Abstract
Background: Osteosarcoma (OS) is one of the most common bone tumors in adolescents and young adults. Emerging evidence suggested ncRNA (lncRNA and miRNA) are closely associated with cell progression, apoptosis and autophagy. However, the role of regulatory network between ncRNA and mRNA in OS has not been fully verified. Methods: lncRNA XIST, miRNA expression were detected by qRT-PCR. The protein expression of LC3, p62, AKT, p-AKT, mTOR and p-mTOR was measured by western blot. MTT assay and flow cytometry were applied to measure cell proliferation and apoptosis. Luciferase assay was used to ensure the relationship between lncRNA, miRNA and mRNA. GFP-LC3 cells were observed using fluorescence microscope. Results: XIST expression was up-regulated but miR-375-3p was down-regulated in OS tissues and cells. Luciferase assay results demonstrated that miR-375-3p was a target of XIST and mTOR was a target mRNA of miR-375-3p. In addition, knockdown of XIST and mTOR inhibited OS cell proliferation and autophagy, but induced apoptosis. Knockdown of XIST could reverse the effect of miR-375-3p inhibitor on OS cells. The effects of si-mTOR of OS cells could be reversed by silencing miR-375-3p. Moreover, knockdown of XIST inhibited AKT/mTOR signaling pathway via sponging miR-375-3p. Conclusion: Knockdown of XIST inhibited cell growth and autophagy but induced cell apoptosis by suppressing the AKT/mTOR signaling pathway by sponging miR-375-3p.
Keywords: Osteosarcoma, XIST, miR-375-3p, AKT/mTOR
Introduction
Osteosarcoma (OS) is the most common primary bone tumor in adolescents and young adults, exhibiting osteogenic differentiation and producing malignant bones [1,2]. However, research on the underlying mechanisms of OS is still lacking.
Long non-coding RNAs (lncRNAs) are a class of RNAs, which are ~200 nucleotides in length. Evidence determined that lncRNAs are widely involved in multiple biologic processes, including cell progression, inflammation and tumorigenesis [3-5]. LncRNA-XIST (X inactive-specific transcript) is an oncogenic lncRNA in various cancers, including pancreatic cancer, non-small cell lung cancer, hepatocellular carcinoma, pancreatic cancer and osteosarcoma [6-9]. Some studies showed that LncRNA-XIST was up-regulated in OS and associated with cellular processes [9,10].
MicroRNAs (miRNAs) are ~22 nucleotides small non-coding RNAs that repress gene expression at post-transcription by binding to mRNAs [11]. Additionally, miRNAs, as oncogenes or tumor suppressors, play an important role in cell proliferation, migration, apoptosis, and drug resistance of many cancers [12-15]. For example, miR-155, as an oncogene, is up-regulated in liposarcoma and promotes tumor cell growth through targeting casein kinase-1α [16]. miR-145, as a tumor suppressor, was down-regulated in colon cancer and inhibits cell growth by regulating HDAC4 [17].
Recently, some studies reported that lncRNA act as competing endogenous RNA (ceRNA) with miRNAs by binding miRNAs contained common sites [18]. Until now, many regulatory mechanisms of cell growth have been investigated in OS [19-22], but the role of regulatory network lncRNA, miRNA, and mRNA has not been fully elucidated. In our study, we found that XIST was up-regulated and miR-375-3p was down-regulated in OS, and bioinformatic analysis showed that miR-375-3p was a potential target miRNA of XIST and mTOR was a potential target mRNA of miR-375-3p. In addition, autophagy plays an important role in regulating cellular progression and tumor formation [23]. Therefore, we hypothesized XIST can regulate mTOR expression by sponging miR-375-3p and that the regulatory network was associated with OS cell growth and autophagy.
Materials and methods
Patients and tissues specimens
20 pairs of tissues and adjacent tissues specimens were obtained from 20 OS patients with no chemotherapy or radiation therapy at Affiliated Hospital of Guangdong Medical University. Written informed consent was obtained from patients and their guardians. This study has been approved by Experiments committee of Affiliated Hospital of Guangdong Medical University.
Cell culture
The human OS cell lines MG-63, HOS, U2-OS and Saos-2, and the human normal cell line hFOB1.19 were purchased from the American Type Cultured Collection (Manassas, VA, USA). The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) with 10% fetal bovine serum (FBS, Gemini Bio-Products, West Sacramento, CA), penicillin and streptomycin at 37°C with 5% CO2.
Cell transfection
miR-375-3p mimic, miR-375-3p inhibitor, sh-XIST, pc-XIST and their respective negative controls were obtained from GenePharma (Shanghai, China). si-mTOR, pc-mTOR and their respective negative controls were purchased form from Ribobio (Guangzhou, Guangdong, China). All the vectors and fragments were transfected into OS cell lines using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instruction.
Reverse transcription and quantitative real-time PCR
Total RNA was extracted from tissues and cells by TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. RNA concentration was detected using the NanoDropND-1000 spectrophotometer (NanoDrop Technologies, Wilmintgon, DE, USA). cDNA were reverse transcribed using TaqMan MicroRNA Reverse Transcription Kit for microRNA (Applied Biosystems, Foster City, CA, USA) or M-MLV Reverse Transcriptase (Invitrogen) for mRNA. Then the SYBR® Green (Promega, Madison, WI, USA) was applied to detect the expression of XIST, miR-375-3p and mTOR, according to the manufacturer’s instruction. GAPDH and U6 were used as reference gene for lncRNA, mRNA and miRNA. Quantitative real-time PCR was performed by using an iQTM5 Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The primer: U6: forward 5’-CTCGCTTCGGCAGCACA-3’ and reverse 5’-AACGCTTCACGAATTTGCGT-3’; miR-375-3p: forward 5’-ACACTCCAGCTGGGTTTGTTCGTTCGGCTC-3’ and reverse 5’-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCACGCGA-3’ GAPDH: forward 5’-GCACCGTCAAGGCTGAGAAC-3’ and reverse 5’-ATGGTGGTGAAGACGCCAGT-3’; XIST: forward 5’-ACGCTGCATGTGTCCTTAG-3’ and reverse 5’-GAGCCTCTTATAAGCTGTTTG-3’; mTOR: forward 5’-ACATGCAGCTGTCCTGGTTC-3’ and reverse 5’-TGAGGCTTCTGCATCTCCTT-3’.
Western blot
Total proteins were extracted from tissues and cells with RIPA buffer and separated from using sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA, USA). We blocked the membrane with 5% dried skim milk in TBS and the membrane was incubated with the rabbit anti-LC3, anti-p62, anti-AKT, anti-p-AKT, anti-mTOR and anti-p-mTOR (1:2000 dilution, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and mouse anti-GAPDH antibody (1:2000 dilution, Santa Cruz Biotechnology Inc.) at 4°C overnight. After washing in TBST, the membrane was incubated with HRP-conjugated anti-rabbit IgG secondary antibody (1:2000 dilution, Santa Cruz Biotechnology Inc.) and anti-mouse IgG secondary antibody (1:2000 dilution, Santa Cruz Biotechnology Inc.). The blot was measured using PierceTM ECL western blotting substrate (Thermo Fisher Scientific).
Cell proliferation
2 × 103 cells were seeded into the 96-well cell culture plates (Corning Inc., Corning, NY, USA). Cell proliferation was measured using 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2Htetrazolium bromide (MTT) assay kit (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s protocol. 30 µL serum-free media with MTT solution were added into each well and incubated for 4 h at 37°C. Then 150 ul MTT solvent were added and incubated for 3 h at 37°C. The absorbance at 450 nm was measured using a spectrophotometric microplate reader (Beyotime Institute of Biotechnology, Haimen, China).
Luciferase assay
XIST wild-type (WT) or mutant (MUT) and mTOR wild-type (WT) or mutant (MUT) fragment were amplified and inserted the downstream of the luciferase vector pGL3-control vector (Promega, Madison, WI, USA). Then XIST WT, XIST MUT, mTOR WT and mTOR MUT were co-transfected with miR-375-3p mimic into MG-63 and U2-OS cell lines using Lipofectamine 2000 (Invitrogen). Cell collected at 48 h after transfection, and the luciferase activity was detected using the Dual luciferase reporter assay system (Promega).
Cell apoptosis
Cell apoptosis was detected using flow cytometry and Annexin-V fluorescein isothiocyanate and propidium iodide (FITC-Annexin V/PI) apoptosis detection kit (Life Technology, Waltham, MA, USA). Cells were stained by Annexin-V FITC/PI. Then each sample was measured using flow cytometry.
Microcopy
Cells stably expression GFP-LC3 were cultured. Then the GFP-LC3 cells expressed green fluorescence were observed using Fluorescence microscope (Nikon, Tokyo, Japan).
Statistical analysis
All statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA). All results were showed as mean ± SD (standard deviation) at least three repeated individual experiments. All comparisons groups were analyzed using Student t-test. P-value < 0.5 was considered significant.
Results
XIST expression was up-regulated and miR-375-3p was down-regulated in OS tissues and cells
In our experiments, we obtained tumor tissues and matched adjacent tissues from 20 patients. As shown in Figure 1A and 1B, XIST was up-regulated and miR-375-3p was down-regulated in tumor tissues compared with adjacent tissues. Then to test their role in OS cell lines, we chose hFOB1.19, MG-63, HOS, U2-OS and Saos-2 and measured XIST, miR-375-3p expression by qRT-PCR. The results showed that the expression of XIST was increased and the expression of miR-375-3p was decreased in MG-63, HOS, U2-OS, Saos-2 cell lines compared with hFOB1.19 cell line (Figure 1C and 1D). We selected MG-63 and U2-OS cell lines for following experiments. Meanwhile, miR-375-5p expression was negatively correlated with the expression of XIST in OS tissues (Figure 1E).
Figure 1.
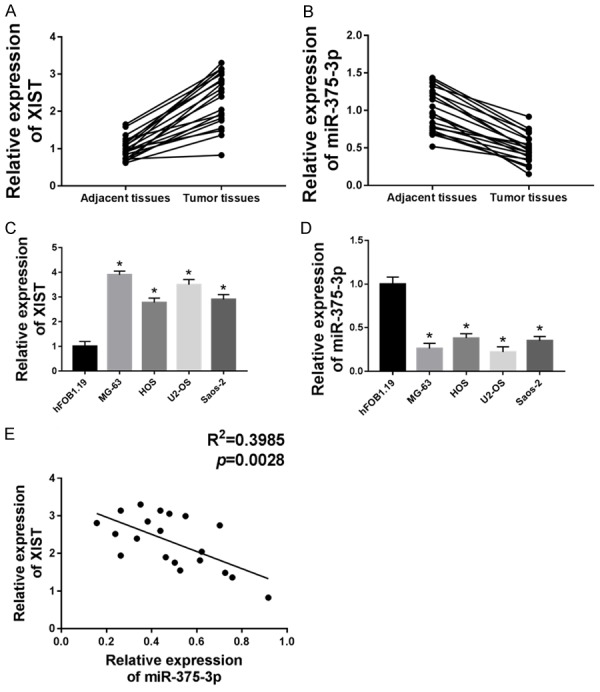
XIST expression was up-regulated and miR-375-3p was down-regulated in OS tissues and cells. (A and B) The expression of XIST (A) and miR-375-3p (B) were detected in tumor tissues and adjacent tissues by qRT-PCR. (C and D) The expression of XIST (C) and miR-375-3p (D) were detected in hFOB1.19, MG-63, HOS, U2-OS and Saos-2 cell lines by qRT-PCR. (E) XIST and miR-375-3p correlation relationship was analyzed by Pearson’s correlation analysis. *P < 0.05.
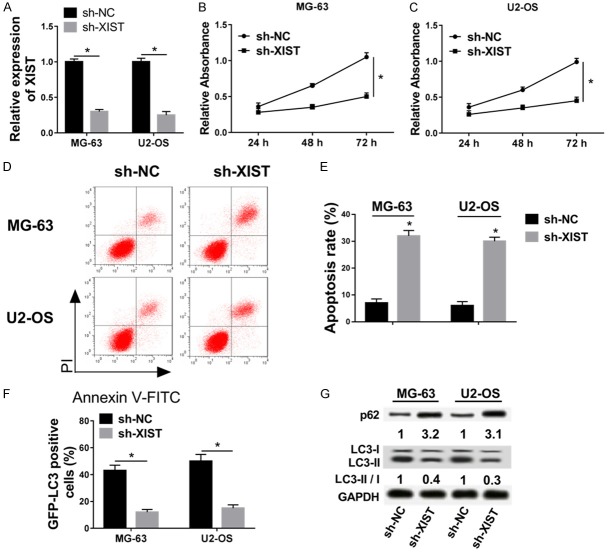
Knockdown of XIST inhibited cell proliferation and autophagy, and induced apoptosis in OS cells
To further access the function of XIST, siRNA was conducted to knock down its expression (Figure 2A). Subsequently, MTT assay showed that knockdown of XIST inhibited cell proliferation in MG-63 and U2-OS cell lines (Figure 2B and 2C). Apoptosis rate of sh-XIST group was significantly higher than control (Figure 2D and 2E). Autophagy plays a critical role in regulating the cell progression in cancers, so we detected the protein expression LC-3 and p62 in OS, which are the important markers of autophagy [24]. As shown in Figure 2F, GFP-LC3 positive cells was significantly lower in sh-XIST group compared with sh-NC groups. In addition, western blot data verified that knockdown of XIST down-regulated the expression of LC3-II/I, and up-regulated p62 expression (Figure 2G). In conclusion, knockdown of XIST inhibited cell proliferation and autophagy, but induced apoptosis in OS cells.
Figure 2.
Knockdown of XIST inhibited cell proliferation and autophagy, but induced apoptosis in OS cells. (A) The expression of XIST was detected in sh-NC and sh-XIST groups of MG-63 and U2-OS cell lines via qRT-PCR. (B and C) Cell proliferation were measured in sh-NC and sh-XIST groups of MG-63 (B) and U2-OS (C) cell lines via MTT assay. (D and E) Cell apoptosis were detected in sh-NC and sh-XIST groups of MG-63 and U2-OS cell lines by flow cytometry. (F) GFP-LC3 positive cells were calculated in sh-NC and sh-XIST groups of MG-63 and U2-OS cell lines. (G) The expression of LC3 and p62 were measured in sh-NC and sh-XIST groups of MG-63 and U2-OS cell lines by western blot. *P < 0.05.
miR-375-3p was a target of XIST
To further explore the relationship of XIST and miR-375-sp, we predicted that miR-375-3p was a candidate miRNA target of XIST in miRCode and miRBase database (Figure 3A). To verify this, we co-transfected the luciferase reporter plasmids XIST-WT or XIST-MUT with miR-NC or miR-375-3p into MG-63 and U2-OS cell lines respectively. The results showed that miR-317-3p decreased the luciferase activity by binding transfection of XIST WT, but not XIST MUT (Figure 3B). Furthermore, we verified that miR-375-3p expression was negatively regulated by XIST (Figure 3C and 3D). With sh-NC or sh-XIST transfected into MG-63 and U2-OS cell lines, we found that the miR-375-3p expression was increased by sh-XIST (Figure 3C). Transfection of miR-375-3p inhibitor decreased the expression of miR-375-3p in MG-63 and U2-OS cell lines, while sh-XIST restored its expression (Figure 3D). Taken together, we proved that miR-375-3p was a target miRNA of XIST.
Figure 3.
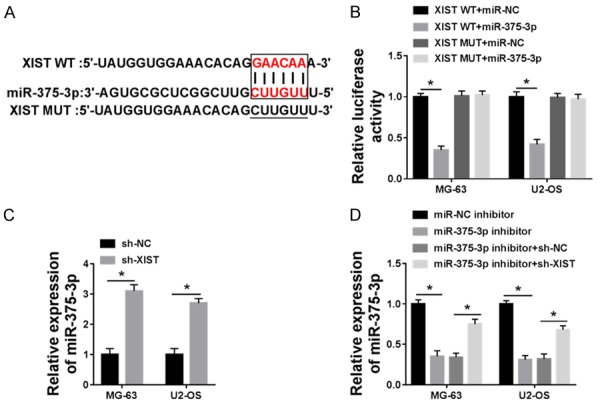
miR-375-3p is a target of XIST. A. miRCode and miRBase prediction of miR-375-3p binding to XIST. B. Luciferase reporter assay was used to detected luciferase activity in XIST WT+miR-NC, XIST WT+miR-375-3p, XIST MUT+miR-NC and XIST MUT+miR-375-3p groups. C. The expression of miR-375-3p was detected in sh-NC and sh-XIST groups by qRT-PCR. D. The expression of miR-375-3p was detected in miR-NC inhibitor, miR-375-3p inhibitor, miR-375-3p inhibitor+sh-NC and miR-375-3p inhibitor+sh-XIST groups by qRT-PCR. *P < 0.05.
Down-regulated XIST reversed the effect of low miR-375-3p expression on OS cells
To explore the function of miR-375-3p in OS cells, we obtained the MG-63 and U2-OS cell lines with transfectionof miR-375-3p inhibitor. As shown in Figure 4A and 4B, MTT assay results showed that cell proliferation were significantly promoted by miR-375-3p inhibitor, whereas knockdown of XIST can reverse the effect of miR-375-3p inhibitor (Figure 4C).
Figure 4.

Knockdown of XIST reversed the effect of miR-375 inhibitor on cell proliferation, autophagy and apoptosis in OS cells. (A and B) Cell proliferation was measured in miR-NC inhibitor, miR-375-3p inhibitor, miR-375 inhibitor+sh-NC and miR-375-3p inhibitor+sh-XIST groups of MG-63 (A) and U2-OS (B) cell lines. (C) Cell apoptosis was detected in miR-NC inhibitor, miR-375-3p inhibitor, miR-375 inhibitor+sh-NC and miR-375-3p inhibitor+sh-XIST groups of MG-63 and U2-OS cell lines by flow cytometry. (D) GFP-LC3 positive cells were calculated in miR-NC inhibitor, miR-375-3p inhibitor, miR-375 inhibitor+sh-NC and miR-375-3p inhibitor+sh-XIST groups of MG-63 and U2-OS cell lines. (E) The expressions of LC3-I, LC3-II and p62 were measured in miR-NC inhibitor, miR-375-3p inhibitor, miR-375 inhibitor+sh-NC and miR-375-3p inhibitor+sh-XIST groups of MG-63 and U2-OS cell lines by western blot. *P < 0.05.
To elucidate whether miR-375-3p downregulation increased cell autophagy, we detected GFP-LC3 positive cells and the expression of related gene. The results determined that GFP-LC3 positive cells in miR-375-3p inhibitor group were obviously higher than that in miR-NC inhibitor group and sh-XIST reversed the effect of miR-375-3p inhibitor (Figure 4D). Meanwhile, downregulation of miR-375-3p increased the protein level of LC3-II/I, and reduced the protein level of p62. However, knockdown of XIST could reverse the effect of miR-375-3p inhibitor on the protein level of LC3-II/I and p62 in MG-63 and U2-OS cell lines (Figure 4E). Therefore, miR-375-3p inhibitor transfection increased OS cells proliferation and autophagy, but decreased apoptosis, which was recovered by XIST knockdown.
mTOR was a target of miR-375-3p
miRNA usually functions to bind with specific mRNA to mediate itsdegradation or transcription [25]. Bioinformatic analysis revealed that mTOR is a potential target gene of miR-375-3p, and miR-375-3p binds to mTOR 3’UTR (Figure 5A). Then mTOR WT or mTOR MUT were co-transfected with miR-NC or miR-375-3p into MG-63 and U2-OS cell lines. Luciferase reporter assay results showed that luciferase activity was significantly decreased in mTOR WT+miR-375-3p group compared with mTOR WT+miR-NC group, while mTOR MUT+miR-375-3p groups showed no difference with mTOR MUT+mTOR MUT+miR-NC group, implying that mTOR was a target of miR-375-3p (Figure 5B). To further clarify our speculation, the protein level of mTOR was measured by western blot. We found that mTOR protein level was remarkably increased by miR-375-3p inhibitor in MG-63 and U2-OS cell lines (Figure 5C). Then, when the si-mTOR and miR-NC inhibitor or si-mTOR and miR-375-3p inhibitor were co-transfected into OS cells, we found that down-regulated miR-375-3p can recovered mTOR expression reduced by si-mTOR (Figure 5D). Therefore, mTOR protein level could be negatively regulated by miR-375-3p and mTOR was a target of miR375-3p.
Figure 5.
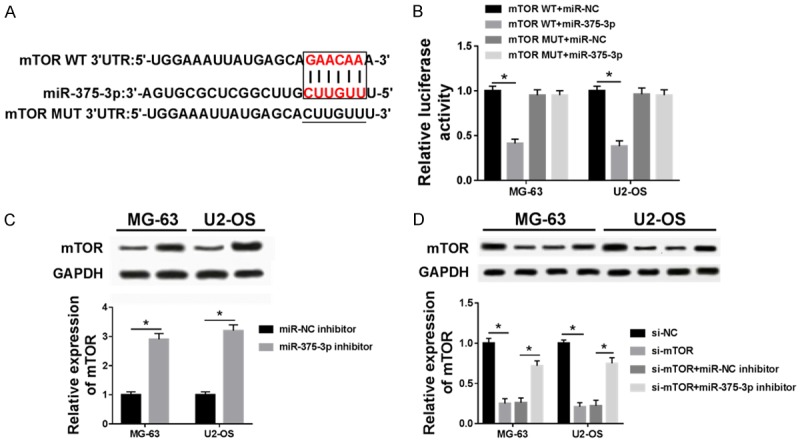
mTOR is a target of miR-375-3p. A. miRCode and miRBase prediction of miR-375-3p binding sites in the 3’UTR of mTOR. B. Luciferase reporter assay was used to detect luciferase activity in mTOR WT+miR-NC, mTOR WT+miR-375-3p, mTOR MUT+miR-NC and mTOR MUT+miR-375-3p groups. C. The expression of mTOR was detected in miR-NC inhibitor and miR-375-3p inhibitor groups. D. The expression of mTOR was detected in si-NC, si-mTOR, si-mTOR+miR-NC inhibitor and si-mTOR+miR-375-3p inhibitor groups. *P < 0.05.
mTOR knockdown inhibited OS cell proliferation and autophagy but induced cell apoptosis in vitro, which was reversed by down-regulating miR-375-3p
To further elucidate the function and underlying mechanism of mTOR, we transfected si-NC, si-mTOR, si-mTOR+miR-NC inhibitor and si-mTOR+miR-375-3p inhibitor into MG-63 and U2-OS cell lines. As shown in Figure 6A and 6B, si-mTOR transfection significantly suppressed cell proliferation, which was recovered by miR-375-3p inhibitor. In addition, knockdown of mTOR significantly induced cell apoptosis and decreased GFP-LC3 positive cells, whereas down-regulated miR-375-3p reversed the effect of low mTOR expression on OS cells (Figure 6C and 6D). As shown in Figure 6E, the protein levels of LC3-I, LC3-II were down-regulated whereas the protein level of p62 was up-regulated in si-mTOR group, but transfection of miR-375-3p inhibitor reversed their expression in OS cells. Taken together, knockdown of mTOR inhibited cell proliferation and autophagy, but increased cell apoptosis rate, whereas the effects of si-mTOR on OS cells could be reversed by down-regulation of miR-375-3p.
Figure 6.
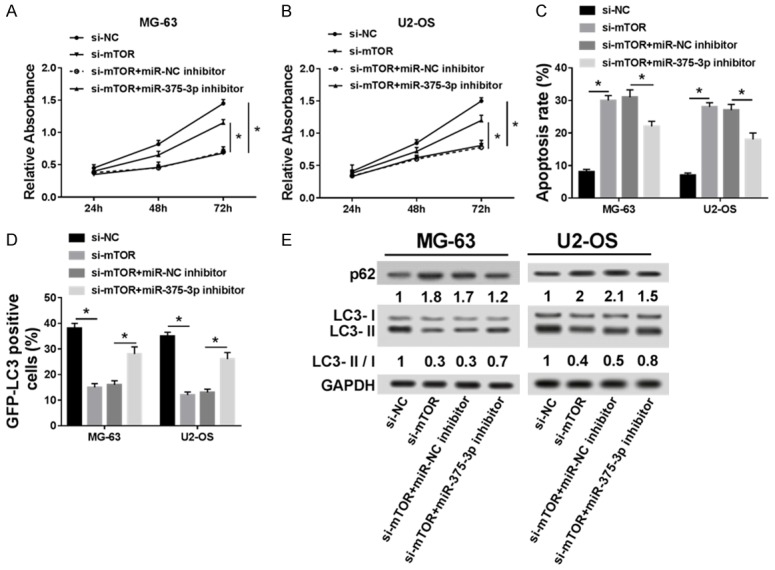
si-mTOR transfection inhibited OS cell proliferation and autophagy and induced cell apoptosis in vitro, which was reversed by down-regulating miR-375-3p. (A and B) Cell proliferation was measured in si-NC, si-mTOR, si-mTOR+miR-NC inhibitor and si-mTOR+miR-375-3p inhibitor groups of MG-63 (A) and U2-OS (B) cell lines. (C) Cell apoptosis was detected in si-NC, si-mTOR, si-mTOR+miR-NC inhibitor and si-mTOR+miR-375-3p inhibitor groups of MG-63 and U2-OS cell lines by flow cytometry. (D) GFP-LC3 positive cells were calculated in si-NC, si-mTOR, si-mTOR+miR-NC inhibitor and si-mTOR+miR-375-3p inhibitor groups of MG-63 and U2-OS cell lines. (E) The expression of LC3-I, LC3-II and p62 were measured in si-NC, si-mTOR, si-mTOR+miR-NC inhibitor and si-mTOR+miR-375-3p groups of MG-63 and U2-OS cell lines by western blot. *P < 0.05.
Knockdown of XIST inhibited AKT/mTOR signaling pathway through regulating miR-375-3p
AKT, p-AKT, mTOR and p-mTOR are key proteins in AKT/mTOR signaling pathway, for which we detected their expression in sh-NC, sh-XIST, sh-XIST+miR-NC inhibitor and sh-XIST+miR-375-3p inhibitor groups by western blot (Figure 7A). Our results demonstrated that down-regulated XIST suppressed the protein level of p-AKT, mTOR and p-mTOR, whereas had no significant effect on AKT. Therefore, the AKT/mTOR signaling pathway was inactivated by sh-XIST. Otherwise, miR-375-3p inhibitor could restore p-AKT, mTOR and p-mTOR expression inhibited by sh-XIST (Figure 7B-E). Therefore, down-regulated XIST inhibited the AKT/mTOR signaling pathway, which was reversed by miR-375-3p silencing.
Figure 7.
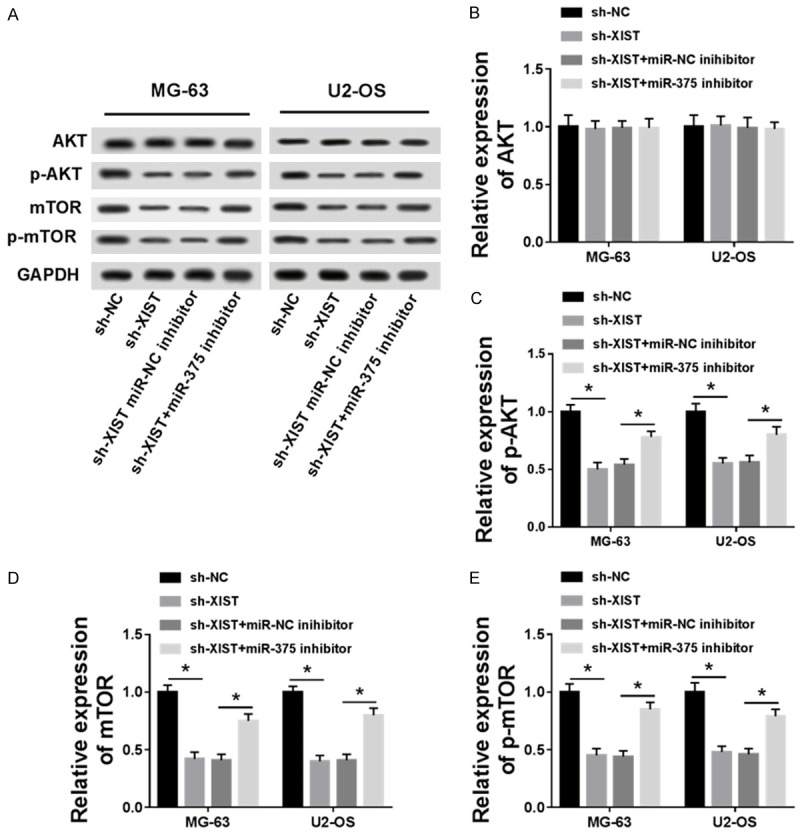
AKT/mTOR signaling pathway was inactivated by knockdown of XIST and restored by miR-375-3p inhibitor in MG-63 and U2-OS cell lines (A). The expression of AKT (B), p-AKT (C), mTOR (D) and p-mTOR (E) in sh-NC, sh-XIST, sh-XIST+miR-NC and sh-XIST+miR-375 inhibitor groups was measured by western blot. *P < 0.05.
Discussion
LncRNA plays an indispensable role in the occurrence and development of tumors. Emerging studies indicated that lncRNAs were widely involved in cell progression, inflammation, immune response, drug resistance and prognosis in cancers [3,22,26]. Of note, lncRNA XIST has been investigated as an oncogenic lncRNA to affect cell growth in several cancers, including OS [7-9]. Previous studies suggested that lncRNA-XIST was up-regulated in OS and XIST/miR-320b/RAP2B axis and XIST/miR-21-5p/PDCD axis affected OS cell proliferation, invasion and metastasis [9,10]. As the regulatory network of lncRNA is very complex and one lncRNA may have multiple targeted miRNAs, the regulatory mechanism of lncRNA complement still needs further research and exploration. In present study, lncRNA-XIST was up-regulated in OS tissues and cells. Besides, inhibition of XIST can reduce cell proliferation and induce cell apoptosis. LC3, as an autophagosome marker, can be incorporated into autophagy protein, which is a critical protein in autophagy [27]. The signaling adaptor p62 is a multidomain protein, which is associated with autophagy and apoptosis in cancers [28]. Thus, we found that knockdown of XIST inhibited cell autophagy and LC3-I and LC3-II; protein level and induced p63 protein level in OS cells.
miR-375-3p is processed from the precursor has-miR-375 and associated with cell growth and is prognostic in cancers [29-31]. For example, Ding et al. showed that miR-375 was down-regulated in gastric cancer and inhibited cell proliferation [29]. As a tumor suppressor, miR-375 is also implicated in OS diagnosis, prognosis and chemosensitivity [32-34]. However, the underlying mechanism has been not fully illuminated. In our study, miR-375-3p was down-regulated in OS tissues and cells and negatively correlated with XIST. Luciferase reporter assay verified that miR-375-3p was a target miRNA of XIST, which was reported for the first time. Moreover, down-regulated miR-375-3p could promote OS cells growth and autophagy but reduce apoptosis which had the opposite effect compared to knockdown of XIST.
Rapamycin (mTOR) was a downstream of AKT, which is a key factor in AKT/mTOR signaling pathway [35,36]. AKT/mTOR signaling pathway plays an important role in cell autophagy, cell cycle progression, and apoptosis in several cancers, such as prostate cancer, endometrial cancer, and OS [19,37,38]. Miwa et al. showed that caffeine contributed to apoptosis via inhibition of AKT/mTOR/S6K, NF-kB and MAPK pathway [39]. In our study, we proved for the first time that mTOR was the target gene of miR-375-3p and verified the function of mTOR in OS. More than that, knockdown of mTOR suppressed OS cells growth and autophagy but promoted apoptosis.
Notably, in our study, miR-375-3p silencing can reverse the effect of XIST knockdown on OS cells. Meanwhile, si-mTOR transfection inhibited OS cells proliferation and autophagy but induced cell apoptosis in vitro, which was reversed by down-regulation of miR-375-3p. Taken together, knockdown of XIST sponged miR-375-3p to inhibit cell growth and autophagy by down-regulating mTOR.
AKT, p-AKT, mTOR and p-mTOR were key proteins in the AKT/mTOR signaling pathway. Also, to further realize whether lncRNA-XIST affect AKT/mTOR signaling pathway, we measured the AKT, p-AKT, mTOR and p-mTOR protein level using western blot and found that down-regulated XIST inhibited p-AKT, mTOR and p-mTOR protein level, whereas it had no significant effect on AKT. In addition, down-regulated miR-375-3p restored p-AKT, mTOR and p-mTOR protein level inhibited by sh-XIST. However, our study showed that the expression of p-AKT, which is the upstream protein of mTOR, was changed by sh-XIST and miR-375-3p. This may be caused by other interactions between miRNAs and their target genes, which remains unclear. Taken together, lncRNA-XIST inhibited AKT/mTOR signaling pathway to affect cell progression by sponging miR-375-3p.
Conclusions
In conclusion, we first elucidated lncRNA-XIST/miR-375-3p/mTOR axis plays an important role in OS cells, and lncRNA XIST promoted OS progression by activating AKT/mTOR signaling pathway through sponging miR-375-3p.
Acknowledgements
This study was approved by the Science and Technology Planning Project of Guangdong Province (Grant No.: 2017ZC0311).
Disclosure of conflict of interest
None.
References
- 1.Cortini M, Avnet S, Baldini N. Mesenchymal stroma: role in osteosarcoma progression. Cancer Lett. 2017;405:90–99. doi: 10.1016/j.canlet.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:421–436. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Yu B, Li J, Su L, Yan M, Zhu Z, Liu B. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5:2318–2329. doi: 10.18632/oncotarget.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rapicavoli NA, Qu K, Zhang J, Megan M, Remi-Martin L, Chang HY. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. Elife. 2013;2:e00762. doi: 10.7554/eLife.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang YR, Zang SZ, Zhong CL, Li YX, Zhao SS, Feng XJ. Increased expression of the lncRNA PVT1 promotes tumorigenesis in non-small cell lung cancer. Int J Clin Exp Path. 2014;7:6929–6935. [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang YL, Li XB, Hou YX, Fang NZ, You JC, Zhou QH. The lncRNA XIST exhibits oncogenic properties via regulation of miR-449a and Bcl-2 in human non-small cell lung cancer. This article has been corrected since advanced online publication, and an erratum is also printed in this issue. Acta Pharmacol Sin. 2017;38:371–381. doi: 10.1038/aps.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong Q, Zhang S, Liang C, Zhang Y, Kong Q, Chen S, Qin J, Jin Y. LncRNA XIST functions as a molecular sponge of miR-194-5p to regulate MAPK1 expression in hepatocellular carcinoma cell. J Cell Biochem. 2017;119:4458–4468. doi: 10.1002/jcb.26540. [DOI] [PubMed] [Google Scholar]
- 8.Wei W, Liu Y, Lu Y, Yang B, Tang L. LncRNA XIST promotes pancreatic cancer proliferation through miR-133a/EGFR. J Cell Biochem. 2017;118:3349–3358. doi: 10.1002/jcb.25988. [DOI] [PubMed] [Google Scholar]
- 9.Lv GY, Miao J, Zhang XL. Long non-coding RNA XIST promotes osteosarcoma progression by targeting ras-related protein RAP2B via miR-320b. Oncol Res. 2018;26:837–846. doi: 10.3727/096504017X14920318811721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rui Z, Tian X. Long non-coding RNA XIST regulates PDCD4 expression by interacting with miR-21-5p and inhibits osteosarcoma cell growth and metastasis. Int J Oncol. 2017;51:1460–1470. doi: 10.3892/ijo.2017.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurtan AM, Sharp PA. The role of miRNAs in regulating gene expression networks. J Mol Biol. 2013;425:3582–3600. doi: 10.1016/j.jmb.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ping H, Jie X, Liu S. MiR-139-3p induces cell apoptosis and inhibits metastasis of cervical cancer by targeting NOB1. Biomed Pharmacother. 2016;83:850–856. doi: 10.1016/j.biopha.2016.07.050. [DOI] [PubMed] [Google Scholar]
- 13.Zhong S, Li W, Chen Z, Xu J, Zhao J. MiR-222 and miR-29a contribute to the drug-resistance of breast cancer cells. Gene. 2013;531:8–14. doi: 10.1016/j.gene.2013.08.062. [DOI] [PubMed] [Google Scholar]
- 14.Tang H, Deng M, Tang Y, Xie X, Guo J, Kong Y, Ye F, Su Q, Xie X. miR-200b and miR-200c as prognostic factors and mediators of gastric cancer cell progression. Clin Cancer Res. 2013;19:5602–5612. doi: 10.1158/1078-0432.CCR-13-1326. [DOI] [PubMed] [Google Scholar]
- 15.Xu D, Takeshita F, Hino Y, Fukunaga S, Kudo Y, Tamaki A, Matsunaga J, Takahashi R, Takata T, Shimamoto A. miR-22 represses cancer progression by inducing cellular senescence. J Cell Biol. 2011;193:409–424. doi: 10.1083/jcb.201010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang P, Bill K, Liu J, Young E, Peng T, Bolshakov S, Hoffman A, Song Y, Demicco EG, Terrada DL, Creighton CJ, Anderson ML, Lazar AJ, Calin GG, Pollock RE, Lev D. MiR-155 is a liposarcoma oncogene that targets casein kinase-1α and enhances β-catenin signaling. Cancer Res. 2012;72:1751–1762. doi: 10.1158/0008-5472.CAN-11-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu G, Yu W, Zhang M, Yin R, Wu Y, Liu Q. MicroRNA-145-3p suppresses proliferation and promotes apotosis and autophagy of osteosarcoma cell by targeting HDAC4. Artif Cells Nanomed Biotechnol. 2018;46:579–586. doi: 10.1080/21691401.2018.1464459. [DOI] [PubMed] [Google Scholar]
- 18.Qu J, Li M, Zhong W, Hu C. Competing endogenous RNA in cancer: a new pattern of gene expression regulation. Int J Clin Exp Med. 2015;8:17110–17116. [PMC free article] [PubMed] [Google Scholar]
- 19.Li E, Zhao Z, Ma B, Zhang J. Long noncoding RNA HOTAIR promotes the proliferation and metastasis of osteosarcoma cells through the AKT/mTOR signaling pathway. Exp Ther Med. 2017;14:5321–5328. doi: 10.3892/etm.2017.5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren YF, Zhang TH, Zhong S, Zhao YT, Lv YN. miR144 suppresses proliferation and induces apoptosis of osteosarcoma cells via direct regulation of mTOR expression. Oncol Lett. 2018;15:1163–1169. doi: 10.3892/ol.2017.7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang G, Nishimoto K, Zhou Z, Hughes D, Kleinerman ES. miR-20a encoded by the miR-17-92 cluster increases the metastatic potential of osteosarcoma cells by regulating Fas expression. Cancer Res. 2012;72:908–916. doi: 10.1158/0008-5472.CAN-11-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang CL, Zhu KP, Ma XL. Antisense lncRNA FOXC2-AS1 promotes doxorubicin resistance in osteosarcoma by increasing the expression of FOXC2. Cancer Lett. 2017;396:66–75. doi: 10.1016/j.canlet.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Koren I, Kimchi A. Promoting tumorigenesis by suppressing autophagy. Science. 2012;338:889–890. doi: 10.1126/science.1230577. [DOI] [PubMed] [Google Scholar]
- 24.Shibuya K, Yamada T, Ichimura K. Autophagy regulates progression of programmed cell death during petal senescence in Japanese morning glory. Autophagy. 2009;5:546–547. doi: 10.4161/auto.5.4.8310. [DOI] [PubMed] [Google Scholar]
- 25.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 26.Chen Z, Yu C, Zhan L, Pan Y, Chen L, Sun C. LncRNA CRNDE promotes hepatic carcinoma cell proliferation, migration and invasion by suppressing miR-384. Am J Cancer Res. 2016;6:2299–2309. [PMC free article] [PubMed] [Google Scholar]
- 27.Kuma A, Matsui M, Mizushima N. LC3, an autophagosome marker, can be incorporated into protein aggregates independent of autophagy: caution in the interpretation of LC3 localization. Autophagy. 2007;3:323–328. doi: 10.4161/auto.4012. [DOI] [PubMed] [Google Scholar]
- 28.Moscat J, Diazmeco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137:1001–1004. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding L, Xu Y, Zhang W, Deng Y, Si M, Du Y, Yao H, Liu X, Ke Y, Si J. MiR-375 frequently downregulated in gastric cancer inhibits cell proliferation by targeting JAK2. Cell Res. 2010;20:784–793. doi: 10.1038/cr.2010.79. [DOI] [PubMed] [Google Scholar]
- 30.Wang F, Li Y, Zhou J, Xu J, Peng C, Ye F, Shen Y, Lu W, Wan X, Xie X. miR-375 is down-regulated in squamous cervical cancer and inhibits cell migration and invasion via targeting transcription factor SP1. Am J Pathol. 2011;179:2580–2588. doi: 10.1016/j.ajpath.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Yan Z, Zhang J, Gong L, Li W, Cui J, Liu Y, Gao Z, Li J, Shen L. Combination of hsa-miR-375 and hsa-miR-142-5p as a predictor for recurrence risk in gastric cancer patients following surgical resection. Ann Oncol. 2011;22:2257–2266. doi: 10.1093/annonc/mdq758. [DOI] [PubMed] [Google Scholar]
- 32.Hu W, Xiao Z. Formononetin induces apoptosis of human osteosarcoma cell line U2OS by regulating the expression of Bcl-2, Bax and MiR-375 in vitro and in vivo. Cell Physiol Biochem. 2015;37:933–939. doi: 10.1159/000430220. [DOI] [PubMed] [Google Scholar]
- 33.Liu W, Zhao X, Zhang YJ, Fang GW, Xue Y. MicroRNA-375 as a potential serum biomarker for the diagnosis, prognosis, and chemosensitivity prediction of osteosarcoma. J Int Med Res. 2018;46:975–983. doi: 10.1177/0300060517734114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi ZC, Chu XR, Wu YG, Wu JH, Lu CW, Lü RX, Ding MC, Mao NF. MicroRNA-375 functions as a tumor suppressor in osteosarcoma by targeting PIK3CA. Tumour Biol. 2015;36:8579–8584. doi: 10.1007/s13277-015-3614-9. [DOI] [PubMed] [Google Scholar]
- 35.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Gao N, Zhang Z, Jiang BH, Shi X. Role of PI3K/AKT/mTOR signaling in the cell cycle progression of human prostate cancer. Biochem Biophys Res Commun. 2003;310:1124–1132. doi: 10.1016/j.bbrc.2003.09.132. [DOI] [PubMed] [Google Scholar]
- 37.Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, Sun Y, Ouyang X, Gerald WL, Cordon-Cardo C. Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest. 2008;118:3051–3064. doi: 10.1172/JCI34764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slomovitz BM, Coleman RL. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin Cancer Res. 2012;18:5856–64. doi: 10.1158/1078-0432.CCR-12-0662. [DOI] [PubMed] [Google Scholar]
- 39.Miwa S, Sugimoto N, Yamamoto N, Shirai T, Nishida H, Hayashi K, Kimura H, Takeuchi A, Igarashi K, Yachie A. Caffeine induces apoptosis of osteosarcoma cells by inhibiting AKT/mTOR/S6K, NF-κB and MAPK pathways. Anticancer Res. 2012;32:3643–3649. [PubMed] [Google Scholar]