Abstract
This study aimed to investigate the effect of long non-coding RNA nuclear enriched abundant transcript 1 (lnc-NEAT1) on mouse mesangial cells (MMCs) proliferation, apoptosis, fibrosis as well as inflammation in diabetic nephropathy (DN). MMCs (SV40 MES13 cells) were cultured under 30 mM glucose to construct DN cellular model (high glucose (HG) group); meanwhile, MMCs cultured under 5.6 mM glucose (normal glucose (NG) group) and 5.6 mM glucose plus 24.4 mM 3-O-methyl-D-glucose (osmotic control (OC) group) served as controls, and lnc-NEAT1 expression was determined by qPCR assay. Lnc-NEAT1 interference plasmids and control interference plasmids were transfected into DN cellular model as Sh-NEAT1 group and Sh-NC group. Cell proliferation, apoptosis, fibrosis, and inflammation were detected using Counting Kit-8, Annexin V/propidium iodide, western blot and quantitative polymerase chain reaction assays. Lnc-NEAT1 expression was elevated in HG group compared to NG group and OC group. Cell proliferation was decreased, and proliferative marker protein Cyclin D1 and proliferating cell nuclear antigen expressions also decreased in Sh-NEAT1 group compared to Sh-NC group. For cell apoptosis, apoptosis rate was increased, and apoptotic protein Cleaved Caspase3 expression enhanced but anti-apoptosis protein Bcl-2 expression decreased in Sh-NEAT1 group compared to Sh-NC group. For fibrosis markers (including fibronectin and collagen I) and inflammatory cytokines (including tumor necrosis factor-α, interleukin-1β and interleukin-6), their expressions were reduced in Sh-NEAT1 group compared to Sh-NC group. Lnc-NEAT1 is overexpressed, and its downregulation inhibits cell proliferation, fibrosis, and inflammation but promotes cell apoptosis in HG-induced MMCs DN cellular model.
Keywords: Long non-coding RNA, enriched abundant transcript 1, diabetic nephropathy, proliferation, apoptosis, fibrosis, inflammatory cytokines
Introduction
Diabetic nephropathy (DN), with the functional derangement and structural remodeling of kidney triggered by hyperglycemic injury, is one of the most frequent microvascular complications of diabetes patients and the leading cause of end-stage renal disease worldwide, and is characterized by a series of pathologic abnormalities such as glomerular hypertrophy, mesangial proliferation, thickening of the glomerular basement membrane and accumulation of the extracellular matrix [1-6]. According torecent reports, around 30%-40% diabetes patients tend to develop DN, and about 50% of DN patients would further develop end-stage renal disease (ESRD), which has the requirement of dialysis or renal transplantation [7-9]. Prevention or counteraction of these disease progression is based on the well understanding of underlying mechanisms of DN [10]. Although several molecular events such as the amplification and perpetuation of inflammatory reactions, oxidative stress, activation of sorbitol-aldose reductase, elevated production of advanced glycation end-products, raised activity of intracellular protein kinase C and altered expression of cellular cyclin-dependent kinases have been revealed to participate in the pathophysiology of DN, the mechanism in DN is still poorly understood due to its complicated pathogenesis [1,7,8,11]. Thus, further exploration of underlying mechanism for DN progression is of great importance, which might provide novel strategies for preventing or treating DN.
Long non-coding RNA (lncRNA), a subgroup of RNAs with insufficient protein coding capability, is composed of more than 200 nucleotides [12,13]. Increasing studies have shown that lncRNA plays crucial roles in multiple biologic processes (including regulation of gene expression, recruitment of chromatin modification, X chromosome inactivation, chromosome recombination and protein folding). Also, some of them are identified as important regulators in the pathologic processes of several endocrine and metabolic diseases, such as diabetes, osteoporosis and fatty liver disease [14-16]. LncRNA nuclear enriched abundant transcript 1 (lnc-NEAT1), located on chromosome 11q13.1, works as an important structural component of paraspeckles [17]. Majority of previous studies focus on the role of lnc-NEAT1 in different carcinomas, and identify its ability to affect cell activities via targeting miRNAs or regulating signaling pathways in cancer progression [18-20]. Recently, some recent studies disclose lnc-NEAT1 is aberrantly expressed in diabetes mice, moreover, there are some studies show that lnc-NEAT1 has proinflammatory influence through affecting inflammatory pathways such as activation of mitogen-activated protein kinase (MAPK) pathways or Toll-like receptor 3 (TLR3)-p38 pathway in some inflammatory diseases [21,22]. Considering inflammation is a key pathogenic mechanism in DN and lnc-NEAT1 is involved in inflammatory processes, we hypothesized that lnc-NEAT1 might participate in the pathology of DN, while the underlying mechanism of lnc-NEAT1 in DN was not deeply investigated [23,24]. In this study, we constructed a DN cellular model with mouse mesangial cells (MMCs) under high glucose (HG) condition, and investigated the effect of lnc-NEAT1 on cell proliferation, apoptosis, fibrosis as well as inflammation in DN.
Materials and methods
Cells source
MMCs cell line (SV40 MES13 cells) was purchased from Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China).
Cells culture
SV40 MES13 cells were cultured in 90% Dulbecco’s modified eagle medium (DMEM) (Gibco, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA) in a humidified incubator under 95% air and 5% CO2 at 37°C.
Measurement of lnc-NEAT1 expression in SV40 MES13 cells under HG condition
30 mM glucose (Sigma, USA), 5.6 mM glucose, 5.6 mM glucose plus 24.4 mM 3-O-methyl-D-glucose (Wako, Japan) were added to treat SV40 MES13 cells for 96 h, and these cells were divided into three groups accordingly as HG group, normal glucose group (NG group) and osmotic control group (OC group). Quantitative polymerase chain reaction (qPCR) was subsequently performed to measure lnc-NEAT1 expression in each group.
Construction and transfection of lnc-NEAT1 interference plasmids
Lnc-NEAT1 interference plasmids and control interference plasmids were constructed by Shanghai GenePharma Bio-Tech Company (Shanghai, China). These plasmids were transfected into SV40 MES13 cells under HG condition as Sh-NEAT1 and Sh-NC groups.
Measurement of cell proliferation
Cell proliferation ability was measured using Counting Kit-8 (CCK-8) (Sangon, China) at 0 h, 24 h, 48 h and 72 h after transfection according to the instructions of manufacturer. In addition, cell proliferative markers (Cyclin D1 and proliferating cell nuclear antigen (PCNA)) were also measured using qPCR and western blot at 48 h after transfection.
Measurement of apoptosis
Cell apoptosis rate was measured using Annexin V (AV) apoptosis detection kit with propidium iodide (PI) (BD, USA) at 24 h after transfection according to the instructions of manufacturer. In addition, apoptotic markers (Cleaved Caspase3 (C-Caspase3) and Bcl-2) were also measured using western blot at 24 h after transfection.
Measurement of cell fibrosis
To determine the effect of lnc-NEAT1 inhibition on MMCs, cell fibrosis markers (Fibronectin (FN) and collagen I) were measured using qPCR and western blot at 24 h after transfection.
Measurement of inflammatory cytokines
Inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6 were measured using qPCR and western blot at 24 h after transfection.
Western blot
Total proteins were extracted from each group of cells with 1 mL RIPA (Sigma, USA) on ice and shocked every 5 mins during 30 mins for complete pyrolysis. Then, protein concentration was determined by Bicinchoninic Acid Kit (Sigma, USA), followed by thermal denaturation that performed at 98°C for 5 mins. Subsequently, 20 μg proteins were added to NuPAGE Bis-Tris protein gel (Invitrogen, USA), and after electrophoresis was finished, proteins were transferred to polyvinylidene fluoride membranes (Milipore, USA). 5% skim milk was used to block the membranes at 37°C for 1 h, and membranes were incubated with the corresponding primary antibodies overnight at 4°C. Then, membranes were further incubated with secondary antibodies at room temperature for 2 h. Lastly, the bands were visualized using EasyBlot ECL kit (Sangon, China). The antibodies applied in western blot assay are listed in Table 1.
Table 1.
Antibodies applied in western blot
| Antibody | Company | Dilution |
|---|---|---|
| Primary Antibody | ||
| Cyclin D1 Rabbit mAb | Cell Signaling Technology (USA) | 1:1000 |
| PCNA Rabbit mAb | Cell Signaling Technology (USA) | 1:1000 |
| Rabbit monoclonal to Fibronectin | Abcam (UK) | 1:1000 |
| Rabbit polyclonal to Collagen I | Abcam (UK) | 1:1000 |
| Cleaved Caspase-3 Rabbit mAb | Cell Signaling Technology (USA) | 1:1000 |
| Caspase-3 Rabbit mAb | Cell Signaling Technology (USA) | 1:1000 |
| Bcl 2 Rabbit mAb | Cell Signaling Technology (USA) | 1:1000 |
| TNF-α Rabbit mAb | Cell Signaling Technology (USA) | 1:1000 |
| IL-1β Rabbit mAb | Cell Signaling Technology (USA) | 1:1000 |
| IL-6 Rabbit mAb | Cell Signaling Technology (USA) | 1:1000 |
| GAPDH Rabbit mAb | Cell Signaling Technology (USA) | 1:1000 |
| Secondary Antibody | ||
| Goat Anti-Rabbit IgG H&L (HRP) | Cell Signaling Technology (USA) | 1:4000 |
qPCR
The qPCR process was as follows: (1) total RNA was extracted by PureZOL RNA isolation reagent (Bio-Rad, USA); (2) reverse transcription to cDNA was performed with 1 μg total RNA using iScript™ Reverse Transcription Supermix (Bio-Rad, USA); (3) TB Green™ Fast qPCR Mix (Takara, Japan) was applied to perform the qPCR, and qPCR amplification was conducted at 95°C for 3 mins, followed by 40 cycles of 95°C for 5 s, 61°C for 10 s, and then 72°C for 30 s. Finally, the qPCR results were calculated by 2-ΔΔCt formula, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal references. The primers used in qPCR assay were listed in Table 2.
Table 2.
Primers applied in qPCR
| Gene | Forward Primer (5’-3’) | Reverse Primer (5’-3’) |
|---|---|---|
| Lnc-NEAT1 | TGAGTAGTGGAAGCAGGAGGAT | GGAGGCAAGGACGAGACAGA |
| Cyclin D1 | CCAGAGGCGGATGAGAACAAG | GCGGTAGCAGGAGAGGAAGT |
| PCNA | GCCGAGACCTTAGCCACATTG | ATGGTTACCGCCTCCTCTTCTT |
| FN | CAGTAGAAGGCAGTAGCACAGA | CCAGACACCACACTATCAGGAG |
| Collagen I | CTCGTGGATTGCCTGGAACA | GCACCAACAGCACCATCGT |
| TNF-α | CGTGGAACTGGCAGAAGAGG | TCAGTAGACAGAAGAGCGTGGT |
| IL-1β | TCTCGCAGCAGCACATCAAC | TGTTCATCTCGGAGCCTGTAGT |
| IL-6 | CTTCCATCCAGTTGCCTTCTTG | GTAATTAAGCCTCCGACTTGTGAA |
| GAPDH | GAGTCCACTGGCGTCTTCAC | ATCTTGAGGCTGTTGTCATACTTCT |
Statistics
Statistics was conducted using SPSS 21.0 Software (IBM, USA) and graphs were drawn using GraphPad 6.01 Software (GraphPad Software, USA). Data were presented as mean ± standard deviation. Comparison among groups was detected using One-way ANOVA followed by Turkey’s multiple comparison test, and comparison between two groups was detected using t test. P value < 0.05 was considered as significant in this study.
Results
Comparison of lnc-NEAT1 expression among HG group, OC group and NG group
Lnc-NEAT1 expression was remarkably elevated in HG group compared to NG group (P < 0.001), and it was also greatly higher in HG group than that in OC group (P < 0.001), while no difference of lnc-NEAT1 expression was found between OC group and NG group. These results indicated that lnc-NEAT1 was overexpressed in DN cellular model (Figure 1).
Figure 1.
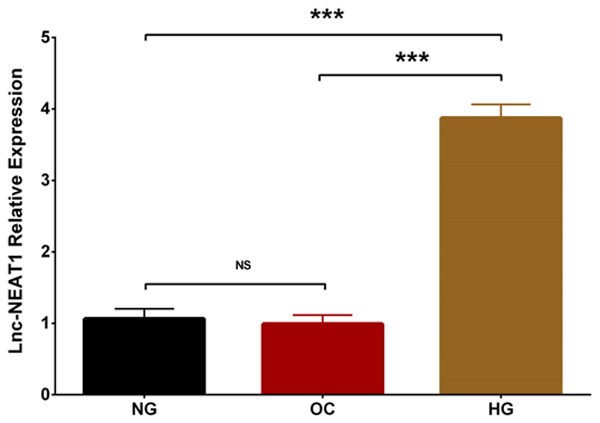
Lnc-NEAT1 expression in NG group, OC group and HG group. Lnc-NEAT1 expression was undifferentiated in OC group compared to NG group, while it was higher in HG group compared to NG group and elevated in HG group compared to OC group. Lnc-NEAT1, long non-coding RNA nuclear enriched abundant transcript 1; NG, normal glucose; OC, osmotic control; HG, high glucose. Comparison among groups was detected using One-way ANOVA followed by Turkey’s multiple comparison test. P value < 0.05 was considered significant. ***P < 0.001.
Comparison of lnc-NEAT1 expression after plasmids transfection in DN cellular model
In order to determine the effect of lnc-NEAT1 in DN, we transfected lnc-NEAT1 interference plasmids and control plasmids into DN cellular model. Lnc-NEAT1 expression in Sh-NEAT1 group was dramatically reduced in Sh-NEAT1 group compared to Sh-NC group (P < 0.001) (Figure 2), suggesting the successful transfection of lnc-NEAT1 interference plasmids into DN cellular model.
Figure 2.
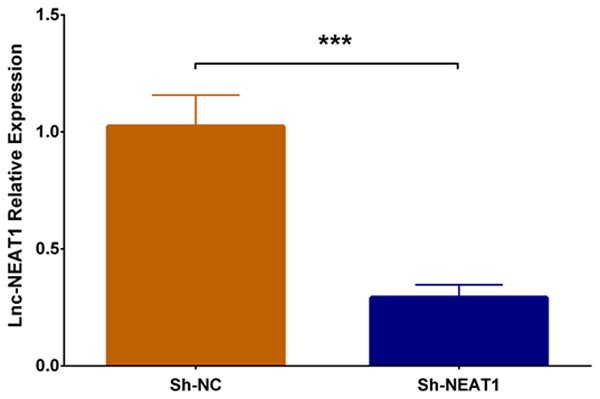
Lnc-NEAT1 expression in DN cellular model after transfection with lnc-NEAT1 interference plasmids. Lnc-NEAT1 expression was decreased in Sh-NEAT1 group compared to Sh-NC group. Lnc-NEAT1, long non-coding RNA nuclear enriched abundant transcript 1. Comparison between two groups was determined by t test. P value < 0.05 was considered significant. ***P < 0.001.
Comparison of cell proliferation between Sh-NEAT1 group and Sh-NC group
In order to investigate the function of lnc-NEAT1 downregulation on cell proliferation in DN, we performed CCK-8 assay and found that cell proliferation was decreased in Sh-NEAT1 group at 48 h (P < 0.05) and 72 h (P < 0.05) compared to Sh-NC group (Figure 3A). Besides, western blot disclosed that expressions of both Cyclin D1 protein and PCNA protein were reduced in Sh-NEAT1 group compared to Sh-NC group (Figure 3B), and qPCR assay displayed that expressions of Cyclin D1 mRNA and PCNA mRNA were lower in Sh-NEAT1 group compared to Sh-NC group (P < 0.01) (Figure 3C, 3D). These results suggested that lnc-NEAT1 downregulation repressed cell proliferation in the DN cellular model.
Figure 3.
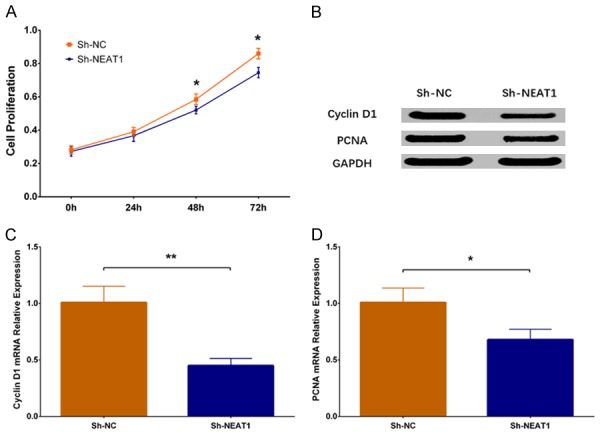
Cell proliferation in Sh-NEAT1 group and Sh-NC group. Compared to Sh-NC group, cell proliferation rate was lower in Sh-NEAT1 group (A). Expressions of cell proliferative markers including Cyclin D1 and PCNA were decreased in Sh-NEAT1 group (B-D). NEAT1, nuclear enriched abundant transcript 1; PCNA, proliferating cell nuclear antigen. Comparison between two groups were determined by t test. P value < 0.05 was considered significant. *P < 0.05; **P < 0.01.
Comparison of cell apoptosis between Sh-NEAT1 group and Sh-NC group
Cell apoptosis rate was increased in Sh-NEAT1 group than that in Sh-NC group (P < 0.001) (Figure 4A, 4B). Western blot revealed that expression of apoptotic protein C-Caspase3 was enhanced in Sh-NEAT1 group compared to Sh-NC group, while expression of anti-apoptosis protein Bcl 2 was decreased in Sh-NEAT1 group compared to Sh-NC group (Figure 4C). These results indicated that lnc-NEAT1 downregulation inhibited cell proliferation in a DN cellular model.
Figure 4.
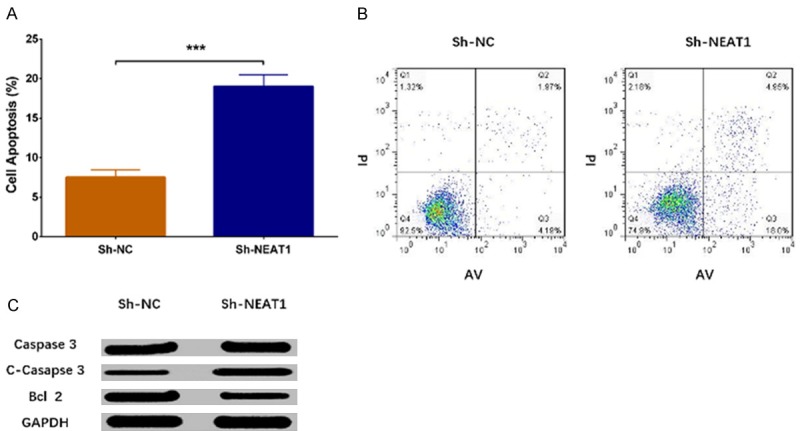
Cell apoptosis in Sh-NEAT1 group and Sh-NC group. As to cell apoptosis, AV/PI assay revealed that cell apoptosis rate was elevated in Sh-NEAT1 group compared to Sh-NC group (A, B). Moreover, western blot displayed that apoptotic protein C-Caspase3 expression was increased but anti-apoptosis protein Bcl 2 expression was decreased in Sh-NEAT1 group compared to Sh-NC group (C). NEAT1, nuclear enriched abundant transcript 1; AV/PI, Annexin V propidium iodide. Comparison between two groups was determined by t test. P value < 0.05 was considered significant. ***P < 0.001.
Comparison of cell fibrosis between Sh-NEAT1 group and Sh-NC group
Expression of cell fibrosis marker mRNAs were measured by qPCR assay, which displayed that FN mRNA expression (P < 0.05) (Figure 5A) and collagen I mRNA expression (P < 0.01) (Figure 5B) were decreased in Sh-NEAT1 group compared to Sh-NC group. Besides, expressions of fibrotic proteins were assessed by western blot, which showed that FN protein expression and collagen I protein expression were also reduced in the Sh-NEAT1 group compared to the Sh-NC group (Figure 5C), suggesting that lnc-NEAT1 downregulation inhibited fibrosis in a DN cellular model.
Figure 5.
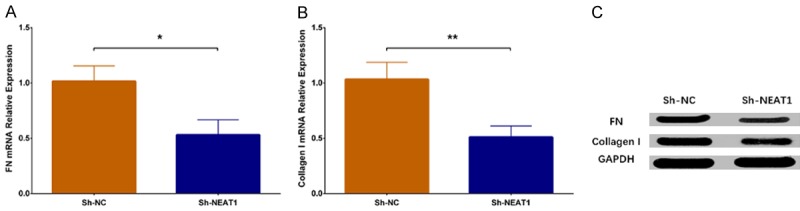
Expression of cell fibrosis markers in Sh-NEAT1 group and Sh-NC group. The qPCR assay disclosed that miRNA expressions of cell fibrosis markers including FN and Collagen Ⅰ were reduced in the Sh-NEAT1 group compared to the Sh-NC group (A, B), and western blot revealed that expressions of FN and Collagen Ⅰ proteins were lower in the Sh-NEAT1 group compared to Sh-NC group (C). NEAT1, nuclear enriched abundant transcript 1; qPCR, quantitative polymerase chain reaction (qPCR); FN, fibronectin. Comparison between two groups was determined by t test. P value < 0.05 was considered significant. *P < 0.05; **P < 0.01.
Comparison of inflammatory cytokines expressions between Sh-NEAT1 group and Sh-NC group
The qPCR assay revealed that expressions of inflammatory cytokines including TNF-α (P < 0.01), IL-1β (P < 0.01) and IL-6 (P < 0.05) were reduced in the Sh-NEAT1 group compared to Sh-NC group (Figure 6A-C). Furthermore, western blot disclosed that protein expressions of TNF-α, IL-1β and IL-6 were attenuated in Sh-NEAT1 group than those in the Sh-NC group (Figure 6D). All these results indicated that lnc-NEAT1 downregulation lowered the inflammation level in a DN cellular model.
Figure 6.
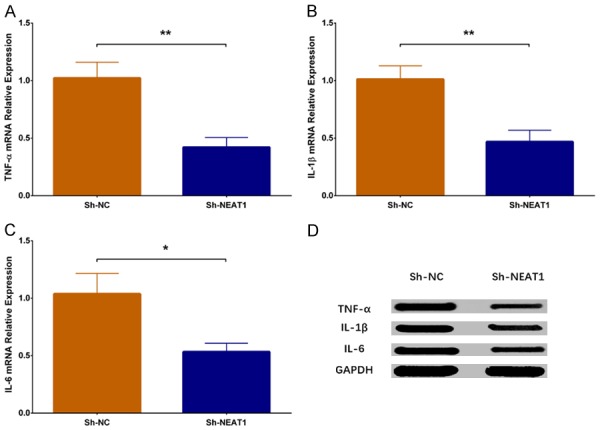
Expressions of inflammatory cytokines in Sh-NEAT1 group and Sh-NC group. TNF-α mRNA expression was lower in the Sh-NEAT1 group compared to the Sh-NC group (A). IL-1β mRNA expression was decreased in the Sh-NEAT1 group compared to the Sh-NC group (B). IL-6 mRNA expression was reduced in Sh-NEAT1 group compared to Sh-NC group (C). Protein expressions of inflammatory cytokines including TNF-α, IL-1β and IL-6 were reduced in the Sh-NEAT1 group compared to the Sh-NC group (D). NEAT1, nuclear enriched abundant transcript 1; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6. Comparison between two groups was determined by t test. P value < 0.05 was considered significant. *P < 0.05; **P < 0.01.
Discussion
In this study, we found that: (1) lnc-NEAT1 was overexpressed and its downregulation repressed cell proliferation but enhanced apoptosis in a DN cellular model; (2) lnc-NEAT1 downregulation decreased fibrotic marker expression in a DN cellular model; (3) lnc-NEAT1 downregulation reduced inflammatory cytokine expressions in a DN cellular model.
LncRNA, which has been identified to have a vital role in a wide spectrum of diseases, typically acts as scaffolds for multiple proteins and forms complexes to regulate gene or protein expression [25-30]. Emerging studies have disclosed that the lncRNA dysregulation which responds to hyperglycemia may enhance the metabolic impairment and renal lesions in DN [9,25,31-33]. For instance, lnc-ASncmtRNA-2 is upregulated in DN mice and HG-treated MMCs, and it promotes glomerular fibrosis through upregulating expression of pro-fibrotic factor transforming growth factor β1 (TGFβ1) [25]. Besides, lnc-LINC00968 represses p21 in DN cellular model (MMCs under HG condition) via binding with zeste homolog 2 (EZH2), thereby facilitates cell proliferation and fibrosis [32]. Furthermore, lnc-Gm4419 knockdown inhibits cell proliferation, represses expressions of pro-inflammatory cytokines (including monocyte chemoattractant protein-1 (mcp-1), IL-1β and TNF-α), and decreases expressions of fibrosis biomarkers (including FN and collagen IV) through reducing activation of nuclear factor kappa light-chain enhancer of activated B cells (NF-κB)/NACHT, leucine-rich repeats (LRR) and (pyrin domain) PYD domain-containing protein 3 (NLRP3) inflammasome signaling pathway in DN cellular model (MMCs under HG condition) [33]. These previous studies reveal that lncRNA may be involved in the pathology of DN and they present potentials to serve as treatment targets.
Lnc-NEAT1, which is transcribed by RNA polymerase II from a common promoter and widely expressed in mammalian cells, functions as scaffolds of nuclear bodies [17,34-36]. Recently, emerging evidence shows lnc-NEAT1 plays a crucial role in regulating gene expression and consequently mediates pathophysiologic processes by affecting cell proliferation or apoptosis in several diseases such as diabetes, traumatic brain injury, as well as multiple cancers [23,37-40]. Whereas, limited information of lnc-NEAT1 in diabetes was found. One previous study conducted microarray analyses on diabetic Akita mice heart and displayed that lnc-NEAT1 expression is downregulated [23]. However, another study disclosed that lnc-NEAT1 is overexpressed and it promotes cell apoptosis as well as autophagy in diabetic rats with myocardial ischemia reperfusion injury [41]. Hence, these previous data reveal that the influence of lnc-NEAT1 in diabetes or diabetic complications is still controversial, and the underlying mechanism of lnc-NEAT1 in DN is rarely disclosed. Since inflammation is the important pathogenetic mechanism in DN and lnc-NEAT1 has potential to serve as a pro-inflammatory factor; and lnc-NEAT1 expression is dysregulated in diabetic rats in the aforementioned previous studies, we suspected that lnc-NEAT1 might act as a key component in the pathology of DN. In the present study, we measured lnc-NEAT1 expression in MMCs (SV40 MES13 cells) with different glucose conditions, and we found that lnc-NEAT1 expression was increased in the HG group compared to the OC group and NG group, indicating that lnc-NEAT1 was overexpressed in the DN cellular model. Moreover, we conducted CCK-8 assay, AV/PI assay and western blot (detection of apoptotic markers) to investigate the influence of lnc-NEAT1 on cell proliferation and apoptosis in DN, and we observed that lnc-NEAT1 downregulation inhibited cell proliferation but enhanced cell apoptosis in DN cellular model. Our data suggested that lnc-NEAT1 might serve as a potential target for preventing or treating the abnormally increase of MMCs in DN.
Some previous studies have proposed that inhibition of renal fibrosis is important to alleviate the functional deterioration in DN, while the effect of lnc-NEAT1 on fibrosis is seldom reported. One related investigation in liver fibrosis mice displays that silencing of lnc-NEAT1 reduces collagen I expression by targeting miR-122 and Kruppel-like factor 6 in liver fibrosis mice, suggesting that lnc-NEAT1 may play a pro-fibrogenic role in liver fibrosis [9,42-44]. As to the influence of lnc-NEAT1 on fibrosis in DN, no previous studies have been found. In our present study, we found that lnc-NEAT1 downregulation lowered the fibrotic markers’ expressions including FN and collagen I in DN cellular model, which might provide evidence to explore novel preventative or therapeutic measures in inhibiting fibrosis of DN.
Inflammation is involved in the pathogenesis of diabetes, and elevated inflammatory cytokines are revealed to be associated with the development of microvascular diabetic complications, including DN [45,46]. In previous studies, one study discloses that lnc-NEAT1 knockdown reduces several inflammatory cytokines’ expressions including IL-6 and IL-8 in osteoarthritis synoviocytes [47]. Also, a study observes that lnc-NEAT1 downregulation suppresses the inflammatory response through modulating the intestinal epithelial barrier and regulating exosome-mediated polarization of macrophages in inflammatory cell line models [48]. Another study reveals that silencing of lnc-NEAT1 reduces the expressions of inflammatory cytokines including IL-6 and C-X-C motif chemokine ligand 10 (CXCL10) in monocytes from patients with systemic lupus erythematosus [21]. Furthermore, an interesting study has identified the proinflammatory role of lnc-NEAT1 as a factor of the innate immune response in normal cells through activating the genes transcription by sequestration of transcriptional factor splicing factor proline/glutamine-rich (SFPQ) [49]. These previous data reveal the pro-inflammatory influence of lnc-NEAT1 in some inflammatory diseases, while evidence of lnc-NEAT1 affecting inflammatory response in DN is limited. In this present study, we used qPCR and western blot to explore the influence of lnc-NEAT1 downregulation on inflammatory cytokines including TNF-α, IL-1β and IL-6. We found that lnc-NEAT1 downregulation reduced the expressions of TNF-α, IL-1β and IL-6 in a DN cellular model, indicating that lnc-NEAT1 aggravated the inflammatory response. This might shed light on the application of lnc-NEAT1 downregulation in prevention or treatment of DN.
In conclusion, lnc-NEAT1 is overexpressed, and its downregulation inhibits cell proliferation, fibrosis, and inflammation but promotes cell apoptosis in a HG-induced MMCs DN cellular model.
Acknowledgements
This study was supported by New Drug Research Foundation of Heilongjiang University of Chinese Medicine (No. 035149), and National Traditional Chinese Medicine Clinical Research Base Foundation (No: 2015D04).
Disclosure of conflict of interest
None.
References
- 1.Han Q, Zhu H, Chen X, Liu Z. Non-genetic mechanisms of diabetic nephropathy. Front Med. 2017;11:319–332. doi: 10.1007/s11684-017-0569-9. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharjee N, Barma S, Konwar N, Dewanjee S, Manna P. Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: an update. Eur J Pharmacol. 2016;791:8–24. doi: 10.1016/j.ejphar.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Zhou L, Xu DY, Sha WG, Shen L, Lu GY. Long non-coding RNA MALAT1 interacts with transcription factor Foxo1 to regulate SIRT1 transcription in high glucose-induced HK-2 cells injury. Biochem Biophys Res Commun. 2018;503:849–855. doi: 10.1016/j.bbrc.2018.06.086. [DOI] [PubMed] [Google Scholar]
- 4.Bjornstad P, Cherney D, Maahs DM. Early diabetic nephropathy in type 1 diabetes: new insights. Curr Opin Endocrinol Diabetes Obes. 2014;21:279–286. doi: 10.1097/MED.0000000000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mudaliar H, Pollock C, Panchapakesan U. Role of toll-like receptors in diabetic nephropathy. Clin Sci (Lond) 2014;126:685–694. doi: 10.1042/CS20130267. [DOI] [PubMed] [Google Scholar]
- 6.Sharma D, Bhattacharya P, Kalia K, Tiwari V. Diabetic nephropathy: new insights into established therapeutic paradigms and novel molecular targets. Diabetes Res Clin Pract. 2017;128:91–108. doi: 10.1016/j.diabres.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Feng Y, Chen S, Xu J, Zhu Q, Ye X, Ding D, Yao W, Lu Y. Dysregulation of lncRNAs GM5524 and GM15645 involved in highglucoseinduced podocyte apoptosis and autophagy in diabetic nephropathy. Mol Med Rep. 2018;18:3657–3664. doi: 10.3892/mmr.2018.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol. 2011;6:395–423. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magee C, Grieve DJ, Watson CJ, Brazil DP. Diabetic nephropathy: a tangled web to unweave. Cardiovasc Drugs Ther. 2017;31:579–592. doi: 10.1007/s10557-017-6755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maestroni S, Zerbini G. Glomerular endothelial cells versus podocytes as the cellular target in diabetic nephropathy. Acta Diabetol. 2018;55:1105–1111. doi: 10.1007/s00592-018-1211-2. [DOI] [PubMed] [Google Scholar]
- 11.Papadopoulou-Marketou N, Kanaka-Gantenbein C, Marketos N, Chrousos GP, Papassotiriou I. Biomarkers of diabetic nephropathy: a 2017 update. Crit Rev Clin Lab Sci. 2017;54:326–342. doi: 10.1080/10408363.2017.1377682. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Kan QE, Su Y, Man H. Long non-coding RNA CASC2 improves diabetic nephropathy by inhibiting JNK pathway. Exp Clin Endocrinol Diabetes. 2018 doi: 10.1055/a-0629-9958. [DOI] [PubMed] [Google Scholar]
- 13.Cui Z, Ren S, Lu J, Wang F, Xu W, Sun Y, Wei M, Chen J, Gao X, Xu C, Mao JH, Sun Y. The prostate cancer-up-regulated long noncoding RNA PlncRNA-1 modulates apoptosis and proliferation through reciprocal regulation of androgen receptor. Urol Oncol. 2013;31:1117–1123. doi: 10.1016/j.urolonc.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 14.Wu QY, Li X, Miao ZN, Ye JX, Wang B, Zhang F, Xu RS, Jiang DL, Zhao MD, Yuan FL. Long non-coding RNAs: a new regulatory code for osteoporosis. Front Endocrinol (Lausanne) 2018;9:587. doi: 10.3389/fendo.2018.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun C, Liu X, Yi Z, Xiao X, Yang M, Hu G, Liu H, Liao L, Huang F. Genome-wide analysis of long noncoding RNA expression profiles in patients with non-alcoholic fatty liver disease. IUBMB Life. 2015;67:847–852. doi: 10.1002/iub.1442. [DOI] [PubMed] [Google Scholar]
- 16.Sathishkumar C, Prabu P, Mohan V, Balasubramanyam M. Linking a role of lncRNAs (long non-coding RNAs) with insulin resistance, accelerated senescence, and inflammation in patients with type 2 diabetes. Hum Genomics. 2018;12:41. doi: 10.1186/s40246-018-0173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, Li J, Chen C, Zhang R, Wang K. Pan-cancer analysis of long non-coding RNA NEAT1 in various cancers. Genes Dis. 2018;5:27–35. doi: 10.1016/j.gendis.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu X, Li Z, Zheng H, Chan MT, Wu WK. NEAT1: a novel cancer-related long non-coding RNA. Cell Prolif. 2017;50 doi: 10.1111/cpr.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao J, Zhang Y, Yang J, He S, Li M, Yan S, Chen Y, Qu C, Xu L. NEAT1 regulates pancreatic cancer cell growth, invasion and migration though mircroRNA-335-5p/c-met axis. Am J Cancer Res. 2016;6:2361–2374. [PMC free article] [PubMed] [Google Scholar]
- 20.Fang L, Sun J, Pan Z, Song Y, Zhong L, Zhang Y, Liu Y, Zheng X, Huang P. Long non-coding RNA NEAT1 promotes hepatocellular carcinoma cell proliferation through the regulation of miR-129-5p-VCP-IkappaB. Am J Physiol Gastrointest Liver Physiol. 2017;313:G150–G156. doi: 10.1152/ajpgi.00426.2016. [DOI] [PubMed] [Google Scholar]
- 21.Zhang F, Wu L, Qian J, Qu B, Xia S, La T, Wu Y, Ma J, Zeng J, Guo Q, Cui Y, Yang W, Huang J, Zhu W, Yao Y, Shen N, Tang Y. Identification of the long noncoding RNA NEAT1 as a novel inflammatory regulator acting through MAPK pathway in human lupus. J Autoimmun. 2016;75:96–104. doi: 10.1016/j.jaut.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Santoro M, Nociti V, Lucchini M, De Fino C, Losavio FA, Mirabella M. Expression profile of long non-coding RNAs in serum of patients with multiple sclerosis. J Mol Neurosci. 2016;59:18–23. doi: 10.1007/s12031-016-0741-8. [DOI] [PubMed] [Google Scholar]
- 23.Kesherwani V, Shahshahan HR, Mishra PK. Cardiac transcriptome profiling of diabetic akita mice using microarray and next generation sequencing. PLoS One. 2017;12:e0182828. doi: 10.1371/journal.pone.0182828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327–340. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 25.Gao Y, Chen ZY, Wang Y, Liu Y, Ma JX, Li YK. Long non-coding RNA ASncmtRNA-2 is upregulated in diabetic kidneys and high glucose-treated mesangial cells. Exp Ther Med. 2017;13:581–587. doi: 10.3892/etm.2017.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang W, Zhang D, Ma X. RNA-sequencing reveals genome-wide long non-coding RNAs profiling associated with early development of diabetic nephropathy. Oncotarget. 2017;8:105832–105847. doi: 10.18632/oncotarget.22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kornienko AE, Guenzl PM, Barlow DP, Pauler FM. Gene regulation by the act of long non-coding RNA transcription. BMC Biol. 2013;11:59. doi: 10.1186/1741-7007-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, Blencowe BJ, Prasanth SG, Prasanth KV. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrieri C, Cimatti L, Biagioli M, Beugnet A, Zucchelli S, Fedele S, Pesce E, Ferrer I, Collavin L, Santoro C, Forrest AR, Carninci P, Biffo S, Stupka E, Gustincich S. Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature. 2012;491:454–457. doi: 10.1038/nature11508. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, Chen X, Wang M, Yao D, Chen T, Yan Q, Lu W. Long non-coding RNA CYP4B1-PS1-001 inhibits proliferation and fibrosis in diabetic nephropathy by interacting with nucleolin. Cell Physiol Biochem. 2018;49:2174–2187. doi: 10.1159/000493821. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Yu Z, Meng X, Yu P. LncRNA LINC00968 accelerates the proliferation and fibrosis of diabetic nephropathy by epigenetically repressing p21 via recruiting EZH2. Biochem Biophys Res Commun. 2018;504:499–504. doi: 10.1016/j.bbrc.2018.08.048. [DOI] [PubMed] [Google Scholar]
- 33.Yi H, Peng R, Zhang LY, Sun Y, Peng HM, Liu HD, Yu LJ, Li AL, Zhang YJ, Jiang WH, Zhang Z. LincRNA-Gm4419 knockdown ameliorates NF-kappaB/NLRP3 inflammasome-mediated inflammation in diabetic nephropathy. Cell Death Dis. 2017;8:e2583. doi: 10.1038/cddis.2016.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawaguchi T, Hirose T. Chromatin remodeling complexes in the assembly of long noncoding RNA-dependent nuclear bodies. Nucleus. 2015;6:462–467. doi: 10.1080/19491034.2015.1119353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chujo T, Yamazaki T, Hirose T. Architectural RNAs (arcRNAs): a class of long noncoding RNAs that function as the scaffold of nuclear bodies. Biochim Biophys Acta. 2016;1859:139–146. doi: 10.1016/j.bbagrm.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Yu B, Shan G. Functions of long noncoding RNAs in the nucleus. Nucleus. 2016;7:155–166. doi: 10.1080/19491034.2016.1179408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choudhry H, Albukhari A, Morotti M, Haider S, Moralli D, Smythies J, Schodel J, Green CM, Camps C, Buffa F, Ratcliffe P, Ragoussis J, Harris AL, Mole DR. Tumor hypoxia induces nuclear paraspeckle formation through HIF-2alpha dependent transcriptional activation of NEAT1 leading to cancer cell survival. Oncogene. 2015;34:4546. doi: 10.1038/onc.2014.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chakravarty D, Sboner A, Nair SS, Giannopoulou E, Li R, Hennig S, Mosquera JM, Pauwels J, Park K, Kossai M, MacDonald TY, Fontugne J, Erho N, Vergara IA, Ghadessi M, Davicioni E, Jenkins RB, Palanisamy N, Chen Z, Nakagawa S, Hirose T, Bander NH, Beltran H, Fox AH, Elemento O, Rubin MA. The oestrogen receptor alpha-regulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nat Commun. 2014;5:5383. doi: 10.1038/ncomms6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong J, Jiang L, Huang Z, Zhang H, Cheng C, Liu H, He J, Wu J, Darwazeh R, Wu Y, Sun X. The long non-coding RNA Neat1 is an important mediator of the therapeutic effect of bexarotene on traumatic brain injury in mice. Brain Behav Immun. 2017;65:183–194. doi: 10.1016/j.bbi.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Cai Y, Zheng L, Zhang Z, Lin X, Jiang N. Long noncoding RNA NEAT1 regulate papillary thyroid cancer progression by modulating miR-129-5p/KLK7 expression. J Cell Physiol. 2018;233:6638–6648. doi: 10.1002/jcp.26425. [DOI] [PubMed] [Google Scholar]
- 41.Ma M, Hui J, Zhang QY, Zhu Y, He Y, Liu XJ. Long non-coding RNA nuclear-enriched abundant transcript 1 inhibition blunts myocardial ischemia reperfusion injury via autophagic flux arrest and apoptosis in streptozotocin-induced diabetic rats. Atherosclerosis. 2018;277:113–122. doi: 10.1016/j.atherosclerosis.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 42.Wang M, Wang S, Yao D, Yan Q, Lu W. A novel long non-coding RNA CYP4B1-PS1-001 regulates proliferation and fibrosis in diabetic nephropathy. Mol Cell Endocrinol. 2016;426:136–145. doi: 10.1016/j.mce.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 43.Alvarez ML, Khosroheidari M, Eddy E, Kiefer J. Role of microRNA 1207-5P and its host gene, the long non-coding RNA Pvt1, as mediators of extracellular matrix accumulation in the kidney: implications for diabetic nephropathy. PLoS One. 2013;8:e77468. doi: 10.1371/journal.pone.0077468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu F, Jiang Z, Chen B, Dong P, Zheng J. NEAT1 accelerates the progression of liver fibrosis via regulation of microRNA-122 and Kruppel-like factor 6. J Mol Med (Berl) 2017;95:1191–1202. doi: 10.1007/s00109-017-1586-5. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Zeng L, Cao C, Lu C, Lian W, Han J, Zhang X, Zhang J, Tang T, Li M. Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Exp Cell Res. 2017;350:327–335. doi: 10.1016/j.yexcr.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Navarro-Gonzalez JF, Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 47.Wang Q, Wang W, Zhang F, Deng Y, Long Z. NEAT1/miR-181c regulates osteopontin (OPN)-mediated synoviocyte proliferation in osteoarthritis. J Cell Biochem. 2017;118:3775–3784. doi: 10.1002/jcb.26025. [DOI] [PubMed] [Google Scholar]
- 48.Liu R, Tang A, Wang X, Chen X, Zhao L, Xiao Z, Shen S. Inhibition of lncRNA NEAT1 suppresses the inflammatory response in IBD by modulating the intestinal epithelial barrier and by exosome-mediated polarization of macrophages. Int J Mol Med. 2018;42:2903–2913. doi: 10.3892/ijmm.2018.3829. [DOI] [PubMed] [Google Scholar]
- 49.Imamura K, Akimitsu N. Long non-coding RNAs involved in immune responses. Front Immunol. 2014;5:573. doi: 10.3389/fimmu.2014.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]


