Abstract
Gestational diabetes mellitus (GDM) is a common metabolic disease during pregnancy with serious harm. However, the pathogenesis of GDM has not been thoroughly studied. Recent reports have shown that microRNAs (miRNAs) are associated with GDM, but the mechanisms remain unclear. This study aimed to investigate the role of miR-142-3p in β cells of GDM. We established GDM mouse models by injecting streptozotocin (STZ) to extract embryonic tissue, peripheral blood and pancreas. qRT-PCR was used to detect the expression of miR-142-3p and FOXO1. 5-ethynyl-2’-deoxyuridine (EDU) staining and flow cytometry were used to measure cell proliferation and apoptosis. Western blot analysis was used to determine the expression of proliferation and apoptosis-related proteins. Dual-luciferase reporter assay was used to assess the target relationship between miR-142-3p and FOXO1. The results showed that miR-142-3p was up-regulated in embryonic tissue and peripheral blood of GDM model mice. Overexpression of miR-142-3p and knockdown of FOXO1 both promoted INS-1 cell proliferation, inhibited apoptosis, increased proliferating cell nuclear antigen (PCNA) and Bcl-2 expression, as well as reduced the expression level of p27, Bax and cleaved caspase-3. There are binding sites between miR-142-3p and FOXO1, which is miR-142-3p directly regulated FOXO1 expression. Moreover, above increases and decreases induced by miR-142-3p were attenuated by FOXO1 overexpression. In conclusion, miR-142-3p promotes the survival of pancreatic β cells through targeting FOXO1 in GDM. This study suggests that targeted regulation of miR-142-3p/FOXO1 might be a new strategy for the treatment of GDM.
Keywords: miR-142-3p, pancreatic β cell, FOXO1, gestational diabetes mellitus (GDM)
Introduction
Gestational diabetes mellitus (GDM) is a disease of abnormal glucose metabolism that occurs during pregnancy [1]. Most patients with GDM can return to normal shortly after delivery, but others can also be converted to diabetes [2,3]. A study reported that GDM is closely associated with obesity and type II diabetes [4]. GDM has serious short-term and long-term adverse effects on pregnant women and fetuses, which not only causes preeclampsia, excessive amniotic fluid, dystocia and postpartum hemorrhage in pregnant women, but also causes neonatal hypoglycemia, malformation, cardiac insufficiency and hypocalcemia in neonates [5]. Therefore, the studies of GDM have been highly valued by researchers globally in recent years [6]. MicroRNAs (miRNAs) are a class of important gene expression regulators of 22nt that widely exist in eukaryotes [7]. Numerous studies have found that miRNAs play crucial roles in the pathogenesis of diabetes on proliferation, apoptosis and survival of pancreatic β cells, such as miR-375, miR-204, miR-184 and miR-503 [8-12]. These miRNAs are highly expressed both in vivo and in vitro, and control the production and secretion of insulin, thereby regulating the function of β cells [9-12]. MiR-142-3p, a prognostic biomarker, has been reported to inhibit the growth of cancer cells in various cancers, such as colon cancer, esophageal squamous cell carcinoma, and lung cancer [13-15]. Esguerra et al. discovered that miR-142-3p is overexpressed in islet tissue of non-obese type II diabetic rat models [16]. Xu et al. also found that miR-142-3p is highly expressed in GDM cells [17]. However, the specific regulatory mechanism of miR-142-3p in GDM has not been studied in depth.
Forkhead box protein O1 (FOXO1), the earliest transcription factor found in the FOXO subfamily, is located at 13q14.1 and encodes 655 amino acids (AA) [18]. It not only promotes adipocyte differentiation and negatively regulates skeletal muscle production, but also plays essential effects on insulin in pancreatic β cells and adipocytes [19]. FOXO1 is widely expressed in β cells and regulates the occurrence of diabetes through transcriptional accommodation and signaling pathways [18]. Moreover, FOXO1, also as a pro-inflammatory factor, strengthens pro-inflammatory cytokines expression in GDM cells [20]. Recently, Lou et al. reported that down-regulation of miR-142-5p could promote hepatocellular carcinoma (HCC) cell growth by regulating FOXO expression [21]. However, the co-regulatory influence of miR-142-3p and FOXO1 in GDM is still lacking.
Therefore, in this study, we predicted the targeting relationship and binding site of miR-142-3p and FOXO1 by bioinformatics analysis. Moreover, we established GDM mouse models to detect the expression of miR-142-3p and FOXO1 and the effect of pancreatic β cells, thus exploring the possible mechanisms of the two in GDM and providing a new idea for the treatment of GDM.
Materials and methods
Cell culture
Rat insulin cell line INS-1 (GDC192) was purchased from China Center for Type Culture Collection (CCTCC; Wuhan, China) and cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, California, USA) containing 10% fetal bovine serum (FBS; Gibco, California, USA), 1 mmol/L sodium pyruvate (Gibco, California, USA), 10 mmol/L 4-(2-hydroxyerhyl) piperazine-1-erhanesulfonicacid (HEPES; Gibco, California, USA), 50 μmol/L β-mercaptoethanol (Gibco, California, USA), 100 U/mL penicillin (Gibco, California, USA), and 100 mg/mL streptomycin (Gibco, California, USA). Human embryonic kidney cell line HEK 293-T was purchased from American Type Culture Collection (ATCC; CRL-157; Manassas, USA) and cultured in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, California, USA) containing 10% FBS. All cells were incubated at 37°C in a humidified atmosphere with 5% CO2.
GDM mouse model establishment
The 3-4 weeks old C57BL/6 mice weighing 15-25 g (45 males and 90 females) were purchased from the Guangdong Medical Laboratory Animal Center (Foshan, China) and fed in a 12 h light environment at 20-25°C. After male and female mice were caged at 2:1, the pudendal embolus was examined on the second day. If the pudendal embolus was found, the mating was successful. Female mice mating for 6 d were randomly divided into 2 groups and fasted for 10 h. GDM group was intraperitoneally injected with 0.25% streptozotocin (STZ) solution (Sigma-Aldrich, St. Louis, USA) at 80 mg/kg for 3 consecutive days. The control group was intraperitoneally injected with the same amount of normal saline. Blood samples were collected from the tail veins in both groups. Importantly, the modeling was successfully established when the blood glucose of mice were higher than 11 mmol/L within 48 h. Subsequently, 6 mice in each group were sacrificed for 18 d of pregnancy, the peripheral blood and placental tissues were pre-treated for subsequent experimental detection and pancreas was taken to extract β cells. All animal experiments were approved by the Ethical Committee for Animal Experiment of Nanjing Medical University.
Pancreatic β cells extraction
Isolated pancreatic tissues were digested with pre-cooled Hanks solution and Collagenase (Roche, Basel, Switzerland) and mixed with 84%, 67% and 50% Histopaque solution (Sigma-Aldrich, St. Louis, USA) in turn to aspirate the cell precipitates. The precipitates were re-dissolved and suspended in DMEM with 5.6 mmol/L glucose (Gibco, California, USA), 10% FBS, 100 U/mL penicillin and 100 mg/mL streptomycin to purify and authenticate pancreatic cells. The pancreatic β cells were cultured in DMEM at 37°C.
Cell transfection
miR-142-3p mimic, miR-142-3p inhibitor and the negative control (NC or NC inhibitor) were provided by Sigma-Aldrich (St. Louis, USA). Recombinant plasmid pcDNA-FOXO1 and pcDNA-3.1 were constructed by Sangon Biotech Co., Ltd (Shanghai, China) and transfected into INS-1 cells by the use of diluted Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, US). The cells were subsequently cultured in an incubator containing 5% CO2 at 37°C and harvested after 48 h post-transfection for further analysis.
Quantitative real time polymerase chain reaction (qRT-PCR)
Total RNA was extracted by Trizol (Thermo Fisher Scientific, Waltham, USA), and RNA concentration was determined by a spectrophotometer (Thermo Fisher Scientific, Waltham, USA). cDNA was reversely transcribed by oligo dT (Thermo Fisher Scientific, Waltham, USA). qPCR was conducted by SYBR Green qPCR Mix Kit (MedChemExpress, New Jersey, USA). The sequences of miR-142-3p were 5’-UGUAGUGUUUCCUACUUUAUGGA-3’ (Forward) and 5’-CAUAAAGUAGGAAACACUACAUU-3’ (Reward). The sequences of FOXO1 were 5’-AAAGGATCCATGGCCGAGGCGCCTCAG-3’ (Forward) and 5’-AAAACTAGTTCAGCCTGACACCCAGCTA-3’ (Reward). The relative expression of each target gene was quantified by 2-ΔΔCt method [22].
Cell proliferation
Cells (4×103-1×105 cells/well) were cultured in 96-well plates. The 5-ethynyl-2’-deoxyuridine (EDU) solution (Thermo Fisher Scientific, Waltham, USA) was diluted with the cell culture medium. After incubating the culture medium with 100 μL of diluted solution for 2 h, cells were washed and fixed in phosphate buffer saline (PBS) for 30 min. We added 2 mg/mL glycine (Solarbio, Beijing, China) and 0.5% Triton X (Solarbio, Beijing, China) for 10 min, and re-incubated with fluorochrome (Thermo Fisher Scientific, Waltham, USA) for 30 min in a decolorized shaking bed at room temperature without light. Finally, cells were observed by a fluorescence microscope (Leica Microsystems, Weitzlar, Germany).
Cell apoptosis
INS-1 cells (4×103-1×105 cells/well) were cultured in 96-well plates, centrifuged for 5 min, and washed in cold PBS three times. On the basis of Annexin-V-FITC Cell Apoptosis Detection Kit (Thermo Fisher Scientific, Waltham, USA), cells were added to 150 μL binding buffer and 5 μL Annexin-V-FITC and incubated at room temperature for 15 min with 5 μL Propidium (PI) dye. Apoptosis was detected by flow cytometry (Attune NxT, Thermo Fisher Scientific, Waltham, USA).
Western blot
Cells were lysed by RIPA lysis buffer (Sigma-Aldrich, St. Louis, USA). Protein extract was determined by bicinchoninic acid (BCA) method. The primary antibodies of proliferating cell nuclear antigen (PCNA; ab92552), p27 (ab32034), B-cell lymphoma-2 (Bcl-2; ab59348), Bax (ab182733), cleaved caspase-3 (ab49822), FOXO1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; ab181602, all from Abcam, Cambridge, UK) were diluted 500 times and incubated with 0.5% BSA at 4°C. Horseradish peroxide (HRP) labeled goat anti-rabbit or anti-mouse IgG (ab191866 or ab205719; 1:1000; Abcam, Cambridge, UK) was incubated at room temperature for 2 h after washing. Bio-rad microscopic imaging system (Hercules, USA) was used to obtain the image, and the results were analyzed by Image J (NIH Image, Bethesda, USA).
Dual-luciferase assay
miR-142-3p mimic or miR-142-3p inhibitor and FOXO1-wild type (wt) or FOXO1-mutant (mut) were co-transfected into 293T cells. Dual Luciferase Reporter Gene Assay Kit (Promega, Shanghai, China) was adopted to assess the relationship of miR-142-3p and FOXO1 following the instructions.
Statistical analysis
All data were processed by SPSS 21.0 statistical software (SPSS Inc., Chicago, USA) and expressed in the form of mean ± standard deviation (SD). Differences between two groups were analyzed by means of Studentt-test. Statistical significance was considered as P<0.05.
Results
miR-142-3p expression was increased in GDM mouse model
In order to assess the expression of miR-142-3p in GDM, we first established a GDM mouse model to extract placental tissues and peripheral blood. The qRT-PCR results showed that miR-142-3p expression was clearly up-regulated in both placental tissues (Figure 1A, P<0.01) and peripheral blood (Figure 1B, P<0.001) of the GDM model.
Figure 1.
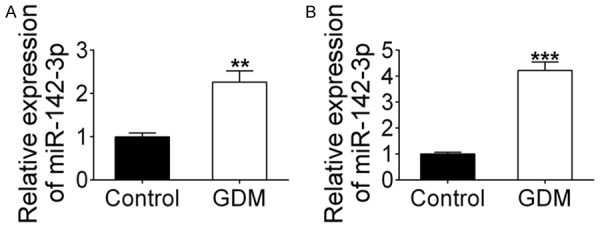
Up-regulation of miR-142-3p expression in GDM mouse model. The expression of miR-142-3p was detected in (A) placental tissues and (B) peripheral blood of GDM mice via qRT-PCR. **indicates P<0.01, ***indicates P<0.001.
Overexpression of miR-142-3p promoted β cell survival
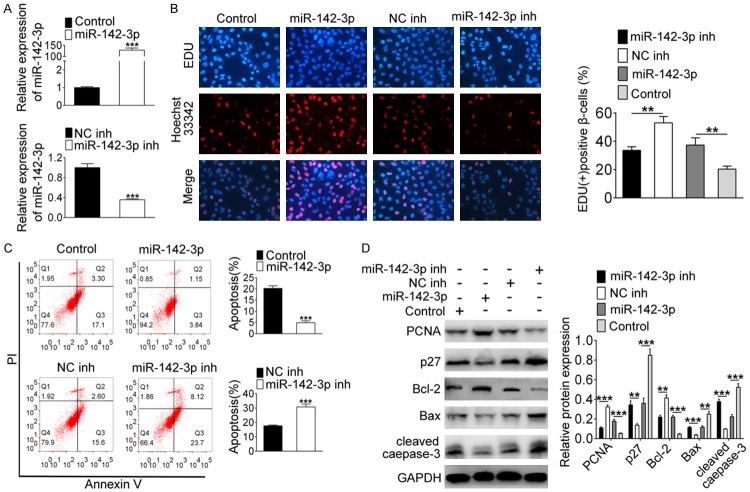
To research the role of miR-142-3p in GDM, miR-142-3p mimics and inhibitors were transfected into INS-1 cells. qRT-PCR results showed that the expression level of miR-142-3p was significantly up-regulated in the mimic group, while it was clearly down-regulated in the miR-142-3p inhibitor group, indicating that the transfections of miR-142-3p mimics and inhibitors were successful (Figure 2A, P<0.001). In Figure 2B, the results of EDU staining showed that the positive β cells was increased when miR-142-3p was overexpressed, and distinctly decreased after silencing miR-142-3p (P<0.01), indicating that miR-142-3p promoted β cell proliferation. On the contrary, the number of apoptotic cells was less in the miR-142-3p mimic group than the control group, and increased in the miR-142-3p, inhibitor group compared with NC inhibitor group (P<0.001), suggesting that miR-142-3p inhibited β cell apoptosis (Figure 2C). As shown in Figure 2D, the expression of PCNA and Bcl-2 were up-regulated after overexpression of miR-142-3p, but the expressions of p27, Bax and cleaved caspase-3 were down-regulated after knocking down miR-142-3p (P<0.001). Above data demonstrated that overexpression of miR-142-3p could promote β cell survival.
Figure 2.
Overexpression of miR-142-3p promotes β cell survival. Cells were transfected with miR-142-3p inhibitor or miR-142-3p mimic. A. The expression of miR-142-3p was detected by qRT-PCR. B. The detection of cell proliferation was performed by EDU staining. C. Flow cytometry analyzed cell apoptosis. D. The protein level of PCNA, Bcl-2, p27, Bax and cleaved caspase-3 were assessed by western blot. **Indicates P<0.01, ***indicates P<0.001.
miR-142-3p targeted to regulate FOXO1 expression
To explore the mechanisms of miR-142-3p regulating the survival of β cells, we used TargetScan (http://www.targetscan.org/vert_72/) to predict the molecules that might bind to miR-142-3p. The results displayed that miR-142-3p and FOXO1 might have binding sites (Figure 3A). Subsequently, we carried out dual-luciferase reporter assay to verify the interaction between miR-142-3p and FOXO1. The results showed that the luciferase activity was clearly decreased when FOXO1-wt was co-transfected with miR-142-3p mimics, and increased in the FOXO1-wt and miR-142-3p inhibitor group (Figure 3A, P<0.001). However, there was no change in the luciferase activity of FOXO1-mut and miR-142-3p mimic (or inhibitor) co-transfection (Figure 3A). Also, we detected FOXO1 expression at the protein level. Western blot showed that the expression of FOXO1 was down-regulated when overexpression of miR-142-3p, conversely, was up-regulated after knocking down miR-142-3p (P<0.01). These together suggested that miR-142-3p could specifically regulate the expression of FOXO1.
Figure 3.
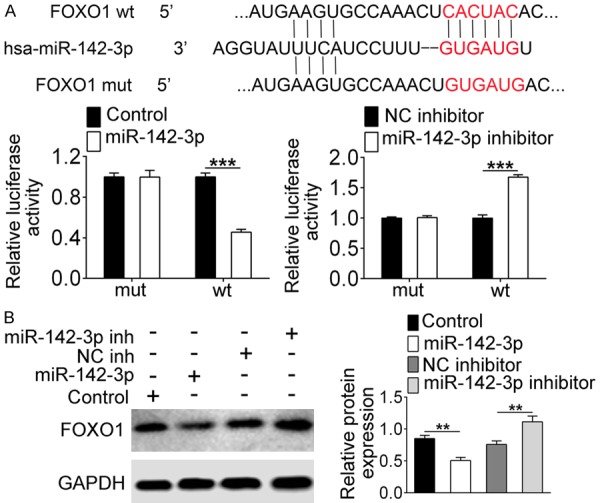
miR-142-3p regulates FOXO1 expression. A. TargetScan predicted the potential binding sites of miR-142-3p and FOXO1. Dual-luciferase reporter assay detected the luciferase activity of co-transfection of miR-142-3p mimics or inhibitor and FOXO1-wt or FOXO1-mut. B. Western blot analyzed the expression of FOXO1. **Indicates P<0.01, ***indicates P<0.001.
Overexpression of FOXO1 inhibited β cell survival
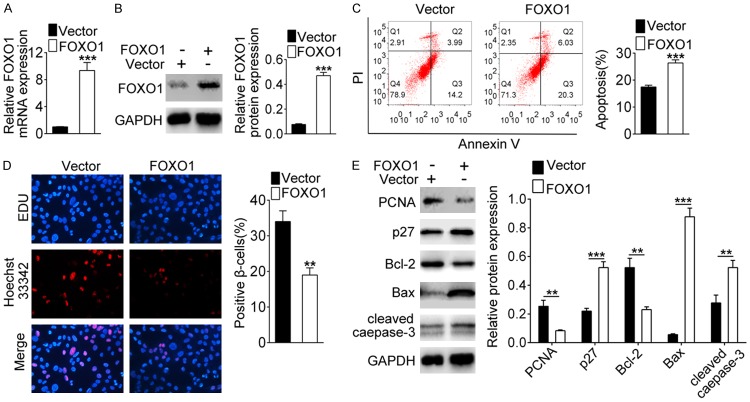
For evaluating the effect of FOXO1 on the cell survival, pcDNA-FOXO1 was transfected into INS-1 cells. After 48 h of transfection, the expression level of FOXO1 was detected through western blot and qRT-PCR assay. The results showed that FOXO1 expression was up-regulated in the pcDNA-FOXO1 group compared with the vector group, indicating that the transfection of FOXO1 was successful (Figure 4A and 4B, P<0.001). After that, flow cytometry and EDU staining showed that overexpression of FOXO1 added apoptotic cell number and reduced positive cell number in NIS-1 cells (Figure 4C and 4D, P<0.001), suggesting that FOXO1 inhibited cell proliferation and promoted apoptosis. In Figure 4E, the results of western blot displayed that the expression of PCNA and Bcl-2 were down-regulated, and the expression of p27, Bax and cleaved caspase-3 was up-regulated when FOXO1 overexpression (P<0.001). These data demonstrated that overexpression of FOXO1 could inhibit the survival of β-cells.
Figure 4.
Overexpression of FOXO1 inhibits β cell survival. Cells were transfected with pcDNA-FOXO1. A, B. The expression of FOXO1 was detected by qRT-PCR and western blot. C. Flow cytometry analyzed cell apoptosis. D. EDU staining tested cell proliferation. E. Western blot assessed PCNA, Bcl-2, p27, Bax, and cleaved caspase-3 expression. **indicates P<0.01, ***indicates P<0.001.
miR-142-3p promoted β cell survival by regulating the expression of FOXO1
To investigate the effect of miR-142-3p and FOXO1 on GDM, pancreatic β cells were isolated from GDM model mice and co-transfected with miR-142-3p mimics and pcDNA-FOXO1. From Figure 5A, we see that the transfection had been completed efficiently. Then, EDU staining results showed that the promoting effect of miR-142-3p on cell proliferation was weakened when overexpression of FOXO1 simultaneously (Figure 5B, P<0.01). On the contrary, on the decrease of apoptosis was attenuated when co-overexpression of FOXO1 and miR-142-3p (Figure 6A, P<0.01). In addition, Figure 6B showed that the expressions of PCNA and Bcl-2 were up-regulated when miR-142-3p only was overexpressed, while the expression of p27, Bax and cleaved caepase-3 were down-regulated. These changes were reversed in the miR-142-3p + FOXO1 group.
Figure 5.
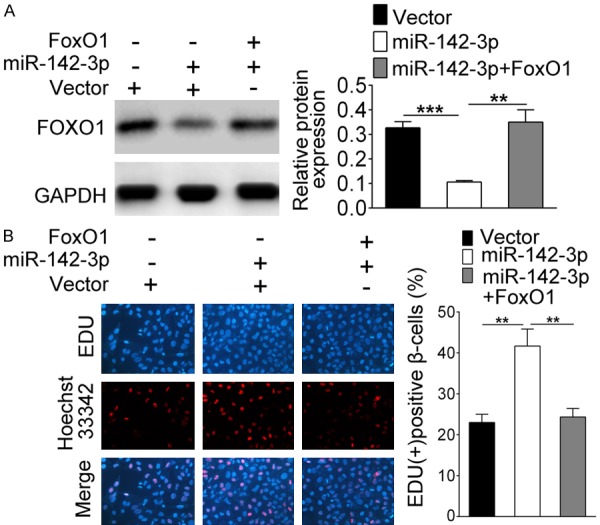
miR-142-3p promotes β cell proliferation by regulating the expression of FOXO1. Cells were co-transfected with miR-142-3p mimics and/or pcDNA-FOXO1. A. The expression of FOXO1 was detected by western blot. B. EDU staining detected cell proliferation. **Indicates P<0.01, ***indicates P<0.001.
Figure 6.
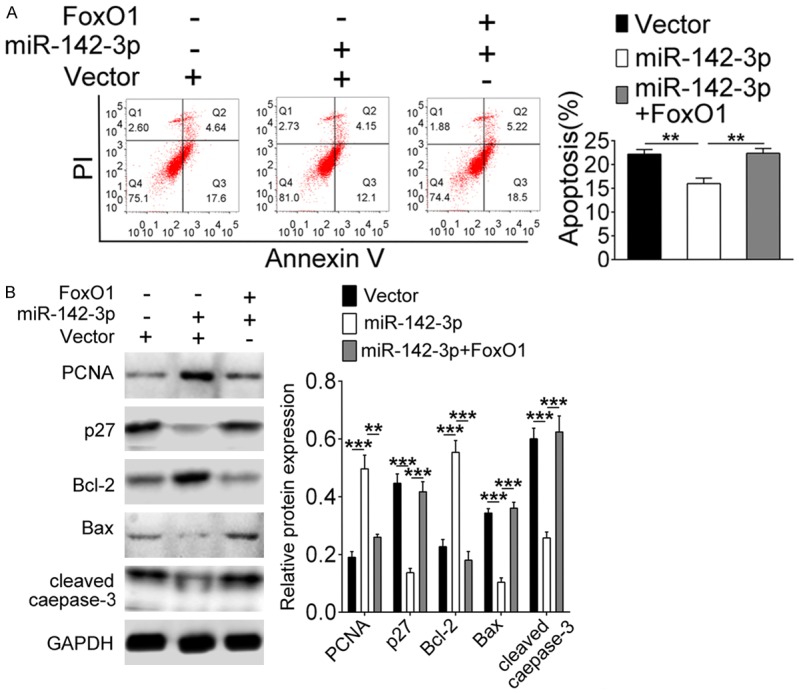
miR-142-3p promotes β cell survival by regulating the expression of FOXO1. Cells were co-transfected with miR-142-3p mimics and/or pcDNA-FOXO1. A. Flow cytometry analyzed cell apoptosis. B. Western blot assessed PCNA, Bcl-2, p27, Bax, and cleaved caspase-3 expression. **Indicates P<0.01, ***indicates P<0.001.
Discussion
GDM is a common metabolic disorder during pregnancy with an incidence of 4 to 15% [23]. miRNAs have been shown to play an important role in metabolic and developmental regulation in all kinds of diabetes [9-11]. Zhao et al. found that the expression of miRNAs in serum of patients with GDM for the first time in 2011 [24]. Subsequently, a report said that miR-503 is up-regulated in placental tissues and peripheral blood of GDM patients, and could target mTOR to disrupt pancreatic β cell function [17]. In addition, Esguerra et al. also found that miR-142-3p was highly expressed in GDM [16]. In our study, we established GDM mouse model to extract placental tissues and peripheral blood to test the expression level of miR-142-3p, and found that miR-142-3p was highly up-regulated in GDM model mice. This result was consistent with previous research.
A study has shown that insulin resistance during pregnancy is the basis of GDM [25]. The GDM patients may have decreased β cell function when insulin resistance increases during pregnancy [25]. Other study found that the reduction of insulin-stimulated glucose treatment in GDM patients precedes the decrease of insulin response, suggesting that the defect of β cells are associated with chronic insulin resistance, which can be stabilized or ameliorated by improving insulin resistance [26]. Furthermore, overexpression of miR-375 in β cells was related to the inhibition of insulin secretion and the promotion of proliferation [9]. miR-184 could regulate the function of pancreatic β cells [11]. Therefore, we selected rat insulin cell line INS-1 to detect the influence of miR-142-3p in β cells survival in vitro, and found that miR-142-3p increased cell proliferation and inhibited apoptosis. These results suggested that overexpression of miRNA-142-3p might contribute to the treatment of GDM.
MicroRNA-142-3p has been extensively studied in cancers, and shows that it regulates the growth and development of cancer cells through regulating some target genes, such as CD133, ATP-binding cassette sub-family G member 2 (ABVG2) and transforming growth factor-βR1 (TGF-βR1) [14,15]. Therefore, in this study, we studied the target gene of miR-142-3p for searching the mechanism of the action of miR-142-3p on β cells. By using bioinformatics analysis, we found that there are potential binding sites between miR-142-3p and FOXO1. Moreover, dual-luciferase reporter assay confirmed that miR-142-3p targeted the regulation of FOXO1 expression.
Transcription factor FOXO1 is one of the major downstream targets of AKT activation, and regulates cell cycle, survival, and energy generation [27]. It has been reported that the AKT pathway can phosphorylate FOXO1 to regulate the proliferation and apoptosis of pancreatic β cells after activation by glucose-dependent insulinotropic polypeptide (GIP) [28]. Then, GIP could induce FOXO1 nucleation, leading to the down-regulation of apoptotic gene Bax and promoting the survival of β cells [28]. Above studies were similar to our results. In this study, we found that FOXO1 strengthened apoptosis and reduced proliferation of INS-1 cells, suggesting that FOXO1 could inhibit β cells survival. Recently, Lou et al. showed that down-regulation of miR-142-5p promotes the growth of HCC cell via regulating FOXO expression [21]. Hence, we speculated that the role of miR-142-3p might be related to the regulation of FOXO1, and was verified by replenishment experiments in pancreatic β cells from GDM model mice. Finally, the results displayed that the addition of cell proliferation and the reduction of apoptosis induced by miR-142-3p were abolished when FOXO1 overexpression, indicating that miR-142-3p could regulate the survival of β cells in GDM through FOXO1.
In conclusion, this study demonstrated that miR-142-3p promotes pancreatic β cell survival through targeting FOXO1 in GDM, and provides a new idea for the treatment of GDM. However, we have conducted a simple study on the influence and regulation mechanism of miR-142-3p on GDM, and need more in vivo experiments to perfect our argument and explore more relevant GDM-related miRNAs and target genes in future work.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China under contract NO. 81472199 and by the National Natural Science Foundation of Jiangsu Province of China under contract NO. BK20141162.
Disclosure of conflict of interest
None.
References
- 1.Drouin P, Blickle JF, Charbonnel B, Eschwege E, Guillausseau PJ, Plouin PF, Daninos JM, Balarac N, Sauvanet JP. Diagnosis and classification of diabetes mellitus: the new criteria. Diabetes Metab. 1999;25:72–83. [PubMed] [Google Scholar]
- 2.Boyle KE, Hwang H, Janssen RC, DeVente JM, Barbour LA, Hernandez TL. Gestational diabetes is characterized by reduced mitochondrial protein expression and altered calcium signaling proteins in skeletal muscle. PLoS One. 2014;9:e106872. doi: 10.1371/journal.pone.0106872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Sullivan KE, Hurley ET, Hurley JP. Understanding complete pathologic response in oesophageal cancer: implications for management and survival. Gastroenterol Res Pract. 2015;2015:518281. doi: 10.1155/2015/518281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vivas Y, Diez-Hochleitner M, Izquierdo-Lahuerta A, Corrales P, Horrillo D, Velasco I, Martinez-Garcia C, Campbell M, Sevillano J, Ricote M, Ros M, Ramos MP, Medina-Gomez G. Peroxisome proliferator activated receptor gamma 2 modulates late pregnancy homeostatic metabolic adaptations. Mol Med. 2016;22:724–736. doi: 10.2119/molmed.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103:176–185. doi: 10.1016/j.diabres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19113342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Li J, Ding X, He M, Cheng SY. microRNA and cancer. AAPS J. 2010;12:309–317. doi: 10.1208/s12248-010-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filios SR, Shalev A. β-Cell microRNAs: small but powerful. Diabetes. 2015;64:3631–3644. doi: 10.2337/db15-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poy MN, Hausser J, Trajkovski M, Braun M, Collins S, Rorsman P, Zavolan M, Stoffel M. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc Natl Acad Sci U S A. 2009;106:5813–5818. doi: 10.1073/pnas.0810550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu G, Chen J, Jing G, Shalev A. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat Med. 2013;19:1141–1146. doi: 10.1038/nm.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tattikota SG, Rathjen T, McAnulty SJ, Wessels HH, Akerman I, van de Bunt M, Hausser J, Esguerra JL, Musahl A, Pandey AK, You X, Chen W, Herrera PL, Johnson PR, O’Carroll D, Eliasson L, Zavolan M, Gloyn AL, Ferrer J, Shalom-Feuerstein R, Aberdam D, Poy MN. Argonaute2 mediates compensatory expansion of the pancreatic beta cell. Cell Metab. 2014;19:122–134. doi: 10.1016/j.cmet.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruix J, Han KH, Gores G, Llovet JM, Mazzaferro V. Liver cancer: approaching a personalized care. J Hepatol. 2015;62:S144–156. doi: 10.1016/j.jhep.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin RJ, Xiao DW, Liao LD, Chen T, Xie ZF, Huang WZ, Wang WS, Jiang TF, Wu BL, Li EM, Xu LY. MiR-142-3p as a potential prognostic biomarker for esophageal squamous cell carcinoma. J Surg Oncol. 2012;105:175–182. doi: 10.1002/jso.22066. [DOI] [PubMed] [Google Scholar]
- 14.Shen WW, Zeng Z, Zhu WX, Fu GH. MiR-142-3p functions as a tumor suppressor by targeting CD133, ABCG2, and Lgr5 in colon cancer cells. J Mol Med (Berl) 2013;91:989–1000. doi: 10.1007/s00109-013-1037-x. [DOI] [PubMed] [Google Scholar]
- 15.Lei Z, Xu G, Wang L, Yang H, Liu X, Zhao J, Zhang HT. MiR-142-3p represses TGF-beta-induced growth inhibition through repression of TGFbetaR1 in non-small cell lung cancer. FASEB J. 2014;28:2696–2704. doi: 10.1096/fj.13-247288. [DOI] [PubMed] [Google Scholar]
- 16.Esguerra JL, Bolmeson C, Cilio CM, Eliasson L. Differential glucose-regulation of microRNAs in pancreatic islets of non-obese type 2 diabetes model goto-kakizaki rat. PLoS One. 2011;6:e18613. doi: 10.1371/journal.pone.0018613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu K, Bian D, Hao L, Huang F, Xu M, Qin J, Liu Y. microRNA-503 contribute to pancreatic beta cell dysfunction by targeting the mTOR pathway in gestational diabetes mellitus. EXCLI J. 2017;16:1177–1187. doi: 10.17179/excli2017-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116:2464–2472. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Jin B, Sun L, Yang H, Cao X, Zhang G. The expression of FoxO1 in placenta and omental adipose tissue of gestational diabetes mellitus. Exp Clin Endocrinol Diabetes. 2014;122:287–294. doi: 10.1055/s-0034-1371830. [DOI] [PubMed] [Google Scholar]
- 21.Lou K, Chen N, Li Z, Zhang B, Wang X, Chen Y, Xu H, Wang D, Wang H. MicroRNA-142-5p overexpression inhibits cell growth and induces apoptosis by regulating FOXO in hepatocellular carcinoma cells. Oncol Res. 2017;25:65–73. doi: 10.3727/096504016X14719078133366. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Wang XS, Gong JN, Yu J, Wang F, Zhang XH, Yin XL, Tan ZQ, Luo ZM, Yang GH, Shen C, Zhang JW. MicroRNA-29a and microRNA-142-3p are regulators of myeloid differentiation and acute myeloid leukemia. Blood. 2012;119:4992–5004. doi: 10.1182/blood-2011-10-385716. [DOI] [PubMed] [Google Scholar]
- 24.Zhao C, Dong J, Jiang T, Shi Z, Yu B, Zhu Y, Chen D, Xu J, Huo R, Dai J, Xia Y, Pan S, Hu Z, Sha J. Early second-trimester serum miRNA profiling predicts gestational diabetes mellitus. PLoS One. 2011;6:e23925. doi: 10.1371/journal.pone.0023925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lv M, Zhang X, Jia H, Li D, Zhang B, Zhang H, Hong M, Jiang T, Jiang Q, Lu J, Huang X, Huang B. An oncogenic role of miR-142-3p in human T-cell acute lymphoblastic leukemia (T-ALL) by targeting glucocorticoid receptor-alpha and cAMP/PKA pathways. Leukemia. 2012;26:769–777. doi: 10.1038/leu.2011.273. [DOI] [PubMed] [Google Scholar]
- 26.Winhofer Y, Handisurya A, Tura A, Bittighofer C, Klein K, Schneider B, Bieglmayer C, Wagner OF, Pacini G, Luger A, Kautzky-Willer A. Osteocalcin is related to enhanced insulin secretion in gestational diabetes mellitus. Diabetes Care. 2010;33:139–143. doi: 10.2337/dc09-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 28.Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ. Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic beta-cells (INS-1) J Biol Chem. 2002;277:49676–49684. doi: 10.1074/jbc.M208756200. [DOI] [PubMed] [Google Scholar]