Abstract
Inflammatory myofibroblastic tumor (IMT) is a neoplasm composed of spindled neoplastic myofibroblasts admixed with reactive lymphoplasmacytic cells, plasma cells, and/or eosinophils, which has an intermediate biological behavior. An IMT variant with plump round epithelioid or histiocytoid tumor cells, recognized as epithelioid inflammatory myofibroblastic sarcoma (EIMS), has a more clinically aggressive progression. To the best of our knowledge, only about 40 cases of EIMS have previously been reported in limited literature. Here, we report here a case of unusual EIMS with a relative indolent clinical behavior. We reviewed the literature for 18 similar cases. The patients present with a highly aggressive inflammatory myofibroblastic tumor characterized by round or epithelioid morphology, prominent neutrophilic infiltrate, and positive staining of ALK with RANBP2-ALK gene fusion or RANBP1-ALK gene fusion, or EML4-ALK gene fusion. Our case is the first case of primary stomach EIMS. Moreover, the mechanisms of the rare entity have not been widely recognized and require further study. Early accurate diagnosis and complete resection of this tumor is necessary. Some researchers suggest expression of PD-L1 may provide new strategies for ALK-targeted therapy.
Keywords: Inflammatory myofibroblastic tumor, epithelioid inflammatory myofibroblastic sarcoma, RANBP2-ALK gene fusion, RANBP1-ALK gene fusion, morphology, immunostaining, poor prognosis
Introduction
Inflammatory myofibroblastic tumor (IMT) is a distinctive neoplasm composed of myofibroblastic and fibroblastic spindled cells accompanied by an inflammatory infiltrate of plasma cells, lymphocytes, and/or eosinophils, which has intermediate biological behavior [1,2]. It most often occurs in soft tissue and the abdominopelvic region, lung, mediastinum and retroperitoneum, and usually affects children and adolescents [3]. Cytoplasmic reactivity for anaplastic lymphoma kinase (ALK) protein is detectable in 50-60% of cases, which is triggered by clonal rearrangements of ALK gene located on chromosome 2p23 [1,4].
The epithelioid variant of IMT, known as epithelioid inflammatory myofibroblastic sarcoma (EIMS), is first described by Marino-Enriquez [5] and is clinically aggressive and has a poor prognosis. It often occurs in the abdominal cavity, mesentery, omentum majus, and affects mostly adults, and men more frequently than women [5]. In contrast to the conventional spindle-cell IMT, EIMS is mainly consisted of round-to-epithelioid cells, with a loose or myxoid stroma, containing abundant neutrophilic inflammmatory infiltrates [6-10]. Immunostain for ALK typically localizes to the nuclear membrane, and occasionally presents a cytoplasmic pattern with perinuclear accentuation [11]. Several studies revealed that EIMS has a RANBP2-ALK gene fusion or RANBP1-ALK gene fusion [12,13]. Researchers speculated the RANBP1-ALK fusion leads to cytoplasmic and perinuclear ALK expression, and RANBP2-ALK fusion results from nuclear membranous ALK expression pattern in EIMS. Both are associated with an aggressive clinical course [1,14,15]. To the best of our knowledge, only about 40 cases have been reported in the English literature. In this study, we describe an additional case of EIMS with clinical, pathologic and immunohistochemical analysis.
Materials and methods
Our case was obtained from the Department of Ningbo Clinical Pathological Diagnosis Center, China. Glass slides and paraffin blocks were available from the specimens of stomach in January 2018. Follow-up time was 10 months. Two expert pathologists (S.Y.W and C.X.H) confirmed a consensus diagnosis of epithelioid inflammatory myofibroblastic sarcoma, according to the 2018 (2nd edition) by Wang Jian on soft tissue pathology. The Research Ethics Committee of Ningbo reviewed and approved the study according to the principles expressed in the Declaration of Helsinki.
Immunohistochemical staining was performed on deparaffinized tissue sections using a labeled streptavidin-biotin peroxidase or polymer detection system on an automated immunostaining module (Dako), according to the instructions of the manufacturers. Antibodies are detailed in Table 1. Appropriate positive and negative controls were used for each antibody. Tumor reactivity for immunohistochemical antibodies was scored as follows: -, all tumor cells were negative; +, 5-25% of tumor cells were positive; ++, 26-50% of tumor cells were positive; and +++, >50% of tumor cells were positive. Only tumor cells with distinct nuclear staining for ERG, Fli-1, Ki67, S-100, INI1 and TFE3; distinct cell membrane staining for CK (pan), CK5/6, CD30 and D2-40; distinct cell cytoplasmic staining for Melanoma, SMA and Calponin; distinct nuclear and/or cytoplasm staining for anaplastic lymphoma kinase (ALK) were recorded as positive; and distinct cell membrane and/or cytoplasm staining for EMA, CD117, DOG-1, CD31 and CD34 were recorded as positive.
Table 1.
Antibodies used for immunohistochemical staining
| Antibody | Clone number | Source | Dilution |
|---|---|---|---|
| CK (pan) | AE1/AE3 | MAB, Fuzhou, China | Ready to use |
| EMA | E29 | Dako | Concentrated |
| SMA | 1A4 | MAB, Fuzhou, China | Ready to use |
| S-100 | 4c4.9 | MAB, Fuzhou, China | Ready to use |
| CD117 | C-kit | Dako | Concentrated |
| CD30 | Ber H2 | Dako | 1:400 |
| CD34 | QBEnd/10 | MAB, Fuzhou, China | Ready to use |
| CD31 | JC/70A | MAB, Fuzhou, China | Ready to use |
| ERG | EP111 | ZM. Beijing, China | Ready to use |
| Dog-1 | SP31 | MAB, Fuzhou, China | Ready to use |
| D2-40 | D2-40 | MAB, Fuzhou, China | Ready to use |
| Calponin | CALP | MAB, Fuzhou, China | Ready to use |
| Desmin | D33 | Dako | Concentrated |
| ALK | ALK1 | Dako, Glostrup, Denmark | 1:100 |
| Ki-67 | MIB-1 | Dako, Glostrup, Denmark | 1:100 |
| Melanoma | HMB45 | MAB, Fuzhou, China | Ready to use |
| Ck5/6 | D5/16B4 | MAB, Fuzhou, China | Ready to use |
| INI-1 | MRQ-27 | MAB, Fuzhou, China | Ready to use |
| TFE3 | MRQ-37 | MAB, Fuzhou, China | Ready to use |
Results
Clinical features
Clinical features of all cases are summarized in Table 2. Our patient is a 35-year-old female who was hospitalized due to generalized weakness and loss of appetite for 17 days in January 2018. Electron gastroscopy revealed an elevated lesion at the greater curvature of the gastric body. CT examination showed a solid mass in the greater curvatureof the gastric body and no clear demarcation to the mucosa, with the dimension of 4.0 × 2.0 cm. When strengthen scan was performed, the mass showed non-uniform intensification (Figure 1A and 1B). The resection of the tumor together with part of the stomach was conducted. Hypersensitive c-reactive protein was elevated and measured 17.13 mg/L. There was no family history of genetic disease. The patient was not subjected to further chemotherapy or radiation therapy. The patient is currently well and close follow-up would be required to ascertain the behavior.
Table 2.
Clinical features of 18 cases of epithelioid inflammatory myofibroblastic sarcoma
| Author, Year | Case | Age/Sex | Site | Size (cm) | Treatment | Recurrence or metastasis | Follow-up Status (m) |
|---|---|---|---|---|---|---|---|
| Yu et al., 2016 | 1 | 37 y/F | Rectum | 5 | SE | No recurrence and metastasis | 8 (NED) |
| 2 | 55 y/M | Mesentery of ileum | 11 | SE, CT | Recurred at 2 m | 10 (NED) | |
| 3 | 22 y/M | Mesentery of colon | 20 | SE, ALKi | Recurred at 2 m | 14 (AWD) | |
| 4 | 58 y/F | Omentum | 5.5 | SE, CT | Recurred at 2 m | 8 (DOD) | |
| 5 | 15 y/F | Transverse colon | 10 | SE | No recurrence and metastasis | 7 (NED) | |
| Telugu et al., 2017 | 6 | 3 y/F | Suprarenel | 15 | SE, CT | Metastasis to liver and lung | 8 (DOD) |
| Jiang et al., 2017 | 7 | 45 y/M | Abdominal cavity | 20 | SE, CT | Metastasis to liver, spleen, small intestine et al. | 1 (DOD) |
| Lee et al., 2018 | 8 | 42 y/M | Abdominal cavity | NA | NA | Recurred at 8 m | 40 (AWD) |
| 9 | 34 y/M | Liver | NA | NA | Recurred at 5 m | 5 (DOD) | |
| 10 | 62 y/M | Abdominal cavity | NA | NA | NA | 2 (DOD) | |
| 11 | 76 y/F | Abdominal cavity | NA | NA | NA | 4 (DOD) | |
| 12 | 30 y/M | Abdominal cavity | NA | NA | NA | 8 (DOD) | |
| 13 | 26 y/M | Abdominal cavity | NA | NA | Recurred at 7&16 m | 16 (AWD) | |
| 14 | 39 y/M | Abdominal cavity | NA | NA | Recurred at 10 m | 10 (AWD) | |
| 15 | 7 m/M | Abdominal cavity | NA | NA | NA | 36 (DOD) | |
| 16 | 16 y/F | Lung | NA | NA | Recurred at 1 m | 48 (AWD) | |
| Du et al., 2018 | 17 | 26 y/M | Pelvic cavity | 5 | SE, CT | NA | 8 (DOD) |
| Hong et al., 2018 | 18 | 15 y/F | Ovary | NA | ALKi | Multiple recurrences | 24 (AWD) |
| Current case | 19 | 35 y/F | Stomach | NA | No | No | 11 (NED) |
NA not available, SE surgical excision, CT chemotherapy, ALKi ALK inhibitor, DOD died of disease, NED no evidence of disease, AWD alive with disease.
Figure 1.
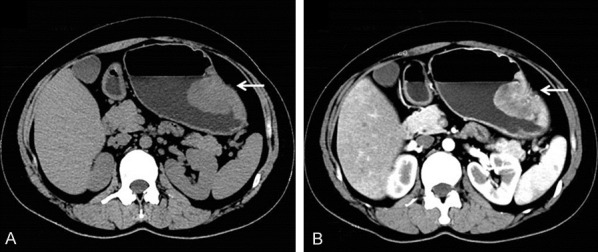
CT scan. A. Computer tomography revealed a heterogeneous-density occupation in the big bend of the gastric body (arrows); B. When strengthen scan was performed, the mass showed non-uniform intensification (arrows).
Histopathology
Tumor was in irregular shape with the size of 5.0 × 4.0 × 2.0 cm. On cut section, the tumor was grayish-yellow to gray red in color, moderate or fleshy in consistency. Macroscopically, the tumor was located on the mucosa and submucosa and invaded into the muscularis. Marked necrosis could be easily seen (Figure 2A). Tumor cells were predominantly composed of non-cohesive sheets or clusters of round to epithelioid cells, embedded in a loose or myxoid stroma, with abundant neutrophilic granulocyte infiltrating into the stroma (Figure 2A, 2B). On high power, tumor cells were characterized by abundant eosinophilic cytoplasm, and obvious vesicular nuclei with prominent nucleoli (Figure 2C). Ganglion cell-like cells with abundant basophilic cytoplasm, obvious vesicular nuclei and prominent nucleoli also could be seen (Figure 2D). Mitotic activity was 2-7/10HPF. Atypical forms were also present. Less than 10% of spindle cells were seen in the tumor and lymphocytes were also observed.
Figure 2.
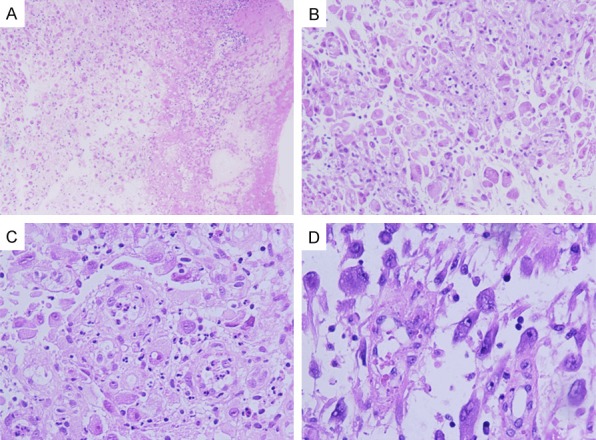
Microscopic features of stomach EIMS. A. Marked necrosis (magnification, × 50). B. Tumor cells were predominantly composed of non-cohesive sheets or clusters of round to epithelioid cells and embedded in a loose or myxoid stroma, with abundant neutrophilic granulocyte (magnification, × 100). C. Epithelioid cells were characterized by abundant eosinophilias cytoplasm, obvious vesicular nuclei with prominent nucleoli (magnification, × 200). D. Ganglion cell-like cells with abundant basophilic cytoplasm, obvious vesicular nuclei and prominent nucleoli (magnification, × 200).
Immunohistochemical studies
The results of immunostaining are shown in Table 3 and Figure 3. Epithelioid cells in all specimens expressed ALK, most cases presented nuclear membrane staining, but some cases presented cytoplasmic staining and/or perinuclear accentuation. Epithelioid cells displayed variable staining for SMA and desmin. Our case diffusely expressed ALK with a distinctive cytoplasmic pattern and showed moderate intensity (Figure 3A). In addition, epithelioid cells were locally positive for calponin, diffusely expressed SMA, and INI1 (Figure 3B-D). Immunostain of ERG was localized in vessels but not tumor cells (Figure 3E). The Ki67 proliferative index was 15-20% (Figure 3F). Irregular epithelioid cells were negative for CD30, HMB45, TFE3, CD117, DOG-1, EMA (Figure 3G-L).
Table 3.
Reported cases and our case with Immunohistochemical and genetic features
| Case | Immunohistochemical features | ALK | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||
| Desmin | SMA | H-caldesmon | CD30 | CK | EMA | S-100 | Myogenin | IHC | FISH | RT-PCR | |||||
| 1 | + | - | - | - | - | NA | - | - | NM+ | + | NA | ||||
| 2 | + | - | NA | - | - | - | - | - | NM+ | + | NA | ||||
| 3 | + | - | - | - | - | - | - | - | PN+ | + | NA | ||||
| 4 | + | Focal+ | - | - | Focal+ | NA | - | - | NM+ | + | NA | ||||
| 5 | - | + | NA | - | - | - | - | - | NM+ | + | NA | ||||
| 6 | NA | NA | NA | NA | NA | NA | NA | NA | + | NA | NA | ||||
| 7 | NA | NA | NA | NA | NA | NA | NA | NA | NM+ | + | RANBP2-ALK | ||||
| 8 | NA | NA | NA | NA | NA | NA | NA | NA | NM+ | + | RANBP2-ALK | ||||
| 9 | NA | NA | NA | NA | NA | NA | NA | NA | Cytoplasmic, PN+ | + | RANBP1-ALK | ||||
| 10 | NA | NA | NA | NA | NA | NA | NA | NA | NM+ | + | RANBP2-ALK | ||||
| 11 | NA | NA | NA | NA | NA | NA | NA | NA | NM+ | + | NP | ||||
| 12 | NA | NA | NA | NA | NA | NA | NA | NA | Cytoplasmic, PN+ | + | RANBP1-ALK | ||||
| 13 | NA | NA | NA | NA | NA | NA | NA | NA | Cytoplasmic, PN+ | + | RANBP1-ALK | ||||
| 14 | NA | NA | NA | NA | NA | NA | NA | NA | Cytoplasmic, PN+ | + | RANBP1-ALK negative | ||||
| 15 | NA | NA | NA | NA | NA | NA | NA | NA | Cytoplasmic | + | RANBP1-ALK negative | ||||
| 16 | + | + | NA | NA | NA | NA | NA | NA | NM+ | + | RANBP2-ALK | ||||
| 17 | + | - | - | - | + | - | - | NA | NM+ | + | RANBP2-ALK | ||||
| 18 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | RANBP2-ALK | ||||
| 19 | Focal+ | Focal+ | NP | - | - | - | - | NP | Cytoplasmic | NP | NP | ||||
PN, perinuclear accentuation. NP, not provided/performed (including uninformative analyses). NM, nuclear membrane staining. NA, not available.
Figure 3.
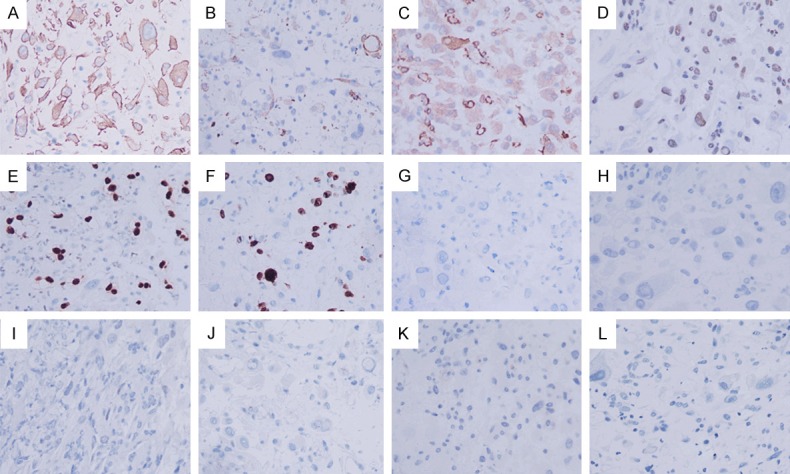
Immunohistochemical staining appearance of our case. A. Epithelioid cells diffusely expressed ALK with a distinctive cytoplasmic pattern and showed moderate intensity. B-D. Epithelioid cells were locally positive for calponin, diffusely expressed SMA and INI1. E. Vessels were positive for ERG. F. The Ki67 proliferative index was 15-20%. G-L. Irregular epithelioid cells showed negative for CD30, HMB45, TFE3, CD117, DOG-1, EMA.
Discussion
EIMS is an extremely rare disease first described and nominated by Marino-Enriquez in 2011, which is characterized by an epithelioid morphology accompanied with prominent neutrophilic inflammatory infiltrate [5]. EIMS was included in the 2012 (4th edition) World Health Organization (WHO) tumor classification of soft tissue, and seems to portend more aggressive clinical behavior. Eighteen other cases have been reported in recent 3 years, Clinicopathological, immunohistochemical and genetic characteristics are summarized in Tables 2 and 3. Of these 19 cases, 15 (79%) patients were adults (range from 22 years to 76 years), and the other 4 patients were children and adolescents. Eight patients were female and eleven were male. The tumors of 18 patients were located in abdominal cavity, including rectum, mesentery of ileum/colon, omentum, transverse colon, adrenal, liver, and ovary, and in lung. EIMS occurred mainly in adults and had a male predilection, and almost was intraabdominal locations, consisted with previous literature reports. On the contrary, there was a slight female predominance of the conventional spindle-cell IMT. All cases appeared to have morphologic features of large round to epithelioid cells with vesicular nuclei, large prominent nucleoli and abundant of amphophilic or eosinophilic cytoplasm, and epithelioid cells bathed in the myxoid stroma with significant inflammatory infiltrates frequently composed of neutrophils.
Immunohistochemically, all tumors almost were found to be positive for ALK with diverse expression patterns, shown in Table 2. Of these 19 cases, 53% (10/19) of cases displayed ALK nuclear membrane staining pattern. However, several cases (21%, 4/19) had a perinuclear staining pattern of ALK. Our case and one other case demonstrated a cytoplasmic staining pattern. In addition, 32% (7/19) of cases were negative for CD30. Of note, FISH assay, PCR assay or gene sequencing is helpful to confirm the diagnosis of EIMS. ALK rearrangement has been confirmed by FISH assay in 16 cases, except for our case and other two cases. In previous studies, RANBP2-ALK fusion was detected in EIMS, accompanying with nuclear membrane or perinuclear staining pattern, and nearly all cases containing RANBP2-ALK fusion gene demonstrated an aggressive behavior [5,13,16-19]. Researchers inferred that RANBP2-ALK fusion gene might be a potential molecular mechanism for the rapid growth and recurrence of EIMS [1]. EML4-ALK fusion gene was first demonstrated by Jiang and his colleagues [7], with the awareness of a possible link between the EML4-ALK fusion gene and aggravated clinical manifestation. Moreover, Lee et al. first discovered another novel ALK fusion partners-RANBP1 in 2018 [15], shown in Table 2. They described EIMS with RANBP1-ALK fusion exhibited cytoplasmic ALK expression and had clinically-aggressive progression. They speculated that RANBP1-ALK was a recurrent oncogenic mechanism in clinically aggressive EIMS [15]. In addition, RRBP1-ALK had specificity in EMIS and was not found in other 100 ALK-positive cancers, such as lung adenocarcinoma, anaplastic large-cell lymphoma, epithelioid fibrous histiocytoma, and conventional IMT [15].
Diagnosis of EIMS is difficult, given its rarity and morphology. In our case, the first differential diagnosis is poorly differentiated carcinoma (PDC), owing to the tumor location in stomach. Both have large round to epithelioid cells with vesicular nuclei, large prominent nucleoli, and abundant eosinophilic cytoplasm. Immunohistochemically, PDC is strongly positive for AE1/AE3, EMA, and CK7, but ALK is absent. Importantly, RANBP1-ALK or RANBP2-ALK by RT-PCR gene fusion can be detected by PT-PCR in EIMS but not in PDC. In addition, epithelioid gastrointestinal stromal tumor (GIST) is also easily confused with EIMS, due to the morphologic overlap. Epithelioid GIST stains positively for CD117, DOG-1 and CD34, but negatively for ALK staining. Moreover, mutations of c-kit and PDGF-α (platelet derived growth factor-α) can be detected in epithelioid GIST. Meanwhile, other tumors with epithelioid morphology also need to be discriminated from EIMS, such as anaplastic large cell lymphoma (ALCL), malignant mesothelioma, solid variant of alveolar rhabdomyosarcoma, epithelioid leiomyosarcoma, epithelioid malignant peripheral nerve sheath tumor, melanoma, myxoid/round cell liposarcoma, and myxofibrosarcoma. An appropriate immunohistochemical panel of satins and FISH assay, PCR assay or gene sequencing are crucial in making the correct diagnosis.
EIMS is an aggressive neoplasm with poor prognosis. Nine of 18 patients died of disease [1,6-8,15], 6 were alive with disease [15,20], and only 3 remained well with no evidence of disease [1]. The mean time of overall survival was 13 months. Nine of 18 reported patients were confirmed with recurrence, and 2 developed distant metastases (1 to liver and lung, 1 to liver, spleen, small bowel). However, there is no optimal treatment for EIMS. Surgical resection remains as the main method. Postoperative adjuvant chemotherapy and immunotherapies have not yet been clearly identified, owing to the available experiences are very limited. Five patients were treated with chemotherapy. Only one patient remained alive with no evidence of disease at 10-month follow-up [1]. Four patients died with recurrence or metastasis at 8 months, 8 months, 1 month, and 8 months, respectively [1]. The data indicate chemotherapy has no distinct effect in the control of aggressive progression of EIMS. Notably, ALK inhibitor-crizotinib has been given in the treatment of EIMS with effectiveness in several studies [1,5,18,21,22]. In our summarized cases, two patients remained alive with disease at 14 months and 24 months follow-up [1,20]. Recently, DU et al. found programmed death-ligand 1 (PD-L1) was diffusely positive in their case [6]. The PD-1/PD-L1 axis has a significant role in the immune antitumor response [23-25]. Therefore, they expect novel inmmunomodulatory therapies targeting the PD-1/PD-L1 pathway could be established.
In conclusion, EIMS is considered as a distinct variant of IMT, which mainly consists of cells with a round or epithelioid morphology, with a high potential recurrence and a poor prognosis. The mechanisms of the rare entity have not been widely recognized, and require further study. Detection of ALK gene rearrangement is helpful for pathologists to make a correct diagnosis of EIMS and provides a reliable reference for ALK-targeted therapy. Expression of PD-L1 was found in EIMS recently, which probably could provide new strategies for ALK-targeted therapy.
Disclosure of conflict of interest
None.
References
- 1.Yu L, Liu J, Lao IW, Luo Z, Wang J. Epithelioid inflammatory myofibroblastic sarcoma: a clinicopathological, immunohistochemical and molecular cytogenetic analysis of five additional cases and review of the literature. Diagn Pathol. 2016;11:67. doi: 10.1186/s13000-016-0517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai Y, Jiang M, Liang W, Chen F. Incomplete intestinal obstruction caused by a rare epithelioid inflammatory myofibroblastic sarcoma of the colon: a case report. Medicine (Baltimore) 2015;94:e2342. doi: 10.1097/MD.0000000000002342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg AE. WHO classification of soft tissue and bone, fourth edition: summary and commentary. Curr Opin Oncol. 2013;25:571–573. doi: 10.1097/01.cco.0000432522.16734.2d. [DOI] [PubMed] [Google Scholar]
- 4.Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59:2776–2780. [PubMed] [Google Scholar]
- 5.Marino-Enriquez A, Wang WL, Roy A, Lopez-Terrada D, Lazar AJ, Fletcher CD, Coffin CM, Hornick JL. Epithelioid inflammatory myofibroblastic sarcoma: an aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol. 2011;35:135–144. doi: 10.1097/PAS.0b013e318200cfd5. [DOI] [PubMed] [Google Scholar]
- 6.Du X, Gao Y, Zhao H, Li B, Xue W, Wang D. Clinicopathological analysis of epithelioid inflammatory myofibroblastic sarcoma. Oncol Lett. 2018;15:9317–9326. doi: 10.3892/ol.2018.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang Q, Tong HX, Hou YY, Zhang Y, Li JL, Zhou YH, Xu J, Wang JY, Lu WQ. Identification of EML4-ALK as an alternative fusion gene in epithelioid inflammatory myofibroblastic sarcoma. Orphanet J Rare Dis. 2017;12:97. doi: 10.1186/s13023-017-0647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Telugu RB, Prabhu AJ, Kalappurayil NB, Mathai J, Gnanamuthu BR, Manipadam MT. Clinicopathological study of 18 cases of inflammatory myofibroblastic tumors with reference to ALK-1 expression: 5-year experience in a tertiary care center. J Pathol Transl Med. 2017;51:255–263. doi: 10.4132/jptm.2017.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang N, Yang QJ, Deng YT, Feng X, Xia HS, Zhang YG, Wang MW, Wu D, Zhou H, Guo F. [Epithelioid inflammatory myofibroblastic sarcoma of small bowel mesentery: report of a case] . Zhonghua Bing Li Xue Za Zhi. 2017;46:201–202. doi: 10.3760/cma.j.issn.0529-5807.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Fu X, Jiang J, Tian XY, Li Z. Pulmonary epithelioid inflammatory myofibroblastic sarcoma with multiple bone metastases: case report and review of literature. Diagn Pathol. 2015;10:106. doi: 10.1186/s13000-015-0358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J, Jiang G, Zhang D, Zhang L, Xu J, Li S, Li W, Ma Y, Zhao A, Zhao Z. Epithelioid inflammatory myofibroblastic sarcoma with recurrence after extensive resection: significant clinicopathologic characteristics of a rare aggressive soft tissue neoplasm. Int J Clin Exp Pathol. 2015;8:5803–5807. [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Q, Kan Y, Zhao Y, He H, Kong L. Epithelioid inflammatory myofibroblastic sarcoma treated with ALK inhibitor: a case report and review of literature. Int J Clin Exp Pathol. 2015;8:15328–15332. [PMC free article] [PubMed] [Google Scholar]
- 13.Cook JR, Dehner LP, Collins MH, Ma Z, Morris SW, Coffin CM, Hill DA. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol. 2001;25:1364–1371. doi: 10.1097/00000478-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Hallin M, Thway K. Epithelioid inflammatory myofibroblastic sarcoma. Int J Surg Pathol. 2019;27:69–71. doi: 10.1177/1066896918767557. [DOI] [PubMed] [Google Scholar]
- 15.Lee JC, Li CF, Huang HY, Zhu MJ, Mariño-Enríquez A, Lee CT, Ou WB, Hornick JL, Fletcher JA. ALK oncoproteins in atypical inflammatory myofibroblastic tumours: novel RRBP1-ALK fusions in epithelioid inflammatory myofibroblastic sarcoma. J Pathol. 2017;241:316–323. doi: 10.1002/path.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimbara S, Takeda K, Fukushima H, Inoue T, Okada H, Shibata Y, Katsushima U, Tsuya A, Tokunaga S, Daga H, Okuno T, Inoue T. A case report of epithelioid inflammatory myofibroblastic sarcoma with RANBP2-ALK fusion gene treated with the ALK inhibitor, crizotinib. Jpn J Clin Oncol. 2014;44:868–871. doi: 10.1093/jjco/hyu069. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Yin WH, Takeuchi K, Guan H, Huang YH, Chan JK. Inflammatory myofibroblastic tumor with RANBP2 and ALK gene rearrangement: a report of two cases and literature review. Diagn Pathol. 2013;8:147. doi: 10.1186/1746-1596-8-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butrynski JE, D’Adamo DR, Hornick JL, Dal Cin P, Antonescu CR, Jhanwar SC, Ladanyi M, Capelletti M, Rodig SJ, Ramaiya N, Kwak EL, Clark JW, Wilner KD, Christensen JG, Janne PA, Maki RG, Demetri GD, Shapiro GI. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363:1727–1733. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Z, Hill DA, Collins MH, Morris SW, Sumegi J, Zhou M, Zuppan C, Bridge JA. Fusion of ALK to the Ran-binding protein 2 (RANBP2) gene in inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2003;37:98–105. doi: 10.1002/gcc.10177. [DOI] [PubMed] [Google Scholar]
- 20.Fang H, Langstraat CL, Visscher DW, Folpe AL, Schoolmeester JK. Epithelioid inflammatory myofibroblastic sarcoma of the ovary with RANB2-ALK fusion: report of a case. Int J Gynecol Pathol. 2018;37:468–472. doi: 10.1097/PGP.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 21.Dymond S, Haselgrove M, McGregor A. Clever crows or unbalanced birds? Proc Natl Acad Sci U S A. 2013;110:E336. doi: 10.1073/pnas.1218931110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tothova Z, Wagner AJ. Anaplastic lymphoma kinase-directed therapy in inflammatory myofibroblastic tumors. Curr Opin Oncol. 2012;24:409–413. doi: 10.1097/CCO.0b013e328354c155. [DOI] [PubMed] [Google Scholar]
- 23.Stenehjem DD, Tran D, Nkrumah MA, Gupta S. PD1/PDL1 inhibitors for the treatment of advanced urothelial bladder cancer. Onco Targets Ther. 2018;11:5973–5989. doi: 10.2147/OTT.S135157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguiar PN Jr, De Mello RA, Hall P, Tadokoro H, Lima Lopes G. PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: updated survival data. Immunotherapy. 2017;9:499–506. doi: 10.2217/imt-2016-0150. [DOI] [PubMed] [Google Scholar]
- 25.Zandberg DP, Strome SE. The role of the PD-L1: PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2014;50:627–632. doi: 10.1016/j.oraloncology.2014.04.003. [DOI] [PubMed] [Google Scholar]


