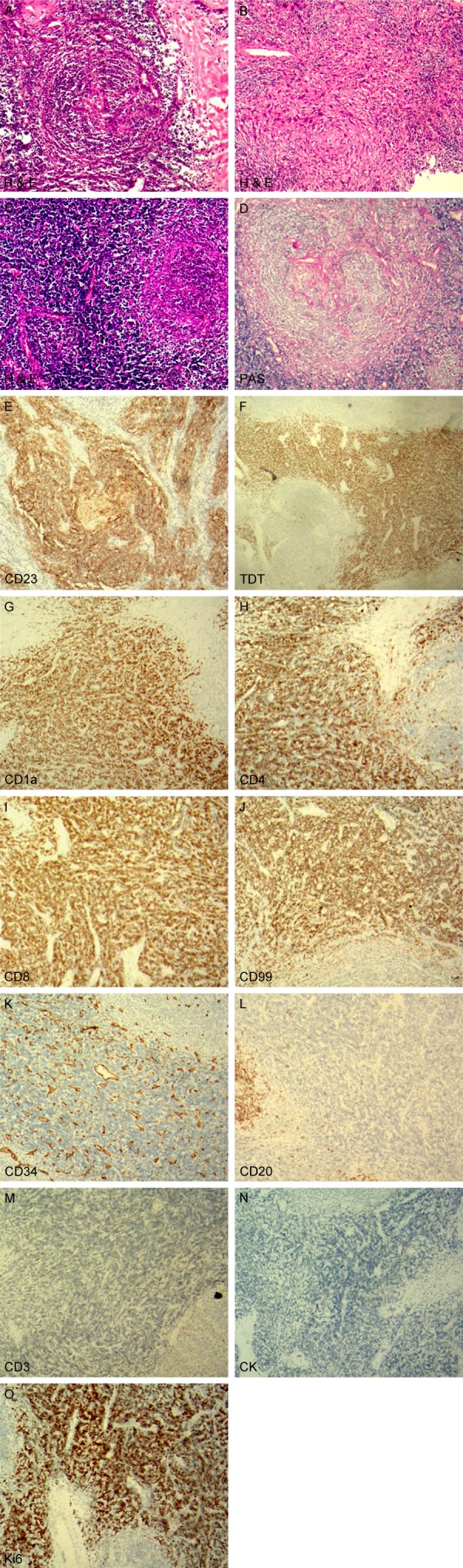
Microscopic features of the tumor. (A-C) H&E staining presented follicular germinal center hyaline vessels and the mantle zone onion-like hyperplasia (A), and focal slight atypia spindle cells arranged in a swirling pattern (B). A large number of immature small lymphocytes infiltrated in the interfollicular areas and spindle cells (C); (D) PAS staining depicted hyaline vessels in the follicular germinal center; (E-O) Immunohistochemical staining appearance of the tumors.
PAS staining and immunophenotype: PAS staining depicted hyaline vessels in the follicular germinal center (Figure 2D). Immunophenotypically, the proliferative spindle cells were positive for CD23, and CD21 (Figure 2E). The immature small lymphocytes in the interfollicular area and around the spindle cells were positive for TDT (Figure 2F), CD1a (Figure 2G), CD4 (Figure 2H), CD8 (Figure 2I), and CD99 (Figure 2J), and negative for CD34 (Figure 2K), CD20 (Figure 2L), and CD3 (Figure 2M), which is consistent with normal cortical thymocytes at the intermediate stage of Tcell differentiation but without thymic epithelial cells (negative for CK) (Figure 2N). The ki67 index was 80% (Figure 2O).
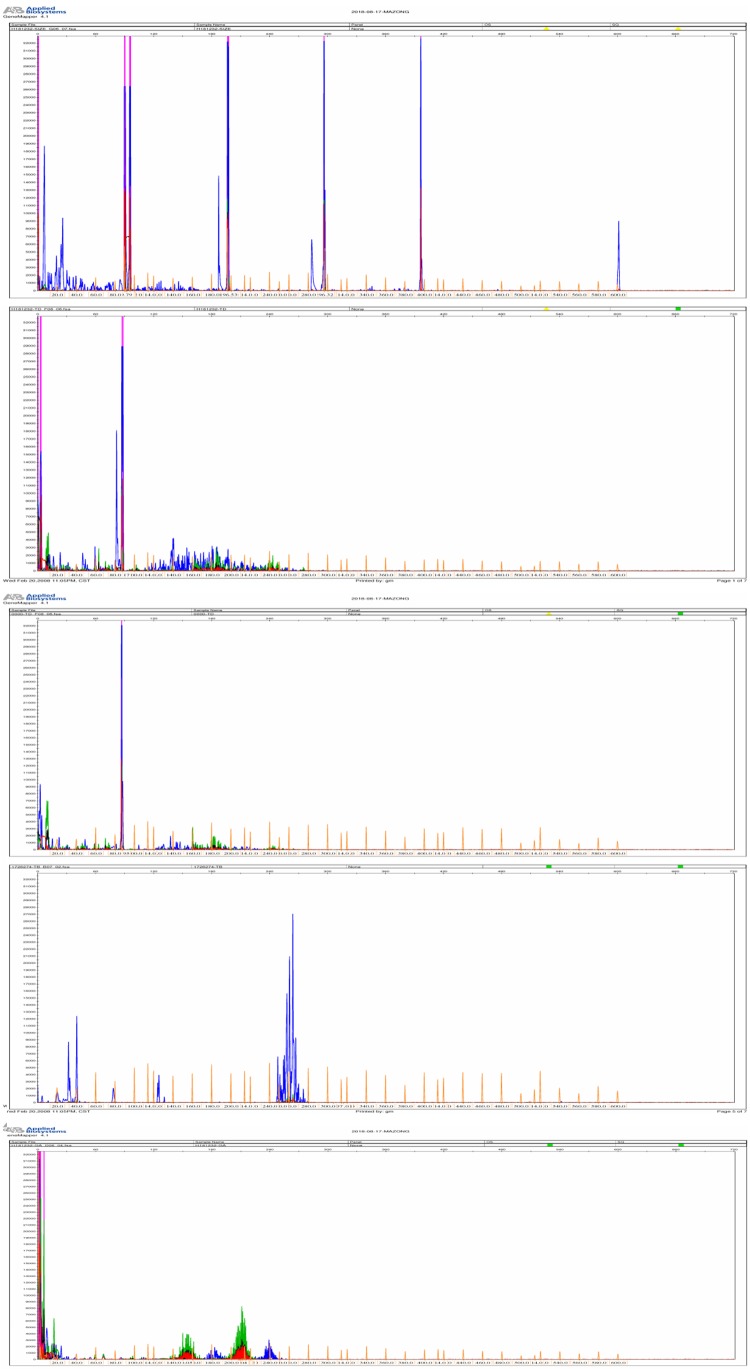
The TCR gene rearrangement studies proved that there was no clonal rearrangement in the TCRγ gene and TCRβ genes (Figure 3).
Figure 3.
The TCR gene rearrangement studies proved that there was no clonal rearrangement in the TCRγ gene and TCRβ gene.
To sum up, we made a diagnosis of indolent T-lymphoblastic proliferation associated with hyaline vascular Castleman disease and low grade follicular dendric cell sarcoma.
Clinical follow-up
We followed up the patient for 6 months and found no relapse or other lymphadenopathy without any treatment.
Discussion
IT-LBP is a non-neoplastic lesion, with the immature TDT+T cell nonclonal proliferation from the extrathymic lymphoid tissues, consistent with the cortical thymocyte immunophenotype (TDT+CD3+CD99+CD4+CD8+CD1a+CD34-), absence of thymic epithelium. In clinical practice, it is easily misdiagnosed as T-lymphoblastic lymphoma. Furthermore, ITLBP can be associated with CD, FDCS, AITL, HCC, ACC, and so on. Ohgami et al. [2]. discovered the number of TDT+T cells/HPF was various depending on the associated tumors, the number of TDT+T cells/HPF was more in association with both CD and FDCS (>1000/HPF, P=0.011), compared in CD, FDCS and reactive follicular hyperplasia. The reasons for this difference is unclear.
Up to now, a total of 15 cases have been reported since the first report of IT-LBP by Velankar et al. (Table 1). According to Table 1, it can be seen that IT-LBP is more prone to occur in lymph nodes, and mostly associated with CD. The ratio of male to female was 9:6, the age of onset was 13-71 years, and the median age was 40.4 years. Of these cases, two cases were misdiagnosed as T lymphoblastic lymphoma and accepted unnecessary chemotherapy. In the literature, the prognosis of IT-LBP is good only by surgical excision without any treatment. Only one patient died of sarcoma without treatment. Another case relapsed from a 13-year-old girl in inguinal lymph node, which was reported by Kansal in 2015. Tumor relapsed for three times in the original site within five years. There was no malignant indicator of having a relapse. The pathogenesis of IT-LBP remains unclear. Some scholars believe that it is related to autoimmune disorders and cytokine homeostasis changes, such as the IL-6 promoting the release of immature thymic cells home to lymph nodes [6]. We hypothesize that it may also be related to the associated tumors which can stimulate the secretion of certain chemokines to promote immature TDT+T cells proliferation. Of course, these are only hypotheses, and the mechanism needs further study.
Table 1.
Clinical characteristics of 15 patients with indolent T-lymphoblastic proliferation
| Feature | N (%) |
|---|---|
| Age | 40.4 (13~71 years) |
| Gender | |
| Male | 9 (60%) |
| Female | 6 (40%) |
| Associated disease | |
| None [1,3] | 2 (13%) |
| CD [2,4-6] | 4 (26%) |
| HCC [7,8] | 2 (13%) |
| ACC [2,9] | 2 (13%) |
| FDCS [10] | 1 (7%) |
| MG [11] | 1 (7%) |
| Upper respiratory tract illness [12] | 1 (7%) |
| AITL [2] | 1 (7%) |
| CD+FDCS [13] | 1 (7%) |
| Primary site | |
| Lymph node | 6 (40%) |
| Upper aerodigestive tract | 2 (13.3%) |
| Liver | 2 (13.3%) |
| Parotid gland | 2 (13.3%) |
| Retroperitoneal mass | 2 (13.3%) |
| Abdominal mass | 1 (6.7%) |
| Follow-up (months) | 50 (1 month~192 months) |
| Alive | 14 (93.3%) |
| Die | 1 (6.7%) |
CD: Castleman disease; HCC: hepatocellular carcinoma; ACC: acinic cell carcinoma; FDCS:follicular dendric cell sarcoma; MG: myasthenia gravis; AITL: angioimmunoblastic T-cell lymphoma.
Ohgami et al. [14]. have proposed diagnostic criteria for IT-LBP in 2013, including these points: (1) TDT+/CD3+ T cell in sheets or dense clusters primarily in interfollicular regions; (2) Preservation of general follicular lymphoid architecture; (3) Small-sized to medium-sized T cells without significant morphologic atypia; (4) No aberrant antigen expression; (5) Nonclonal TDT+ T cells; (6) No associated thymic epithelium; (7) Clinical evidence of indolence, >6 mo follow-up without significant progression in the absence of treatment.
With IT-LBP, a major concern is misdiagnosis as T-lymphoblastic lymphoma for the high ki67 index. The TCR gene rearrangement studies can avoid a misdiagnosis. In addition, another main differential diagnosis is thymoma, which can be ruled out by CK staining. Negative results for CK can demonstrate an absence of thymic epithelium
In our case, the primary physician misdiagnosed T-lymphoblastic lymphoma (T-LBL) for the high ki67 index. Fortunately, we stopped the error by searching literature. Our case of IT-LBP occurred in the adrenal gland, which has not been reported by now, and was associated with both CD and LG-FDCS, a rare combination of findings. Microscopically, the atrophic follicular germinal center showed hyaline vessels, slightly atypical spindle cell sheet hyperplasia, and dense immature small lymphocyte clusters hyperplasia in the interfollicular zone (>1000/HPF, consistent with Ohgami’s findings) [12]. Immunohistochemistry showed CD21 and CD23 positive in spindle cells, TDT+CD99+CD4+CD8+CD1a+ in immature small lymphocytes, but CD34-CD3-CD20-. According to the diagnostic criteria, the immature small lymphocytes should be positive for both TDT and CD3, but CD3 expression was absent in our case. In earlier studies, Strauchen [11] had reported to be lacking CD3 and CD79a expression in immature small lymphocytes due to the immature differentiation, this view explained why CD3 expression was absent in our case. Molecular studies confirm that immature lymphocytes are not clonal. Histomorphology, immunophenotype and molecular research support the diagnosis of IT-LBP associated with HV-CD and LG-FDCS.
In summary, we report a case of IT-LBP associated with HV-CD and LG-FDCS, along with a literature review, to deepen the understanding of IT-LBP. It will help avoid misdiagnosis and overtreatment to understand IT-LBP and its common associated diseases for clinicians and pathologists.
Acknowledgements
We thank technical experts from Daping Hospital affiliated to the Army Medical University for the molecular analysis.
Disclosure of conflict of interest
None.
References
- 1.Velankar MM, Nathwani BN, Schlutz MJ, Bain LA, Arber DA, Slovak ML, Weiss LM. Indolent T-lymphoblastic proliferation:report of a case with a 16-year course without cytotoxic therapy. Am J Surg Pathol. 1999;23:977–981. doi: 10.1097/00000478-199908000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Ohgami RS, Zhao S, Ohgami JK, Leavitt MO, Zehnder JL, West RB, Arber DA, Natkunam Y, Warnke RA. TDT+T-lymphoblastic populations are increased in castleman disease, in castleman disease in associated with follicular dendritic cell tumors, and in angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2012;36:1619–1628. doi: 10.1097/PAS.0b013e318264e223. [DOI] [PubMed] [Google Scholar]
- 3.Yang F, Liu T, Zhao H, Hu Z, Xiao L, Liu Y, Wang X, Li Z. Indolent T-lymphoblastic proliferation: report of a case involving the upper aerodigestive tract. Int J Clin Exp Pathol. 2014;7:6350–6356. [PMC free article] [PubMed] [Google Scholar]
- 4.Qian YW, Weissmann D, Goodell L, August D, Strair R. Indolent T-lymphoblastic proliferation in castleman lymphadenopathy. Leuk Lymphoma. 2009;50:306–308. doi: 10.1080/10428190802645079. [DOI] [PubMed] [Google Scholar]
- 5.Woo CG, Huh J. TDT+ T-lymphoblastic proliferation in castleman disease. J Pathol Transl Med. 2015;49:1–4. doi: 10.4132/jptm.2014.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kansal R, Nathwani BN, Yiakoumis X, Moschogiannis M, Sachanas S, Stefanaki K, Pangalis GA. Exuberant cortical thymocyte proliferation mimicking T-lymphoblastic lymphoma within recurrent large inguinal lymph node masses of localized castleman disease. Hum Pathol. 2015;46:1057–1061. doi: 10.1016/j.humpath.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Wang ZM, Xiao WB, Zheng SS, Sun K, Wang LJ. Hepatocellular carcinoma with indolent T-lymphoblastic proliferation. Leuk Lymphoma. 2006;47:2424–2426. doi: 10.1080/10428190600822151. [DOI] [PubMed] [Google Scholar]
- 8.Eun S, Jeon YK, Jang JJ. Hepatocellular carcinoma with immature T-cell (T-lymphoblastic) proliferation. J Korean Med Sci. 2010;25:309–312. doi: 10.3346/jkms.2010.25.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasuda H, Tsutsui M, Ota Y, Tanaka M, Komatsu N. Indolent T-lymphoblastic proliferation concomitant with acinic cell carcinoma mimicking T-lymphoblastic lymphoma: case report and literature review. Histopathology. 2018;72:862–866. doi: 10.1111/his.13433. [DOI] [PubMed] [Google Scholar]
- 10.Kim WY, Kim H, Jeon YK, Kim CW. Follicular dendritic cell sarcoma with immature T-cell proliferation. Hum Pathol. 2010;41:129–133. doi: 10.1016/j.humpath.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Strauchen JA. Indolent T-lymphoblastic proliferation: report of a case with an 11-year history and associated with myasthenia gravis. Am J Surg Pathol. 2001;25:411–415. doi: 10.1097/00000478-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Ohgami RS, Sendamarai AK, Atwater SK, et al. Indolent T-lymphoblastic proliferation with disseminated multimodal involvement and partial CD33 expression. Am J Surg Pathol. 2014;38:1298–1304. doi: 10.1097/PAS.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 13.Quesada AE, Young KH, Medeiros LJ, Thakral B. Indolent T-lymphoblastic proliferation associated with low grade follicular dendritic cell sarcoma and castleman disease. Pathology. 2018;50:351–352. doi: 10.1016/j.pathol.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Ohgami RS, Arber DA, Zehnder JL, Natkunam Y, Warnke RA. Indolent T-lymphoblastic proliferation (iT-LBP): a review of clinical and pathologic features and distinction from malignant T-lymphoblastic lymphoma. Adv Anat Pathol. 2013;20:137–140. doi: 10.1097/PAP.0b013e31828d17ec. [DOI] [PubMed] [Google Scholar]