Abstract
Large cell neuroendocrine carcinoma (LCNEC) of the endometrium is a rare and highly malignant neoplasm with no characteristic findings in terms of clinical manifestations, diagnostic imaging, or pathology, and thus, the definitive preoperative diagnosis of LCNEC is difficult. A 61-year-old postmenopausal woman presented with low abdominal pain and a rapidly growing uterine mass without postmenopausal bleeding. Magnetic resonance imaging of the pelvis revealed an enlarged uterus with a 7.5 cm mass. Intraoperative frozen examination revealed a malignant tumor, and accordingly, cytoreductive surgery was performed. Microscopically, the tumor showed extensive necrosis, hemorrhage, and an organoid nesting pattern of large cells. Immunohistochemistry revealed tumor cells were diffusely positive for the neuroendocrine markers CD56 and synaptophysin. Thus the tumor was diagnosed as LCNEC of endometrium. Postoperatively, the disease pursued a progressive course and relapsed even after repeated multiple chemotherapy courses. The patient succumbed to the disease 23 months after surgery. We present a case of LCNEC of the endometrium with a high Ki-67 index that exhibited a rapidly progressive course. LCNEC should be considered when a rapidly growing uterine tumor is detected.
Keywords: Large cell neuroendocrine carcinoma, endometrium, neuroendocrine markers
Introduction
Large cell neuroendocrine carcinoma (LCNEC) originates predominantly from lungs and is only occasionally encountered in gastrointestinal or genitourinary tracts. In particular, LCNEC of endometrium is an extremely rare and highly aggressive malignancy. Neuroendocrine tumors are classified into two categories based on tumor grade as well-differentiated or poorly differentiated NEC, and the latter is subcategorized as small or large cell NEC. Approximately 90 cases of small cell NEC, the majority in endometrium, have been reported to date [1], but fewer than 20 cases of LCNEC of endometrium have been reported (Table 1) [2]. Characteristically, LCNEC is composed of large tumor cells that exhibit neuroendocrine differentiation [3]. Due to its rarity, no standard therapy has been established for LCNEC of endometrium and its prognosis is poor. Here, we present a case of LCNEC of the endometrium with a high Ki-67 index that exhibited a rapidly progressive course.
Table 1.
Large cell neuroendocrine carcinoma of the endometrium
| Case | Age | Presenting symptom | Preop. presumptive diagnosis | Histology | Surgery | Immuno-profile | Initial stage | Adjuvant treatment | Survival | Recurrence/Persistence | Metastasis site | Reference | |
|
| |||||||||||||
| RT | CTx | ||||||||||||
|
| |||||||||||||
| Survival, unknown | |||||||||||||
| 1 | 88 | PMB | NA | L/SCNEC + endometrioid | TAH, BSO, PLND | NSE, CGA, CD56 | IIIC | RT | None | Alive (1 mo) | No | None | 13 |
| 2 | 79 | PMB | NA | LCNEC + endometrioid | TAH, BSO, Biopsies | NSE, CGA, CD56 | IIIA | RT | None | Alive (2 mo) | No | None | 13 |
| 3 | 59 | AGC | NA | LCNEC + serous | RH, BSO, OMT, P/PALND | NSE, SNP, CD56 | IIIB | RT | Unspecified | Alive (5 mo) | No | None | 7 |
| 4 | 70 | PMB | NA | Pure LCNEC | TAH, BSO, OMT | SNP, CGA, CD56 | IB | None | EP | Alive (6 mo) | Yes | Liver | 7 |
| Abd. pain | Paraaortic LN | ||||||||||||
| 5 | 42 | AUB | NA | Pure LCNEC | RH | SNP, CGA, CD56 | IC | None | Platinum based | Alive (9 mo) | No | None | 7 |
|
| |||||||||||||
| Survival ≤ 12 months | |||||||||||||
| 6 | 73 | Lumbago | LCNEC | Pure LCNEC | None | NSE, SNP, CGA#2 | IVB | None | None | Died (1 mo) | NA | Bone, SCN | 7 |
| Abd. distention | |||||||||||||
| 7 | 52 | PMB | MMMT | Pure LCNEC | None | SNP, CD56 | IIIC2 | None | None | Died (1 mo) | NA | NA | 3 |
| 8 | 71 | PMB | NA (extensive necrosis) | Pure LCNEC | RH, BSO, OMT, P/PALND | SNP, CGA, CD56 | IVB | None | Planned EP | Died (1 mo) | NA | NA | 6 |
| 9 | 80 | PMB | NA | LCNEC + endometrioid | TAH, BSO, PLND | NSE, CGA | IC | None | None | Died (5 mo) | Yes | Unspecified | 12 |
| 10 | 51 | PMB, AGC | MMMT | Pure LCNEC | TAH, BSO, OMT | SNP, CGA, CD56 | IVB | None | EP, Ifosfamide + cisplatin | Died (9 mo) | Yes | Intraabd. | 7 |
| Ut. mass | |||||||||||||
| 11 | 52 | Abd. pain | Endometrial stromal sarcoma | Pure LCNEC | TAH, BSO, P/PALND | SNP, CGA, CD56 | IIIC2 | CCRT | None | Died (10 mo) | Yes | Upper abdomen | 4 |
| Rapid uterine enlargement | |||||||||||||
| 12 | 52 | PMB | Pure LCNEC | TAH, BSO | NSE, SNP | IC | RT | EP | Died (10 mo) | Yes | Brain, Lung | 11 | |
| 13 | 59 | PMB | Poorly diff. endometrial carcinoma | Pure LCNEC | TAH, BSO, OMT | NSE, SNP, CGA, CD56 | IIIC2 | RT | TC, PLD, EP + Oct | Died (12 mo) | Yes | None | 7 |
|
| |||||||||||||
| Survival > 12 months | |||||||||||||
| 14 | 50 | PMB | NA | Pure LCNEC | TAH, BSO, OMT, P/PALND | NSE, SNP | IIIC | RT | EC | Alive (12 mo) | No | None | 12 |
| 15 | 40 | AUB | Sarcomatous undiff. carcinoma | LCNEC + sarcomatoid | TAH, BSO, OMT, PLND | NSE, SNP, CD56 | IB | None | None | Alive (16 mo) | No | None | 7 |
| 16 | 73 | PMB | Poorly diff. adenoca. | Pure LCNEC | TAH, BSO, OMT, P/PALND | SNP, CGA, CD56 | IIIC1 | None | IP | Alive (13 mo) | Yes | Paraaortic LN | 7 |
| 17 | 51 | Ut. mass | NA | LCNEC + endometrioid | RH, BSO, OMT, PALND | SNP, CGA, CD56 | IIIA | None | IP | Alive (20 mo) | No | None | 2 |
| 18 | 77 | PMB | NA | LCNEC + endometrioid | TAH, BSO | NSE, SNP, CGA, CD56 | IIB | RT | None | Died (23 mo) | NA | NA | 12 |
| Present | 61 | Abd. pain | Uterine sarcoma | Pure LCNEC | TAH, BSO, OMT | SNP, CD56 | IIIB | RT | EP, IP, FOLFIRI | Died (23 mo) | Yes | Pelvic LN | Ours |
| Rapid uterine enlargement | |||||||||||||
PMB, postmenopausal bleeding; MMMT, malignant mixed Mullerian tumor; AUB, abnormal uterine bleeding; AGC, atypical glandular cells; CGA, Chromogranin A; CT, chemotherapy; B, vaginal brachytherapy, EC, etoposide/carboplatin; EP, etoposide/cisplatin; IP, irinotecan/cisplatin; FOLFIRI, fluorouracil, leucovorin, and irinotecan; N/A, not available or not applicable; NSE, neuron-specific enolase; Oct, octreotide; OMT, omentectomy; RH, radical hysterectomy; PLD, pegylated doxorubicin; PLND, pelvic lymph node dissection; PMB, postmenopausal bleeding; P/PALND, pelvic and paraaortic lymph node dissection; RT, radiotherapy; SNP, synaptophysin; TC, paclitaxel/carboplatin; WP, whole pelvic radiotherapy; mo, month; diff, differentiated; L/SCNEC, large and small-cell neuroendocrine carcinoma.
Case presentation
A 61-year-old postmenopausal woman (gravida 2, para 2) presented with low abdominal pain and a rapidly growing uterine mass, but without abnormal vaginal discharge or bleeding. The patient had a history of surgery for rectal cancer and subsequent radiotherapy at age 53 and had been followed for several years. Abdominopelvic computed tomography (CT) conducted 11 months prior to this presentation revealed no uterine mass lesion. Transvaginal ultrasonography and pelvic magnetic resonance imaging (MRI) both depicted an enlarged uterus with an ill-defined mass measuring 7.5 × 6.3 cm abutting the rectum. Axial and sagittal T2-weighted images revealed uterine mass of intermediate signal intensity, and contrast-enhanced fat-suppressed T1-weighted images exhibited enhancement of the mass (Figure 1). Office endometrial biopsy failed due to cervical stenosis. Laboratory tests, which included CA125, CEA, and AFP, were unremarkable.
Figure 1.
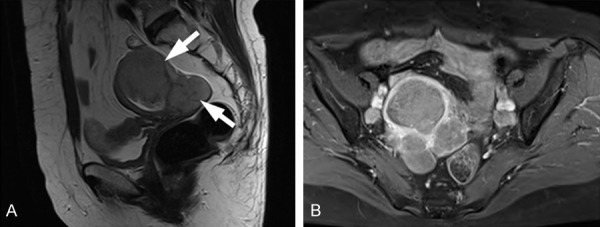
MRI features of large cell neuroendocrine carcinoma of the endometrium in a 61-year-old woman. A. Sagittal T2-weighted images showing uterine and parauterine masses (arrows) of intermediate signal intensity. B. Contrast-enhanced fat-suppressed T1-weighted image showing mass enhancements.
Under suspicion of uterine sarcoma based on imaging findings, the patient underwent exploratory laparotomy, during which intraoperative frozen examination of the uterine mass revealed malignancy. Accordingly, total abdominal hysterectomy, bilateral salpingo-oophorectomy, and infracolic omentectomy were performed. Grossly, multiple ill-defined white solid masses were observed in the uterine corpus that extended to uterine serosa, cervix, and right ovary (Figure 2A). Cut surfaces exhibited focal hemorrhage and necrosis, and microscopically, the tumors showed extensive necrosis, hemorrhage, and an organoid nesting pattern of large cells on hematoxylin and eosin stained sections (Figure 2B). Tumor cells were present in parametrium, cervix, and right ovary, but were not observed in omentum. Final FIGO stage was IIIB. Immunohistochemistry revealed tumor cells were diffusely positive for the neuroendocrine markers CD56 and synaptophysin (Figure 2C and 2D), positive in some areas (~5%) for WT-1, CD10, and pancytokeratin, and negative for ER, PR, p53, and SMA. The Ki-67 proliferation index was > 50%. Based on these histological and immunohistochemical features, the tumor was diagnosed as LCNEC of the endometrium. After six cycles of adjuvant chemotherapy with cisplatin (100 mg/m2, day 1) and etoposide (100 mg/m2, days 1-3) and stereotactic body radiation therapy (SBRT, 34Gy), the patient was disease-free, but at 12 months postoperatively, follow-up CT revealed enlarged retroperitoneal lymph nodes. Thereafter, relapse and metastasis occurred despite repeated multiple chemotherapy regimens (etoposide-cisplatin, irinotecan-cisplatin), and FOLFIRI (fluorouracil, leucovorin, irinotecan) (Figure 3). The disease followed a persistent, progressive course and the patient succumbed to the disease 23 months after surgery.
Figure 2.
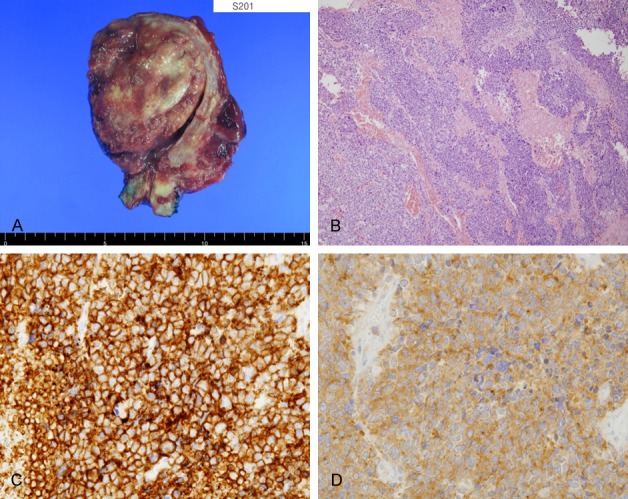
Histology and immunohistochemical findings. (A) Gross appearance of a surgical specimen showing a large cell neuroendocrine carcinoma of endometrium. Cut surfaces of the masses showed focal hemorrhage and necrosis, and the masses deeply invaded myometrium and extended to uterine serosa. (B) Microscopically, tumors showed extensive necrosis and an organoid nesting pattern of large cells (H&E, ×100). Immunohistochemistry revealed diffuse positivity for CD56 (C, membranous) and synaptophysin (D, cytoplasmic).
Figure 3.
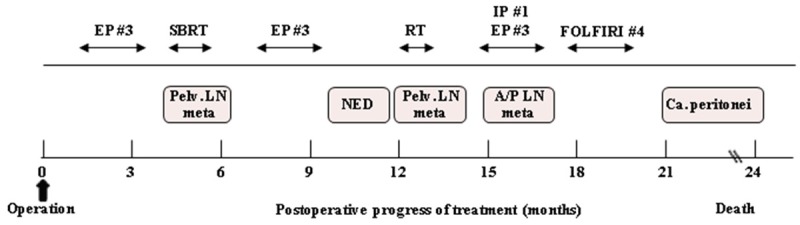
Clinical course and therapeutic regimens. EP, etoposide/cisplatin; IP, irinotecan/cisplatin, FOLFIRI, fluorouracil, leucovorin and irinotecan, SBRT, stereotactic body radiation therapy; Pelv, pelvic; NED, no evidence of disease; A/P, abdominopelvic; Ca. peritonei, carcinomatosis peritonei.
Discussion
Neuroendocrine carcinomas (NECs) arising from endometrium are a rare type of endometrial carcinoma with a non-endometrioid histology and account for less than 1% of all endometrial carcinomas [4]. However, due to their aggressive nature, LCNECs have a poor clinical prognosis. Furthermore, definitive preoperative diagnosis and optimal management are challenging because of the lack of specific symptoms and the rarity of LCNEC.
To date, only 19 case reports (including our case) have been issued on LCNEC of the endometrium (Table 1). Median age of reported cases was 62 years (range 40-88 years) and the disease mainly affected older postmenopausal women. The most common symptom (14 of 19 cases, 74%) was abnormal vaginal bleeding, including postmenopausal bleeding. However, the symptoms exhibited by endometrial LCNEC are nonspecific and similar to those of other uterine tumors. As occurred in our case, some cases showed rapid uterine enlargement during follow up for benign disease [4]. In our case, postmenopausal bleeding was not present. Notably, a large growing mass, which was not detected previously by follow-up CT for rectal cancer, newly appeared 11 months later. Thus the possibility of LCNEC should be considered when a rapidly growing uterine tumor is detected.
No diagnostic criteria have been proposed for endometrial LCNEC to date. As in cases of the lung, pathologic and immunohistochemical assessments are required to make a diagnosis of LCNEC of endometrium. It may be defined as large-cell carcinoma with a neuroendocrine morphology that expresses neuroendocrine markers, such as synaptophysin, chromogranin A, and/or CD56 [5]. Histologic characteristics typically include cellular features of abundant cytoplasm with a granular eosinophilic or basophilic appearance, large nuclei, clear nucleolus, and ≥ 10 mitoses/high power field. In addition, high grade scant stroma, ulceration, marked necrosis, and neuroendocrine morphologic arrangement (trabeculae, organoid nesting, palisading, or a rosette-like growth pattern) were reported in the majority of cases [6].
Since endometrial LCNEC closely resembles other endometrial cancers, including poorly differentiated adenocarcinoma, undifferentiated carcinoma, and MMMT [7], it is difficult to distinguish it from other endometrial cancers based on pathologic features alone. Of the 19 reported cases, preoperative diagnosis was achieved in only one case (Table 1). Immunohistochemical assay is the most useful tool for confirming a diagnosis of uterine LCNEC and positivity for one or more neuroendocrine markers is required [7]. LCNEC is known to exhibit high neuroendocrine immunoreactivity rates, that is, 80-90% for CD56, 50-60% for synaptophysin, and 80-85% for chromogranin A [8]. Previous cases have been positive for at least two neuroendocrine markers (Table 1), and in our case, the tumor was diffusely and strongly positive for synaptophysin and CD56 (Figure 2).
The radiologic findings of uterine LCNEC have not been well-described. Makihara et al. described the MRI features of two cases of pathologically proven LCNEC of uterine endometrium, but findings were nonspecific and mimicked those of other uterine malignancies like poorly differentiated endometrial adenocarcinoma and uterine sarcoma [9]. Nevertheless, preoperative MRI can be useful for assessing disease extent. In our patient, no preoperative diagnosis was made based on MRI findings.
Data regarding the optimal management of uterine LCNEC are scarce due to the limited number of reports available. The disease has been treated in the same manner as other endometrial carcinomas, that is, by surgery, chemotherapy and radiotherapy, and thus, it appears that a multi-modality approach is required. Standard surgical procedures for endometrial carcinomas include total hysterectomy, bilateral salpingo-oophorectomy, and lymph node dissection, though omentectomy is performed in cases with a non-endometrioid histology. Similar surgical procedures have been adopted in most patients with uterine LCNEC.
In previous case reports, adjuvant chemotherapy and/or radiotherapy have been either performed or planned. Significant response to cisplatin/etoposide-based chemotherapy has been reported in SCNEC of the lung [10], and in another case, LCNEC of the lung and digestive tract showed response to irinotecan/cisplatin therapy [11]. Therefore, these combinations are generally used as adjuvant therapies for uterine LCNEC. In some cases, postoperative pelvic radiotherapy provided some therapeutic benefit [7]. In our case, repeated multiple chemotherapy (cisplatin/etoposide, irinotecan/cisplatin, and FOLFIRI) and radiotherapy were administered (Figure 3), and a transient disease-free status was achieved, but FDG-PET/CT at 12 months after surgery revealed increased FDG uptake in pelvic lymph nodes, suggesting rapid progression.
Generally, stage is a key prognostic factor of carcinomas, and of the 19 cases of endometrial LCNEC reported to date, 8 cases (42%) wererapidly progressing with a survival of ≤ 12 months and 13 (42%) were of advanced stage (stages III or IV) (Table 1) This suggests the prognosis of LCNEC of endometrium is poor. Division of the 19 cases into 3 groups according to survival (≤ 12 months, > 12 months, or unknown due to short follow up), revealed percentages of advanced stage disease (stage III or IV) were similar in the three groups, that is, 75% (6/8) in the ≤ 12-month group, 67% (4/6) in the > 12-month group and 60% (3/5) in the unknown group. Furthermore, even in two patients with early stage disease (IC), brain and lung metastases occurred at six and four months after initial diagnosis [12]. In another stage IC case, the patient concerned died of disseminated malignancy at 5 months postoperatively [13]. Accordingly, based on these results, stage is not a significant prognostic factor in endometrial LCNEC.
In conclusion, LCNEC arising from uterine endometrium is a rare, highly aggressive, and rapidly progressive tumor. We recommend endometrial LCNEC be included in the differential diagnosis of rapidly growing endometrial tumors in postmenopausal women. This tumor is difficult to diagnose because its imaging findings are non-specific and because no pathologic criteria have been proposed to date. Nevertheless, neuroendocrine morphology and tumor immunohistochemical characteristics should be determined to facilitate the differential diagnosis of endometrial malignancies. Here, we report a case of LCNEC of endometrium with a high Ki-67 index and that exhibited a rapidly progressive course. We encourage clinicians to report cases of uterine LCNEC to guide its optimal adjuvant management.
Disclosure of conflict of interest
None.
References
- 1.Matsumoto H, Nasu K, Kai K, Nishida M, Narahara H, Nishida H. Combined large-cell neuroendocrine carcinoma and endometrioid adenocarcinoma of the endometrium: a case report and survey of related literature. J Obstet Gynaecol Res. 2016;42:206–10. doi: 10.1111/jog.12881. [DOI] [PubMed] [Google Scholar]
- 2.Ogura J, Adachi Y, Yasumoto K, Okamura A, Nonogaki H, Kakui K, Yamanoi K, Suginami K, Koyama T, Ikehara S. Large-cell neuroendocrine carcinoma arising in the endometrium: a case report. Mol Clin Oncol. 2018;8:575–8. doi: 10.3892/mco.2018.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gardner GJ, Reidy-Lagunes D, Gehrig PA. Neuroendocrine tumors of the gynecologic tract: a society of gynecologic oncology (SGO) clinical document. Gynecol Oncol. 2011;122:190–8. doi: 10.1016/j.ygyno.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi A, Yahata T, Nanjo S, Mizoguchi M, Yamamoto M, Mabuchi Y, Yagi S, Minami S, Ino K. Rapidly progressing large-cell neuroendocrine carcinoma arising from the uterine corpus: a case report and review of the literature. Mol Clin Oncol. 2017;6:881–5. doi: 10.3892/mco.2017.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG, editors. WHO Classification of Tumours of Lung, Pleura, Thymus and Heart. 4th edition. Lyon: IARC; 2015. Large cell neuroendocrine carcinoma; pp. 69–72. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen ML, Han L, Minors AM, Bentley-Hibbert S, Pradhan TS, Pua TL, Tedjarati SS. Rare large cell neuroendocrine tumor of the endometrium: a case report and review of the literature. Int J Surg Case Rep. 2013;4:651–5. doi: 10.1016/j.ijscr.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tu YA, Chen YL, Lin MC, Chen CA, Cheng WF. Large cell neuroendocrine carcinoma of the endometrium: a case report and literature review. Taiwan J Obstet Gynecol. 2018;57:144–9. doi: 10.1016/j.tjog.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 8.Ono K, Yokota NR, Yoshioka E, Noguchi A, Washimi K, Kawachi K, Miyagi Y, Kato H, Yokose T. Metastatic large cell neuroendocrine carcinoma of the lung arising from the uterus: a pitfall in lung cancer diagnosis. Pathol Res Pract. 2016;212:654–7. doi: 10.1016/j.prp.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Makihara N, Maeda T, Nishimura M, Deguchi M, Sonoyama A, Nakabayashi K, Kawakami F, Itoh T, Yamada H. Large cell neuroendocrine carcinoma originating from the uterine endometrium: a report on magnetic resonance features of 2 cases with very rare and aggressive tumor. Rare Tumors. 2012;4:e37. doi: 10.4081/rt.2012.e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K, Tamura T, Yamamoto S, Saijo N Japan Clinical Oncology Group. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2001;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 11.Tanimoto H, Hamasaki A, Akimoto Y, Honda H, Takao Y, Okamoto K, Teramoto M, Teramoto H, Kaneko M, Oshita T. A case of large cell neuroendocrine carcinoma (LCNEC) of the uterine cervix successfully treated by postoperative CPT-11+CDDP chemotherapy after non-curative surgery. Gan To Kagaku Ryoho. 2012;39:1439–41. [PubMed] [Google Scholar]
- 12.Erhan Y, Dikmen Y, Yucebilgin MS, Zekioglu O, Mgoyi L, Terek MC. Large cell neuroendocrine carcinoma of the uterine corpus metastatic to brain and lung: case report and review of the literature. Eur J Gynaecol Oncol. 2004;25:109–12. [PubMed] [Google Scholar]
- 13.Mulvany NJ, Allen DG. Combined large cell neuroendocrine and endometrioid carcinoma of the endometrium. Int J Gynecol Pathol. 2008;27:49–57. doi: 10.1097/pgp.0b013e31806219c5. [DOI] [PubMed] [Google Scholar]


