Abstract
Gastric cancer is the fifth most lethal carcinoma in the world. Genetic and epigenetic factors transform the normal cells into malignant cells and lead to tumor development. MicroRNA (miRNA), a small non-coding RNA which functions in RNA silencing and post-transcriptional regulation of gene expression, is closely associated with cancer initiation and propagation, including stomach cancer. In this study, for the first time, we report miR-539-3P, as a tumor suppressor, was down-regulated in gastric cancer both in vivo and in vitro. In addition, dysfunction of miR-539-3P regulates gastric cancer cell proliferation and invasion. Bioinformatics analysis revealed CTBP1 is the direct target of miR-539-3P and high expression of CTBP1 faciliates the progression of gastric carcinoma through promoting the epithelial to mesenchymal transition (EMT). Overall, these results indicate that epigenetic regulation of CTBP1 through miR-539-3P is critical to gastric cancer and provide a new insight into gastric cancer diagnosis, treatment and prognosis.
Keywords: Gastric cancer, miR-539, CTBP1, malignant proliferation, invasion, epithetial to mesenchymal transition (EMT)
Introduction
Gastric cancer, accouting for 9% of cancer death globally, originates from gastric mucous membrane [1]. Epidemiologic data show that gastric cancer most affects men [2]. In China, 324439 people per year ultimately die from gastric cancer [3]. Many factors, including infections, smoking, diet and genetics are involved in gastric cancer development [4]. Especially, Helicobacter pylori infection is the leading risk factor in 80% of gastric cancer and cagA protein may responsible for the malignant effects of Helicobacter pylori on normal gastric epithelial cells. Clearance of Helicobacter pylori seemsto prevent gastric cancer [5]. Unfortunately, owing to ignorable symptoms, gastric cancer is commonly diagnosed at advanced stage. Surgery, chemotherapy, and radiation therapy are the main treatments [6,7]. Recent studies showed immunotherapy and gene therapy also improved the outcomes of gastric cancer treatment [8].
Of note, given the irreversible malignancy of gastric cancer at advanced stage, investigators have turned to early detection of gastric carcinoma. Diagnostic markers, such as several cytokines, antigens, virus and specific RNAs were all applicable to gastric cancer detection and treatments [9-11]. miRNAs [12], a small non-coding RNA molecule, are capable of reversibly interfering with the transcription and translation of the genes without altering DNA sequence, i.e., miRNA first cleave the mRNA strand into two pieces, and then destabilize the target mRNA by shortening its poly (A) tail and eventually cause athe deficiency of mRNAs translation [13]. In gastric cancer, miRNA also plays a pivotal role as either tumor suppressor or tumorigenesis enhancer., Oncogenic miRNA, such as miR-21 overexpression decreased the sensitivity of gastric cells to 5-fluorouridine and promoted gastric cancer malignancy [14,15]. In contrast, miR-188 and miR-144 showed inhibitory effects on gastric cancer cells through inhibiting cell proliferation, invasion, or other mechanisms [16].
Previous work reported miR-539 functions as a tumor suppressor in various cancers including breast cancer [17], lung cancer [18], colorectal cancer [19], hepatocellular carcinoma [20] and osteosarcoma [21]. However, the role of miR-539 in gastric cancer remains unclear. Therefore, in this study, we focus on whether miR-539-3P is involved in the gastric cancer, and the mechanism. Gastric tumor tissues were collected to examine the expression of miR-539-3P. Interestingly, functional assays revealed that miR-539-3P suppressed malignant proliferation and invasion of SGC-7901 cells through targeting to CTBP1, which is a human protein bound a PLDLS motif in the C-terminus of adenovirus E1A proteins and widely expressed from embryo to terminally differentiated cells [28]. Several papers reported that CTBP1 is associated with cancer [29]. Also, morphologic change after transfection of miR-539-mimics or miR-539-inhibitor prompted us to study the role of EMT in gastric cancer. These results suggest that miR-539-3P could be a novel marker for gastric cancer diagnosis as well as provide a new druggable locus for translational research and clinical treatment.
Materials and methods
Patients and tissue samples
Gastric cancer tissues and the paired normal tissues from 30 advanced stage patients were collected at the Tongren Hospital. We immediately froze all samples in liquid nitrogen and stored at -80°C. All these patients received no radiotherapy before surgery. In addition, this project had permission of the patients and was approved by the ethics committee of Tongren Hospital. All procedures performed in the studies involved human participants.
Cell culture
Human gastric cancer cells, (SGC-7901 ,100674) and normal gastric epithelial cells (GES-1, 28201), were purchased from the Bena Culture Collection Co., Ltd (Jiangsu, China). Cells were cultured according to the manufacturer’s instructions. Briefly, we cultured these cells with Dulbecco’s modified Eagle’s medium (DMEM). Additionally, 10% FBS and 1% penicillin/streptomycin were added (Gibco, Rockville, MD, USA) to culture SGC-7901 cell lines. Cell incubator in a humidified atmosphere containing 5% CO2 was applicable for all cell culture.
RNA extraction and real-time PCR analysis
We used the mirVana miRNA kit (Takara, Dalian, China) to extract total RNA from cultured cells following the manufacturer’s instructions. For the detection of CTBP1 and miR-539-3P expression, PrimeScript RT reagent kit (Takara, Dalian, China) was applicable for synthesizing the first-strand cDNA. The expression of CTBP1 was quantified by Real-time PCR Mixture assays (Takara). GAPDH was used as the internal control. The primers of CTBP1 were: Forward, 5’-CGACCCTTACTTGTCGGATGG-3’, and reverse, 5’-TTGACGGTGAAGTCGTTGATG-3’; and GAPDH primers was: Forward, 5’-GGAG CGAGATCCCTCCAAA A T-3’, Reverse 5’-G G C T G T T G T C A T A C T T C T C A T G G-3’.
Cell transfection
Plasmid (CTBP1), miR-539-3P mimic and its corresponding miRNA negative control (miR-NC), miR-539-3P inhibitor and its control (NC) were purchased from Hanbio Co., Ltd. (Shanghai, China). Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), a common transfection reagent, was performed to efficiently transfect miR-539-3P in SGC-7901 cells according to the manufacturer’s instructions.
MTT assay
We used the MTT assay kit (Thermo, USA) to assess the viability of SGC-7901 cells transfected with or without miR-539-3P. In brief, we added 20 µl MTT reagent to each well and then these cells were incubated at 37°C for 2 hours. we aspirated the medium and added 100 ul DMSO to dissolve the formazan crystals. Microplate reader was used to examine the absorbance at 570 nm.
Colony formation assay
After transfection, we seeded 1×103 cells in a 6-well plate for 24 h culture. Next, two week cells culture was performed in DMEM medium. Of note, DMEM contained 10% FBS. Then colonies were washed with PBS for three times. In addition, methanol and 0.1% crystal violet were used to fix and stain for 30 min. Finally, we counted the number of colonies with >50 cells.
Luciferase reporter assay
The human CTBP1 3’-UTR, which contains the miR-539-3P binding site, was amplified by PCR and cloned into the pGL3-control vector (Ambion) at the NheI and XhoI sites. The resultant reporter plasmid was titled as CTBP1-Wt-3’-UTR. SGC-7901 cells were cultured in 6-well plates and transfected with miR-539-3P mimic or inhibitor, then used for luciferase assays. The CTBP1-Wt-3’-UTR reporter plasmid (100 ng/well) and the pRL-TK luciferase reporters (25 ng/well) were transfected into the cells using Lipofectamine 2000 (Invitrogen). Dual-Luciferase Reporter Assay kit (Promega, USA) was used to examine luciferase activity levels according to the manufacturer’s instructions. Renilla-luciferase was used for normalization.
Western blot assay
Expression of CTBP1 and EMT associated markers were determined by western blot. Briefly, we first lysed the SGC-7901 cells with RIPA buffer. Primary antibodies, such as rat anti CTBP1 (Santa Cruz, 1:500), mouse anti N-cadherin and mouse anti E-cadherin (Santa Cruz, 1:1000) were integrated with the targeted protein with incubation at room temperature for 1-2 h. Secondary antibodies conjugated with HRP label, were used to detect the expression of CTBP1, N-cadherin, and E-cadherin through chemiluminescence reagent. Mouse GAPDH was used as the loading control.
Cell invasion assay
The capacity of gastric cancer cell invasion was evaluated by transwell chambers (BD Biosiciences). In brief, 5×104 SGC-7901 cells were placed into the upper chamber with matrigel (BD, USA) for 24 hours. We then added the 10% FBS as chemoattractant in the bottom chamber. After 48 hours, we removed the upper chamber cells with a cotton swab and fixed the bottom chamber cells with 70% ethanol for 30 minutes. Finally, these cells were stained with 0.2% crystal violet for 10 minutes. Microscope (Olympus, Japan) was used to count the fixed cells of five randomly selected fields.
Data analysis
Data are shown as mean ± standard deviation. Statistical analyses between two groups were performed using Student’s t-test using SPSS 16.0 (SPSS Science, Chicago, IL); and, statistical analyses between multiple groups were performed using one way analysis of variance followed by the least significant difference post hoc test. Differences with values of P<0.05 were regarded as significant.
Results
The expression of miR-539-3P is significantly decreased in gastric cancer
Considering that miR-539 plays an important role in many cancers, in this study, we first examined the expression of miR-539-3P in gastric cancer tissues. 30 tumor tissues from gastric cancer patients and adjacent normal tissues are colletced for quanification of miR-539-3P. qRT-PCR results show that miR-539-3P is significantly down-regulated in gastric cancer tissues (Figure 1A). Next, in vitro cell lines, such as SGC-7901 (huamn gastric cancer cell) and GES-1 (normal gastric epithelial cell), were used to confirm the down-regulation of miR-539-3P expression. As expected, miR-539-3P was decreased in gastric cancer cells (Figure 1B). These results suggestthat miR-539 is a tumor suppressor in gastric cancer.
Figure 1.
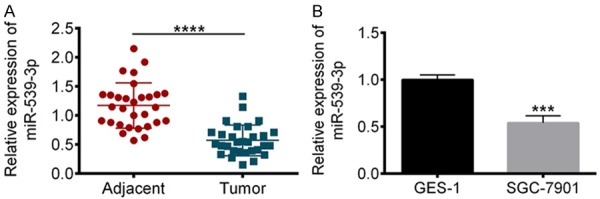
miR-539-3P expression is significantly decreased in gastric cancer tissue samples and SGC-7901 cells. A. RT-PCR results revealed that expression of miR-539-3P in gastric cancer patients was significantly lower than normal tissues. B. in SGC-7901 cells, miR-539-3P was also significantly decreased. ***P<0.001, ****P<0.0001 vs. control.
Down-regulation of miR-539-3P promotes gastric cancer cell malignancy
To assess the function of miR-539-3P in gastric cancer cells, we constructed the gastric cancer cells lines transfected with miR-539-3P-mimics or miR-539-3P inhibitor. Transfection efficiency was examined by RT-PCR (Figure 2A). Previous work reported that miR-539 regulated various cancer cell proliferation and invasion [17,18], therefore, in this study, we suspected that miR-539-3P is also closely associated with SGC-7901 cell proliferation and invasion. MTT assay and clone formation experiments indicated miR-539-3P overexpression inhibits gastric cancer cell proliferation and miR-539-3P inhibition facilitates SGC-7901 cell malignant proliferation (Figure 2B, 2C). On the other hand, miR-539-3P overexpression suppressed the gastric cancer cells invasion (Figure 2D).
Figure 2.
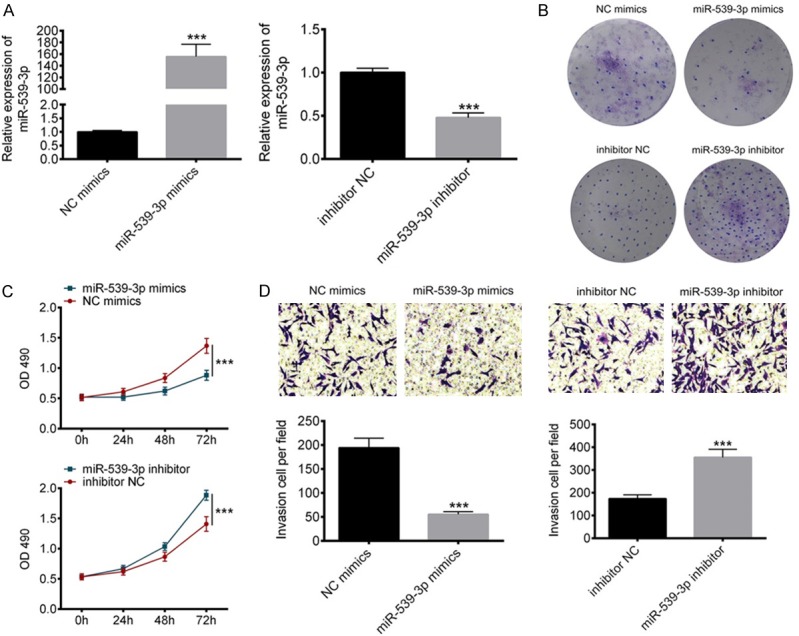
Overexpression of miR-539-3P inhibits gastric cancer cell proliferation and invasion. A. SGC-7901 cells were transfected with miR-539-3P mimics or inhibitor respectively. Transfection efficiency was examined by RT-PCR. B. Clone formation assay revealed that miR-539-3P inhibited SGC-7901 cell proliferation. C. MTT assay showed SGC-7901 cells transfected with miR-539-3P mimics had restricted proliferation capacity, whereas inhibition of miR-539-3P increased SGC-7901 cell proliferation. D. Inhibition of miR-539-3P promoted SGC-7901 cell invasion and overexpression of miR-539-3P prevented the invasion of SGC-7901 cells.
CTBP1 is a target of miR-539-3P
To further study the function of miR-539-3P, we screened the target genes of miR-539-3P. CTBP1, owing to its pitvotal role in gastric cancer malignancy, got our attention (Figure 3A). Luciferase reporter assay further showed the interaction between miR-539-3P and CTBP1 (Figure 3B). Gastric cancer cells transfected with miR-539-3P mimics or inhibitor were established to assess the relationship between miR-539-3P and CTBP1. RT-PCR results showed that overexpression of miR-539-3P suppresses CTBP1 expression and inhibition of miR-539-3P promotes CTBP1 expression (Figure 3C).
Figure 3.
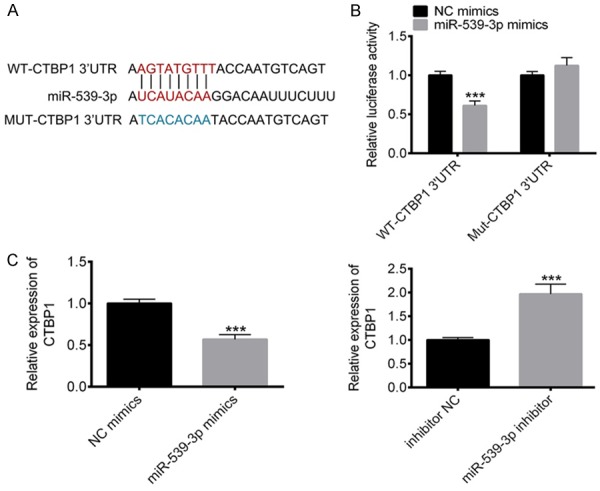
CTBP1 is the direct target of miR-539-3P. A. Bioinformatics analysis revealed that CTBP1 was the target of miR-539-3P. B. Lucifierase reporter assays confirmed the interaction between miR-539-3P and CTBP1. C. RT-PCR result showed a negative correlation between CTBP1 and miR-539-3P. ***P<0.001 vs. control.
Exogenous expression of CTBP1 reverses the effects of miR-539-3P overexpression on SGC-7901 cell proliferation and invasion
To further assess the correlation between CTBP1 and miR-539-3P, we hypothesized that exogenous expression of CTBP1 could reverse the effects induced by overexpression of miR-539-3P in gastric cancer cells. As expected, the expression of CTBP1 was reduced in gastric cancer cells overexpressing miR-539-3P. In addition, gastric cancer cell medium supplemented with CTBP1 significantly elevated the expression of CTBP1 in SGC-7901 cells. Interestingly, elevated expression of CTBP1 reversed the inhibitory effects of miR-539-3P on gastric cancer cell proliferation and invasion (Figure 4A-C). These results demonstrated miR-539-3P, negatively regulating CTBP1, manipulated malignant behavior.
Figure 4.
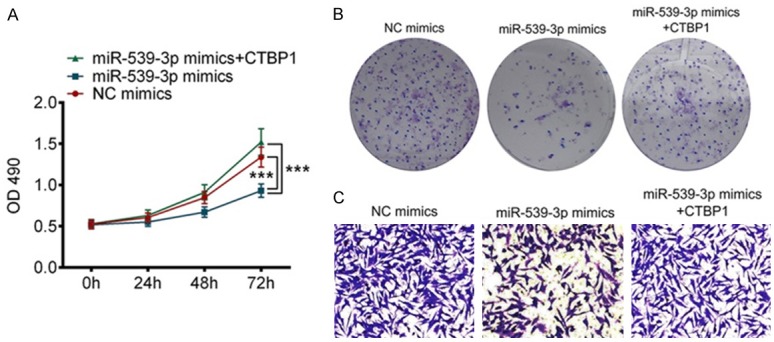
Exogenous expression of CTBP1 reversed the effects caused by miR-539-3P overexpression. A, B. Exogenous expression of CTBP1 promoted SGC-7901 cell proliferation. C. Exogenous expression of CTBP1 enhanced the capacity of gastric cancer cell invasion. ***P<0.001 vs. NC.
Dysfunction of miR-539-3P regulates gastric cancer malignancy through EMT
Interestingly, we observed that SGC-7901 cells transfected with miR-539-3P inhibitor showed vast morphologic variation into mesenchymal phenotype (Figure 2D). We hypothesized that EMT was critical to miR-539-3P dysfunction in gastric cancer. Therefore, we next checked the expression of EMT markers in SGC-7901 cell lines and found mesenchymal markers, such as vimentin and N-cadherin were all elevated, whereas epithelial markers like E-cadherin were downregulated (Figure 5A, 5B). Overexpression of miR-539-3P repressed EMT, and exogenous expression of CTBP1 in gastric cancer cells reactivated EMT.
Figure 5.
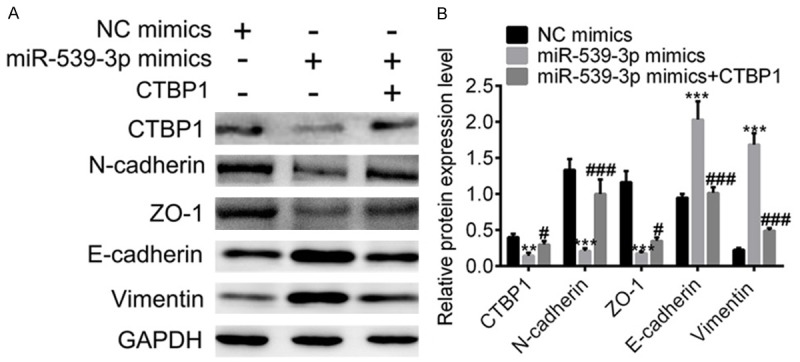
EMT is activated in SGC-7901 cells. A. After transfection with miR-539-3P, the expressions of CTBP1 and epithelial markers were downregulated, whereas mesenchymal gene expression was increased. Exogenous expression of CTBP1 could reverse this effect. B. Statistical data of EMT-associated gene expression.
Discussion
Epigenetic regulation, such as miRNA, lncRNA, and DNA methylation plays an important role in gastric cancer [22,23]. In this study, we focus on the role of miR-539-3P in gastric cancer. miR-539-3P, considered a tumor suppressor, is involved in many diseases including several cancers, rheumatoid arthritis, and chronic neuropathic patin. Mechanically, there are at least three mechanisms of miR-539 dysfunction in cancer. First, miR-539 regulates the capacity of gastric cancer cell proliferation, invasion and migration. For example, Ye et al. reported that miR539 respressed renal cell proliferation and induced apoptosis [24]. Yang et al. indicated that miR-539 inhibited triple-negative breast cancer cell migration [17,25] and others demonstrated that miR-539 inhibited glioma cell invasion [25]. miR-539 inhibits the EMT in cancer cells. Cao et al. implied that EMT is responsible for esophageal cancer cell malignancy and overexpression of miR-539 inhibited EMT through regulating twist-related protein 1 [26]. Finally, miR-539 showed inhibitory effects on tumor growth through decreasingarsenic trioxide resistance which is critical to cancer invasion and recurrence [27].
In gastric cancer, whether miR-539 also plays an important role remains unknown. Therefore, we first examined the expression of miR-539-3P in tumor tissues from gastric cancer patients at advanced stage. As expected, the expression of miR-539-3P was significantly decreased in gastric carcinoma. In vitro study also demonstrated the down regulation of miR-539-3P in gastric cancer cells. Consequently, we hypothesized that miR-539-3P regulated cancer cellproliferation and invasion. Results showed down-regulation of miR-539-3P facilitated gastric cancer malignancy. Further, we screened the targeted genes of miR-539-3P by targetscan and CTBP1 was one of the target genes [28]. CTBP1 has reported to be closely associated with gastric cancer. miR-644a was demonstrated to inhibit CTBP1 expression and suppressed gastric cancer cell proliferation and invasion [29]. Other investigators found that knocking down CTBP1 sensitized gastric cancer cells to chemotherapeutic drugs in gastric cancer [30]. Consequently, further studies demonstrated the interaction of miR-539-3P and CTBP1. Exogenous expression of CTBP1 reversed the effects of miR-539-3P overexpression on gastric cancer cell malignant proliferation and invasion.
Interestingly, we observed that gastric cancer cells transfected with miR-539-3P inhibitor showed morphology like mesenchymal cells. Therefore, we hypothesized EMT might be involved in gastric cancer malignancy. EMT is a process by which epithelial cells lose their cell polarity and cell-cell adhesion and gain migratory and invasive properties to become mesenchymal cells [31]. EMT is a common mechanism of cancer metastasis and invasion. Li et al. suggested miR-34a regulated hypoxia induced EMT and facilitated colorectal cancer malignancy [32]. Others indicated that miR-200 regulated ZEB2 expression and affected acute myeloid leukemia. Fischer et al. intriguingly argued that EMT is not required for lung cancer metastasis but contributes to chemoresistance [33]. In line with these results, we reported SGC-7901 cells expressed more mesenchymal cell markers but decreased epithelial cell markers. Overexpression of miR-539-3P could inhibit CTBP1 and ameliorated EMT.
Conclusion
In summary, our research first reports a novel miRNA in regulation of gastric cancer cell proliferation and invasion. Identification of miR-539-3P could not only be a prediction marker for gastric cancer development but also provide a new druggable locus for gastric cancer.
Acknowledgements
This study was supported by Shanghai Key Specialty-General Surgery Project (No. ZK2015A25).
Disclosure of conflict of interest
None.
References
- 1.Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–71. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 2.Higginson J. International agency for research on cancer. Berlin Heidelberg: Springer; 2009. [Google Scholar]
- 3.Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12:17–20. doi: 10.3748/wjg.v12.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee YY, Derakhshan MH. Environmental and lifestyle risk factors of gastric cancer. Arch Iran Med. 2013;16:358–365. [PubMed] [Google Scholar]
- 5.Ohara S. Infection and the development of gastric cancer. Keio J Med. 2002;51(Suppl 2):63–8. doi: 10.2302/kjm.51.supplement2_63. [DOI] [PubMed] [Google Scholar]
- 6.Chen K, Xu XW, Zhang RC, Pan Y, Wu D, Mou YP. Systematic review and meta-analysis of laparoscopy-assisted and open total gastrectomy for gastric cancer. World J Gastroenterol. 2013;19:5365. doi: 10.3748/wjg.v19.i32.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pretz JL, Wo JY, Mamon HJ, Kachnic LA, Hong TS. Chemoradiation therapy: localized esophageal, gastric, and pancreatic cancer. Surg Oncol Clin N Am. 2013;22:511–524. doi: 10.1016/j.soc.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Romano F, Uggeri F, Nespoli L, Gianotti L, Garancini M, Maternini M, Nespoli A, Uggeri F. Gastric cancer immunotherapy: an overview. J Cancer Ther. 2013;04:1018–1036. [Google Scholar]
- 9.Cao XY, Jia ZF, Jin MS, Cao DH, Kong F, Suo J, Jiang J. Serum pepsinogen II is a better diagnostic marker in gastric cancer. World J Gastroenterol. 2012;18:7357–7361. doi: 10.3748/wjg.v18.i48.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin VY, Ng EK, Chan VW, Kwong A, Chu KM. A three-miRNA signature as promising non-invasive diagnostic marker for gastric cancer. Mol Cancer. 2015;14:1–9. doi: 10.1186/s12943-015-0473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thong-Ngam D, Tangkijvanich P, Lerknimitr R, Mahachai V, Theamboonlers A, Poovorawan Y. Diagnostic role of serum interleukin-18 in gastric cancer patients. World J Gastroenterol. 2006;12:4473–4477. doi: 10.3748/wjg.v12.i28.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng AM, Byrom MW, Shelton J, Ford LP. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005;33:1290–1297. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNA and siRNA. Cell. 2009;136:642–55. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z, Li Z, Gao C, Chen P, Chen J, Liu W, Xiao S, Lu H. miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab Invest. 2008;88:1358–66. doi: 10.1038/labinvest.2008.94. [DOI] [PubMed] [Google Scholar]
- 15.Hu N, Yin JF, Ji Z, Hong Y, Wu P, Bian B, Song Z, Li R, Liu Q, Wu F. Strengthening gastric cancer therapy by trastuzumab-conjugated nanoparticles with simultaneous encapsulation of Anti-MiR-21 and 5-fluorouridine. Cell Physiol Biochem. 2017;44:2158–2173. doi: 10.1159/000485955. [DOI] [PubMed] [Google Scholar]
- 16.Yao Q, Gu A, Wang Z, Xue Y. MicroRNA-144 functions as a tumor suppressor in gastric cancer by targeting cyclooxygenase-2. Exp Ther Med. 2018;15:3088–3095. doi: 10.3892/etm.2018.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang ZX, Zhang B, Wei J, Jiang GQ, Wu YL, Leng BJ, Xing CG. MiR-539 inhibits proliferation and migration of triple-negative breast cancer cells by down-regulating LAMA4 expression. Cancer Cell Int. 2018;18:16. doi: 10.1186/s12935-018-0512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao X, Li S, Li W, Wang G, Zhao W, Han J, Diao C, Wang X, Zhang M. MicroRNA-539 suppresses tumor cell growth by targeting the WNT8B gene in Non-small cell lung cancer. J Cell Biochem. 2017 doi: 10.1002/jcb.26634. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Wen D, Li S, Jiang W, Zhu J, Liu J, Zhao S. miR-539 inhibits human colorectal cancer progression by targeting RUNX2. Biomed Pharmacother. 2017;95:1314–1320. doi: 10.1016/j.biopha.2017.09.044. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Hong W, Zhou C, Jiang Z, Wang G, Wei G, Li X. miR-539 inhibits FSCN1 expression and suppresses hepatocellular carcinoma migration and invasion. Oncol Rep. 2017;37:2593–2602. doi: 10.3892/or.2017.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirghasemi A, Taheriazam A, Karbasy SH, Torkaman A, Shakeri M, Yahaghi E, Mokarizadeh A. Down-regulation of miR-133a and miR-539 are associated with unfavorable prognosis in patients suffering from osteosarcoma. Cancer Cell Int. 2015;15:1–5. doi: 10.1186/s12935-015-0237-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Calcagno DQ, de Arruda Cardoso Smith M, Burbano RR. Cancer type-specific epigenetic changes: gastric cancer. New York: Springer; 2015. [DOI] [PubMed] [Google Scholar]
- 23.Guo M, Yan W. Epigenetics of gastric cancer. New York: Springer; 2015. [DOI] [PubMed] [Google Scholar]
- 24.Ye Z, Gui D. miR-539 suppresses proliferation and induces apoptosis in renal cell carcinoma by targeting high mobility group A2. Mol Med Rep. 2018;17:5611–5618. doi: 10.3892/mmr.2018.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quan J, Qu J, Zhou L. MicroRNA-539 inhibits glioma cell proliferation and invasion by targeting DIXDC1. Biomed Pharmacother. 2017;93:746–753. doi: 10.1016/j.biopha.2017.06.097. [DOI] [PubMed] [Google Scholar]
- 26.Cao Z, Zheng X, Cao L, Liang N. MicroRNA-539 inhibits the epithelial-mesenchymal transition of esophageal cancer cells by twist-related protein 1-mediated modulation of melanoma associated antigen A4 (MAGEA4) Oncol Res. 2018;26:529–536. doi: 10.3727/096504017X14972679378357. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Zhu C, Zhou R, Zhou Q, Chang Y, Jiang M. microRNA-539 suppresses tumor growth and tumorigenesis and overcomes arsenic trioxide resistance in hepatocellular carcinoma. Life Sci. 2016;166:34–40. doi: 10.1016/j.lfs.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Furusawa T, Moribe H, Kondoh H, Higashi Y. Identification of CtBP1 and CtBP2 as corepressors of zinc finger-homeodomain factor δEF1. Mol Cell Biol. 1999;19:8581–8590. doi: 10.1128/mcb.19.12.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Yan X, Ren L, Li Y. miR-644a inhibits cellular proliferation and invasion via suppression of CtBP1 in gastric cancer cells. Oncol Res. 2018;26:1–8. doi: 10.3727/096504016X14772410356982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bizama C, Benavente F, Salvatierra E, Gutiérrezmoraga A, Espinoza JA, Fernández EA, Roa I, Mazzolini G, Sagredo EA, Gidekel M. The low-abundance transcriptome reveals novel biomarkers, specific intracellular pathways and targetable genes associated with advanced gastric cancer. Int J Cancer. 2014;134:755–64. doi: 10.1002/ijc.28405. [DOI] [PubMed] [Google Scholar]
- 31.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Rokavec M, Jiang L, Horst D, Hermeking H. Antagonistic effects of p53 and HIF1A on microRNA-34a regulation of PPP1R11 and STAT3 and hypoxia-induced epithelial to mesenchymal transition in colorectal cancer cells. Gastroenterology. 2017;153:505–520. doi: 10.1053/j.gastro.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 33.Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El RT, Ryu S, Troeger J. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–6. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]


