Abstract
Gastric cancer (GC) is one of the leading malignancies worldwide and is also a leading cause of cancer-related mortality. Micro RNA (miRNA) is a group of short non-coding RNAs modulating gene expression through targeting the 3’UTR of genes. Peripheral blood exosome miRNAs are relatively stable and might be used as biomarkers for clinical diagnosis. In this study, we found a reduced level of miR-129-3p in GC tumor when compared with adjacent non-tumor tissues, and the peripheral blood exosome miR-129-3p level was also reduced when compared with healthy controls. Subsequently, we identified that miR-129-3p repressed the expression of SUMO-activating enzyme subunit 1 (SAE1) directly through targeting 3’UTR. miR-129-3p also inhibited the sumoylation modification of XRCC4 which disturbed the nuclear localization of XRCC4 and induced more DNA damage in GC cells. Furthermore, overexpression of miR-129-3p induced more cell apoptosis and inhibited GC cell proliferation, migration and invasion, indicating miR-129-3p is a powerful anti-tumor miRNA and has potential for GC treatment.
Keywords: microRNA, DNA repair, XRCC4, sumoylation
Introduction
Accurate replication and segregation of the genome to the next generation are essential for the survival of an organism. However, normal metabolic activities and environmental factors such as radiation and reactive oxygen can induce DNA damage. Thus, cells developed several precisely modulated DNA repair systems to fix single-strand breaks and double-strand breaks (DSBs). Unlike the homologous recombination (HR) pathway that repairs DSBs only when a sister chromatid is available, the NHEJ pathway repairs DSBs by relying on short homologous sequences present on the single-stranded tails of the DNA ends and working through the overall cell cycle duration [1]. It is believed that the cascades of posttranslational modifications, including phosphorylation, ubiquitylation, sumoylation and parylation are involved in the DSB repair regulation system [2].
Sumoylation is a protein modification process by which substrates are modified by covalently conjugated small ubiquitin-like modifier (SUMO) that regulates their degradation or sub-cellular localization. Similar to ubiquitination, sumoylation consists of a cascade of enzymes including E1 SUMO activating enzymes, E2 conjugating enzyme, and E3 SUMO ligases [3].
miRNAs are a group of short non-coding RNA regulators, modulating gene expression by binding with the 3’UTR of the target genes. A miRNA is first transcribed as primary miRNA and then is processed into a shorter form molecule in the cell nucleus, called precursor miRNA. After transportation into the cytoplasm, the precursor miRNA is finally processed into the mature miRNA by endoribonuclease Dicer. miRNAs play important roles in maintaining normal human body physiologic conditions, but abnormal miRNA expression has been found related to human diseases including malignant tumors [4,5].
miR-129-5p and miR-129-3p are two products from the same precursor miR-129, and both of their expressions are repressed ingastric cancer [6,7]. However, the function of miR-129-3p during carcinogenesis is not well understood. In this study, we first examined the expression of miR-129-3p in peripheral blood and gastric cancer tissue samples. After functional studies in vitro, we found miR-129-3p is an important NHEJ pathway regulator through controlling the sumoylation system.
Materials and methods
Cohorts
A total of 50 specimens of primary gastric adenocarcinoma and corresponding adjacent non-tumorous gastric tissue samples were obtained between 2013 and 2015 at Qingdao University affiliated Qingdao Municipal Hospital. A part of each tissue sample was subject to formalin fixation and paraffin-embedding. Another part of each tissue sample was immediately snap-frozen in liquid nitrogen and stored in a refrigerator at -80°C. All the 50 matched fresh frozen gastric adenocarcinoma tissues and adjacent non-cancerous tissues were selected for RNA extraction and qRT-PCR. Plasma control samples were obtained from 50 healthy participants who were age and sex matched.
RNA extraction
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. RNA concentration and purity were determined by a model ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). Samples only with absorbance ratios 260 nm/280 nm of ~2.0, and 260 nm/230 nm of 1.9-2.2 were considered for inclusion in the study.
miRNA quantification
Quantitative RT-PCR analysis was used to determine the relative level of miR-129-5p, miR-129-1-3p and miR-129-2-3p. The levels were determined by TaqMan miRNA RT-Real Time PCR according to the manufacturer’s instruction. U6 small nuclear RNA was used for normalization. Each sample in each group was measured three times and the experiment was repeated at least three times.
Immunoblotting
Protein was extracted from cells using RIPA buffer (Abcam, Cambridge, MA, USA), and quantified using a protein quantitation kit (Abcam, Cambridge, MA, USA). The samples were boiled in sodium dodecyl sulfate/β-mercaptoethanol sample buffer, and 20 μg samples were loaded into each lane of 10% polyacrylamide gels. After electrophoresis, the proteins were blotted onto a polyvinylidene fluoride membrane (Amersham Pharmacia Biotech, St. Albans, Herts, UK) by electrophoretic transfer. The membrane was incubated with rabbit anti-SAE1 monoclonal antibody (Abcam, Cambridge, MA, USA), rabbit anti-SUMO1 monoclonal antibody (Abcam, Cambridge, MA, USA), rabbit anti-SUMO2/3 polyclonal antibody (Abcam, Cambridge, MA, USA), rabbit anti-XRCC4 polyclonal antibody (Abcam, Cambridge, MA, USA), rabbit anti-Lamin B1 polyclonal antibody (Abcam, Cambridge, MA, USA), rabbit anti-PARP1 monoclonal antibody (Abcam, Cambridge, MA, USA), or mouse anti-β-actin monoclonal antibody (Santa Cruz Biotechnology , Santa Cruz, CA, USA) overnight at 4°C. The specific protein-antibody complex was detected by using horseradish peroxidase conjugated rabbit anti-mouse IgG. Detection by the chemiluminescence reaction was carried out using ECL kit (Pierce, Appleton, WI, USA). The β-actin signal was used as a loading control.
Cell culture
Human gastric cancer cells SGC-7901, AGS and human embryonic kidney cell HEK293T were cultured in DMEM medium containing 10% fetal calf serum (Hyclone), 100 IU/mL penicillin and 100 µg/mL streptomycin.
Dual luciferase assays
To generate 3’-UTR luciferase reporter, a 422 bp segment of 3’UTR from SAE1 was cloned downstream of the firefly luciferase gene into the pmirGLO plasmid (Promega, Madison, WI USA). miRNA mimics and inhibitors were synthesized by GenePharma Co., Ltd (Shanghai, China). For the luciferase reporter assay, HEK293T cells were seeded in 48-well plates. Luciferase reporter vectors were co-transfected with miRNA mimic or inhibitor using lipofectamine 2000 (Invitrogen). Two days after transfection, cells were harvested and assayed with the Dual-Luciferase Assay kit (Promega, Madison, WI USA). Each treatment was performed in triplicate in three independent experiments. The results are expressed as relative luciferase activity (Firefly Luciferase/Renilla Luciferase).
Cell proliferation assay
Cell proliferation was estimated by using the MTS Assay Kit (Abcam, Cambridge, MA, USA). SGC-7901 or AGS cells were seeded in wells of 96-well plates (1×104) in DMEM medium and allowed to attach overnight. The cells were then transfected with miR-129-3p mimic for 48 hours and then 20 μl MTS were added to each well. The cells were incubated for 2 h and the absorbance was recorded at 570 nm with a 96-well plate reader.
Cell migration and invasion assay
A typical transwell assay (6.5 mm diameter, 8 µm pore size; Thermo Fisher Scientific, Carlsbad, CA, USA) was used. Cells (1×104) in 200 µL serum-free medium were seeded in the top chamber and 500 µL of medium with a high concentration of serum was added to the bottom chamber. After 12 h, the filters was then removed and submerged in 4% paraformaldehyde for 15 min and cells on the upper surface were removed using a cotton swab. The cells on the lower surface were stained with hematoxylin. Ten random fields were selected to determine the average number of cells per view field.
For the cell invasion assay, transwell membranes were pre-coated with 24 µg/µL Matrigel (BD Bioscience, Franklin Lakes, NJ, USA) and incubated for 8 h at 37°C. Cells (2×104) in 200 µL serum-free medium were seeded in the top chamber and 500 µL medium with a high concentration of serum was added to the bottom chamber. After 12 h, the cells adhering to the lower surface of the filter were counted.
Statistical analyses
Data were analyzed using SPSS Statistical Package version 16 (SPSS Inc., Chicago, IL, USA). Two-tailed Student’s t-test was used to calculate statistical significance between the two comparator groups. The correlation analysis was analyzed by χ2-analysis. The findings were considered significant at a p-value <0.05.
Results
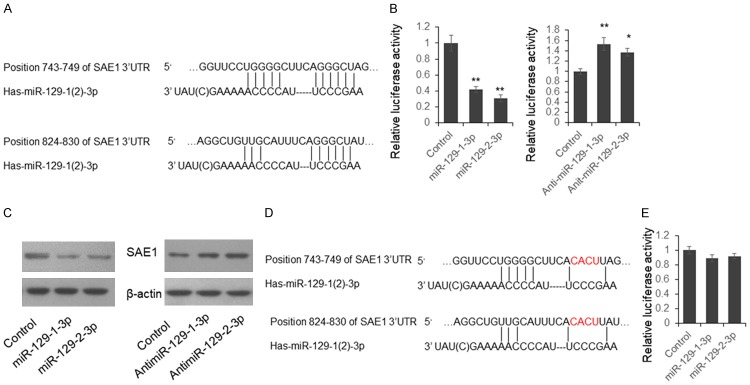
To investigate the roles of miR-129 during the carcinogenesis of gastric cancer, we first examined the expression of miR-129-3p in the tumor and plasma exosome samples from patients with gastric cancer. We found miR-129-3p level in the tumor samples is significantly down regulated when compared with adjacent non-cancerous tissues (Figure 1A). The plasma exosome miR-129-3p level was also significantly reduced in the GC patients when compared with healthy controls (Figure 1B). To further understand the meaning of miR-129-3p reduction in gastric cancer, we first checked the predicted target gene of miR-129-3p (http://www.targetscan.org/vert_70/). We found SUMO-activating enzyme subunit 1 (SAE1) is a potential target of miR-129-3p and there are two predicted target sites in the 3’UTR of SAE1 (Figure 2A).
Figure 1.
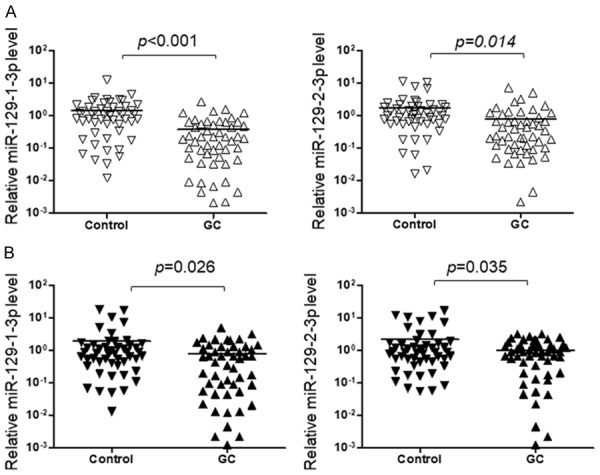
The level of miR-129-1-3p and miR-129-2-3p was reduced in GC patients. Total RNA was extracted from 50 paired tumor and non-tumor tissue samples, and plasma exosome samples from 50 GC patients and 50 healthy controls. The level of miR-129-1-3p and miR-129-2-3p was quantified by qRT-PCR and results were analyzed by Student’s t-test. *P<0.05, **P<0.01.
Figure 2.
SAE1 is a direct target of miR-129-1-3p and miR-129-2-3p. A. Predicted interaction between miR-129-3p and 3’UTR of SAE1 mRNA. B. Dual-luciferase assay. C. Immunoblotting. SGC-7901 cells were transfected with miR-129-1(2)-3p mimics or inhibitors for 48 hours. The endogenous SAE1 level was determined by immunoblotting. D. A schematic diagram for mutant reporter vector construction. E. Dual-luciferase assay using the mutant reporter vector. Results were analyzed by Student’s t-test. *P<0.05, **P<0.01.
To confirm the direct interaction between miR-129-3p and SAE1 3’UTR, we cloned the 422 bp segment of 3’UTR of SAE1 containing two miR-129-3p target sites, into the pmirGLO vector, following the firefly luciferase coding region to form the SAE1 reporter vector. HEK293T cells were transfected with miR-129-3p mimics or inhibitors with SAE1 reporter vector for 48 hours. Cells were lysed and the luciferase activities were detected. As shown in Figure 2B, the relative luciferase activities were significantly repressed by miR-129-1(2)-3p mimics, and up-regulated by miR-129-1(2)-3p inhibitors. Meanwhile, the SAE1 protein level was significantly inhibited by miR-129-1(2)-3p mimics and up-regulated by miR-129-1(2)-3p inhibitors in SGC-7901 cells (Figure 2C). When 8 nucleotides in the miR-129-3p target region were mutated, the luciferase activity was not repressed by miR-129-3p mimics. These results indicated that miR-129-1(2)-3p repress SAE1 expression through targeting 3’UTR.
SUMO1 activating enzyme subunit 1 (SAE1) forms a heterodimer with UBA2 functions as SUMO activating enzyme initiating the protein sumoylation process. Since miR-129-3p directly represses SAE1 expression, we would like to know whether cell sumoylation system was modulated by miR-129-3p. First, we detected the cell sumoylation conditions in the SGC-7901 cells and found that the total sumoylation condition was repressed by miR-129-3p (Figure 3A left panel). When the cells were co-transfected with miR-129-3p and SAE1 expression plasmid, the repressed sumoylation was totally rescued, indicating that miR-129-3p repressed cell sumoylation conditions through targeting SAE1 (Figure 3A right panel). It is known that XRCC4 is a key component in the DNA double strand break repair pathway which can be modified by SUMO, and this modification regulates XRCC4 subcellular localization function [8]. We did the immunoprecipitation using SUMO1 and SUMO2/3 antibody, and detected the endogenous sumoylated XRCC4 by immunoblotting. As shown in Figure 3B (left panel), the sumoylated XRCC4 level was significantly reduced. When the cells were co-transfected with miR-129-3p and SAE1 expression plasmid, the sumoylated XRCC4 level was not significantly changed (Figure 3B right panel). Meanwhile, in the miR-129-3p treated cells, XRCC4 was blocked in the cytoplasm which induced more DNA damage (increased γH2AX signal) and increased cleaved PARP1 and caspase-3 level (Figure 4 left panel). However, no significant difference was found in the levels of γH2AX, cleaved PARP1 and caspase-3 level, in the cells transfected by miR-129-3p and SAE1 expression plasmid (Figure 4 right panel).
Figure 3.
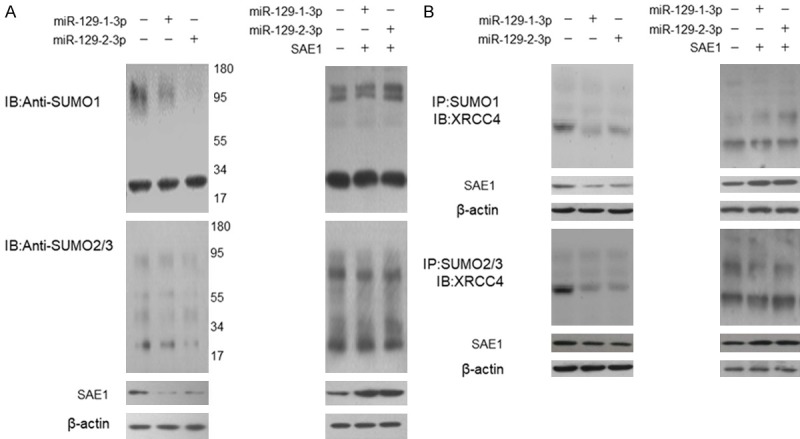
miR-129-1(2)-3p represses XRCC4 sumoylation by targeting SAE1. A. SGC-7901 cells were treated with miR-129-3p mimic for 48 hours and the endogenous sumoylation condition was examined by immunoblotting. For the rescue experiment, SGC-7901 cells were transfected with miR-129-3p mimic and SAE1 expression plasmid for 48 hours. The control cells were transfected with control RNA oligo and empty vector. The endogenous sumoylation condition was examined by immunoblotting. B. SGC-7901 cells were transfected with miR-129-3p mimics for 48 hours and the sumoylated proteins were precipitated using SUMO1 or SUMO2/3 antibody. The sumoylated XRCC4 was detected by immunoblotting. For the rescue experiment, SGC-7901 cells were transfected with miR-129-3p mimic and SAE1 expression plasmid for 48 hours. The cells transfected by control RNA oligo and empty vector were used as controls.
Figure 4.
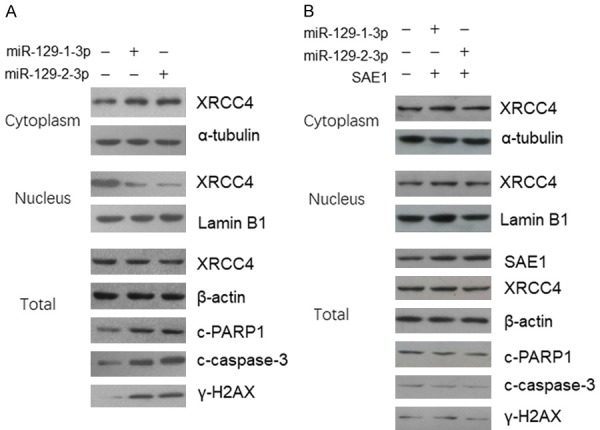
miR-129-1(2)-3p represses XRCC4 translocation and induces DNA damage by targeting SAE1. A. SGC-7901 cells were treated with miR-129-3p for 48 hours and then the proteins in the cell nucleus and cytoplasm were extracted separately. The XRCC4 level was determined by immunoblotting. B. SGC-7901 cells were co-transfected with miR-129-3p and SAE1 expression plasmid for 48 hours and then the proteins in the cell nucleus and cytoplasm were extracted separately. The XRCC4 level was determined by immunoblotting.
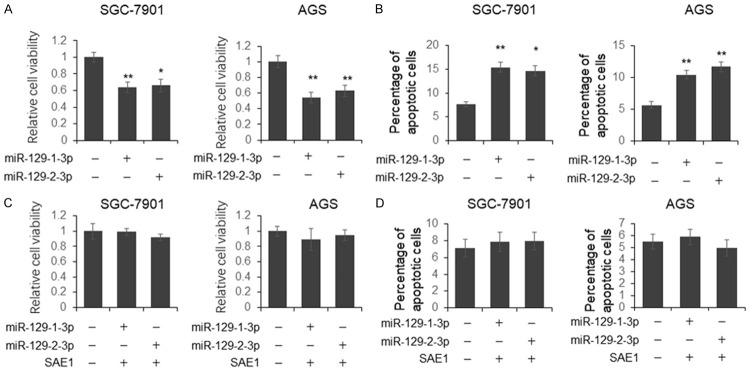
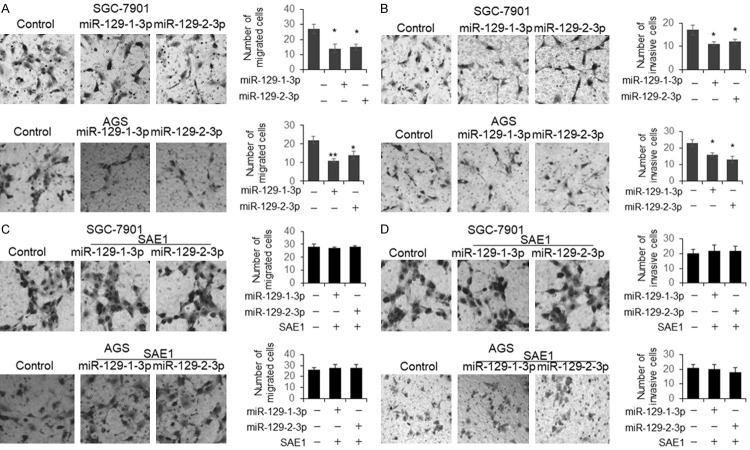
To further understand whether miR-129-3p has the potential to be used for GC treatment, SGC-7901 and AGS cells were transfected with miR-129-1(2)-3p for 48 hours, followed by cell viability and apoptosis analysis. We found that the cell viability was significantly reduced and the number of apoptotic cells was increased in the miR-129-1(2)-3p treated cells (Figure 5A and 5B). However, the co-transfection with SAE1 expression plasmid totally rescued the function of miR-129-1(2)-3p on cell viability and apoptosis (Figure 5C and 5D). The capacity of cell migration and invasion was examined by transwell assay. As shown in Figure 6A and 6B, the number of migrated and invasive cells treated by miR-129-1(2)-3p was significantly reduced. However, no significant difference was found in the cells co-transfected with miR-129-1(2)-3p and SAE1 expression plasmid (Figure 6C and 6D).
Figure 5.
miR-129-1(2)-3p represses GC cell proliferation and induces more cell apoptosis. SGC-7901 and AGS cells were transfected with miR-129-1(2)-3p for 48 hours. The cell viability was determined using MTS assay kit (A); the apoptotic cell number was quantified using flow cytometry after PI and FITC-Annexin V staining (B). For the rescue experiment, SGC-7901 and AGS cells were co-transfected with miR-129-1(2)-3p and SAE1 expression vector for 48 hours. The cell viability was determined using MTS assay kit (C); the apoptotic cell number was quantified using flow cytometry after PI and FITC-Annexin V staining (D).
Figure 6.
miR-129-1(2)-3p represses GC cells migration and invasion by targeting SAE1. SGC-7901 and AGS cells were transfected with miR-129-1(2)-3p for 48 hours. The cell migration (A) and invasion (B) capacity were examined by transwell assay. For the rescue experiment, SGC-7901 and AGS cells were co-transfected with miR-129-1(2)-3p and SAE1 expression vector for 48 hours (C, D). Results were analyzed by Student’s t-test.
Discussion
miRNA is a group of short non-coding RNAs modulating gene expression through interaction with the 3’UTR of a target gene. Peripheral blood miRNAs are mostly protected by exosomes and other extracellular vesicles, which are stable and can be used as a biomarker for clinical diagnosis [9,10]. In this study, we first examined the expression of miR-129-1(2)-3p in the GC tumor and normal control samples and confirmed the downregulation of miR-129-1(2)-3p in the GC tumors. Subsequently, we found reduced miR-129-1(2)-3p level in the plasma exosomes from patients with GC when compared with healthy controls. These data indicated that miR-129-1(2)-3p can be used as a biomarker for GC diagnosis.
Post-translational modification by SUMOs is very important in the DNA damage response (DDR), with various DNA repair factors being SUMOylated in response to DNA damage [11]. XRCC4, one of the key regulators of NHEJ pathway, was confirmed to be sumoylated in eukaryotic cells, and the SUMOs modification is necessary for XRCC4 nuclear localization [8]. In this study, we confirmed that SAE1, the SUMO activating enzyme, is a direct target of miR-129-1(2)-3p. Meanwhile, miR-129-1(2)-3p overexpression induced more DNA damage which directly leads to cell death, suggesting that miR-129-1(2)-3p is a potential drug for GC treatment.
In conclusion, we constructed the regulatory relationship between miR-129-1(2)-3p and SAE1 level in GC cells, unveiling part of the miR-129-1(2)-3p function during the pathogenesis of GC. These findings may give insight into understandingGC pathogenesis and provide new biomarkers for clinical diagnosis.
Acknowledgements
This study is supported by the general research project of Qingdao Municipal Health and Family Planning Commission and the project No.: 17-3-3-35-nsh.
Disclosure of conflict of interest
None.
References
- 1.Uckelmann M, Sixma TK. Histone ubiquitination in the DNA damage response. DNA Repair (Amst) 2017;56:92–101. doi: 10.1016/j.dnarep.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Morris JR, Garvin AJ. Sumo in the DNA double-stranded break response: similarities, differences, and cooperation with ubiquitin. J Mol Biol. 2017;429:3376–3387. doi: 10.1016/j.jmb.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Himmels SF, Sartori AA. Controlling DNA-end resection: an emerging task for ubiquitin and sumo. Front Genet. 2016;7:152. doi: 10.3389/fgene.2016.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao NB, He YF, Li XQ, Wang K, Wang RL. The role of mirna and lncrna in gastric cancer. Oncotarget. 2017;8:81572–81582. doi: 10.18632/oncotarget.19197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuzaki J, Ochiya T. Circulating micrornas and extracellular vesicles as potential cancer biomarkers: a systematic review. Int J Clin Oncol. 2017;22:413–420. doi: 10.1007/s10147-017-1104-3. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Z, Wang H, Li Y, Hou Z, Ma N, Chen W, Zong Z, Chen S. Mir-129-5p is down-regulated and involved in migration and invasion of gastric cancer cells by targeting interleukin-8. Neoplasma. 2016;63:673–680. doi: 10.4149/neo_2016_503. [DOI] [PubMed] [Google Scholar]
- 7.Yu X, Song H, Xia T, Han S, Xiao B, Luo L, Xi Y, Guo J. Growth inhibitory effects of three mir-129 family members on gastric cancer. Gene. 2013;532:87–93. doi: 10.1016/j.gene.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 8.Yurchenko V, Xue Z, Sadofsky MJ. Sumo modification of human xrcc4 regulates its localization and function in DNA double-strand break repair. Mol Cell Biol. 2016;26:1786–1794. doi: 10.1128/MCB.26.5.1786-1794.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salehi M, Sharifi M. Exosomal mirnas as novel cancer biomarkers: challenges and opportunities. J Cell Physiol. 2018;233:6370–6380. doi: 10.1002/jcp.26481. [DOI] [PubMed] [Google Scholar]
- 10.Xu Z, Yan Y, Zeng S, Dai S, Chen X, Wei J, Gong Z. Circular rnas: clinical relevance in cancer. Oncotarget. 2018;9:1444–1460. doi: 10.18632/oncotarget.22846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cipolla L, Maffia A, Bertoletti F, Sabbioneda S. The regulation of DNA damage tolerance by ubiquitin and ubiquitin-like modifiers. Front Genet. 2016;7:105. doi: 10.3389/fgene.2016.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]