Abstract
IGF-1R is expressed abnormally in osteosarcoma (OS) and could participate in its progression. In this study, we aimed to explore the effect of the IGF-1R inhibitor PQ401 as a treatment for OS. The relative expression of IGF-1R in OS patient tumors and the U2OS cell line were determined by qRT-PCR and by accessing information in a public database. Inhibition of cell proliferation by PQ401 was determined by MTT assay. Cell migration under low concentration treatment of PQ401 was carried out by transwell and wound healing assays. PQ401 induction of OS cell apoptosis was investigated by flow cytometry. Tumorigenesis under PQ401 treatment was evaluated by a colony formation assay. Finally, downstream blockage of the IGF-1R pathway was verified by western blotting. Our results show that the expression of IGF-1R was remarkably higher in OS cells, particularly in U2OS, than in other cancer-type cell lines. The inhibition of the IGF-1R pathway by PQ401 exhibited significant anticancer activity in the U2OS cell line in not only proliferation but also migration and colony formation. In addition, PQ401 is a strong inducer of OS cell apoptosis. Furthermore, western blotting was used to demonstrate that the IGF-1R related downstream pathway, including total ERK1/2, was significantly inhibited by PQ401. Thus, IGF-1R inhibition may represent a novel treatment for OS.
Keywords: PQ401, IGF-1R, IGF-1R inhibitor, osteosarcoma, metastasis
Introduction
Osteosarcoma (OS), one of the most frequent types of primary malignant bone neoplasms, occurs in both children and adolescents [1]. The most common and effective therapeutic strategy for patients with OS is orthopedic surgical intervention. However, the recurrence and metastasis rates are quite high, resulting in a low survival rate [2]. In attempt to address this, chemotherapy and occasionally radiotherapy are employed; however, once the patient develops metastasis, particularly in the lungs, those treatments always fail [3]. Therefore, novel therapeutics treatments that target the molecular pathway regulating the metastasis of OS are urgently needed.
Receptor tyrosine kinases (RTKs) play a critical and essential role in cancers, including osteosarcoma, and have become ideal targets for anti-cancer therapy [4]. IGF-1R, an important RTK, is believed to regulate cancer cell survival, apoptosis and motility [5,6]. However, research focused on using IGF-1R inhibitors for OS as a cancer treatment is still quite limited.
A novel inhibitor of IGF-1R, PQ401, which is a diarylurea compound serving as a specific IGF-1R inhibitor, was evaluated in certain cancers as a possible anti-cancer drug. However, no evidence concerning the effect of PQ401 on osteosarcoma has thus far been identified. Therefore, in the present study, we aimed to study the anticancer activity of PQ401 using osteosarcoma cell lines and provide evidence for its use in osteosarcoma treatment.
Materials and methods
Cell lines, cell culture and chemicals
The U2OS and 143B cell lines were obtained from the American Type Culture Collection (ATCC, USA). Cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Gibco, USA), 100 U/ml penicillin and 100 U/ml streptomycin (Gibco, USA) in a humidified incubator with 5% CO2 at 37°C. PQ401 (Sigma-Aldrich, USA) was diluted to a stock concentration of 1 mM in PBS containing 2% DMSO.
Patient samples
Tissue from five OS patients with clinical diagnosis of potential metastasis was biopsied and confirmed by a definite pathology report. All patients were provided with and signed consent forms. Aliquots of the biopsy samples were stored at -80°C for further analysis. This study was conducted in accordance with the regulations of the human ethics committee of Harbin Medical University.
MTT assay
The MTT assay was performed to determine the chemosensitivity of osteosarcoma cells. In brief, a total of 5×103 U2OS or 143B cells per well were seeded in 96-well plates with 100 µl culture medium and cultured for 24 h. Then, the cells were exposed to different concentrations of PQ401 in 1% FBS medium for another 48 h. 100 µl medium containing MTT (5 mg/ml) was added to each well, and the cells were incubated at 37°C for additional 4 h. Afterwards, 150 µl DMSO was added, and the cells were incubated with gentle shaking for 15 min. The absorbance of each well at 490 nm was then determined by a microplate reader. Cisplatin (Sigma, USA) was used as a positive drug control for the MTT test and cell culture medium was used as a negative control for this assay. For IC50 time-dependent test, the cells were treated for 24 h, 48 h and 72 h.
Wound healing assay
The wound healing assay was used to measure U2OS cell migration under the treatment of PQ401. A total of 4×105 cells/well were seeded into 6-well plates with 10% FBS culture medium overnight. On the next day, a wound scratch was made by scraping a micropipette tip across the cell monolayer in the center. After removing the dislodged cells by gentle washing with culture medium, cells were treated with PQ401 in 5% FBS culture medium for 48 h. At 0 h and 48 h, five representative images of the wound-healing were taken. Cell migration was measured by the closure area measured by ImageJ [7].
Transwell assay
The transwell migration assay was performed to investigate the effects of PQ401 on the migration of U2OS cells. Cells were collected and resuspended in serum-free culture medium. A total of 1×104 cells in 200 µl serum-free culture medium containing PQ401 were seeded into the upper chamber, while 600 µl culture medium supplemented with 10% FBS was added to the lower chamber. Following 24 h of incubation at 37°C, the upper chamber was carefully scraped off using a cotton swab. The cells that migrated were stained with 0.5% crystal violet, and five randomized visual fields were counted. The number of cells was quantified using ImageJ.
Apoptosis assay
A total of 1×106 U2OS cells/well were seeded in 6-well plates with 2 ml culture medium and cultured for 24 h. The cells were treated with PQ401 at 5 µM and 10 µM in cell culture medium containing 0.05% DMSO for another 24 h prior to harvesting. Then, the cells were washed twice with ice-cold PBS, and their concentrations were adjusted to 1×106/ml in binding buffer. 200 µl PBS containing cells was taken from the suspension and incubated for 30 min with 5 µl Annexin V-FITC, and then with 5 µl PI. FACS analysis for annexin-V and PI staining was performed using a C6 flow cytometer.
Colony formation assay
Briefly, 3000 cells were seeded into a 100 mm cell culture dish overnight in fresh cell culture medium. The next day, the cells were treated with PQ401 at 0 µM, 5 µM, or 10 µM in cell culture medium with 0.05% DMSO for 10 days. Then, cells were fixed in cold methanol and stained with 0.1% crystal violet for 10 min. After washing twice with PBS, a picture of the whole plate was taken, and the occupied area was quantified by ImageJ.
Western blot
U2OS cells were lysed in RIPA containing 1% protease inhibitor and 1% phosphatase inhibitor. Briefly, solubilized proteins were quantified using the colorimetric DC protein assay (Bio-Rad, USA). Then, 20 µg protein was loaded for electrophoresis in a 10% SDS-PAGE gel and transferred onto a PVDF membrane (Bio-Rad, USA). The primary antibodies against p-IGF-1R (1:1000, Santa Cruz, USA), total-ERK1/2 (1:2000, Santa Cruz, USA), p-AKT (1:1000, Santa Cruz, USA), beta-actin (1:2000, Santa Cruz, USA) were diluted in the blocking buffer. The membrane was incubated with primary antibody at 4°C overnight. Detection of bands was carried out using chemiluminescence by ChemiDoc (Bio-Rad, USA).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA of patient samples, as well as the U2OS and 143B cell lines, was extracted by using Trizol reagent (Invitrogen, USA) and then reverse transcribed into cDNA using the Prime-Script RT-PCR kit (Takara, Japan). The expression levels of IGF-1R were determined using SYBR-Green Master Mix (Roche, USA) according to the manufacturer’s protocol. GAPDH was used as the internal control for quantification. A total of 1 µg cDNA was used in the qPCR reactions using an ABI7500 Fast Real-time PCR system (Applied Biosystems, USA). Data were analyzed using the 2-ΔΔCt method [8]. The primer sequences were as follows:
IGF-1R forward, 5’AGGATATTGGGCTTTACAACCTG3’; reverse, 5’GAGGTAACAGAGGTCAGCATTT-T3’; GAPDH forward, 5’TGCACCACCAACTGCTTA3’; reverse, 5’GGATGCAGGGATGATGTTC3’.
Public database extraction
The human protein atlas database (https://www.proteinatlas.org/) was used to extract the IGF-1R expression profile of cell lines [9].
Statistical analysis
Data are presented as the mean ± standard deviation. The differences between groups were compared with Student’s t-test or one-way analysis of variance using Prizm GraphPad (Version 7). Each experiment was performed in triplicate unless otherwise stated. A p-value <0.05 was considered to indicate a significant difference.
Results
PQ401 chemical information and IGF-1R expression in OS patients
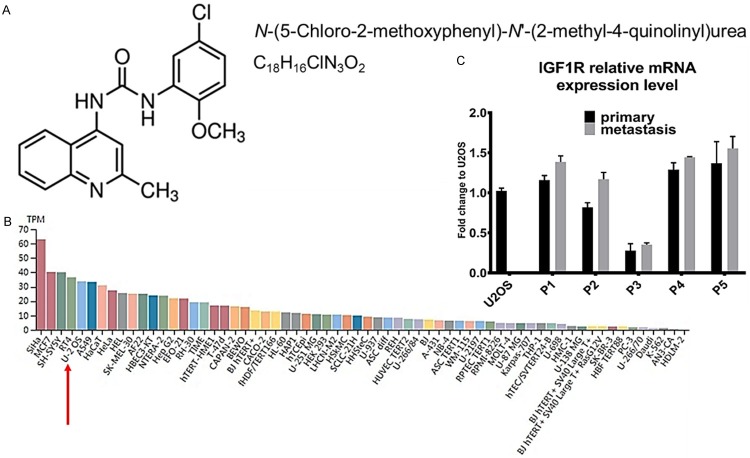
We extracted IGF-1R expression levels from the human protein atlas database for all available cell lines. Similar to MCF7, which is an IGF-1R inhibitor sensitive cancer cell line, we found that, for U2OS cells, the IGF-1R transcriptional expression level is in the top list (Figure 1B). As an IGF-1R inhibitor, PQ401 might affect cancer cell proliferation and migration if the tumor cells expressed high levels of IGF-1R. To this end, we chose the U2OS cell line (which has higher IGF-1R expression than the 143B cell line) as our in vitro model to test the relative effect of PQ401. In addition, we collected samples from five patients with a diagnosis of late-stage OS and quantified IGF-1R mRNA from both primary and metastasis sites for comparison with the U2OS cell line (Figure 1C). All clinical parameters of the OS patients were listed in Table 1.
Figure 1.
PQ401 chemical structure and IGF-1R expression in cell lines and OS patient samples. A. PQ401 chemical structure, International Union of Pure and Applied Chemistry name and structural formula. B. Public data extracted from human protein atlas. Red arrow highlights U2OS cell line IGF-1R expression. TPM: Transcripts per million. C. Five OS patients’ relative IGF-1R expression normalized to U2OS cells in both primary and metastasis sites. GAPDH is used as an internal reference.
Table 1.
Clinical characteristics of the OS patients
| Patient | Gender | Age | P site | M site | H-Grade | TNM | Cancer stage |
|---|---|---|---|---|---|---|---|
| Case 1 | M | 22 | Distal Femur | Lung | G2 | T1NxM1a | IV-A |
| Case 2 | F | 66 | Distal Femur | Lung | G3 | T1N1M1a | IV-A |
| Case 3 | F | 59 | Proximal Tibia | Lung | G1 | T1NxM1a | IV-A |
| Case 4 | M | 64 | Distal Femur | Lung, Brain | G3 | T1N1M1b | IV-B |
| Case 5 | M | 17 | Distal Femur | Lung | G3 | T1N1M1a | IV-A |
Abbreviations: P: primary; M: metastasis; H-Grade: Histology Grade; TNM: T: Tumor; N: Lymph Node; M: Metastasis.
PQ401 exerts antiproliferative effects on U2OS cells
To evaluate the effect of PQ401 in proliferation, U2OS and 143B cells were treated with different concentrations of PQ401 and the cell viability was assessed using the MTT assay. The results showed that PQ401 exhibited significant suppression of proliferation activity on the U2OS cells, which acted in a dose-dependent manner (Figure 2A). However, PQ401 did not show the same effect on low-IGF-1R 143B cells. (Figure 2B). The IC50 of PQ401 for suppression of OS cell proliferation in our 48 h MTT test was 5 µM. In addition, cisplatin, a traditional chemotherapy drug, was used in our test as a positive control drug. Compared with cisplatin, the single drug applicant of PQ401 in vitro did not exhibit a greater inhibition of proliferation in the high IGF-1R U2OS cell line. We measured IC50 concentrations for a range of incubation times; the results showed that longer time treatment (72 h) could significantly decrease the U2OS cell viability (Figure 2C). However, PQ401 only inhibited 143B cell line viability by approximately 20% and showed no time-dependent behavior (Figure 2C).
Figure 2.
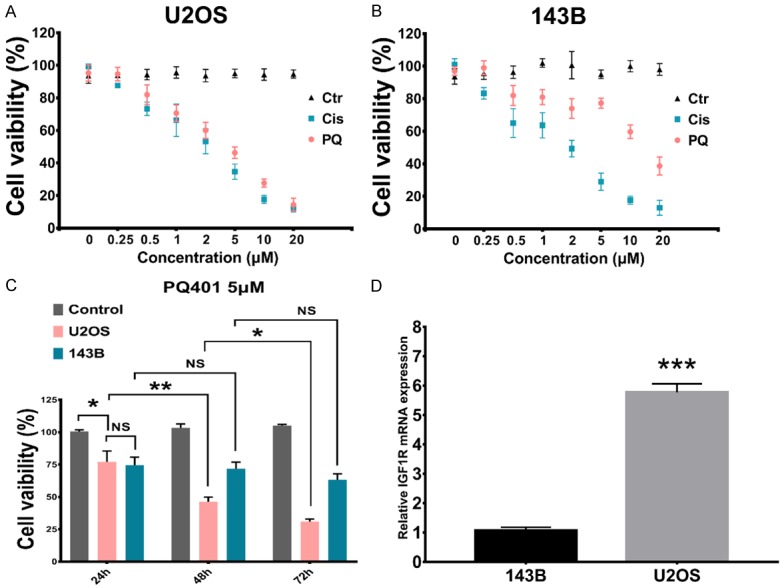
PQ401 inhibits U2OS and 143B cell proliferation. A. U2OS cell cells were treated with PQ401 (0.25 to 20 µM) for 48 h followed by application of the MTT assay. Culture medium was used as the blank control and indicated as 0 µM. Cisplatin was used as positive control and applied at indicated concentrations. B. PQ401 effect on 143B cell line. C. Percentage of cell viability of U2OS and 143B cell lines was significantly decreased with PQ401 treatment at a concentration of 5 µM at the indicated time point compared with the culture medium control group. At 24, 48, and 72 h, the cell viability was assessed. D. Real-time PCR for the relative IGF1R expression between the two selected cell lines. The means and error bars (standard deviation) were derived from experiments using triplicate samples. (***P<0.001).
Inhibition of IGF-1R significantly enhances induction of apoptosis in osteosarcoma cells by PQ401
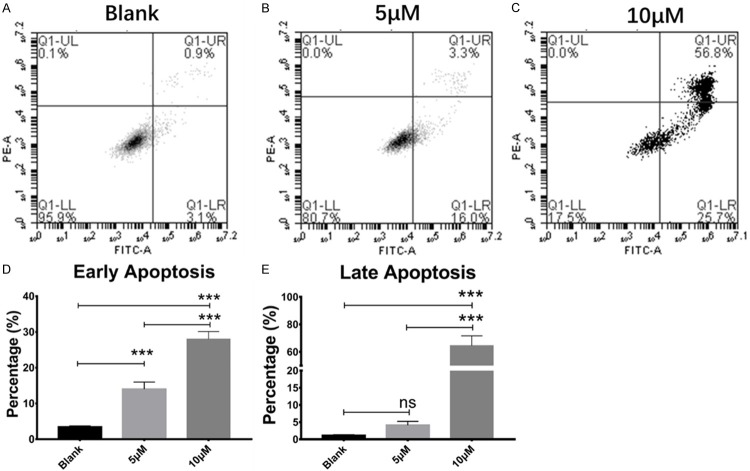
To access the effect of PQ401 on apoptosis induction in OS cells, U2OS cancer cells were treated with PQ401 at IC50 concentration (5 µM) and 10 µM, and then subjected to Annexin-V/PI staining and analysis by flow cytometry. It was observed that PQ401 induced apoptosis in a dose-dependent manner. Our results clearly show that both early and late apoptosis were increased after PQ401 treatment (Figure 3B, 3C).
Figure 3.
PQ401 induced U2OS cell apoptosis in a dose-dependent manner. Cells were treated with indicated concentrations of PQ401 for 24 h and stained with Annexin-V/PI. A. A representative flow cytometry result of U2OS in negative control cell culture medium. B. With treatment of PQ401 at 5 µM. C. With treatment of PQ401 at 10 µM. D, E. Statistical analysis showed that PQ401 signifcantly increased both early and late apoptosis in U2OS cells at 10 µM. However, with PQ401 treatment at 5 µM for 24 h, there is no signifcant difference in late apoptosis compared with blank control. Each experiment was performed in triplicate. NS = not signifcant, ***P<0.001.
PQ401 significantly inhibits osteosarcoma cell migration
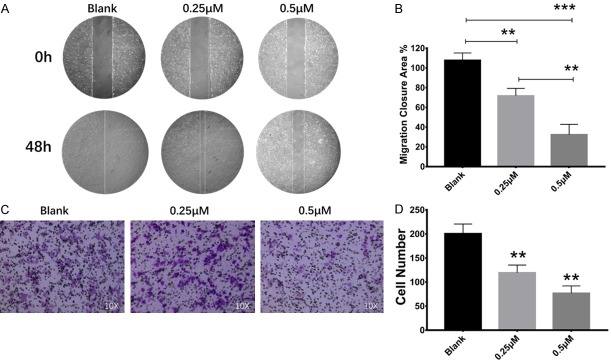
The cell migration capability was tested using wound healing and transwell assays. Our findings demonstrated that OS cancer cell migration was significantly decreased with low concentrations of PQ401 (0.25 µM and 0.5 µM, Figure 4A, 4C). The inhibition of cell migration at 0.5 µM was stronger than 0.25 µM in both wound healing and transwell assays (Figure 4B, 4D).
Figure 4.
PQ401 inhibited U2OS cell migration at low concentration. A. Wound healing assay: 0 h picture represents starting point of wound gap. The 48 h picture represents final migration status. The front line was marked with white. B. Statistical result of migration closure area compared with starting point. C. Transwell assay: the migrated cells were stained in crystal violet and counted using ImageJ. D. Statistical results of transwell assay compared with negative control (Blank). (*P<0.05; **P<0.01; ***P<0.001).
Inhibition of clonogenesis and the IGF-1R related pathway by PQ401
Colony formation inhibition by PQ401 was chosen to evaluate the effect of IGF-1R on clonogenesis. Our findings show that the U2OS cell colony formation capability was significantly decreased with the treatment of PQ401 at the IC50 (5 µM) and at 10 µM (Figure 5B, 5C). In addition, exposure of U2OS cells to 5 µM of PQ401 for 4 h resulted in a significant reduction in phosphorylation of IGF-1R and AKT (Figure 5E). For total extracellular-signal-regulated kinase 1/2 (ERK1/2), which is related to cell proliferation and colony formation, the protein expression level was also significantly decreased.
Figure 5.
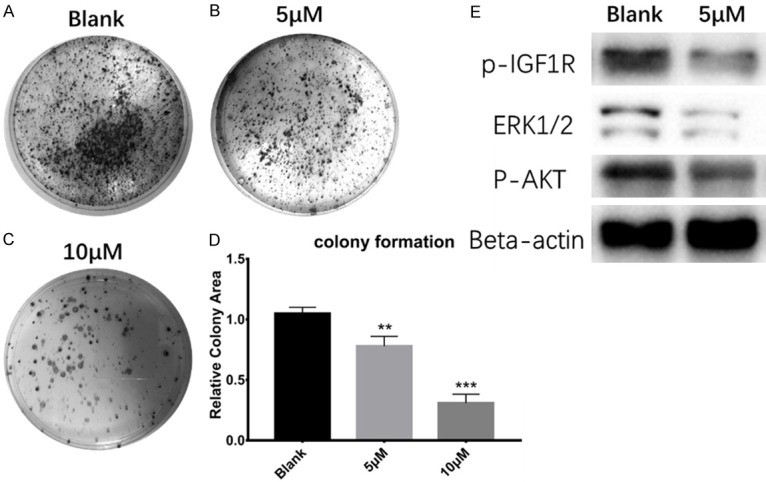
PQ401 inhibited clonogenesis, blocked IGF-1R phosphorylation and suppressed related downstream pathway. A. Colony formation phenotype for U2OS cells in cell culture medium (negative control). B. In the presence of PQ401 at 5 µM. C. In the presence of PQ401 at 10 µM. D. Statistical results of colony formation assay. E. Western blot result for the IGF-1R relative pathway, (**P<0.01; ***P<0.001).
Discussion
Current strategies for osteosarcoma treatment mainly focus on surgery, chemotherapy, or rarely, radiation therapy. Chemotherapy is still widely used for osteosarcoma patients with metastasis. However, the treatment outcome is usually poor due to early chemoresistance [10]. Therefore, identification of novel targets, with potential for effective management of aggressive osteosarcoma, is urgently needed. IGF-1R is a transmembrane tyrosine kinase receptor of the insulin receptor family, which regulates tumor proliferation, apoptosis, invasion, and metastasis [11-14]. The aberrant expression of IGF-1R has been confirmed in many human cancers, including osteosarcoma [15]. It is believed that IGF-1R overexpression contributes to cancer metastasis and chemotherapy resistance [16-18]. Thus, IGF-1R is emerging as an anti-cancer target in OS.
Thorsten et al. used tissue microarray to identify IGF-1 expression levels in 66 OS patients, and their results suggested that high IGF-1/IGF-1R expression is significantly associated with metastasis and a worse clinical outcome [19]. They also demonstrated that IGF-1/IGF-1R axis activation could identify patients with metastasis and thus poor response to chemotherapy. In our study, we not only showed that the U2OS cell line has the top level of IGF-1R expression in the available cell lines described in the human protein atlas but also verified that the IGF-1R expression was approximately 8 times higher than that of the 143B OS cell line. In addition, the response of OS cell lines to PQ401 shows an IGF-1R expression-level dependent manner; that is, the higher the level of IGF-1R expression is, the better the anti-cancer effect of PQ401. In clinical samples, we found that metastasis site clinical samples have higher IGF-1R mRNA expression than primary site samples. These findings indicated that IGF-1R inhibitor treatment could be an effective therapy for OS and especially for inhibition of metastasis. Interestingly, the only clinical sample with a relatively low expression level of IGF-1R was assessed to be histopathology grade 1. The association between IGF-1R expression and histopathology grade needs to be further elucidated.
PQ401, a novel diarylurea compound, was previously found to have anti-cancer drug properties in glioma and breast cancer [20,21]. However, there are no reports describing the effect of PQ401 as a putative chemotherapy drug in osteosarcoma cells. Thus, we first explored the therapeutic potential of PQ401 to inhibit IGF-1R function as a treatment for human osteosarcoma. Our results showed that PQ401 effectively suppressed osteosarcoma cell growth, migration and colony formation in vitro, as well as induced apoptosis in vitro. We found that PQ401 inhibited U2OS cell viability almost as effective as cisplatin. However, in a relatively low-IGF-1R OS cell line (143B), the inhibition effect of PQ401 was significantly reduced compared with cisplatin. It is widely believed that dysregulated apoptotic pathways play major roles in carcinogenesis. In the present study, we also observed that PQ401 can significantly cause U2OS cell apoptosis and clonogenesis at the IC50 concentration with the blockage of IGF-1R phosphorylation and related downstream signaling.
Taken together, our results suggest that PQ401 may be a promising drug candidate for clinical chemotherapy for OS patients with metastasis. Higher IGF-1R level OS patients may benefit more from PQ401 treatment.
Acknowledgements
We are very grateful for the sincere help and technical support by the Department of Pathology and Department of Operating Room. This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81670459) to Maomao Zhang and the National Natural Science Foundation of China (Grant No. 81572472 and No. 81773161) to Mian Guo.
Disclosure of conflict of interest
None.
References
- 1.Vijayakumar V, Lowery R, Zhang X, Hicks C, Rezeanu L, Barr J, Giles H, Vijayakumar S, Megason G. Pediatric osteosarcoma: a single institution’s experience. South Med J. 2014;107:671–675. doi: 10.14423/SMJ.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 2.Friebele JC, Peck J, Pan X, Abdel-Rasoul M, Mayerson JL. Osteosarcoma: a meta-analysis and review of the literature. Am J Orthop (Belle Mead NJ) 2015;44:547–553. [PubMed] [Google Scholar]
- 3.Shankar GM, Clarke MJ, Ailon T, Rhines LD, Patel SR, Sahgal A, Laufer I, Chou D, Bilsky MH, Sciubba DM, Fehlings MG, Fisher CG, Gokaslan ZL, Shin JH. The role of revision surgery and adjuvant therapy following subtotal resection of osteosarcoma of the spine: a systematic review with meta-analysis. J Neurosurg Spine. 2017;27:97–104. doi: 10.3171/2016.12.SPINE16995. [DOI] [PubMed] [Google Scholar]
- 4.Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- 5.Simpson A, Petnga W, Macaulay VM, Weyer-Czernilofsky U, Bogenrieder T. Insulin-like growth factor (IGF) pathway targeting in cancer: role of the IGF axis and opportunities for future combination studies. Target Oncol. 2017;12:571–597. doi: 10.1007/s11523-017-0514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park BR, Lee SA, Moon SM, Kim CS. Anthricin induced caspase dependent apoptosis through IGF1R/PI3K/AKT pathway inhibition in A549 human nonsmall lung cancer cells. Oncol Rep. 2018;39:2769–2776. doi: 10.3892/or.2018.6333. [DOI] [PubMed] [Google Scholar]
- 7.Nunes JP, Dias AA. ImageJ macros for the user-friendly analysis of soft-agar and wound-healing assays. Biotechniques. 2017;62:175–179. doi: 10.2144/000114535. [DOI] [PubMed] [Google Scholar]
- 8.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 9.Thul PJ, Akesson L, Wiking M, Mahdessian D, Geladaki A, Ait Blal H, Alm T, Asplund A, Bjork L, Breckels LM, Backstrom A, Danielsson F, Fagerberg L, Fall J, Gatto L, Gnann C, Hober S, Hjelmare M, Johansson F, Lee S, Lindskog C, Mulder J, Mulvey CM, Nilsson P, Oksvold P, Rockberg J, Schutten R, Schwenk JM, Sivertsson A, Sjostedt E, Skogs M, Stadler C, Sullivan DP, Tegel H, Winsnes C, Zhang C, Zwahlen M, Mardinoglu A, Ponten F, von Feilitzen K, Lilley KS, Uhlen M, Lundberg E. A subcellular map of the human proteome. Science. 2017;356 doi: 10.1126/science.aal3321. [DOI] [PubMed] [Google Scholar]
- 10.Cassier P, Pissaloux D, Alberti L, Ray-Coquard I, Blay JY. Targeted treatment of rare connective tissue tumors and sarcomas. Bull Cancer. 2010;97:693–700. doi: 10.1684/bdc.2010.1115. [DOI] [PubMed] [Google Scholar]
- 11.Yeo CD, Kim YA, Lee HY, Kim JW, Lee SH, Kim SJ, Kwon SS, Kim YH, Kim SC. Inhibiting IGF-1R attenuates cell proliferation and VEGF production in IGF-1R over-expressing EGFR mutant non-small cell lung cancer cells. Exp Lung Res. 2017;43:29–37. doi: 10.1080/01902148.2017.1282994. [DOI] [PubMed] [Google Scholar]
- 12.Tu C, Wang F, Wan J. MicroRNA-381 inhibits cell proliferation and invasion in endometrial carcinoma by targeting the IGF-1R. Mol Med Rep. 2018;17:4090–4098. doi: 10.3892/mmr.2017.8288. [DOI] [PubMed] [Google Scholar]
- 13.Subramani R, Lopez-Valdez R, Arumugam A, Nandy S, Boopalan T, Lakshmanaswamy R. Targeting insulin-like growth factor 1 receptor inhibits pancreatic cancer growth and metastasis. PLoS One. 2014;9:e97016. doi: 10.1371/journal.pone.0097016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svalina MN, Kikuchi K, Abraham J, Lal S, Davare MA, Settelmeyer TP, Young MC, Peckham JL, Cho YJ, Michalek JE, Hernandez BS, Berlow NE, Jackson M, Guillaume DJ, Selden NR, Bigner DD, Nazemi KJ, Green SC, Corless CL, Gultekin S, Mansoor A, Rubin BP, Woltjer R, Keller C. IGF1R as a key target in high risk, metastatic medulloblastoma. Sci Rep. 2016;6:27012. doi: 10.1038/srep27012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paz K, Hadari YR. Targeted therapy of the insulin-like growth factor-1 receptor in cancer. Comb Chem High Throughput Screen. 2008;11:62–69. doi: 10.2174/138620708783398313. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y, Roth M, Piperdi S, Montoya K, Sowers R, Rao P, Geller D, Houghton P, Kolb EA, Gill J, Gorlick R. Insulin-like growth factor 1 receptor and response to anti-IGF1R antibody therapy in osteosarcoma. PLoS One. 2014;9:e106249. doi: 10.1371/journal.pone.0106249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu D, Shibuya H, Miura M. Roles of the insulin-like growth factor I receptor C-terminus in cellular radioresistance. Biochem Biophys Res Commun. 2003;311:174–178. doi: 10.1016/j.bbrc.2003.09.195. [DOI] [PubMed] [Google Scholar]
- 18.Luk F, Yu Y, Walsh WR, Yang JL. IGF1R-targeted therapy and its enhancement of doxorubicin chemosensitivity in human osteosarcoma cell lines. Cancer Invest. 2011;29:521–532. doi: 10.3109/07357907.2011.606252. [DOI] [PubMed] [Google Scholar]
- 19.Jentzsch T, Robl B, Husmann M, Bode-Lesniewska B, Fuchs B. Worse prognosis of osteosarcoma patients expressing IGF-1 on a tissue microarray. Anticancer Res. 2014;34:3881–3889. [PubMed] [Google Scholar]
- 20.Zhou X, Zhao X, Li X, Ping G, Pei S, Chen M, Wang Z, Zhou W, Jin B. PQ401, an IGF-1R inhibitor, induces apoptosis and inhibits growth, proliferation and migration of glioma cells. J Chemother. 2016;28:44–49. doi: 10.1179/1973947815Y.0000000026. [DOI] [PubMed] [Google Scholar]
- 21.Gable KL, Maddux BA, Penaranda C, Zavodovskaya M, Campbell MJ, Lobo M, Robinson L, Schow S, Kerner JA, Goldfine ID, Youngren JF. Diarylureas are small-molecule inhibitors of insulin-like growth factor I receptor signaling and breast cancer cell growth. Mol Cancer Ther. 2006;5:1079–1086. doi: 10.1158/1535-7163.MCT-05-0397. [DOI] [PubMed] [Google Scholar]