Abstract
Pituitary adenoma is a common intracranial tumor, but the underlying molecular carcinogenesis mechanisms remain unclear. Accumulative evidence has demonstrated that aberrant expression of microRNAs (miRNAs) is an important feature of cancer. The aim of the current study was to explore the role of miR-137 in pituitary tumor. The expression level of miR-137 in pituitary tumor tissues was measured by quantitative RT-PCR. Then the effects of miR-137 upregulation/downregulation on the proliferation and invasion as well as the potential molecular mechanisms were further investigated. Our results showed that the expression level of miR-137 was significantly reduced in pituitary tumor tissues compared to normal controls. Ectopic expression of miR-137 inhibited the proliferation and invasion of pituitary tumor cells, while miR-137 suppression promoted the proliferation and invasion capacity of cancer cells. Bioinformatic analysis of the downstream targets of miR-137 revealed that many enriched gene ontology functions and pathways were closely associated with carcinogenesis. Mechanically, AKT2 was demonstrated to be a direct downstream target of miR-137. The expression level of miR-137 was negatively correlated with AKT2 in pituitary tumor tissues. Taken together, miR-137 plays a tumor suppressive role in pituitary adenoma through regulating AKT2.
Keywords: Pituitary adenoma, miR-137, AKT2, proliferation, invasion
Introduction
Pituitary adenomas are common neoplasms, accounting for approximately 25% of all intracranial tumors [1]. Although the majority of pituitary adenomas are benign and slow-growing malignancy, patients might suffer from severe headache, visual problems, and hormone-associated symptoms [2]. Some pituitary adenomas are aggressive, leading to serious morbidity or even mortality. The etiology for the tumorigenesis of pituitary adenoma remains poorly understood. Therefore, elucidating the underlying molecular mechanisms of pituitary adenoma contributes to its early detection, treatment and prognosis.
MicroRNAs (miRNAs) are a class of small, endogenous non-coding RNA with 22-nt in length. They can regulate gene expression by binding the 3’-untranslated region (UTR) of their target mRNAs, leading to the translation inhibition or mRNA degradation [3]. miRNAs have been shown to be involved in numerous biologic processes such as proliferation, differentiation, apoptosis and development [4]. Deviant levels of miRNAs have been closely linked to various human disorders including cancer [5]. miRNA might be either a tumor suppressor or oncogenic promoter during tumorigenesis of cancer. For instance, the expression level of miR-16 was downregulated in pituitary tumor tissues, and reduced miR-16 was associated with unfavorable clinical outcome. In addition, overexpression of miR-16 inhibited cancer cell proliferation and promoted apoptosis [6], indicating that miR-16 acts as a tumor suppressor gene in pituitary tumor. The miR-106b levels were remarkably increased in pituitary adenoma. Downregulation of miR-106b significantly reduced the proliferation and invasion capacity of pituitary adenoma cells [7], suggesting that miR-106b plays an oncogenic role in the progression of pituitary adenoma.
miR-137, a nervous system enriched miRNA, is located within a long non-coding host gene named MIR137HG. The downstream targets of miR-137 are closely linked with cell cycle, proliferation, and differentiation [8]. Deregulation of miR-137 has been reported in various tumor types such as pancreatic cancer, melanoma, and lung cancer [9-11]. However, its role in pituitary tumor is poorly known. The current study aimed to elucidate the expression pattern and the regulatory mechanisms of miR-137 in pituitary tumor.
Materials and methods
Patient samples and cell culture
A total of forty patients with pituitary tumors were enrolled in this study. Another 20 normal tissue samples obtained from head trauma surgery were recruited as the control group. There were 27 patients with non-invasive pituitary tumors and 13 patients with invasive pituitary tumors respectively. The study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University, and all the patients gave written informed consent.
The human pituitary adenoma cell line HP-75 was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). HP-75 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. They were incubated at 37°C in a humidified atmosphere with 5% CO2.
Cell transfection
All RNA oligonucleotides were synthesized by Shanghai GenePharma Co., Ltd (Shanghai, China). The HP-75 cells were transfected with either miR-137 mimic, miR-137 inhibitor, and their respective controls (negative control mimic/negative control inhibitor) with Lipofectamine® RNAiMAX Transfection Reagent (Invitrogen, Carlsbad, CA, USA).
Cell count
The transfected cells were harvested with 0.5% trypsin and suspended in complete culture medium. They were seeded at a density of 1 × 105 cells per well in 12-well plated. Cell counts were performed following 24, 48, 72 and 96 h of culture using a hemocytometer under a light microscope. The trypan blue was used to exclude the dead cells.
CCK-8 assay
The transfected cells were harvested and seeded at a density of 3 × 103 cells per well in 96-well plates. Briefly, 10 µl CCK-8 reagent (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added to culture medium and incubated at 37°C for 4 h. Absorbance was measured at a wavelength of 450 nm following 24, 48, 72 and 96 h of culture with a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
Transwell invasion assay
The effects of miR-137 upregulation/downregulation were assessed using Matrigel-coated transwell chamber with a pore size of 8 µm (Corning Inc., Corning, NY, USA). Briefly, 2 × 104 transfected cells in 200 µl of DMEM were seeded into the upper chamber. Then 800 µl complete culture medium was added to the lower chamber. After 36 h of incubation, the cells remaining in the upper chamber were removed with cotton swab. Those have invaded to the lower chamber were fixed with 100% cold methanol and stained with crystal violet. The results were visualized and counted under a microscope.
Quantitative RT-PCR
Total RNA was isolated from cells and tissues using the TRIzol reagent (Takara, Dalian, China) according to the manufacturer’s instruction. The SYBR PrimeScript RT-PCR kit (Takara) was used for reverse-transcribing the RNA to cDNA. Quantitative RT-PCR was conducted using SYBR® Premix Ex TaqTM II (Takara) on an ABI 7500 fast real-time PCR system (Applied Biosystems, Waltham, MA, USA). U6 and β-actin were used as the endogenous control for miR-137 and AKT2 respectively. Relative fold changes were calculated using the 2-ΔΔCT method. The primers used were as follows: miR-137-forward, 5’-TTATTGCTTAAGAATACGCG-3’; miR-137-reverse, 5’-TCGTATCCAGTGCAGGGTC-3’; U6-forward, 5’-GCTTCGGCAGCACATATACTAAAAT-3’; U6-reverse, 5’-CGCTTCACGAA-TTTGCGTGTCAT-3’; AKT2-forward, 5’-TCACATGGATTTGGAGGCACGCC-3’; AKT2-reverse, 5’-TACTGCTTTTGTGCCCCAGGTGG-3’; ACTB-forward 5’-AAGGCCAACC-GTGAAAAGAT-3’; ACTB-reverse 5’-GTGGTACGACCA GAGGCATAC-3’.
Luciferase reporter assay
Cells were co-transfected with pMIR-AKT2-3’-UTR wild-type vector or pMIR-AKT2-3’-UTR mutant vector and miR-137 mimic or NC mimic using Lipofectamine® 2000 (Invitrogen). Following incubation for 48 h, the luciferase reporter activity was determined using a Dual Luciferase Reporter Assay Kit (Promega, Madison, WI, USA) based on the manufacturer’s instructions. Renilla luciferase activity was normalized to Firefly luciferase expression.
Statistical analysis
All data were expressed as the mean ± standard deviation. All statistical analyses were performed using Graphpad Prism version 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). The differences between two groups were analyzed with the Student’s t-test. Correlation analysis was performed to determine the association between miR-137 and AKT2 expression level in the pituitary tumor tissues. The Database for Annotation, Visualization and Integrated Discovery was used for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis. P values < 0.05 were considered significant.
Results
miR-137 was downregulated in pituitary tumor tissues
We first compared the miR-137 level between pituitary tumor tissues and the normal control tissues. Our results showed that the expression level of miR-137 was dramatically reduced in pituitary tumor tissues compared to the normal control tissues (***P < 0.001) (Figure 1A). Interestingly, miR-137 levels were significantly lower in invasive pituitary tumor than non-invasive pituitary tumor (***P < 0.001) (Figure 1B).
Figure 1.
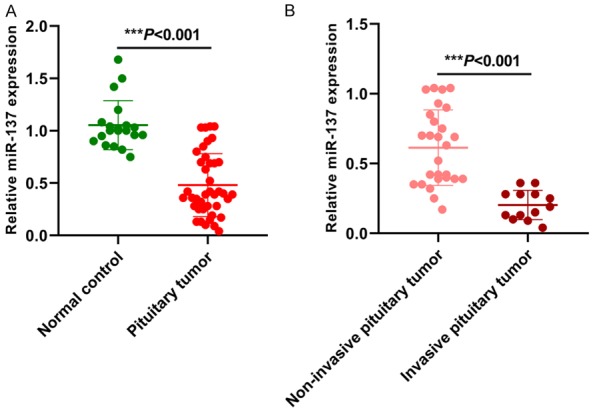
Expression pattern of miR-137 in pituitary tumor tissues.
Effects of miR-137 upregulation on the proliferation and invasion of pituitary tumor cells
Quantitative RT-PCR was performed to evaluate the transfection efficiency of miR-137 mimic. The expression level of miR-137 was significantly higher in the miR-137 mimic transfected cancer cells compared with the NC mimic transfected cells (***P < 0.001) (Figure 2A). The cell count assay showed that the number of cells was markedly lower in the miR-137 mimic group than the control group at day 3 and 4 (***P < 0.001) (Figure 2B). Similarly, the OD values were lower in the miR-137 mimic group than the control group at day 2, 3 and 4 (**P < 0.01; ***P < 0.001) (Figure 2C). The matrigel invasion assay revealed that miR-137 mimic transfected cancer cells had lower invasive capacity than the NC mimic transfected cells (***P < 0.001) (Figure 2D, 2E).
Figure 2.
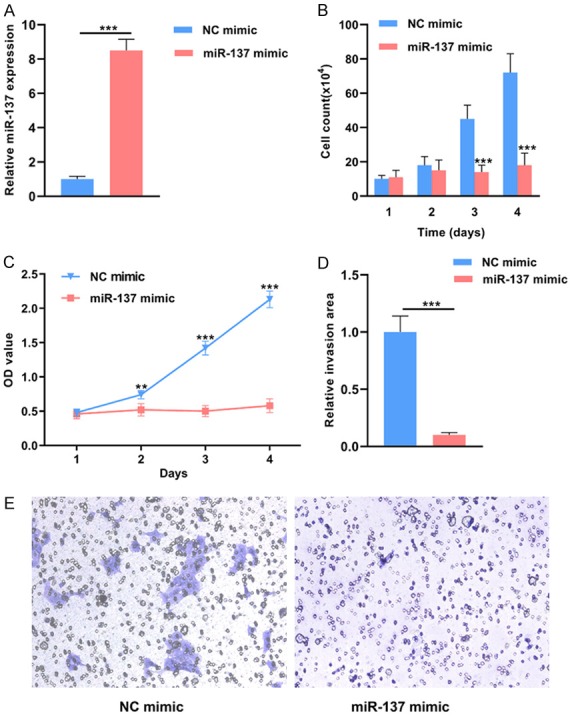
Effects of miR-137 overexpression on the proliferation and invasion of pituitary tumor cells.
The effects of miR-137 downregulation on the proliferation and invasion of pituitary tumor cells
miR-137 level was significantly lower in the miR-137 inhibitor group compared to the NC inhibitor group (***P < 0.001) (Figure 3A). The cell count assay demonstrated that the number of cells was higher in the miR-137 inhibitor group than the control group at day 2, 3 and 4 (*P < 0.05, **P < 0.01) (Figure 3B). The OD values were higher in miR-137 inhibitor group than the control group at day 2, 3 and 4 (*P < 0.05, **P < 0.01) (Figure 3C). The matrigel invasion assay showed that miR-137 inhibitor transfected cancer cells had higher invasive capacity than the NC inhibitor transfected cells (**P < 0.01) (Figure 3D, 3E).
Figure 3.
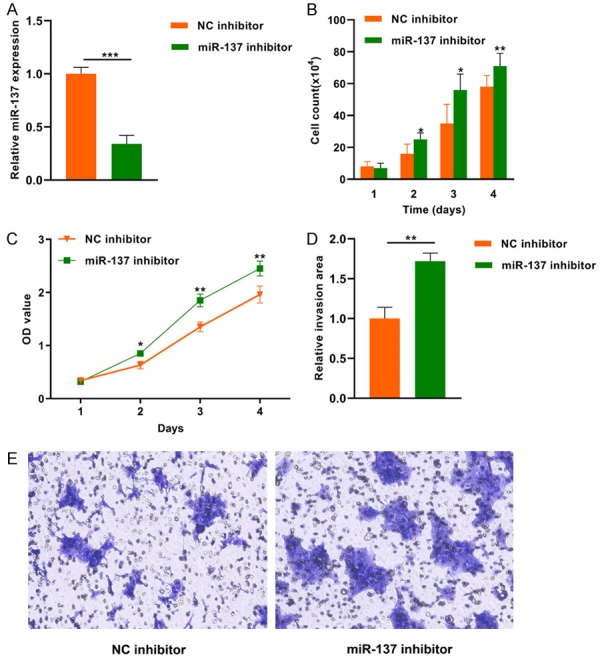
Effects of miR-137 downregulation on the proliferation and invasion of pituitary tumor cells.
GO and KEGG analysis of the downstream targets of miR-137
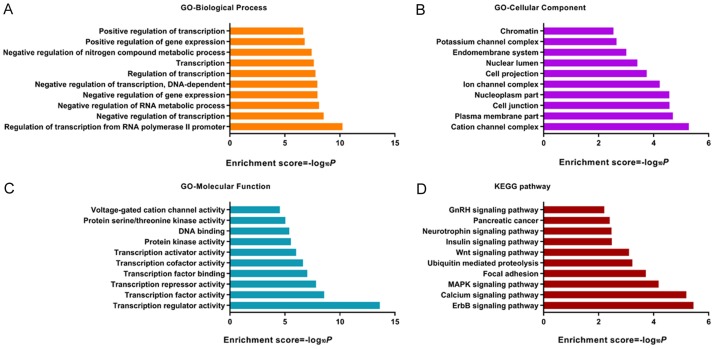
GO and KEGG analysis of the downstream targets of miR-137 were performed. Our results showed that regulation of transcription from RNA polymerase II promoter, negative regulation of transcription, negative regulation of RNA metabolic process, negative regulation of gene expression, negative regulation of transcription, DNA-dependent, regulation of transcription, transcription, negative regulation of nitrogen compound metabolic process, positive regulation of gene expression and positive regulation of transcription were the top enriched biologic processes (Figure 4A). Cation channel complex, plasma membrane part, cell junction, nucleoplasm part, ion channel complex, cell projection, nuclear lumen, endomembrane system, potassium channel complex and chromatin were the top enriched cellular components (Figure 4B). Transcription factor activity, transcription repressor activity, transcription factor binding, transcription cofactor activity, transcription activator activity, protein kinase activity, DNA binding, protein serine/threonine kinase activity and voltage-gated cation channel activity were the top enriched molecular functions (Figure 4C). ErbB signaling pathway, calcium signaling pathway, MAPK signaling pathway, focal adhesion, ubiquitin mediated proteolysis, Wnt signaling pathway, insulin signaling pathway, neurotrophin signaling pathway, pancreatic cancer, and GnRH signaling pathway were the top enriched pathways (Figure 4D).
Figure 4.
GO and KEGG analysis of the downstream targets of miR-137.
AKT2 is a direct downstream target of miR-137
Figure 5A showed that the 3’-UTR of AKT2 was highly complementary to the seed sequence of miR-137. In addition, the 3’-UTR of AKT2 that miR-137 targeted was highly conserved across different species (Figure 5B). For the wild type AKT2 vector, the luciferase reporter assay revealed that the relative luciferase activity was significantly lower in the miR-137 mimic group compared to that in the NC mimic group. However, no significant difference was found for the AKT2 mutant vector between groups (Figure 5C). The expression level of AKT2 was significantly lower in miR-137 mimic group, while higher in miR-137 inhibitor group compared with their respective controls (Figure 5D).
Figure 5.
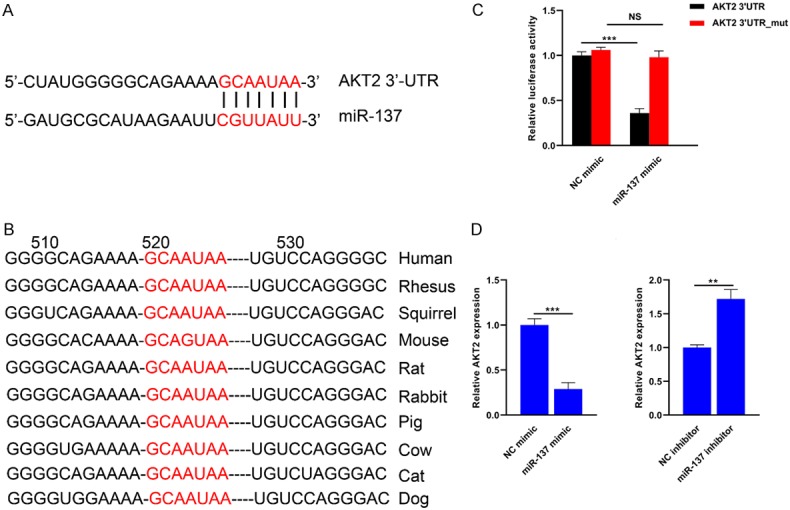
AKT2 is a direct downstream target of miR-137.
miR-137 level was inversely associated with AKT2 in pituitary tumor tissues
We then explored the association between miR-137 and AKT mRNA in the above forty pituitary tumor tissues. Our results showed that a negative correlation was found between miR-137 and AKT mRNA in the pituitary tumor tissues (r = -0.6846, ***P < 0.001) (Figure 6).
Figure 6.
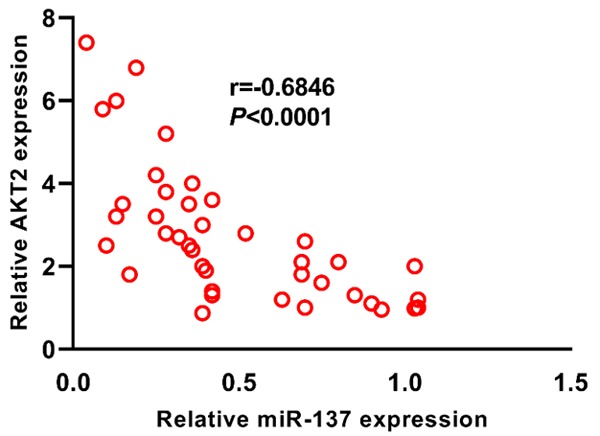
The expression level of miR-137 is negatively associated with AKT2 expression in pituitary tumor tissues.
Discussion
In this study, our results showed that the expression level of miR-137 was significantly downregulated in pituitary tumor tissues, especially those with invasive capacity. In addition, overexpression of miR-137 suppressed the proliferation and invasion of pituitary tumor cells, whereas downregulation of miR-137 promoted malignant behaviors. Bioinformatic analysis of the downstream targets of miR-137 showed that many enriched GO functions and pathways were closely associated with carcinogenesis. Moreover, AKT2 was identified as a direct target of miR-137. Furthermore, the expression level of miR-137 was negatively correlated with AKT2 in pituitary tumor tissues. Collectively, miR-137 acted as a tumor suppressor in pituitary tumor and its downregulation might promote carcinogenesis through upregulating AKT2.
There are three highly conserved members (AKT1, AKT2 and AKT3) in the AKT family [12]. PI3K-AKT activation is regarded as one of the most important pathways responsible for the oncogenic transformation in various types of cancers [13,14]. miR-137 was an upstream regulator of AKT2 in pituitary tumor, which might partially explain the importance of miR-137 in the carcinogenesis of pituitary tumor. One limitation of our study was the small sample size. Further studies with larger patient cohort are warranted to validate our findings. In addition, animal studies are needed to explore the role of miR-137 in vivo.
Consistent with our findings, miR-137 was hypermethylated in colorectal cancer (CRC), and ectopic expression of miR-137 suppressed the proliferation of CRC cells by regulating LSD-1, indicating that miR-137 functioned as a tumor suppressor gene in CRC [15]. Similarly, Langevin et al. reported that aberrant miR-137 promoter methylation was frequently detected in head and neck squamous cell carcinoma, and the methylation status was associated with prognosis [16]. The expression level of miR-137 was markedly downregulated in lung cancer cell lines. Overexpression of miR-137 restrained the proliferation of lung cancer cells in vitro and tumor growth in vivo. In addition, cdc42 and cdk6 were demonstrated to be downstream targets of miR-137 [17]. Low miR-137 level was strongly correlated with unfavorable clinical outcome of patients with neuroblastoma. Upregulation of miR-137 in neuroblastoma cell lines decreased cell proliferation and viability as well as promoted apoptosis by regulating KDM1A [18]. Cheng et al. reported that the miR-137 level was lower in the gastric cancer tissues and cell lines compared to their respective controls. In addition, miR-137 overexpression suppressed the proliferation, migration, and invasion of gastric cancer cells in vitro, and tumor growth in vivo. Moreover, ectopic expression of COX2 could partially rescue the tumor suppressive effects of miR-137, indicating that COX2 was a functional downstream target of miR-137 [19]. Interestingly, Li et al. showed that the serum miR-134 level was reduced in patients with glioblastoma. Low serum miR-137 was strongly linked with worse clinical parameters and overall survival [20]. Further studies are warranted to explore the expression pattern of miR-137 in the biofluids of patients with pituitary tumor and its potential clinical significance.
Although most studies found that miR-137 functioned as a tumor suppressor gene in most tumor types, miR-137 might also promote tumor progression. For instance, miR-137 was found to be a target of Slug, a known epithelial mesenchymal transition regulator. Downregulation of miR-137 in lung cancer cells abolished Slug induced invasion and migration, and upregulation of miR-137 promoted invasion by regulating TFAP2C, indicating that miR-137 might function as an oncogene in lung cancer [21]. Ectopic expression of miR-137 promoted the anti-apoptosis and cisplatin resistance capacity of in lung adenocarcinoma cells [22]. The contrary role of miR-137 in different types of cancers or even in the same type of cancer needs further investigation.
In conclusion, this is the first study to explore the role of miR-137 in pituitary tumor. miR-137 is reduced in pituitary tumor tissues and its downregulation is associated with invasiveness. Overexpression of miR-137 suppresses the proliferation and invasion capacity of pituitary tumor cells, and vice versa. AKT2 is a direct target of miR-137. Therefore, targeting miR-137-AKT2 axis might provide a novel therapeutic option for treating pituitary tumor.
Acknowledgements
This study was supported by the Natural Science Fund of Jiangxi Province (2014ZBAB205019), Jiangxi Province health planning committee scientific and technological plan (20165116), the National Natural Science Foundation of China (81560220), the Youth Science Fund of Jiangxi Province (20151BAB215014), the Key Project of Jiangxi Youth Science Foundation (20171ACB21054).
Disclosure of conflict of interest
None.
References
- 1.Gillam MP, Molitch ME, Lombardi G, Colao A. Advances in the treatment of prolactinomas. Endocr Rev. 2006;27:485–534. doi: 10.1210/er.2005-9998. [DOI] [PubMed] [Google Scholar]
- 2.Chanson P, Salenave S. Diagnosis and treatment of pituitary adenomas. Minerva Endocrinol. 2004;29:241–275. [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM, Zhang GZ. Biological functions of microRNAs: a review. J Physiol Biochem. 2011;67:129–139. doi: 10.1007/s13105-010-0050-6. [DOI] [PubMed] [Google Scholar]
- 5.Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol. 2012;6:590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu B, Liu GL, Yu F, Li WJ, Xiang XX, Xiao HZ. MicroRNA-16/VEGFR2/p38/NF-κB signaling pathway regulates cell growth of human pituitary neoplasms. Oncol Rep. 2018;39:1235–1244. doi: 10.3892/or.2018.6227. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Z, Zhang Y, Zhang Z, Yang Y, Song T. Effect of miR-106b on invasiveness of pituitary adenoma via PTEN-PI3K/AKT. Med Sci Monit. 2017;23:1277–1285. doi: 10.12659/MSM.900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahmoudi E, Cairns MJ. MiR-137: an important player in neural development and neoplastic transformation. Mol Psychiatry. 2017;22:44–55. doi: 10.1038/mp.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding F, Zhang S, Gao S, Shang J, Li Y, Cui N, Zhao Q. MiR-137 functions as a tumor suppressor in pancreatic cancer by targeting MRGBP. J Cell Biochem. 2018;119:4799–4807. doi: 10.1002/jcb.26676. [DOI] [PubMed] [Google Scholar]
- 10.Peres J, Kwesi-Maliepaard EM, Rambow F, Larue L, Prince S. The tumour suppressor, miR-137, inhibits malignant melanoma migration by targetting the TBX3 transcription factor. Cancer Lett. 2017;405:111–119. doi: 10.1016/j.canlet.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Zhang B, Liu T, Wu T, Wang Z, Rao Z, Gao J. microRNA-137 functions as a tumor suppressor in human non-small cell lung cancer by targeting SLC22A18. Int J Biol Macromol. 2015;74:111–118. doi: 10.1016/j.ijbiomac.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Hers I, Vincent EE, Tavaré JM. Akt signalling in health and disease. Cell Signal. 2011;23:1515–1527. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 14.Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46:372–383. doi: 10.3109/07853890.2014.912836. [DOI] [PubMed] [Google Scholar]
- 15.Balaguer F, Link A, Lozano JJ, Cuatrecasas M, Nagasaka T, Boland CR, Goel A. Epigenetic silencing of miR-137 is an early event in colorectal carcinogenesis. Cancer Res. 2010;70:6609–6618. doi: 10.1158/0008-5472.CAN-10-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langevin SM, Stone RA, Bunker CH, Lyons-Weiler MA, LaFramboise WA, Kelly L, Seethala RR, Grandis JR, Sobol RW, Taioli E. MicroRNA-137 promoter methylation is associated with poorer overall survival in patients with squamous cell carcinoma of the head and neck. Cancer. 2011;117:1454–1462. doi: 10.1002/cncr.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu X, Li Y, Shen H, Li H, Long L, Hui L, Xu W. miR-137 inhibits the proliferation of lung cancer cells by targeting Cdc42 and Cdk6. FEBS Lett. 2013;587:73–81. doi: 10.1016/j.febslet.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Althoff K, Beckers A, Odersky A, Mestdagh P, Köster J, Bray IM, Bryan K, Vandesompele J, Speleman F, Stallings RL, Schramm A, Eggert A, Sprüssel A, Schulte JH. MiR-137 functions as a tumor suppressor in neuroblastoma by downregulating KDM1A. Int J Cancer. 2013;133:1064–1073. doi: 10.1002/ijc.28091. [DOI] [PubMed] [Google Scholar]
- 19.Cheng Y, Li Y, Liu D, Zhang R, Zhang J. miR-137 effects on gastric carcinogenesis are mediated by targeting Cox-2-activated PI3K/AKT signaling pathway. FEBS Lett. 2014;588:3274–3281. doi: 10.1016/j.febslet.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Li HY, Li YM, Li Y, Shi XW, Chen H. Circulating microRNA-137 is a potential biomarker for human glioblastoma. Eur Rev Med Pharmacol Sci. 2016;20:3599–3604. [PubMed] [Google Scholar]
- 21.Chang TH, Tsai MF, Gow CH, Wu SG, Liu YN, Chang YL, Yu SL, Tsai HC, Lin SW, Chen YW, Kuo PY, Yang PC, Shih JY. Upregulation of microRNA-137 expression by Slug promotes tumor invasion and metastasis of non-small cell lung cancer cells through suppression of TFAP2C. Cancer Lett. 2017;402:190–202. doi: 10.1016/j.canlet.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Su TJ, Ku WH, Chen HY, Hsu YC, Hong QS, Chang GC, Yu SL, Chen JJ. Oncogenic miR-137 contributes to cisplatin resistance via repressing CASP3 in lung adenocarcinoma. Am J Cancer Res. 2016;6:1317–1330. [PMC free article] [PubMed] [Google Scholar]