Abstract
Triple-negative breast cancer (TNBC) is associated with epithelial-mesenchymal transition (EMT) and the phenotype of breast cancer stem cells (CSCs). Vasculogenic mimicry (VM) is a novel pattern of tumor blood supply and associated with aggression and metastasis of TNBC. Previous studies have shown that both CSCs and EMT are associated with VM, although the underlying mechanism is yet unclear. The present study aimed to analyze the immunohistochemical (IHC) expression of CSC marker, epithelial cell adhesion molecule (EpCAM), EMT-related markers, including transcription factors (TFs) (Slug, Twist1, and ZEB1), and EMT markers (E-cadherin and vimentin) in 137 TNBC. The expression of these markers was correlated to the clinicopathological features and VM channels of the tumors, including patient overall survival (OS) and disease-free survival (DFS). Furthermore, the expression of EpCAM and EMT-related markers showed a positive correlation with distant metastasis and lymph node metastasis (P < 0.05). A significant association was noted between VM and histological grade (P = 0.007). Moreover, VM showed a significant positive correlation with EpCAM, EMT-associated TFs, and VE-cadherin expression in TNBC. Furthermore, binary logistic analysis showed that VM expression was significantly correlated with lymph node metastasis and distant metastasis (P < 0.05). In survival analysis, the overexpression of EpCAM and ZEB1 predicted a poor prognosis with respect to OS and DFS. In addition, the presence of VM was significantly associated with poor OS and DFS. Multivariate Cox regression analysis revealed that VM expression is an independent prognostic factor for TNBC patients. In summary, VM was confirmed as a potential biomarker for TNBC associated with poor clinical outcomes and tumor metastasis. This study also suggested that EpCAM protein might be involved in VM formation by EMT in TNBC.
Keywords: TNBC, EpCAM, EMT, VM, prognosis, metastasis
Introduction
Breast cancer is the most commonly diagnosed cancer among women and the leading cause of cancer-related deaths in women < 45-year-old in China [1]. Triple-negative breast cancer (TNBC) accounts for approximately 10-15% of all breast cancers that lack estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (Her-2) [2]. Compared to other breast cancers, TNBC has a high incidence among young women; the recurrence and metastasis of cancer have been crucial factors for limited prognosis [3]. Thus, evaluating the mechanism underlying the metastasis is essential for novel treatments of TNBC as well as improving the patient outcome.
Cancer stem cells (CSC) constitute a subpopulation of tumor cells with high self-renewal and tumorigenic ability, suggesting that CSCs might be partially involved in the aggressive behavior of malignant tumors [4]. Notably, the recurrence and metastasis of breast cancer are also based on a limited subpopulation of cells, defined as “breast cancer stem cells” (BCSCs) [5]. Recently, epithelial cell adhesion molecule (EpCAM) has been found to have tumorigenic potential and identified as a CSC marker in several malignancies, including breast cancer [6,7]. EpCAM or CD326 is a 40-kDa type I transmembrane glycoprotein with an extracellular domain, one transmembrane domain, and a cytoplasmic domain consisting of 26 amino acids [8]. In addition, EpCAM is involved in biological processes, such as cell proliferation, migration, adhesion, and cell signaling, as well as in tumor progression [8]. More than 90% of breast cancers express EpCAM protein, indicating that it promotes the proliferation, migration, and invasion of breast cancer [9,10].
Epithelial-mesenchymal transition (EMT) is a physiological mechanism that imparts a mesenchymal phenotype to the epithelial cells, which allows them to detach from primary tumors and metastasize to distant sites [11,12]. EMT can be regulated by a variety of transcription factors (TFs), including Twist, Snail, Slug, and zinc-finger E-box binding homeobox 1 (ZEB1) by binding to E-cadherin promoter and inhibiting its expression, thereby enhancing the invasion capacity of carcinoma cells [12]. Slug, Twist1, and ZEB1 proteins were highly expressed in malignant tumors, as well as closely related to the progression of TNBC [13-15].
Vasculogenic mimicry (VM) was first reported in 1999; it is a new vascularization of highly aggressive melanoma [16]. VM channels are composed of tumor cells without the presence of endothelial cells [16]. Accumulating evidence shows that VM occurs in several aggressive tumors such as melanoma [17], hepatocellular carcinoma [18], and breast carcinoma [19]. It is closely associated with poor prognosis, low differentiation, high clinical stage, and increased metastasis rate of tumors [20]. Moreover, VM is a specific method of blood supply to the tumors independent of endothelial cells and plays a vital role in the metastasis and recurrence of breast cancer [19]. However, only a few studies in TNBC have been conducted with respect to the phenotype, and the mechanism is yet unclear.
Especially, CSC and EMT are considered as the major factors in the correlation between VM and tumor metastasis [17,21,22]; however, the association with TNBC is yet to be elucidated. The present study aimed to observe the distribution characteristics of CSC surface marker EpCAM in TNBC and the correlation with EMT, to assess the influence of EpCAM on VM formation, and to provide a novel direction for investigating the metastasis and prognosis of TNBC.
Material and methods
Patients and tissue samples
All 137 TNBC tissue samples were collected from the Department of Pathology at the First Hospital Affiliated to Bengbu Medical College, China from January 2006 to December 2011. All patients had complete clinical, pathological, and follow-up data, and no distant metastasis prior to surgery. We excluded patients who received preoperative chemotherapy, radiotherapy, targeted therapy, or endocrine treatment. The age of the patients ranged from 26-77 (median age, 55.3 years). The overall survival (OS) was calculated from surgery to death, and the data from patients who died from disease unrelated to TNBC, accident, and those lost to follow-up in December 2016 were excluded (median survival: 57 months; range 6-132 months). Disease-free survival (DFS) was calculated from diagnosis to a regional recurrence or distant metastasis. The histological grade was defined according to the World Health Organization (WHO) Classification of Breast Tumors, 4th Edition (2012) [23]. Other clinicopathological characteristics are listed in Table 1. The present study was approved by the Ethics Committee of the First Hospital Affiliated of Bengbu Medical College and conducted in accordance with the ethical guidelines of the Declaration of Helsinki.
Table 1.
Patient characteristics
| Parameter | Frequency (n) | Percentage (%) |
|---|---|---|
| Age at diagnosis, years | ||
| ≤ 50 | 61 | 44.2 |
| > 50 | 77 | 55.8 |
| Menopausal status | ||
| Premenopausal | 50 | 36.2 |
| Postmenopausal | 88 | 63.8 |
| Tumor size, cm | ||
| ≤ 2 | 43 | 31.2 |
| 2-5 | 84 | 60.9 |
| > 5 | 11 | 8 |
| Histopathology | ||
| Intraductal carcinoma | 1 | 0.7 |
| Invasive ductal carcinoma | 101 | 73.2 |
| Invasive lobular carcinoma | 9 | 6.5 |
| Other types | 27 | 19.6 |
| Histological grade | ||
| I | 3 | 2.2 |
| II | 102 | 73.9 |
| III | 33 | 23.9 |
| Lymph node status | ||
| Positive | 41 | 29.7 |
| Negative | 97 | 70.3 |
| Metastasis | ||
| Absent | 123 | 89.1 |
| Present | 15 | 10.9 |
| Type of surgery | ||
| Breast-conserving surgery | 6 | 4.3 |
| Simple mastectomy | 27 | 19.6 |
| Modifed radical mastectomy | 105 | 76.1 |
| Adjuvant chemotherapy | ||
| Yes | 122 | 88.4 |
| No | 16 | 11.6 |
Immunohistochemistry (IHC) analysis
All specimens were fixed in 10% buffered formalin, embedded in paraffin, and sectioned into 4-μm-thickness slices, which were then deparaffinized and rehydrated with xylene and graded alcohol. Subsequently, the sections were washed in phosphate-buffered saline (PBS, pH 7.2) for 10 min. The endogenous peroxidase activity of the sections was blocked by incubation in 3% H2O2 at room temperature for 10 min and heated to 95°C for 30 min for antigen retrieval. After washing in PBS three times, the sections were blocked in goat serum and incubated with EpCAM (ab71916, 1:50, Abcam), Slug (sc-166476, 1:50, Santa Cruz Biotechnology), Twist1 (sc-15393, 1:200, Santa Cruz Biotechnology), ZEB1 (ab203829, 1:100, Abcam), VE-cadherin (ab232880, 1:50, Abcam) and E-cadherin, Vimentin, CD31 (ready-to-use, MXB Biotechnologies) primary antibodies at 4°C overnight. Subsequently, the slides were incubated with polymer enhancer (reagent A), goat anti-mouse antibody (reagent B) and developed using freshly prepared 3,3’-diaminobenzidine (DAB) substrate. Finally, the sections were counterstained with hematoxylin, dehydrated, air-dried, and mounted. Next, the specimens were subjected to Periodic Acid-Schiff (PAS)-CD31 dual staining to identify the vascular endothelial cells in the glycosylated basement membranes, as well as vascular-like (VM) structures. PBS was used as a negative control, and the known corresponding protein served as the positive control. The sections were observed using a microscope (BX5, Olympus).
Evaluation of immunostaining
All slides were evaluated by two experienced pathologists, blinded to the clinical data or the disease outcome. The immunostaining was determined in 10 fields (100× magnification) for each slide. EpCAM and E-cadherin were localized to the membrane [13,25]. The expression of Vimentin and VE-cadherin was detected in the tumor cell cytoplasm [13]. The expression of the EMT-associated TFs, Slug, Twist1, and ZEB1 was detected on the nucleus [13,14]. The extent and intensity of the expression of EpCAM, EMT-related markers, and VE-cad-herin were evaluated in the carcinoma-involved region. The intensity of the staining was divided into four grades: 0, none; 1, weak; 2, moderate; 3, strong. The extent of staining was also divided into five categories: 0, ≤ 5%; 1, 6-25%; 2, 26-50%; 3, 51-75%; 4, 76-100%. Finally, we determined the score by multiplying the intensity and the extent of staining to produce a range of immunostaining scores from 0-12. The immunostaining was considered positive when scores were ≥ 3.
Statistical analysis
Statistical analysis was performed using SPSS 22.0 software. Fisher’s exact or Pearson Chi-square test was used to analyze the correlation between protein expression and the clinicopathological indices. The correlations between the expressions of these factors were evaluated by Spearman’s correlation analysis. Binary logistic analysis was used to clarify the relative factors for lymph node metastasis and distant metastasis. The univariate survival analysis of OS and DFS was based on the Kaplan-Meier method with log-rank tests. A multivariate Cox regression model was used to analyze the influence of various factors on OS and DFS. The hazard ratio (HR) and 95% confidence intervals (CI) were used for analysis. P < 0.05 was considered to indicate statistical significance.
Results
Expression of EpCAM, EMT-related markers, and VM in TNBC
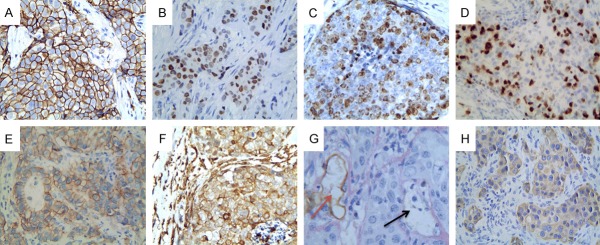
In the present study, EpCAM protein was expressed positively in 63.0% of TNBCs (Figure 1A). EMT-related markers, EMT-associated TFs, Slug, Twist1, and ZEB1 were expressed in 23.9%, 39.1%, and 20.3% of TNBCs, respectively (Figure 1B-D). E-cadherin loss was detected in 70.4% (Figure 1E). On the other hand, Vimentin protein was positively expressed in 36.2% of TNBCs (Figure 1F), VM was expressed positively in 30.4% of TNBCs (Figure 1G), and VE-cadherin, a VM-associated molecule, was positively expressed in 57.8% of TNBCs (Figure 1H).
Figure 1.
Expression of the proteins in TNBC (×400 magnification). (A) Representative case that shows positive staining for EpCAM (A), Slug (B), Twist1 (C), ZEB1 (D), E-cadherin (E) Vimentin (F) and VE-cadherin (H); (G) Positive staining of VM in the TNBC tissue (black arrow is VM structure, red arrow is vessel).
Correlation of EpCAM, EMT-related markers, VM expression and clinicopathological characteristics in TNBC patients
No correlation was established between the expression of EpCAM, EMT-related markers and age, menopausal status, tumor size, and histological grade (P > 0.05). The expression of VM was associated with the histological grade (P = 0.007). The expression of EpCAM, EMT-related markers, such as Slug, ZEB1, and VM showed a positive correlation between distant metastasis and lymph node metastasis (P < 0.05) (Table 2).
Table 2.
The relationship between expression of EpCAM, Slug, Twist1, ZEB1 and VM and clinicopathogical characteristics of TNBC
| Variable | EpCAM expression | P | Slug expression | P | Twist1 expression | P | ZEB1 expression | P | VM expression | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||||||
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | ||||||
| Age at diagnosis, years | |||||||||||||||
| ≤ 50 | 36 | 25 | 0.383 | 17 | 44 | 0.332 | 23 | 38 | 0.76 | 11 | 50 | 0.557 | 22 | 39 | 0.201 |
| > 50 | 51 | 26 | 16 | 61 | 31 | 46 | 17 | 60 | 20 | 57 | |||||
| Menopausal status | |||||||||||||||
| Premenopausal | 34 | 16 | 0.363 | 11 | 39 | 0.691 | 20 | 30 | 0.875 | 10 | 40 | 0.949 | 15 | 35 | 0.933 |
| Postmenopausal | 53 | 35 | 22 | 66 | 34 | 54 | 18 | 70 | 27 | 61 | |||||
| Tumor size, cm | |||||||||||||||
| ≤ 2 | 29 | 14 | 0.536 | 12 | 33 | 0.814 | 18 | 27 | 0.878 | 9 | 36 | 0.375 | 16 | 27 | 0.401 |
| 2-5 | 50 | 34 | 19 | 63 | 31 | 51 | 15 | 67 | 22 | 62 | |||||
| > 5 | 8 | 3 | 2 | 9 | 5 | 6 | 4 | 7 | 4 | 7 | |||||
| Histological grade | |||||||||||||||
| I | 2 | 1 | 0.510 | 1 | 2 | 0.796 | 0 | 3 | 0.284 | 1 | 2 | 0.203 | 0 | 3 | 0.007 |
| II | 67 | 35 | 23 | 79 | 39 | 63 | 17 | 85 | 25 | 77 | |||||
| III | 18 | 15 | 9 | 24 | 15 | 18 | 10 | 23 | 17 | 16 | |||||
| Lymph node metastasis | |||||||||||||||
| Positive | 35 | 6 | < 0.001 | 17 | 25 | 0.007 | 20 | 21 | 0.131 | 14 | 27 | 0.009 | 23 | 18 | < 0.001 |
| Negative | 52 | 45 | 16 | 80 | 34 | 63 | 14 | 83 | 19 | 78 | |||||
| Distant metastasis | |||||||||||||||
| Positive | 14 | 1 | 0.01 | 7 | 8 | 0.029 | 9 | 6 | 0.079 | 7 | 8 | 0.007 | 13 | 2 | < 0.001 |
| Negative | 73 | 50 | 26 | 97 | 45 | 78 | 21 | 102 | 29 | 94 | |||||
Correlations among EpCAM, EMT-related markers, and VM in TNBC
A negative correlation was established between E-cadherin expression and expression of EpCAM, EMT-associated TFs Slug, Twist1, and ZEB1, and VM (r = -0.432, P < 0.001; r = -0.379, P < 0.001; r = -0.291, P = 0.001; r = -0.263, P < 0.001) (Table 3). The correlation analysis revealed that EpCAM was positively associated with EMT-related markers and VM expression (P < 0.05) (Table 3). The presence of VM showed a significantly positive correlation with EpCAM, EMT-associated TFs Slug, Twist1, and ZEB1, and VE-cadherin expression in TNBC (r = 0.213, P = 0.012; r = 0.257, P = 0.002; r = 0.212, P = 0.013; r = 0.254, P = 0.003; r = 0.235, P = 0.006) (Table 3).
Table 3.
Correlation between expression of EpCAM, EMT-related markers, and VM in TNBC
| Variables | EpCAM | Slug | Twist | ZEB1 | VM | E-cadherin | VE-cadherin | Vimentin | |
|---|---|---|---|---|---|---|---|---|---|
| EpCAM | r | 1 | 0.183 | 0.337 | 0.088 | 0.213 | -0.432 | 0.272 | 0.483 |
| p | - | 0.032 | < 0.001 | 0.307 | 0.012 | < 0.001 | 0.001 | < 0.001 | |
| Slug | r | 1 | 0.107 | 0.140 | 0.257 | -0.379 | 0.263 | 0.320 | |
| p | - | 0.210 | 0.103 | 0.002 | < 0.001 | 0.002 | < 0.001 | ||
| Twist1 | r | 1 | 0.002 | 0.212 | -0.291 | 0.160 | 0.199 | ||
| p | - | 0.985 | 0.013 | 0.001 | 0.061 | 0.019 | |||
| ZEB1 | r | 1 | 0.254 | -0.263 | 0.057 | 0.107 | |||
| p | - | 0.003 | 0.002 | 0.505 | 0.212 | ||||
| VM | r | 1 | -0.190 | 0.235 | 0.321 | ||||
| p | - | 0.025 | 0.006 | < 0.001 | |||||
| E-cadherin | r | 1 | -0.191 | -0.434 | |||||
| p | - | 0.025 | < 0.001 | ||||||
| VE-cadherin | r | 1 | 0.296 | ||||||
| p | - | < 0.001 | |||||||
| Vimentin | r | 1 | |||||||
| p | - |
Metastasis analysis
Binary logistic analysis showed that VM and E-cadherin expression correlated significantly with lymph node metastasis (P < 0.05) (Table 4). In addition, VM, E-cadherin expression, and tumor size were significantly associated with distant metastasis (P < 0.05) (Table 4).
Table 4.
Binary logistic analysis of factors affecting lymph node metastasis and distant metastasis
| Variables | HR | 95% CI | P |
|---|---|---|---|
| Lymph node metastasis | |||
| VM | 3.972 | 1.680-9.393 | 0.002 |
| E-cadherin | 0.287 | 0.121-0.685 | 0.005 |
| Distant metastasis | |||
| Tumor size | 0.265 | 0.080-0.872 | 0.029 |
| VM | 22.139 | 4.368-112.209 | < 0.001 |
| E-cadherin | 0.247 | 0.066-0.928 | 0.038 |
Survival analysis
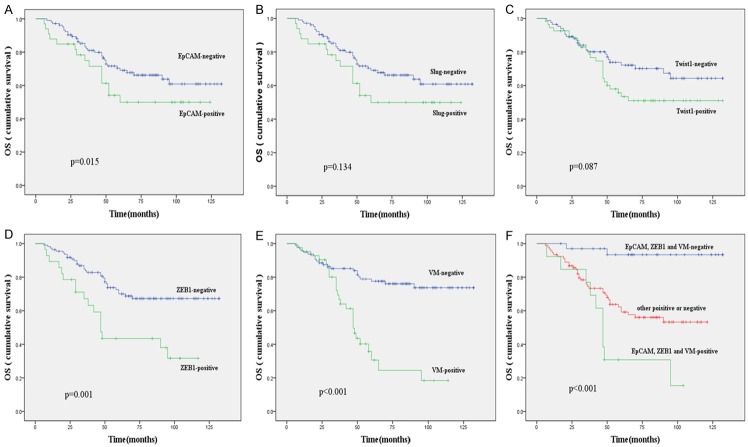
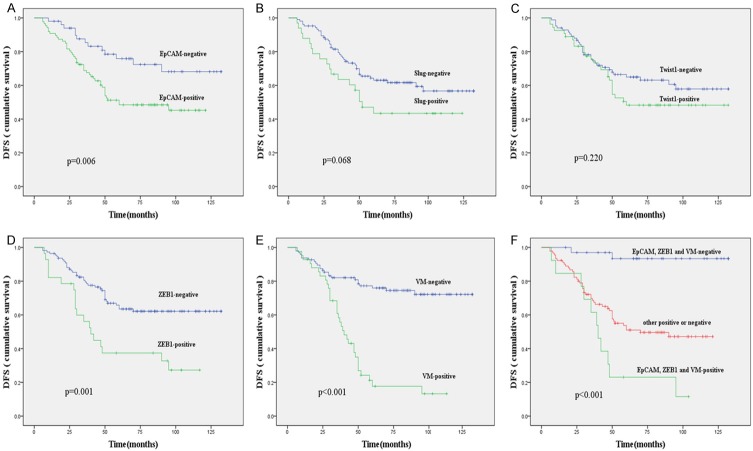
In the univariate analysis, OS and DFS were significantly correlated with the clinicopathological factors, including histological grade (P = 0.005, P = 0.002, respectively), lymph node metastasis (P < 0.001, P < 0.001, respectively), and distant metastasis (P < 0.001, P < 0.001, respectively) (Table 5). The overexpression of EpCAM predicted a poor prognosis with respect to OS and DFS (P = 0.015, P = 0.006 respectively); (Figures 2A and 3A). The expression of ZEB1 was associated with poor OS and DFS of the patients (Figures 2D and 3D) and no survival differences were observed with respect to the expression of other EMT-associated TFs: Slug and Twist1 (Figures 2B, 2C, 3B, 3C). In addition, the presence of VM was significantly associated with the OS and DFS (Figures 2E and 3E). On the other hand, the combination of positive expression of EpCAM, ZEB1, and the presence of VM showed a poorer prognosis as compared to the contrary combination (Figures 2F and 3F). When a multivariate analysis including age, menopausal status, tumor size, histological grade, lymph node metastasis, distant metastasis, and expression of EpCAM, EMT-related markers, VE-cadherin, and VM and the presence of VM (P = 0.005; P = 0.008) and lymph node metastasis (P = 0.024; P = 0.026) remained as independent prognostic factors of OS and DFS (Table 6).
Table 5.
Univariate regression model of prognostic covariates in TNBC patients
| Biomarkers | OS | DFS | ||
|---|---|---|---|---|
|
|
|
|||
| X2 | P | X2 | P | |
| Age | 0.825 | 0.364 | 0.435 | 0.509 |
| Menopausal | 0.009 | 0.924 | 0.156 | 0.693 |
| Size | 0.355 | 0.837 | 1.690 | 0.430 |
| Grade | 10.729 | 0.005 | 12.485 | 0.002 |
| Lymph node metastasis | 21.305 | < 0.001 | 33.04 | < 0.001 |
| Metastasis | 16.980 | < 0.001 | 44.727 | < 0.001 |
| EpCAM | 5.872 | 0.015 | 7.441 | 0.006 |
| Slug | 2.248 | 0.134 | 3.341 | 0.068 |
| Twist | 2.933 | 0.087 | 1.503 | 0.220 |
| ZEB1 | 11.714 | 0.001 | 11.622 | 0.001 |
| VM | 24.036 | < 0.001 | 37.396 | < 0.001 |
| E-cadherin | 7.784 | 0.005 | 3.799 | 0.051 |
| VE-cadherin | 1.225 | 0.268 | 2.362 | 0.124 |
| Vimentin | 6.084 | 0.014 | 7.075 | 0.008 |
Figure 2.
Kaplan-Meier survival plots for overall survival in patients with TNBC. EpCAM (A), Slug expression (B), Twist1 expression (C), ZEB1 expression (D) and VM expression (E); (F) Comprehensive analysis of EpCAM, ZEB1 and VM.
Figure 3.
Kaplan-Meier survival plots for disease-free survival in patients with TNBC. EpCAM (A), Slug expression (B), Twist1 expression (C), ZEB1 expression (D) and VM expression (E); (F) Comprehensive analysis of EpCAM, ZEB1 and VM.
Table 6.
Results of multivariate analyses of OS and DFS time
| Characteristics | Outcome | HR | 95% CI | P |
|---|---|---|---|---|
| Lymph node metastasis | OS | 2.154 | 1.105-4.201 | 0.024 |
| DFS | 2.066 | 1.091-3.913 | 0.026 | |
| VM | OS | 2.667 | 1.352-5.259 | 0.005 |
| DFS | 2.513 | 1.277-4.947 | 0.008 | |
| Metastasis | DFS | 2.342 | 1.151-4.766 | 0.019 |
Discussion
Triple-negative breast cancer (TNBC) is a highly invasive tumor that is more prone to metastasis and recurrence than other types of breast cancers. Based on the poor prognosis and the lack of effective chemotherapy at present, new targeted therapy and biomarkers are an urgent requirement to provide alternatives for treating TNBC patients [24]. Previous studies have shown that cancer stem cells (CSCs) and EMT play a major role in angiogenesis, chemoresistance, invasion, and metastasis of malignant tumors. VM is a specific type of tumor angiogenesis that does not depend on endothelial cells. In addition, a large number of studies suggested that it is closely related to tumor metastasis, albeit the specific mechanism is yet unclear. The present study was undertaken to detect the expression of proteins using CSCs-labeled antibodies, EpCAM and EMT-related TFs, Slug, Twist1, and ZEB1. Moreover, we analyzed the association between CSC proteins, EpCAM, VM and clinicopathological features of TNBC patients. Furthermore, we also evaluated the significance of VM in TNBC metastasis and prognosis.
EpCAM is highly expressed in a variety of epithelial malignancies. It also participates in the proliferation of tumor cells and is vital for maintaining the pluripotency of cancer stem cells [8]. The results of this study showed that EpCAM was highly expressed in TNBC (63%). Soysal et al. [26] reported that the expression rate of EpCAM in 1365 breast cancers was 48% and that in TNBC was 63.8%. In addition, EpCAM was positively correlated with TNBC lymph node metastasis and distant metastasis (Table 2). The study by Maqsoud et al. [25] demonstrated that EpCAM is highly expressed in metastatic lymph nodes of the matched lymph node metastasis, which was in agreement with the current conclusion. Another study found that in TNBC-derived MDA-MB-231 cell line, the expression of phosphoinositide-dependent phospholipase C (PLC-β2) was upregulated and the expression of EpCAM protein and the quality of tumor invasiveness was decreased [7]. Thus, it can be concluded that EpCAM plays a major role in the invasion and metastasis of TNBC.
EMT is a critical mechanism for tumor invasion and metastasis that influences various stages of tumorigenesis and progression. Also, it increases the invasiveness of tumor cells by upregulating the expression of TFs, such as Snail family, Twist family, and ZEB (zinc-finger E-box binding homeobox) family-related proteins with decreased expression of E-cadherin and other cell adhesion molecules [12]. In this study, Spearman’s analysis revealed that the expression of Slug, Twist1, and ZEB1 proteins was negatively correlated with E-cadherin protein expression and positively correlated with Vimentin protein expression (Table 3), suggesting that EMT might exist during the development of TBNC [13]. In addition, Slug, ZEB1, and TNBC were positively correlated with lymph node metastasis and distant metastasis (Table). In the study by Ferrari et al. [27], the expression of Slug in the TNBC cell lines MDA-MB-231 and BT-549 was silenced. As a result, the invasive ability of the tumor decreased, especially the ability to transfer to the bone. Liang et al. [28] silenced the expression of ZEB1 by shRNA technology in the MDA-MB-231 cell line, which in turn, increased the expression of E-cadherin, indicating that the invasion ability of the tumor cell decreased. In addition, the conclusion from the study by Jang et al. [14] was consistent with the results of the present study (Table 5; Figures 2D and 3D) as assessed by IHC. Moreover, ZEB1 was found to be highly expressed in the specific subtype of TNBC and positively correlated with the prognosis of patients, rendering it as a potential prognostic factor.
Blood supply is essential for the growth and invasion of tumors. In addition to the vascular system formed by endothelial cells, the formation of the VM model by tumor cells mimics the endothelial cells, which plays a critical role in tumor progression [29]. In this study, the expression rate of VM in TNBC was found to be 30.4%, which was consistent with that reported previously [30,31]. The current study found that VM was positively correlated with TNBC grading, lymph node metastasis, and distant metastasis (Table 2). On the other hand, binary logistic analysis of lymph node metastasis and distant metastasis found that VM is related to metastasis (Table 4). The above studies suggested that VM plays a major role in the progression of TNBC, especially tumor metastasis; however, the relevant mechanisms are yet unclear. This study also demonstrated that VM was positively correlated with EMT TFs, Slug, Twist1, ZEB1, and Vim, and negatively correlated with E-cadherin expression (Table 3). Consecutively, we also found that the expression of the VM marker protein VE-cadherin [32] was positively correlated with the expression of EMT TF (Table 3). Previous studies also demonstrated a vital role of EMT in the formation of VM [21,22]. In addition, we showed that VM and VE-cadherin are positively correlated with the expression of EpCAM (Table 3). Although only a few studies have addressed EpCAM and VM, recent studies suggested that CSCs might induce VM in malignant tumors [30]. For example, Zhang et al. [33] found that CSC marker proteins, CD133 and CD44, are associated with VM in renal cell carcinoma. Liu et al. [34] demonstrated that VM was positively correlated with CSCs markers c-Myc and Sox2 in breast cancer MDA-MB-231 cell line using 3D culture techniques. Herein, VM, EpCAM, and EMT-related TFs were associated with the progression of TNBC and also associated with each other. In addition, binary logistic analysis revealed that VM was associated with lymph node and distant metastasis (Table 4). Multivariate survival analysis found that only VM and tumor metastasis were independent prognostic factors, suggesting that EMT and EpCAM might be involved in the phasing of tumorigenesis (Table 6). Zhang et al. [31] found that Twist1 is the EMT TF that might promote the formation of VM by inducing CSCs. The authors induced the expression of Twist1 after the application of Sunitinib in the TNBC MCF-7 cell line, resulting in increased expression of CD133 that promoted the formation of VM. In summary, EMT, EpCAM, and VM are involved in tumor progression in TNBC, and EMT might promote the formation of VM by inducing the expression of EpCAM.
In this study, Kaplan-Meier survival analysis noted a positive correlation between EpCAM and TNBC prognosis [26]. Also, EMT-related markers such as Slug, ZEB, E-cadherin, and Vim are associated with the prognosis in patients with TNBC, which is consistent with the results of Zhou et al. [13] and Jang et al. [14] in TNBC analysis. Some studies demonstrated that patients with the expression of VM have a poor prognosis [35]. Comprehensive analysis showed that the survival time of patients with positive expression of EpCAM, ZEB1, and VM in tumor tissues was significantly lower than the negative expression (Figures 2F and 3F). Therefore, EpCAM, EMT, and VM might participate in the progression of tumors and are closely related to the prognosis of patients.
The current studies illustrate that the conventional anti-angiogenic treatment of tumors destroys the development of blood vessels formed by endothelial cells, causing anoxic environment and thus, inhibiting the tumor growth [36]. However, some studies found that hypoxia in TNBC could further stimulate the formation of VM by compensating for tumor blood supply and accelerating the tumor growth and metastasis [24,37]. Therefore, exploring the mechanism of VM invasion and metastasis in TNBC is crucial. Thus, the present study explored the correlation between EMT, CSC, and VM and during the process of tumor development. Nevertheless, further studies require large number of specimens for a molecular level investigation to substantiate the current findings. It is essential to explore the interactions among the molecular crosstalk pathways including CSC, EMT, and VM, to identify key pivotal proteins, and to provide evidence for targeted drug therapy.
Conclusions
In summary, the current data imply that CSC marker EpCAM and EMT-related marker ZEB1 are associated with poor prognosis in TNBC. VM can serve as a potential biomarker for predicting the metastasis and prognosis of TNBC. Moreover, a correlation among EMT, VM, and EpCAM was identified, and EMT might regulate the VM formation by mediating the EpCAM expression in TNBC.
Acknowledgements
This work was supported by the Nature Science Key Program of College and University of Anhui Province (No. KJ2018A0213) and the Science and Technology Funds of Bengbu Medical College (No. BYKF1707).
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Abramson VG, Lehmann BD, Ballinger TJ, Pietenpol JA. Subtyping of triple-negative breast cancer: implications for therapy. Cancer. 2015;121:8–16. doi: 10.1002/cncr.28914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 4.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 5.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, Brown M, Viens P, Xerri L, Bertucci F, Stassi G, Dontu G, Birnbaum D, Wicha MS. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–13. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.González B, Denzel S, Mack B, Conrad M, Gires O. EpCAM is involved in maintenance of the murine embryonic stem cell phenotype. Stem Cells. 2009;27:1782–91. doi: 10.1002/stem.97. [DOI] [PubMed] [Google Scholar]
- 7.Brugnoli F, Grassilli S, Lanuti P, Marchisio M, Al-Qassab Y, Vezzali F, Capitani S, Bertagnolo V. Up-modulation of PLC-β2 reduces the number and malignancy of triple-negative breast tumor cells with a CD133+/EpCAM+ phenotype: a promising target for preventing progression of TNBC. BMC Cancer. 2017;17:617. doi: 10.1186/s12885-017-3592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trzpis M, McLaughlin PM, de Leij LM, Harmsen MC. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol. 2007;171:386–95. doi: 10.2353/ajpath.2007.070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osta WA, Chen Y, Mikhitarian K, Mitas M, Salem M, Hannun YA, Cole DJ, Gillanders WE. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 2004;64:5818–24. doi: 10.1158/0008-5472.CAN-04-0754. [DOI] [PubMed] [Google Scholar]
- 10.Cimino A, Halushka M, Illei P, Wu X, Sukumar S, Argani P. Epithelial cell adhesion molecule (EpCAM) is overexpressed in breast cancer metastases. Breast Cancer Res Treat. 2010;123:701–8. doi: 10.1007/s10549-009-0671-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tania M, Khan MA, Fu J. Epithelial to mesenchymal transition inducing transcription factors and metastatic cancer. Tumour Biol. 2014;35:7335–42. doi: 10.1007/s13277-014-2163-y. [DOI] [PubMed] [Google Scholar]
- 12.Pal M, Bhattacharya S, Kalyan G, Hazra S. Cadherin profiling for therapeutic interventions in Epithelial Mesenchymal Transition (EMT) and tumorigenesis. Exp Cell Res. 2018;368:137–146. doi: 10.1016/j.yexcr.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Zhou S, Sun X, Yu L, Zhou R, Li A, Li M, Yang W. Differential expression and clinical significance of epithelial-mesenchymal transition markers among different histological types of triple-negative breast cancer. J Cancer. 2018;9:604–613. doi: 10.7150/jca.19190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang MH, Kim HJ, Kim EJ, Chung YR, Park SY. Expression of epithelial-mesenchymal transition-related markers in triple-negative breast cancer: ZEB1 as a potential biomarker for poor clinical outcome. Hum Pathol. 2015;46:1267–74. doi: 10.1016/j.humpath.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Martin TA, Goyal A, Watkins G, Jiang WG. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol. 2005;12:488–96. doi: 10.1245/ASO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–52. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W, Zhou P, Meng A, Zhang R, Zhou Y. Down-regulating Myoferlin inhibits the vasculogenic mimicry of melanoma via decreasing MMP-2 and inducing mesenchymal-to-epithelial transition. J Cell Mol Med. 2018;22:1743–1754. doi: 10.1111/jcmm.13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun B, Zhang S, Zhang D, Du J, Guo H, Zhao X, Zhang W, Hao X. Vasculogenic mimicry is associated with high tumor grade, invasion and metastasis, and short survival in patients with hepatocellular carcinoma. Oncol Rep. 2006;16:693–8. [PubMed] [Google Scholar]
- 19.Wagenblast E, Soto M, Gutiérrez-Ángel S, Hartl CA, Gable AL, Maceli AR, Erard N, Williams AM, Kim SY, Dickopf S, Harrell JC, Smith AD, Perou CM, Wilkinson JE, Hannon GJ, Knott SR. A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature. 2015;520:358–62. doi: 10.1038/nature14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Zhang D, Sun B. Vasculogenic mimicry: current status and future prospects. Cancer Lett. 2007;254:157–64. doi: 10.1016/j.canlet.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 21.Fan YL, Zheng M, Tang YL, Liang XH. A new perspective of vasculogenic mimicry: EMT and cancer stem cells (Review) Oncol Lett. 2013;6:1174–1180. doi: 10.3892/ol.2013.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q, Qiao L, Liang N, Xie J, Zhang J, Deng G, Luo H, Zhang J. The relationship between vasculogenic mimicry and epithelial-mesenchymal transitions. J Cell Mol Med. 2016;20:1761–9. doi: 10.1111/jcmm.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 24.Sun H, Zhang D, Yao Z, Lin X, Liu J, Gu Q, Dong X, Liu F, Wang Y, Yao N, Cheng S, Li L, Sun S. Anti-angiogenic treatment promotes triple-negative breast cancer invasion via vasculogenic mimicry. Cancer Biol Ther. 2017;18:205–213. doi: 10.1080/15384047.2017.1294288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abd El-Maqsoud NM, Abd El-Rehim DM. Clinicopathologic implications of EpCAM and Sox2 expression in breast cancer. Clin Breast Cancer. 2014;14:e1–9. doi: 10.1016/j.clbc.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Soysal SD, Muenst S, Barbie T, Fleming T, Gao F, Spizzo G, Oertli D, Viehl CT, Obermann EC, Gillanders WE. EpCAM expression varies significantly and is differentially associated with prognosis in the luminal B HER2(+), basal-like, and HER2 intrinsic subtypes of breast cancer. Br J Cancer. 2013;108:1480–7. doi: 10.1038/bjc.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrari-Amorotti G, Chiodoni C, Shen F, Cattelani S, Soliera AR, Manzotti G, Grisendi G, Dominici M, Rivasi F, Colombo MP, Fatatis A. Calabretta B8 suppression of invasion and metastasis of triple-negative breast cancer lines by pharmacological or genetic inhibition of slug activity. Neoplasia. 2014;16:1047–58. doi: 10.1016/j.neo.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang W, Song S, Xu Y, Li H, Liu H. Knockdown of ZEB1 suppressed the formation of vasculogenic mimicry and epithelial-mesenchymal transition in the human breast cancer cell line MDA-MB-231. Mol Med Rep. 2018;17:6711–6716. doi: 10.3892/mmr.2018.8677. [DOI] [PubMed] [Google Scholar]
- 29.Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156:361–81. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu TJ, Sun BC, Zhao XL, Zhao XM, Sun T, Gu Q, Yao Z, Dong XY, Zhao N, Liu N. CD133+ cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene. 2013;32:544–53. doi: 10.1038/onc.2012.85. [DOI] [PubMed] [Google Scholar]
- 31.Zhang D, Sun B, Zhao X, Ma Y, Ji R, Gu Q, Dong X, Li J, Liu F, Jia X, Leng X, Zhang C, Sun R, Chi J. Twist1 expression induced by sunitinib accelerates tumor cell vasculogenic mimicry by increasing the population of CD133+ cells in triple-negative breast cancer. Mol Cancer. 2014;13:207. doi: 10.1186/1476-4598-13-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirschmann DA, Seftor EA, Hardy KM, Seftor RE, Hendrix MJ. Molecular pathways: vasculogenic mimicry in tumor cells: diagnostic and therapeutic implications. Clin Cancer Res. 2012;18:2726–32. doi: 10.1158/1078-0432.CCR-11-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Sun B, Zhao X, Liu Z, Wang X, Yao X, Dong X, Chi J. Clinical significances and prognostic value of cancer stem-like cells markers and vasculogenic mimicry in renal cell carcinoma. J Surg Oncol. 2013;108:414–9. doi: 10.1002/jso.23402. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Sun B, Liu T, Zhao X, Wang X, Li Y, Meng J, Gu Q, Liu F, Dong X, Liu P, Sun R, Zhao N. Function of AURKA protein kinase in the formation of vasculogenic mimicry in triple-negative breast cancer stem cells. Onco Targets Ther. 2016;9:3473–84. doi: 10.2147/OTT.S93015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao Z, Bao M, Miele L, Sarkar FH, Wang Z, Zhou Q. Tumour vasculogenic mimicry is associated with poor prognosis of human cancer patients: a systemic review and meta-analysis. Eur J Cancer. 2013;49:3914–23. doi: 10.1016/j.ejca.2013.07.148. [DOI] [PubMed] [Google Scholar]
- 36.Cao Y, Arbiser J, D’Amato RJ, D’Amore PA, Ingber DE, Kerbel R, Klagsbrun M, Lim S, Moses MA, Zetter B, Dvorak H, Langer R. Forty-year journey of angiogenesis translational research. Sci Transl Med. 2011;3:114rv3. doi: 10.1126/scitranslmed.3003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]