Abstract
The measurement of analyte concentration is a critical part of successful bioreactor and clinical monitoring. Although strategies exist for measuring the majority of relevant analytes, industrial on-line bioreactor control is carried out primarily by measurement and control of pH, pO2 and, in some cases, cell density. This is because the available technology cannot be easily and inexpensively adapted to (a) measure the analyte in an aseptic manner and/or allow for remote sensing, and (b) measure in real time so that on-line control is possible. Similar issues need to be addressed for biosensors for clinical applications. A rapidly emerging technology that has the potential of meeting these challenges is lifetime-based phase-modulation fluorimetry, an optical technique that uses the measurement of fluorescence lifetime rather than intensity for determining the concentration of an analyte.
Advances in genetic engineering have, over the past two decades, generated a wealth of novel molecules that have redefined the role of microbes in solving environmental, pharmaceutical, industrial and agricultural problems. While some products have entered the marketplace, the difficulties of doing so and of complying with federal mandates of ‘safety, purity, potency, efficacy and consistency’ have shifted the focus from the word ‘genetic’ to the word ‘engineering’. This transition from the laboratory to production – the basis of bioprocess engineering – involves a careful understanding of the conditions most favored for optimal microbial production, and the duplication of these conditions during scaled-up production.
The majority of bioprocesses are based on the cell as the simplest functional unit. The ability of the cell to survive and carry out its task of bioconversion is extremely sensitive to its physiochemical environment. Our ability to control bioconversion is therefore contingent upon how closely we can monitor and control this complex environment. This translates into a need to monitor simultaneously several parameters in real time. On-line monitoring of temperature, pH, pO2, cell density and culture fluorescence using sterilizable devices is currently possible. However, a variety of other parameters that have profound effects on cellular productivities (such as glucose, CO2, ammonium, phosphate and miscellaneous cation and anion levels in the medium), continue to be measured off-line due to technological limitations. The length of time (typically 20 minutes) required for off-line measurements decreases the usefulness of the measurement as well as adding to the labor and other costs. In addition, the need to withdraw samples frequently increases the risk of contamination.
There is, therefore, a strong need to develop robust sensor technology which would permit the real-time monitoring of all these parameters. Ideally, such technology would consist of a single set of instrumentation based on a range of sensors for various analytes, using fluorophores that share the same modulation frequency: this would permit the use of ‘time-share’ electronics for signal transduction and processing for all the sensors. The availability of sensors that can be integrated on-line into the bioreactor, require minimal maintenance and calibration, have high sensitivity and are readily sterilized, would dramatically improve bioprocess management. Although the requirements for clinical monitoring are more stringent and tightly regulated, similar concerns prevail. The technology we describe here, lifetime-based phase-modulation fluorimetry, is particularly applicable to making optical measurements in biological media since it is relatively insensitive to interference. Moreover, the availability of probes based on fluorophores that are excited by red light might enable through-tissue sensing. Consequently, although our discussion focuses on application to bioprocess monitoring, key characteristics of these sensors also make them appropriate for measuring clinically important analytes.
Current technologies
Sensors currently used for monitoring are based on decades-old electrochemical methods (both amperometric and potentiometric). The need to autoclave sensors has prevented the use of other in situ chemical sensors. Moreover, both pH and pO2 electrodes have problems associated with them but continue to be used for lack of an alternative1,2. These problems include calibration drifts, flow dependence, slow response times and electrical interference1–3. Although electrochemical probes have been described for measuring other parameters, such as glucose and CO2 concentrations, similar problems have limited their application in monitoring the dense microbial cultures prevalent in bioreactor cultures4,5.
Fluorescence detection has been extensively investigated as an alternative to electrochemical sensing6–9. This has led to the development of optical probes or ‘optrodes’ as a possible replacement for the widely used electrochemical ‘electrodes’. Of the various schemes for fluorescence sensing, the most studied – steady-state intensity measurements – have not found wide acceptance because of problems of stability and calibration. Intensity measurements are unsuitable for blood or cell culture because of the effects of scattering and absorption by the medium (collectively termed ‘inner filter effects’). An alternative to intensity measurements is the wavelength-ratiometric method in which either (a) the ratio of the intensities at two different emission wavelengths for a single excitation wavelength, or (b) a ratio of the intensities obtained at a single emission wavelength for two different excitation wavelength is plotted as a function of analyte concentration. Wavelength-ratiometric methods are independent of probe concentration and inner filter effects, but have not been applied extensively because of the lack of intensity-ratio probes. The calcium probe Fura-2 is one of the few such probes available10. Ratiometric measurements have been used for on-line pH monitoring in bioreactors11.
The promise of Flow Injection Analysis (FIA) as a means of circumventing the drawbacks of current analytical techniques has not found industrial acceptance either. In FIA, cell-free medium is continuously withdrawn from the culture to monitor concentrations of one or more analytes. The analyte is measured after appropriate dilution to bring its concentration within the sensitivity range of the sensor. By withdrawing cell-free medium, the measurement is not corrupted by the presence of cells, which contribute to sensor fouling, or by inner filter effects. However, fouling of the sampling probe remains a problem12, causing changes in sample volume and consequently uncertainties in the measurement if dilutions are conducted. In addition, the need to have a recycle loop or a penetration into the aseptic bioreactor environment increases contamination risks. Finally, because of the time delay in the recycle loop, FIA is at best a quasi on-line technique with significantly slower response times when compared with traditional on-line methods13.
Fluorescence lifetime measurement
Fluorescence lifetime measurement is distinct from intensity-based methods in that it measures changes in the fluorescence decay time of a fluorophore. Fluorescence is the result of photon emission by molecules in electronically excited states. Following light absorbtion, the fluorescence lifetime of a substance represents the average amount of time a molecule remains in the excited state prior to its return to the ground state. Fluorescence lifetime or decay time typically occurs over tens of nanoseconds. A related phenomenon, phosphorescence14, occurs over much longer time periods (typically milliseconds) but is not likely to provide a general basis for sensing due to the lack of phosphorescence from most chromophores. Of the two available methods for measuring fluorescence lifetimes, time-domain and frequency-domain, the former calls for more expensive instrumentation and complex electronics (Fig. 1a). Fortunately, recent developments in the frequency-domain technique, known as phase-modulation fluorimetry, have made possible reliable and inexpensive measurements of nanosecond lifetimes15. In phase-modulation fluorimetry, sinusoidally modulated light excites a fluorescent chemical probe. The fluorescence emission is thus forced to oscillate at the same frequency. As a result of the analyte-dependent, finite fluorescence lifetime of the probe, the emission is phase shifted and demodulated with respect to the excitation. Both phase and demodulation values provide independent measurements of the analyte concentration, adding to the robustness of the technique. However, to keep instrumentation costs low, generally only phase-sensitive detection is employed.
Figure 1.
(a) Schemes for lifetime-based sensing. In time-domain sensing, the fluorescence intensity decay following excitation by what is ideally an infinitely short pulse of light is collected. The logarithm of the intensity decay is plotted against time, and the lifetime obtained (left). In phase-modulation fluorimetry, the fluorophore is continuously excited by an intensity-modulated light beam. The emission is consequently modulated at the same frequency. However, due to the finite fluorescence lifetime of the fluorophore, the emission is phase shifted (Φ) and demodulated (m = bA/aB) with respect to the excitation. The fluorescence lifetime is calculated from either the phase shift or the demodulation values. (b) Optical oxygen sensor using phase fluorimetry. A blue LED is used as the light source and is amplitude-modulated by a signal derived from a crystal oscillator. The light from the LED is filtered and fed into one of the arms of a bifurcated fiber bundle. The fluorescence is collected from the other arm, and is observed through a filter using a photomultiplier tube or a large-area PIN photodiode. The fluorescent probe immobilized in a silicone rubber membrane is held in front of the common arm of the fiber bundle as shown. The common arm is housed in a stainless steel (SS) tube which sits inside the bioreactor. The optrode end of the sensor (which includes the sensor membrane, SS tube and the fiber bundle) is detachable and can be autoclaved. The phase difference between the signal from the photodetector and the signal generator is obtained by the phase detector. This phase difference, previously calibrated against oxygen partial pressure, is converted into the latter and displayed on the computer using a standard data-acquisition system.
The phase shift (Φ) and the demodulation factor (m) (see Fig. 1a) are related to the fluorescence lifetime (τ) of single exponentially decaying fluorophores according to the relationships:
| (1) |
While single, exponentially decaying fluorophores are rarely encountered in practice, direct calibrations between the analyte concentration and a measured parameter (e.g. Φ) are readily established using calibration methods. Figure 1b illustrates the simplicity of the electronics and optics involved in a phase fluorimetric oxygen sensor. In this case, direct calibration (without first calculating the fluorescence lifetimes) of the observed phase angles against pO2 proved sufficient in developing an effective oxygen-sensing strategy16.
In general, probes with long lifetimes (a few hundred nanoseconds) are preferred for various sensing applications. The primary motivation is that such chemical probes require less expensive detection instrumentation than those with shorter lifetimes. Another advantage that the use of long lifetime metal–ligand complexes confers is off-gating of the prompt autofluorescence from tissue components. Several short-lifetime (a few nanoseconds) probes exist for a number of analytes, but they are not easily packaged as analytical sensors and currently require expensive accessories, such as large mainframe lasers, bulky acoustic or electro-optical modulators and sophisticated software, to make meaningful measurements. This may change in the near future with new compact and inexpensive light sources that are capable of being modulated at high frequencies (≈100 MHz).
Advances in fiber optics, light sources, detectors and other instrumentation (largely due to the defense and communications industries) coupled with the availability of novel synthetic fluorophores continue to make practical applications of this technology possible. Powerful and inexpensive light sources such as red (>600 nm) laser diodes that can be intrinsically modulated to several GHz and the recent availability of high power, modulatable (1000 millicandela, ≈100 MHz) blue gallium nitride light-emitting diodes (LEDs) (45030 nm, Nichia Chemical Industries, Japan) have facilitated the construction of simple, hand-held devices. Other light sources with significant potential are the electroluminescent lamps (400–600 nm) which are inexpensive but have low (<100 kHz) modulation limits and the frequency-doubled sources, where the output of a 850 nm laser diode is frequency doubled to 425 nm in an optical wave guide of potassium titanyl phosphate (KTP) (Pioneer Electronic, Tokyo, Japan, and DuPont Corporation, Wilmington, DE, USA). Recent advances in light detection, the ultra-compact photomultiplier tubes (PMTs) (Hamamatsu Corporation, Bridgewater, NJ, USA) as well as semiconductor detectors such as avalanche photodiodes (APDs) have helped make smaller, more compact detection systems. Although the gain of the APDs (around 100), is lower than the gain of the widely used PMTs, the APDs are significantly cheaper and are adequate for most sensing applications.
Decay-time sensing is advantageous because such measurements are independent of probe concentration, photobleaching and drifts in lamp intensity, are independent of inner filter effects, and do not require dual emission from the probe, all of which are major limitations of current fluorescence-intensity-based optrodes. The recent availability of novel synthetic fluorophores, particularly those with long lifetimes (hundreds of nanoseconds), has further facilitated the early transfer of this powerful technique into practical applications. Within a few years, bioreactor monitoring may well be implemented primarily through optical means. In this review, we have focused primarily upon in situ pO2, pH, pCO2 and glucose measurements, but the methodology is generic and can be readily extended to a variety of other analytes.
Oxygen sensor
Perhaps the most important bioprocess measurement is that of dissolved oxygen tension (pO2). Consequently, much effort in bioreactor design and operation is directed toward the monitoring and control of oxygen supply to the fermenter. Despite its many limitations, the amperometric Clark electrode17 continues to be widely used for this purpose. In this sensor, consumption of oxygen (and electrolyte) is monitored. It therefore suffers from flow dependence, slow response times, calibration drifts and electrical interference. Alternative technologies in this area are long overdue. It is now possible to monitor oxygen tensions through an optical alternative that is comparable in cost and superior in performance to the Clark electrode16.
The simplicity and robustness of phase fluorimetry is perhaps best exemplified by the optical oxygen sensor. The availability of long-lifetime synthetic probes has been particularly instrumental in reducing costs in this case, permitting low-frequency modulation using simple electronics (Fig. 1b). The excitation source is an inexpensive blue LED that is modulated simply by varying its supply current. Phase sensing of oxygen was first described by Wolfbeis and co-workers18. The phase fluorimetric oxygen sensor measures the quenching by oxygen of the transition metal complex, tris [4,7-diphenyl-l,10-phenanthroline] ruthenium (II)2+ (Ref. 19). This transduction scheme generally follows Stern Volmer kinetics15 which predict a first-order-type response to oxygen tension resulting in higher oxygen sensitivities at low oxygen concentrations. The fluorophore is immobilized in a silicone rubber membrane which is an ideal matrix for this application because of its high oxygen solubility and diffusivity. The sensor is equally sensitive to gaseous and dissolved oxygen tensions. The silicone matrix selects for gaseous analytes because of its hydrophobic nature, adding to sensor specificity. In addition, the hydrophobic matrix and the water insolubility of the ruthenium complex add to long-term sensor stability by preventing washing out and leaching of the probe. The sensor performed satisfactorily in a bioreactor environment, was autoclavable and was found to be superior to the Clark electrode in that it is maintenance free, shows no drift in calibration over weeks of operation and has a faster response16.
The recent availability of osmium complexes which have absorbance maxima that are red shifted (greater than 650 nm) has generated the possibility of clinical, non-invasive oxygen sensing, given that most biological tissue, including human skin, does not absorb strongly and has significantly reduced auto-fluorescence when illuminated with light at this wavelength20. We have recently demonstrated through-skin-phase angle sensing of oxygen (unpublished) using the complex [Os(2,2′,2″-terpyridine)22+] (Ref. 21), encouraging the possibility of low-cost transcutaneous sensing of tissue oxygen. For bioprocess applications, the use of red-sensitive probes should permit monitoring oxygen levels inside hollow-fiber and tissue reactors, since red light can penetrate deep into the highly light-scattering environment of these reactors. The clinical assessment of oxygenation in arteries and other tissues is of significant importance in monitoring critically ill patients. Arterial oxygenation problems are a common indication of loss of hemoglobin or of deteriorating lung function. Similarly, low tissue-oxygen indicates tissue hypoxia and loss of function. In general, disturbances in oxygenation result in decreased performance from organs highly dependent on oxygen, such as the heart and brain.
pH sensor
Real-time pH monitoring is necessary for bioreactor process control. However, the pH glass electrodes, which are widely used for this purpose, are far from perfect. In process monitoring, it is rarely possible to measure pH to within 0.25 pH units2. Ground loop currents, interactions between sample and electrolyte solutions, acid and alkali errors and frequent servicing requirements are major limitations of these sensors. Recent improvements in pH electrode design, for example in the Ingold DPAS pre-pressurized gel-filled pH electrode, have improved performance and reduced servicing requirements since there is no need to refill the electrode with electrolyte.
Optical pH sensing using phase-modulation fluorimetry has been demonstrated using fluorescent pH-sensitive dyes22. Newly available pH-sensitive fluorophores (Molecular Probes Inc., Eugene, OR, USA) were found to display large changes in lifetime (phase and modulation) making them suitable for precision pH measurement22. However, these dyes have nanosecond lifetimes, requiring high modulation frequencies (>100 MHz) and blue-green light (450–550 nm) excitation, translating once again into high costs that prevent routine applications.
To develop inexpensive, lifetime-based pH sensors, an alternative transduction strategy compatible with inexpensive red light sources is necessary. Currently, inexpensive red laser diodes (> 635 nm) that permit MHz modulation are readily available. Fluorescence Resonance Energy Transfer (FRET) has been successfully used in developing such a strategy23. This involves using two dyes, a high-quantum-yield fluorescence donor and a pH-sensitive acceptor whose absorbance overlaps significantly with the donor’s emission. FRET is a non-radiative, distance-dependent dipole–dipole-type interaction in which the acceptor quenches the donor fluorescence15,24. Optimal energy transfer is obtained when the donor–acceptor pair are within 40–70 Å of each other. Although this distance (known as the Förster distance of energy transfer) can be fixed by covalently linking the molecules, such linkages require probe-specific chemistries, thus preventing their ready substitution with a variety of dyes.
An identical effect can readily be achieved through co-immobilization of the dyes at concentrations sufficient to mimic the distance requirements. While the O2 probe was easily entrapped in silicone rubber, the pH probes require a more hydrophilic matrix. A recent report describes the use of sol-gel technology in developing FRET-based pH sensors25. Sol-gel is a room-temperature glass-forming technology26 that results in a unique, water-permeable matrix with good optical properties. Such matrices have previously been used to entrap organic dyes (for review see Ref. 27) and have been found to provide an environment that is optically acceptable and chemically inert. Using Texas Red Hydrazide as the donor and Bromothymol blue as the acceptor, entrapment of the dyes in a sol-gel matrix resulted in a unique, chemically active, optical sensing material25. The sensor was compatible with red light sources and, by using thin coatings of this matrix over normal glass substrates, fast (millisecond) response times were observed. The pH-dependent changes in fluorescence intensity and phase angles were reproducible and reversible and the sensor was found to be stable and to retain its pH-sensing capabilities even after repeated autoclaving.
pCO2 sensor
The measurement of pCO2 is essentially that of pH and is often used in determining the metabolic state of the cells in a fermentation and in evaluating the oxygen uptake rate. A pH electrode in contact with a bicarbonate solution and isolated from the sample fluid by a Teflon or plastic membrane is routinely used for this purpose28. CO2 diffuses across the membrane and changes the pH of the bicarbonate solution (Reaction 1) in proportion to the CO2 partial pressure in the sample. Such a system is understandably bulky, has a slow response and has all the drawbacks of a glass pH electrode. This perhaps explains why it is not widely used in biochemical engineering laboratories and process streams despite the considerable metabolic significance of pCO2 measurement5.
| Reaction 1 |
Recently, fiber optic CO2 sensors have been investigated29. These sensors measure the fluorescence response of a pH-sensitive dye in bicarbonate/aqueous systems. Water is an important part of all such sensors, making them bulky. The loss of this water by evaporation severely limits the prolonged use of these sensors in the field and also severely limits their shelf life. In addressing these issues, Mills et al.30 have described a colorimetric CO2 sensor that incorporates all the necessary ingredients, including water molecules, in a hydrophobic (plastic) film with the help of a phase-transfer catalyst30,31. The CO2 diffusing into the plastic matrix generates protons (Reaction 1) which interact with a pH-sensitive dye, thus bringing about a color change. By replacing the colorimetric dye with the energy-transfer probes, described earlier for pH sensing, we were able to convert this sensor into a lifetime-based CO2 sensor (unpublished). Once again, maximum sensitivity at low (below 5%) CO2 concentrations was observed. The sensors demonstrated fast (seconds) response times and a long shelf life. However, prolonged use, over a period of 20–100 hours, led to the irreversible generation of the protonated form of the dye30.
Glucose sensor
Glucose is a key nutrient in practically every bioprocess, which makes its measurement and control highly desirable. Maintaining optimal concentrations of glucose helps to increase bioreactor productivity as it is an important and readily utilizable carbon and energy source. In the clinical setting, the development of improved glucose sensors is clearly a high priority, with the market for non-invasive glucose detection estimated to be in the US$500 million range32. The development of glucose sensor-based closed-loop control systems is a key area of R&D for applications where real-time glucose-sensing technologies are important: for insulin pumps in the development of an artificial pancreas; and, even more importantly, in the monitoring of blood glucose for diabetics.
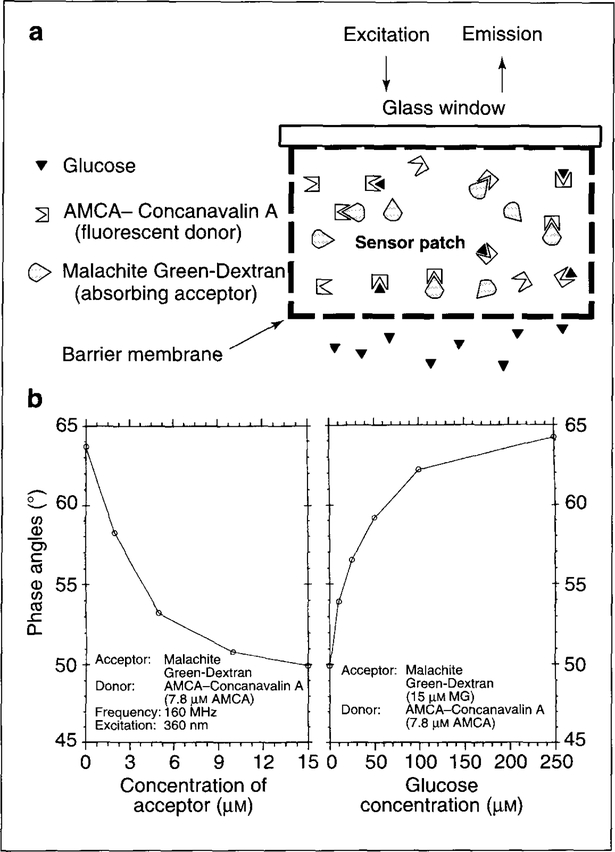
Of the various strategies for glucose measurement (see Refs 33–38) we have concentrated our efforts on a lifetime-based analog of the Schultz sensor37,38. This sensor is based on the competition between glucose and a fluorescently labeled dextran for binding to the active site of the lectin Concanavalin A (Con A) (Fig. 2a). By labeling Con A with a fluorescent donor, and dextran with an absorbing acceptor, Lakowicz and Maliwal4 have demonstrated a lifetime-based glucose competitive assay. The binding of dextran to Con A results in FRET between the donor–acceptor pair and a concomitant decrease in donor intensity and lifetime. When glucose is introduced into the system, it displaces the dextran from the Con A binding site, causing the donor-fluorescence intensity and lifetime to increase, and making it possible to obtain a calibration between glucose concentration and donor lifetime. Figure 2b shows the quenching of fluorescence lifetime of 7-amino-4-methyl-coumarin (AMCA)–Con A upon addition of Malachite Green-Dextran and subsequent reversal of the quenching due to added glucose. Various donor (AMCA, Texas Red Hydrazide, Cascade blue) and acceptor [tetramethyl-rhodamine isothiocyanate (TRITC), Malachite green] combinations were studied, providing varying sensitivities to glucose in the assay system.
Figure 2.
(a) Glucose sensing based on fluorescence resonance energy transfer and lifetime-based phase fluorimetry. When acceptor-labeled dextran binds to the active site of donor-labeled Con A, the donor and acceptor molecules are brought within the Förster distance of energy transfer. FRET occurs, resulting in a decrease in donor fluorescence and lifetime. The labeled dextran and Con A are trapped within a membrane that is permeable to small molecules, including glucose, but which excludes macromolecules present in the bioreactor medium. The sensor element can be mounted on a glass window and glucose can be optically monitored with a fiber bundle from outside the bioreactor. In principle, if a red-sensitive donor is used, the sensor element can be used as a subcutaneous implant for non-invasive glucose monitoring. (b) Left panel: increased acceptor (Malachite Green-Dextran) concentration results in quenching of donor (AMCA–Con A) fluorescence due to specific acceptor binding. Right panel: reversal of quenching by glucose addition. Addition of glucose causes competition between dextran and glucose for Con A active sites, resulting in some dextran being released. There is a glucose concentration-dependent reduction in FRET and increase in donor fluorescence and lifetime. These data are from cuvette-based experiments4.
Experiments using blue-LED-excitable donors, such as the long-lifetime Ru-complexes described earlier, are in progress. Because of the ubiquity of oxygen, available analogs that are relatively oxygen insensitive are being examined. The advantages of such a sensing strategy include a high degree of reversibility, sensitivity to glucose at physiological and typical bioprocess concentrations, and compatibility with fiber optics. There is concern regarding the use of Con A in online in vivo systems due to its toxicity (Con A is a powerful T-cell mitogen39) and the regulatory issues involved. However, in the final sensor configuration (Fig. 2a), the Con A is retained inside a barrier membrane envelope and is present in only very small quantities (a few microliters of a micromolar solution). Both factors (retention behind a barrier membrane and small quantity) help to eliminate the direct interaction of the protein with the macromolecules present in blood or cell culture. The sensor should thus be non-toxic in bioprocess applications, and validated barrier retention procedures should help eliminate toxicity concerns in clinical glucose monitoring.
Recently, bacterial proteins isolated from Escherichia coli and Salmonella typhimurium that specifically bind glucose have become available40. These proteins hold significant promise as a replacement for Con A in developing competitive glucose-binding assays. The low dissociation constants reported for these proteins point towards higher sensitivities and faster response times [glucose binding protein of E. coli 0.17 μM (Ref. 40), and S. typhimurium 0.21 μM (Ref. 40), compared with Concanavalin A 2 mM (Ref. 41)]. More importantly, these proteins are not known to be mitogenic and are possibly safer alternatives to Con A.
Conclusions
The field of lifetime-based sensing has advanced dramatically during the past year. In addition to the probes for O2, pH, pCO2 and glucose, new lifetime probes have become available which change lifetime in response to Ca2+ (Refs 42,43), Mg2+, Na+ (Ref. 44), K+ and Cl− (Ref. 45). Technology based on principles of lifetime-based fluorimetry can be adapted for different applications upon characterization and incorporation of such new probes and development of effective immobilization methods.
The generic nature of this technology will inevitably find applications in other areas (environmental, toxicological and chemical-process monitoring, to name a few). As an example of its versatility, in Fig. 3 we show an application where oxygen levels are being monitored in spinner flasks that have a silicone oxygen sensor patch attached to the interior. Oxygen readings are taken by simply interrogating the patch from outside with a fiber bundle that provides blue excitation light and carries back the emission to a photomultiplier tube (see Fig. 1b). Simultaneous monitoring of several flasks would be feasible by simply using several fiber bundles and a multiplexing device connected to one monitoring instrument. In the bioprocess industry, spinner flasks, roller bottles and shake flasks are commonly employed as ‘bioreactors’, and are currently not instrumented at all. In principle, it should be possible to monitor oxygen levels (and other analytes) economically in all these bioreactors using a similar approach which should lead to more effective process development.
Figure 3.
Spinner flasks used for hybridoma growth were set up with oxygen-sensitive Ru complex-containing patches (orange squares) stuck inside prior to sterilization. Both headspace- and medium-oxygen tensions can be monitored. Monitoring is accomplished by holding the fiber bundle flush against the glass door of the CO2 incubator and directly illuminating the sensor patch.
Importantly, bioprocess monitoring also serves as a proving ground for the ultimate use of these sensors in clinical monitoring and diagnostics. Judicious component selection, low-cost light sources and simple electronics will help reduce instrument costs, as will the use of multiple-analyte sensing. An example would be an instrument such as the combination O2, CO2 and pH sensors for blood-gas monitoring illustrated in Fig. 4, which would require only simple instrumentation and electronics. Similar concepts are also applicable to bioreactor monitoring. Coupled with advances in chemistry, optics and electronics, it is conceivable that the next generation of bioprocess and clinical sensors will move rapidly into the optical realm.
Figure 4.
Conceptual phase-fluorimetric blood-gas instrumentation based on a laser diode light source. This is an example of a multianalyte sensing system using a single set of electronics. On-line sampling is done through a blood-gas catheter. Although long-lifetime fluorophores are desirable for simplifying instrumentation, such probes are not yet available for all analytes. To accommodate shorter-lifetime probes requiring higher modulation frequencies, the fluorimeter uses cross-correlation detection to simplify instrumentation46. By modulating the gain of the photomultiplier tube at the synthesizer frequency plus a small increment (f + Δf), the resulting photocurrent modulates at the lower frequency Δf, but contains the desired phase values. While LEDs can be safely modulated up to 100 MHz, laser diodes permit modulation to several GHz. Both sources are inexpensive. Depending upon the set of fluorescent probes used, either light source can be utilized in such a multianalyte sensor.
Table 1.
Performance comparisons between existing sensors and their phase-fluorimetric alternativesa
| Approximate cost | Sensitivity | Range | Response time | Calibration drifts | |
|---|---|---|---|---|---|
| Oxygen sensor Amperometric | US$2000 (electrode + meter) | 0.01–0.1% of air saturation depending on amplifier used | 0–100% oxygen, linear calibration | <60 s. The response is governed by membrane thickness. Decreasing membrane thickness results in faster response, but introduces increased flow dependence. Thus there is always a compromise between fast response and flow dependence | Less than 2% of reading per week |
| Optical | US$2000b (estimate) | 0.01–0.1% of air saturation depending on sensitivity of phase discriminator | 0–100% oxygen, calibration curve is hyperbolic and at oxygen levels beyond 20% the response is non-linear with lower sensitivity and accuracy. Fortunately, operation at oxygen levels higher than air saturation is rarely required | <60 s. The response is governed by membrane thickness but since the sensor is not flow dependent the membrane thickness can be reduced. On average, the optical sensor showed a 40% faster response when tested under similar conditions | Minimal to none |
| pH sensor Electrochemical | US$500 (electrode + meter) | 0.1 pH units | pH2-pH12 | Seconds. Can be significantly slower in environments where electrode surface is prone to fouling | Less than 10% of the reading per week |
| Optical | US$2000>> (estimate) | 0.1 pH units | About 2 pH units. The actual pH range depends upon the pKa of the dye used | Less than 1 s. Can be slower if subject to fouling | Minimal to none |
| pCO2 sensor Electrochemical | US$2000 (electrode + meter) | Data not available | 0–100% | 1 minute or more (response time more rapid at high CO2 tensions, noticeably slow at low CO2 partial pressures) | Less than 10% of the reading per week |
| Optical | US$2000b (estimate) | 0.01 % at low C02 partial pressures. 0.1% at high CO2 partial pressures | 0–5%. The sensor response saturates and is not useful beyond 5% CO2 | 5 s for increasing CO2, 15 s for decreasing CO2 | Minimal to none |
| Glucose sensor Glucose analyzer (off-line) | US$6000 | 1 mM | 0–140 mM | 1.5 minutes | Regular recalibration recommended |
| Optical | US$2000b (estimate) | 1 mM | 0–250 mM | 5 minutes | Minimal to none (anticipated) |
The data for electrochemical oxygen, pH, CO2 are from Ingold Electrodes Inc. (now Mettler Toledo Inc., Wilmington, MA, USA). The glucose analyzer data is from Yellow Springs Instruments (Yellow Springs, OH, USA).
Same instrument could potentially be used for all four analytes; only the probe molecules and their entrapment matrix need be different.
Acknowledgements
This work was supported by grants (BCS-9157852, BCS-9209157 and BES-9413262) from the National Science Foundation, with support for instrumentation from the National Institutes of Health grants RR-08119 and RR-07510. Matching funds for GR’s Presidential Young Investigator Award were provided by Artisan Industries, Inc. and Genentech, Inc.
Contributor Information
Shabbir B. Bambot, Department of Chemical and Biochemical Engineering and Medical Biotechnology Center of the Maryland Biotechnology Institute, University of Maryland Baltimore County, Baltimore, MD 21228, USA.
Joseph R. Lakowicz, Center for Fluorescence Spectroscopy, Department of Biological Chemistry and Medical Biotechnology Center of the Maryland Biotechnology Institute, University of Maryland at Baltimore, Baltimore, MD 21201, USA.
Govind Rao, Department of Chemical and Biochemical Engineering and Medical Biotechnology Center of the Maryland Biotechnology Institute, University of Maryland Baltimore County, Baltimore, MD 21228, USA..
References
- 1.Lee Y-H and Tsao G (1979) in Advances in Biochemical Engineering, Vol. 13 (Ghose TK, Fiechter A and Blakebrough N, eds), pp. 35–86, Springer-Verlag [Google Scholar]
- 2.McMillan GK (1991) Chem. Eng. Prog 87, 30–37 [Google Scholar]
- 3.Buehler HW and Bucher R (1990) in Sensors in Bioprocess Control (Twork JV and Yacynych AM, eds), pp. 127–171, Marcel Dekker [Google Scholar]
- 4.Lakowicz JR and Maliwal BP (1993) Anal. Chim. Acta 271, 155–164 [Google Scholar]
- 5.Gommers PJF, van Schie BJ, van Dijken JP and Kuenen JG (1988) Biotechnol. Bioeng 32, 86–94 [DOI] [PubMed] [Google Scholar]
- 6.Wolfbeis OS, ed. (1991) Fiber-Optic Chemical Sensors and Biosensors, Vols I and II, CRC Press [Google Scholar]
- 7.Seitz WR (1984) Anal. Chem, 56, 18A–34A [Google Scholar]
- 8.Peterson JI and Vurek GG (1984) Science 224, 123–127 [DOI] [PubMed] [Google Scholar]
- 9.Angel SM (1987) Spectroscopy 2, 38–48 [Google Scholar]
- 10.Tsien RY and Pozzan T (1989) Methods Enzymol. 172, 230–262 [DOI] [PubMed] [Google Scholar]
- 11.Agayn VI and Walt DR (1993) Bio/Technology 11, 726–729 [DOI] [PubMed] [Google Scholar]
- 12.Mattiasson B and HÅkanson H (1993) Trends Biotechnol. 11, 136–142 [DOI] [PubMed] [Google Scholar]
- 13.Scheper T, Plotz F, Mueller C and Hitzmann B (1994) Trends Biotechnol. 12, 42–46 [DOI] [PubMed] [Google Scholar]
- 14.Vanderkooi JM, Maniara G, Green TJ and Wilson DF (1987) J. Biol. Chem 262, 5476–5482 [PubMed] [Google Scholar]
- 15.Lakowicz JR (1983) Principles of Fluorescence Spectroscopy, Plenum Press [Google Scholar]
- 16.Bambot SB, Holavanahali R, Lakowicz JR, Carter GM and Rao G (1994) Biotechnol. Bioeng 43, 1139–1145 [DOI] [PubMed] [Google Scholar]
- 17.Clark LC Jr (1956) Trans. Am. Soc. Artif. Int. Organs 2, 41–54 [Google Scholar]
- 18.Lippitsch ME, Pusterhofer J, Leiner MJP and Wolfbeis OS (1988) Anal. Chim. Acta 205, 1–6 [Google Scholar]
- 19.Lin C-T, Böttcher W, Chou M, Creutz C and Sutin N (1976) J. Am. Chem. Soc 98, 6536–6544 [Google Scholar]
- 20.Lakowicz JR (1992) Laser Focus World 28, 60–80 [Google Scholar]
- 21.Kober EM, Marshall JL, Dressnick WJ, Sullivan BP, Caspar JV and Meyer TJ (1985) Inorg. Chem 24, 2755–2763 [Google Scholar]
- 22.Szmacinski H and Lakowicz JR (1993) Anal. Chem 65, 1668–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lakowicz JR, Szmacinski H and Karakelle M (1993) Anal. Chim. Acta 272, 179–186 [Google Scholar]
- 24.Förster T (1948) Ann. Phys. (Leipzig) 2, 55–75 (Translated by R. S. Knox) [Google Scholar]
- 25.Bambot SB, Sipior J, Lakowicz JR and Rao G (1995) Sensors & Actuators B 22, 181–188 [Google Scholar]
- 26.Brinker CJ and Scherer G (1990) in Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing, Academic Press [Google Scholar]
- 27.Avnir D, Braun S and Ottolenghi M (1991) Nat. Tech. Information Service (NTIS) Publication No. AD-A224, 154 [Google Scholar]
- 28.Severinghaus JW and Bradley AF (1958) J. Appl. Phys 13, 515–520 [DOI] [PubMed] [Google Scholar]
- 29.Wolfbeis OS, Weiss LJ, Leiner MJP and Ziegler WE (1988) Anal. Chem 60, 2028–2030 [Google Scholar]
- 30.Mills A, Chang Q and McMurray N (1992) Anal. Chem 64, 1383–1389 [Google Scholar]
- 31.Mills A and Chang Q (1993) Int. Pat. Appl WO 93/14399 [Google Scholar]
- 32.Amato I (1992) Science 258, 892–8931439802 [Google Scholar]
- 33.Moreno-Bondi MC, Wolfbeis OS, Leiner MJP and Schaffar BPH (1990) Anal. Chem 62, 2377–2384 [DOI] [PubMed] [Google Scholar]
- 34.Trettnak W, Leiner MJP and Wolfbeis OS (1988) Biosensors 4, 15–26 [Google Scholar]
- 35.Trettnak W and Wolfbeis OS (1989) Anal. Chim. Acta 221, 195–203 [Google Scholar]
- 36.Bauer B and Floyd TA (1987) Anal. Chim. Acta 197, 295–301 [Google Scholar]
- 37.Schultz JS (1987) in Biosensors: Fundamentals and Applications (Turner APF, Karube I and Wilson GS, eds), Oxford University Press [Google Scholar]
- 38.Schultz JS, Mansouri S and Goldstein I (1982) Diabetes Care 5, 245–253 [DOI] [PubMed] [Google Scholar]
- 39.Douglas SD, Kamin RM and Fudenberg HH (1969) J. Immunol 103, 1185–1195 [PubMed] [Google Scholar]
- 40.Zukin RS, Strange PG, Heavey LR and Koshland DE (1977) Biochemistry 16, 381–386 [DOI] [PubMed] [Google Scholar]
- 41.Weber SG and Weber A (1993) Anal. Chem 65, 223–230 [Google Scholar]
- 42.Lakowicz JR, Szmacinski H, Nowaczyk K and Johnson ML (1992) Cell Calcium 13, 131–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szmacinski H, Gryczynski I and Lakowicz JR (1993) Photochem. Photobiol 58, 341–345 [DOI] [PubMed] [Google Scholar]
- 44.Szmacinski H and Lakowicz JR (1994) in Probe Design and Chemical Sensing, Topics in Fluorescence Spectroscopy Volume 4 (Lakowicz JR, ed.), pp. 295–334, Plenum Publishing Corporation [Google Scholar]
- 45.Lakowicz JR and Szmacinski H (1993) Sensors and Actuators B 11, 133–143 [Google Scholar]
- 46.Spencer RD and Weber G (1969) Ann. NY Acad. Sci 158, 361–376 [Google Scholar]