Abstract
Breast cancer (BC) is a leading cause of cancer mortality in women worldwide. MAC30/Transmembrane protein 97 (TMEM97) is aberrantly up-regulated in many human carcinoma cells. However, the function of MAC30 in invasion and EMT of BC cells is uncertain. qRT-PCR was used to determine the level of MAC30 in BC tissues and cell lines. si-MAC30 was transfected into BC cells, and the effects of MAC30 silencing on the invasion and EMT were explored by qRT-PCR as well as transwell and western blot assays. Also, we determined the effects of MAC30 silencing on Wnt/β-catenin and PI3K/Akt signaling pathways by western blot. We found that MAC30 is significantly up-regulated in BC tissues and cell lines. Down-regulation of MAC30 expression efficiently inhibited the invasion of BC cells. Furthermore, the EMT of BC cells was also inhibited by down-regulation of MAC30. Finally, we found that MAC30 knockdown inhibited Akt phosphorylation, β-catenin, survivin, and cyclin D1 expressions. To our knowledge, this is the first report investigating the effect of MAC30 on invasion and EMT in BC cells by suppressing Wnt/β-catenin and PI3K/Akt signaling pathways. MAC30 may be a potential therapeutic target for BC.
Keywords: Breast cancer, MAC30, invasion, epithelial-mesenchymal transition
Introduction
Breast cancer (BC) is a leading cause of cancer mortality in women worldwide. Every year, above 1.3 million women are diagnosed with breast cancer and nearly 450,000 women die from it [1]. Metastasis, a process by which cancer cells invade surrounding tissues and migrate to distal organs including lung, liver, brain, bone, and lymph nodes, is a major cause of mortality in breast cancer patients [2]. Since breast cancer is a heterogeneous disease, displaying a variety of histopathologic features, genetic markers and diverse prognostic outcomes [3], exploring the mechanism of cancer metastasis is instrumental to develop molecular targets for clinical treatment and improve survival. Thus, within each treatment modality, ongoing investigations to improve therapeutic benefits continue.
Epithelial-mesenchymal transition (EMT) is a complex molecular program that regulates changes in cell morphology and function during embryogenesis and tissue development. EMT also contributes to tumor progression and metastasis [4]. To date, EMT has been the favored explanation for distant metastases for epithelial cancers including breast cancer [5]. The steps of EMT include a combination of the microenvironment molecules adjusting to accommodate cellular expansion to promote tissue development in either physiologic or pathologic contexts [6-10]. EMT is typically characterized as loss of epithelial cell adhesion protein E-cadherin and cytokeratins, together with the gain of mesenchymal-associated molecules N-cadherin, vimentin, and fibronectin [11]. The process is described as “cadherin switching”, i.e., down-regulation of E-cadherin and up-regulation of N-cadherin [12,13]. EMT is associated with therapy resistance and tumor recurrence. Although the role of EMT in tumor invasion and metastasis is a topic of interest, the molecular mechanism by which EMT is regulated has not been fully understood.
Meningioma-associated protein (MAC30), also known as transmembrane protein 97 (TMEM97), was originally identified as an overexpressed gene in meningiomas [14]. The mRNA level of MAC30 is expressed in multiple normal organs such as the heart, lung, brain, ovary, testis, pregnant uterus, and skeletal muscles [15]. However, MAC30 can act as a tumor suppressor or oncogene in carcinomas. The expression of MAC30 protein was significantly increased in colon, gastric, and esophageal cancers, whereas MAC30 expression was decreased in pancreatic cancer cells of primary tumors and metastases [16]. Moreover, Potapova et al. demonstrated that MAC30 worked to cause growth suppression and induction of apoptosis in prostate carcinoma cells [17]. Because MAC30 is a recently identified protein, its definite function is still unknown.
In this study, we attempted to explore the effects of MAC30 gene silencing on the invasion and EMT of breast cancer MCF-7 and MDA-MB-231 cells and clarify a role for MAC30 and possible mechanisms it is involved in.
Materials and methods
Ethics statement
Approval for this study was obtained from the Ethics Committee of the Affiliated Shengjing Hospital of China Medical University. The participants or their legal guardians provided written informed consent for research.
Human tumor tissue samples
A total of 30 specimens of breast biopsy were obtained from the Affiliated Shengjing Hospital of China Medical University between January 2016 and October 2017. Patients who had a history of other types of cancer or who had received chemotherapy or radiotherapy prior to surgery were excluded from the study. Breast cancer tissues and their corresponding adjacent non-tumor tissues (≥2 cm from tumor margin) were collected. The material was stored at -80°C.
Cell culture and reagents
The normal human breast cell line MCF-10A and breast cancer cell lines MCF7, BT549, Bcap37, and MDA-MB-231 cells were obtained from the ATCC (USA). MCF-10A cells were maintained in Dulbecco’s modified Eagle’s medium/nutrient F12 media (Gibco, USA) supplemented with 10% house serum (Gibco, USA). BT549 and Bcap37 cells were maintained in RPMI-1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA). MDA-MB-231 cells were maintained in L15 medium (Gibco, USA) supplemented with 10% FBS.
Transfection
Transfection was carried out using Lipofectamine 3000 (Invitrogen) according to the manufacturer’s instructions. siRNA for MAC30 (si-MAC30) and siRNA-negative control (si-NC) were synthesized and purified by Gene-Pharma (Shanghai, China). After 48 hours of transfection, the efficiency of knockdown was analyzed by western blot and qPCR.
Western blot analysis
Cells were washed with cold PBS, lysed with ice-cold lysis buffer and incubated on ice for 30 min. Lysates were centrifuged, supernatants were collected, and protein concentration was determined using Bio-Rad Protein Reagents (Bio-Rad, Hercules, CA). Protein lysates (30 μg) were separated by SDS-PAGE, blotted onto membranes, and probed with the appropriate dilution of each primary antibody. Membranes were rinsed and incubated with the appropriate horseradish peroxidase-conjugated secondary antibody, rinsed again, and the bound antibodies were detected using enhanced chemiluminescence (GE Healthcare, Piscataway, NJ) followed by autoradiography in a FluorChemTM 8900 (Alpha Innotech Corporation, San Leandro, CA).
Quantitative reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted from BC tissues and cell lines using TRIzol reagent (ambion; Invitrogen), and reverse transcription was performed using a reverse transcription kit (Cat. no. RR037A; Takara, Tokyo, Japan), following the manufacturers’ instructions. qRT-PCR reactions were performed on an ABI 7500 real-time PCR system (Applied Biosystems, Waltham, MA, USA) using 2× SYBR Premix Ex Taq (Cat. No. RR420A, Takara). The levels of expression were normalized by GADPH levels, respectively. Each sample was run in triplicate. The primer sequences of the genes used in quantitative PCR are listed in Table 1. The relative expression was calculated using the relative quantification equation (RQ) =2-ΔΔCt.
Table 1.
Sequence of primers for qRT-PCR
| Gene | Primer Sequence |
|---|---|
| MAC30 | F: 5’-TACCCAGTCGAGTTTAGAAACCT-3’ |
| R: 5’-TGTCATGGTGTGAACAGAGTAGA-3’ | |
| MMP2 | F: 5’-TACAGGATCATTGGCTACACACC-3’ |
| R: 5’-GGTCACATCGCTCCAGACT-3’ | |
| MMP9 | F: 5’-TGTACCGCTATGGTTACACTCG-3’ |
| R: 5’-GGCAGGGACAGTTGCTTCT-3’ | |
| E-cadherin | F: 5’-TACACTGCCCAGGAGCCAGA-3’ |
| R: 5’-TGGCACCAGTGTCCGGATTA-3’ | |
| N-cadherin | F: 5’-TCAGGCGTCTGTAGAGGCTT-3’ |
| R: 5’-ATGCACATCCTTCGATAAGACTG-3’ | |
| Vimentin | F: 5’-GACGCCATCAACACCGAGTT-3’ |
| R: 5’-CTTTGTCGTTGGTTAGCTGGT-3’ | |
| Twist | F: 5’-GTCCGCAGTCTTACGAGGAG-3’ |
| R: 5’-GCTTGAGGGTCTGAATCTTGCT-3’ | |
| Slug | F: 5’-CGAACTGGACACACATACAGTG-3’ |
| R: 5’-CTGAGGATCTCTGGTTGTGGT-3’ | |
| Snail | F: 5’-TCGGAAGCCTAACTACAGCGA-3’ |
| R: 5’-AGATGAGCATTGGCAGCGAG-3’ | |
| ZEB1 | F: 5’-GATGATGAATGCGAGTCAGATGC-3’ |
| R: 5’-ACAGCAGTGTCTTGTTGTTGT-3’ | |
| GAPDH | F: 5’-GAGTCAACGGATTTGGTCGTATTG-3’ |
| R: 5’-CCTGGAAGATGGTGATGGGATT-3’ |
Transwell invasion assay
Invasion assays were performed in uncoated or Matrigel (BD Biosciences)-coated 24-well Transwell chamber (8-μm pore size, Corning Life Sciences, Corning, NY, USA). Briefly, cells (3×104/well) in 200 μl serum-free medium were seeded into the upper chamber, while 600 μl medium with 10% FBS were added into the lower chamber. After 24 h of incubation at 37°C and 5% CO2, cells remaining in the upper chamber were completely removed using a cotton swab. Cells attached to the bottom of the membranes were fixed with 4% paraformaldehyde and stained with 0.2% crystal violet. Cells were imaged from at least five grids per field. Then the membranes were rinsed with 30% glacial acetic acid. Finally, the wash solution was examined at 540 nm to count the number of glioma cells. All assays were independently repeated three times.
Statistical analysis
All data are presented as means ± SEM. Statistical significance was performed by using the GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA) and determined by Student’s t test. P<0.05 was considered significant.
Results
The MAC30 expression was significantly up-regulated in BC tissues and cell lines
We used qRT-PCR and western blot to detect the expression of MAC30 in BC tissues and the adjacent normal tissues (Figure 1A). Obviously, MAC30 expression was significantly up-regulated in BC tissues. We also used qRT-PCR and western blot to detect the MAC30 expression in BC cell lines such as MCF-7, BT549, Bcap37, MDA-MB-231 and normal human breast cell line MCF-10A. The results are shown in Figure 1B. Compared with MCF-10A, MAC30 expression was significantly increased in BC cells.
Figure 1.
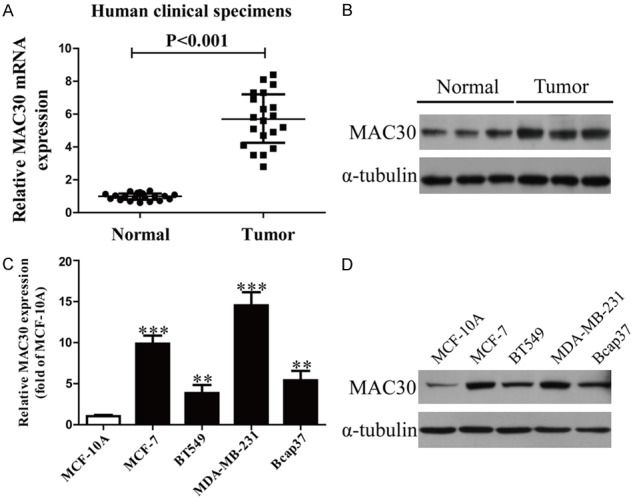
The expression of MAC30 in BC tissues and cell lines. A. mRNA levels of MAC30 in BC tissues and their corresponding adjacent normal tissues. B. Protein expression of MAC30 in BC tissues. C. The mRNA expression of MAC30 analyzed by qRT-PCR in four BC cell lines: MCF-7, BT549, Bcap37, MDA-MB-231 and a normal human breast cell line MCF-10A. D. Protein expression of MAC30 in BC cell lines. All data are presented as the mean ± SEM, n=6. **P<0.01, ***P<0.001 vs. Normal or MCF-10A.
Effect of MAC30 on the invasion and related molecules of BC cells
To study the role of MAC30 in invasion of BC cells, we evaluated the invasive capacities of MCF-7 and MDA-MB-231 cells transfected with si-MAC30 (Figure 2A) by Transwell invasion assays. The results from Transwell assays showed that the invasive capability of MCF-7 and MDA-MB-231 cells was remarkably suppressed in the si-MAC30 group compared to the si-NC group (Figure 2B). These results confirmed that MAC30 might play an important role in inhibiting invasion of BC cells.
Figure 2.
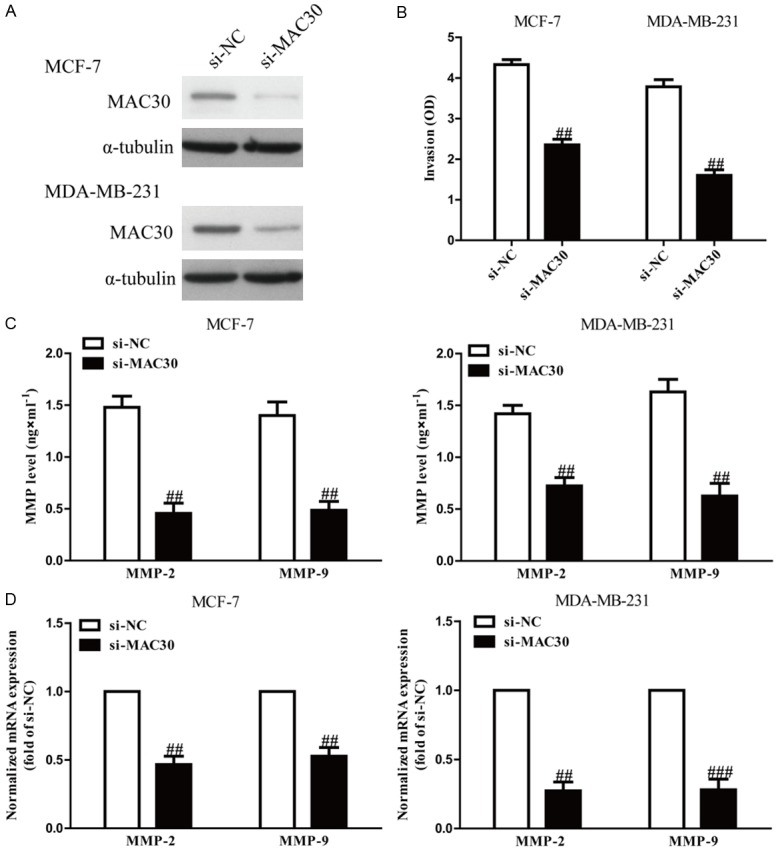
The effects of si-MAC30 on invasion and the expression of related molecules in BC cells. MCF-7 and MDA-MB-231 cells were transfected with si-MAC30 or si-NC for 48 hours. A. The expression of si-MAC30 was analyzed by qRT-PCR and western blot. B. The invasion of BC cells was assessed by a transwell assay. C. Levels of MMP-2 and MMP-9 were detected in the culture supernatants of cultured MCF-7 and MDA-MB-231 cells by ELISA. D. The mRNA levels of MMP-2 and MMP-9 were examined by RT-PCR. All data are presented as mean ± SEM, n=6. ##P<0.01, ###P<0.001 vs. si-NC.
Since MMPs are closely correlated with cell invasion, we determined the expressions of MMP-2 and MMP-9. ELISA showed that knockdown of MAC30 significantly decreased secretion of MMP-2 and MMP-9 in the culture supernatants in MCF-7 and MDA-MB-231 cells (Figure 2C). Furthermore, we examined the expressions of MMP-2 and MMP-9 at the mRNA level by qRT-PCR. After transfection with si-MAC30, the mRNA levels of MMP-2 and MMP-9 were distinctly down-regulated (Figure 2D). Our results suggested that decreased expressions of MMP-2 and MMP-9 might be a mechanism contributing to the inhibitory effect of MAC30 silencing on the invasion of BC cells.
Effects of MAC30 on EMT of BC cells
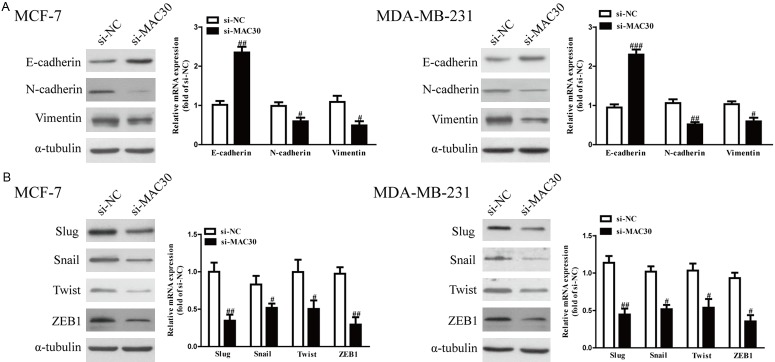
Since EMT is closely related to cancer metastasis, and decreased MAC30 expression could inhibit cell invasion, we investigated the function of MAC30 on EMT in BC cells. We explored the effects of MAC30 on the expressions of EMT markers in MCF-7 and MDA-MB-231 cells using qRT-PCR and western blot. Knockdown of MAC30 in MCF-7 and MDA-MB-231 cells significantly up-regulated the expression of the epithelial marker E-cadherin and down-regulated the expression of mesenchymal markers N-cadherin and vimentin at both the mRNA and protein levels (Figure 3A). Furthermore, the expressions of EMT-related transcription factors in MCF-7 and MDA-MB-231 cells were also detected. Decreased MAC30 expression markedly decreased the mRNA and protein expressions of Slug, Snail, Twist, and ZEB1 in both cell lines (Figure 3B). Altogether, our findings revealed that knockdown of MAC30 could inhibit the EMT of BC cells.
Figure 3.
The effects of si-MAC30 on expressions of EMT-related molecules in BC cells. MCF-7 and MDA-MB-231 cells were transfected with si-MAC30 or si-NC for 48 hours. A. The mRNA and protein levels of E-cadherin, N-cadherin and vimentin were determined by qRT-PCR and western blot, respectively. B. The mRNA and protein levels of Slug, Snail, Twist and ZEB1 were determined by qRT-PCR and western blot. All data are presented as the mean ± SEM, n=6. #P<0.05, ##P<0.01 vs. si-NC.
Down-regulation of MAC30 restrained both PI3K/Akt and Wnt/β-catenin signaling pathways in BC cells
Previous studies have shown that PI3K/Akt and Wnt/β-catenin signaling pathways were implicated in regulating the invasion and EMT of BC cells [18,19]. Therefore, we evaluated the effects of MAC30 on both PI3K/Akt and Wnt/β-catenin signaling pathways. The protein expressions of Akt, p-Akt, β-catenin, and its downstream targets survivin, and cyclin D1 were measured by western blotting. As shown in Figure 4, knockdown of MAC30 significantly decreased the protein expressions of p-Akt, β-catenin, survivin, and cyclin D1 compared with the si-NC group. Our data suggest that MAC30 knockdown could inhibit activation of PI3K/Ak and Wnt/β-catenin in human BC cells.
Figure 4.
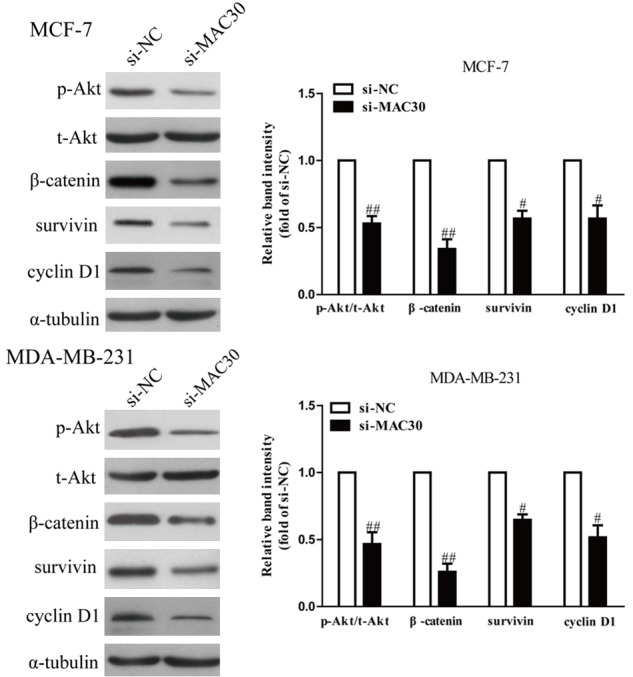
Down-regulation of MAC30 inhibited both PI3K/Akt and Wnt/β-catenin signaling pathways. MCF-7 and MDA-MB-231 cells were transfected with si-MAC30 or si-NC, the p-Akt, t-Akt, β-catenin, survivin and cyclin D1 protein expression levels were analyzed by w and western blotting. All data are presented as mean ± SEM, n=6. #P<0.05, ##P<0.01 vs. si-NC.
Discussion
BC is a serious threat to women’s health and life. In recent years the prevention, screening, early detection plus surgical resection, radiotherapy and chemotherapy, endocrine therapy and targeted therapy have made progress [20-24], effectively improving the 5-year survival rate of BC. The treatment of drug resistance and relapse is still seriously restricting the quality of life of patients with breast carcinoma [25,26]. Therefore, it is meaningful to find new molecular markers and drug targets which can reduce the recurrence rate and mortality rate of BC patients.
EMT, a critical physiologic process during development and wound healing, has been implicated in tumor progression and metastasis, and can lead to increased cellular adhesion, apical-basal polarity, and cellular motility, increasing the potential for invasion/metastasis [27-29]. To date, the epithelial-mesenchymal transition phenomenon has been the favored explanation of distant metastases for epithelial cancers including breast cancer [30]. This phenomenon is characterized by the loss of cell-cell adhesion molecules, down-regulation of epithelial differentiation markers, and transcriptional induction of mesenchymal markers [31]. Loss of the epithelial marker E-cadherin and acquisition of the mesenchymal marker vimentin are considered important characteristics of EMT [32]. E-cadherin, an adhesion molecule expressed in the epithelioid cell phenotype, plays an important role in the process of cancer invasion. Low expression of E-cadherin might significantly enhance the invasion and metastasis of breast cancer [33]. Vimentin, also an important marker of mesenchymal cells, is closely related to invasion, metastasis and EMT of breast cancer cells [34]. It has been reported that MAC30 expression is significantly up-regulated in breast cancer, and associated with disease progression and poor patient outcome [35,36]. However, the exact mechanism of MAC30 on BC metastasis remains unclear. In this study, our results showed that MAC30 knockdown could down-regulate the mRNA and protein levels of MMP-2 and MMP-9 expression. MMP-2 and MMP-9 are the major matrix metalloproteinases (MMPs) mediated by a variety of signal transduction pathways regulating and controlling tumor metastasis [37]. These findings indicated a critical function of MAC30 in degradation of the extracellular matrix. Moreover, the current results indicated that down-regulation of MAC30 effectively increased the expression of E-cadherin and decreased the expressions of N-cadherin and vimentin in both MCF-7 and MDA-MB-231 cells.
Previous studies have demonstrated that the PI3K/Akt pathway plays a critical role in malignant tumor progression, and that activation of Akt is associated with cell proliferation, survival, migration, and invasion [38,39]. Furthermore, the Wnt/β-catenin pathway is one of the key signaling pathways triggering EMT, and its downstream targets are survivin and cyclin D1 [40]. In order to explore the mechanism by which MAC30 promotes invasion and EMT in BC cells, we measured the phosphorylation of Akt to investigate the activation of the PI3K/Akt pathway and the expression of β-catenin, cyclin D1 and survivin to study the activation of the Wnt/β-catenin pathway. Our findings showed that inhibition of MAC30 could reduce the phosphorylation of Akt (Ser473) in both MCF-7 and MDA-MB-231 cells. Our data indicate that expression of β-catenin and its downstream targets cyclin D1 and survivin were significantly suppressed by down-regulation of MAC30. MAC30 may accelerate β-catenin degradation in the cytoplasm, and then suppress the translocation of β-catenin into the nucleus, resulting in inhibition of downstream target expressions. A study has indicated that survivin is an evolutionarily conserved activator of cell migration, invasion, and metastatic dissemination [41]. Cyclin D1 can activate the downstream gene Snail and mediate the occurrence of EMT [42,43]. Therefore, suppression of the Wnt/β-catenin signaling pathway by MAC30 knockdown presumably contributed to inhibition of invasion and EMT of BC cells.
In conclusion, our data show that MAC30 knockdown inhibits invasion and EMT of the breast cancer cells. We also identified a previously unknown function of MAC30, and down-regulation of MAC30 inactivated both the Akt and Wnt/β-catenin signaling pathways. These findings suggested that knockdown of MAC30 suppressed breast cancer metastasis by inhibiting EMT through regulation of both the Akt and Wnt/β-catenin signaling pathways, and may serve as an attractive therapeutic target.
Acknowledgements
Project was supported by Liaoning Provincial Natural Science Foundation of China (NO. 2015020544).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Torrel LA, Bray F, Siegel RL, Ferlay J, Loret-Tieulent J, Jemal Al. Global cancer statistics, 2015. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Peppercorn J, Perou CM, Carey LA. Molecular subtypes in breast cancer evaluation and management: divide and conquer. Cancer Invest. 2008;26:1–10. doi: 10.1080/07357900701784238. [DOI] [PubMed] [Google Scholar]
- 4.Smith BN, Bhowmick NA. Role of EMT in metastasis and therapy resistance. J Clin Med. 2016;5 doi: 10.3390/jcm5020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu YY, Sarkissyan M, Jaydutt V. VadgamaJane Grant-Kels: academic editor epithelial-mesenchymal transition and breast cancer. J Clin Med. 2016;5:13. doi: 10.3390/jcm5020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition. From: dardiovascular development to disease. Circulation. 2012;125:1795–808. doi: 10.1161/CIRCULATIONAHA.111.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–12. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–49. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roussos ET, Keckesova Z, Haley JD, Epstein DM, Weinberg RA, Condeelis JS. AACR special conference on epithelial-mesenchymal transition and cancer progression and treatment. Cancer Res. 2010;70:7360–64. doi: 10.1158/0008-5472.CAN-10-1208. [DOI] [PubMed] [Google Scholar]
- 12.Hazan RB, Qiao R, Keren R, Badano I, Suyama K. Cadherin switch in tumor progression. Ann N Y Acad Sci. 2004;1014:155–63. doi: 10.1196/annals.1294.016. [DOI] [PubMed] [Google Scholar]
- 13.Maeda M, Johnson KR, Wheelock MJ. Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci. 2005;118:873–87. doi: 10.1242/jcs.01634. [DOI] [PubMed] [Google Scholar]
- 14.Murphy M, Pykett MJ, Harnish P, Zang KD, George DL. Identifi cation and characterization of genes differentially expressed in meningiomas. Cell Growth Differ. 1993;4:715–22. [PubMed] [Google Scholar]
- 15.Malhotra K, Luehrsen KR, Costello LL, Raich TJ, Sim K, Foltz L, Davidson S, Xu H, Chen A, Yamanishi DT, Lindemann GW, Cain CA, Madlansacay MR, Hashima SM, Pham TL, Mahoney W, Schueler PA. Identifi cation of differentially expressed mRNAs in human fetal liver across gestation. Nucleic Acids Res. 1999;27:839–47. doi: 10.1093/nar/27.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kayed H, Kleeff J, Ding J, Hammer J, Giese T, Zentgraf H, Büchler MW, Friess H. Expression analysis of MAC30 in human pancreatic cancer and tumors of the gastrointestinal tract. Histol Histopathol. 2004;19:1021–31. doi: 10.14670/HH-19.1021. [DOI] [PubMed] [Google Scholar]
- 17.Potapova O, Anisimov SV, Gorospe M, Dougherty RH, Gaarde WA, Boheler KR, Holbrook NJ. Targets of c-Jun NH(2)-terminal kinase 2-mediated tumor growth regulation revealed by serial analysis of gene expression. Cancer Res. 2002;62:3257–63. [PubMed] [Google Scholar]
- 18.Zheng J, Zhang M, Zhang L, Ding X, Li W, Lu S. HSPC159 promotes proliferation and metastasis by inducing epithelial-mesenchymal transition and activating the PI3K/Akt pathway in breast cancer. Cancer Sci. 2018;109:2153–63. doi: 10.1111/cas.13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z, Ji L, Jiang G, Liu R, Liu Z, Yang Y, Ma Q, Zhao H. FL118, a novel camptothecin analogue, suppressed migration and invasion of human breast cancer cells by inhibiting epithelial-mesenchymal transition via the Wnt/β-catenin signaling pathway. Biosci Trends. 2018;12:40–6. doi: 10.5582/bst.2017.01288. [DOI] [PubMed] [Google Scholar]
- 20.Decker MR, Trentham-Dietz A, Loconte NK, Neuman HB, Smith MA, Punglia RS, Greenberg CC, Wilke LG. The role of intraoperative pathologic assessment in the surgical management of ductal carcinoma in situ. Ann Surg Oncol. 2016;23:2788–94. doi: 10.1245/s10434-016-5192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takashima T, Tokunaga S, Tei S, Nishimura S, Kawajiri H, Kashiwagi S, Yamagata S, Noda S, Nishimori T, Mizuyama Y, Sunami T, Tezuka K, Ikeda K, Ogawa Y, Onoda N, Ishikawa T, Kudoh S, Takada M, Hirakawa K. A phase II, multicenter, single-arm trial of eribulin as first-line chemotherapy for HER2-negative locally advanced or metastatic breast cancer. Springerplus. 2016;5:164. doi: 10.1186/s40064-016-1833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scaranelo AM, Moshonov H, Escallon J. A prospective pilot study of analysis of surgical margins of breast cancers using high-resolution sonography. Springerplus. 2016;5:251. doi: 10.1186/s40064-016-1921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gholami S, Marano A, Chen NG, Aguilar RJ, Frentzen A, Chen CH, Lou E, Fujisawa S, Eveno C, Belin L, Zanzonico P, Szalay A, Fong Y. Erratum to: a novel vaccinia virus with dual oncolytic and anti-angiogenic therapeutic effects against triple-negative breast cancer. Breast Cancer Res Treat. 2016;156:607–8. doi: 10.1007/s10549-016-3767-2. [DOI] [PubMed] [Google Scholar]
- 24.Liu MC, Cortes J, O’Shaughnessy J. Challenges in the treatment of hormone receptor positive, HER2-negative metastatic breast cancer with brain metastases. Cancer Metastasis Rev. 2016;35:323–32. doi: 10.1007/s10555-016-9619-z. [DOI] [PubMed] [Google Scholar]
- 25.Zelnak AB. Special considerations in early-stage breast cancer patients and survivors. Obstet Gynecol Clin North Am. 2013;40:573–82. doi: 10.1016/j.ogc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Zhao L, Niu F, Shen H, Liu X, Chen L, Niu Y. Androgen receptor and metastasis-associated protein-1 are frequently expressed in estrogen receptor negative/HER2 positive breast cancer. Virchows Arch. 2016;468:687–96. doi: 10.1007/s00428-016-1930-0. [DOI] [PubMed] [Google Scholar]
- 27.Blick T, Widodo E, Hugo H, Waltham M, Lenburg ME. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25:629–642. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- 28.Trimboli AJ, Fukino K, de Bruin A, Wei G, Shen L, Tanner SM, Creasap N, Rosol TJ, Robinson ML, Eng C, Ostrowski MC, Leone G. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Res. 2008;68:937–945. doi: 10.1158/0008-5472.CAN-07-2148. [DOI] [PubMed] [Google Scholar]
- 29.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–26. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 30.Matysiak M, Kapka-Skrzypczak L, Jodłowska-Jędrych B, Kruszewski M. EMT promoting transcription factors as prognostic markers in human breast cancer. Arch Gynecol Obstet. 2017;295:817–25. doi: 10.1007/s00404-017-4304-1. [DOI] [PubMed] [Google Scholar]
- 31.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–37. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget. 2015;6:10697–711. doi: 10.18632/oncotarget.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–54. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 34.Paccione RJ, Miyazaki H, Patel V, Waseem A, Gutkind JS, Zehner ZE, Yeudall WA. Keratin down-regulation in vimentin-positive cancer cells is reversible by vimentin RNA interference, which inhibits growth and motility. Mol Cancer Ther. 2008;7:2894–903. doi: 10.1158/1535-7163.MCT-08-0450. [DOI] [PubMed] [Google Scholar]
- 35.Song GQ, Zhao Y. MAC30 knockdown involved in the activation of the Hippo signaling pathway in breast cancer cells. Biol Chem. 2018;399:1305–11. doi: 10.1515/hsz-2018-0250. [DOI] [PubMed] [Google Scholar]
- 36.Xiao M, Li H, Yang S, Huang Y, Jia S, Wang H, Wang J, Li Z. Expression of MAC30 protein is related to survival and clinicopathological variables in breast cancer. J Surg Oncol. 2013;107:456–62. doi: 10.1002/jso.23269. [DOI] [PubMed] [Google Scholar]
- 37.Gao Y, Guan Z, Chen J, Xie H, Yang Z, Fan J, Wang X, Li L. CXCL5/CXCR2 axis promotes bladder cancer cell migration and invasion by activating PI3K/AKT-induced upregulation of MMP2/MMP9. Int J Oncol. 2015;47:690–700. doi: 10.3892/ijo.2015.3041. [DOI] [PubMed] [Google Scholar]
- 38.Yang SX, Polley E, Lipkowitz S. New insights on PI3K/AKT pathway alterations and clinical outcomes in breast cancer. Cancer Treat Rev. 2016;45:87–96. doi: 10.1016/j.ctrv.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabi T, Huwiler A, Zangemeister-Wittke U. AMR-Me inhibits PI3K/Akt signaling in hormone-dependent MCF-7 breast cancer cells and inactivates NF-kappaB in hormone-independent MDA-MB-231 cells. Mol Carcinog. 2014;53:578–88. doi: 10.1002/mc.22012. [DOI] [PubMed] [Google Scholar]
- 40.Lee SC, Kim OH, Lee SK, Kim SJ. IWR-1 inhibits epithelial-mesenchymal transition of colorectal cancer cells through suppressing Wnt/beta-catenin signaling as well as survivin expression. Oncotarget. 2015;6:27146–27159. doi: 10.18632/oncotarget.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehrotra S, Languino LR, Raskett CM, Mercurio AM, Dohi T, Altieri DC. IAP regulation of metastasis. Cancer Cell. 2010;17:53–64. doi: 10.1016/j.ccr.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarkar FH, Li Y, Wang Z, Kong D. The role of nutraceuticals in the regulation of wnt and hedgehog signaling in cancer. Cancer Metastasis Rev. 2010;29:383–94. doi: 10.1007/s10555-010-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmad A, Sarkar SH, Bitar B, Ali S, Aboukameel A, Sethi S, Li Y, Bao B, Kong D, Banerjee S, Padhye SB, Sarkar FH. Garcinol regulates EMT and Wnt signaling pathways in vitro and in vivo, leading to anticancer activity against breast cancer cells. Mol Cancer Ther. 2012;11:2193–201. doi: 10.1158/1535-7163.MCT-12-0232-T. [DOI] [PMC free article] [PubMed] [Google Scholar]