Abstract
Duchenne and Becker muscular dystrophies (DMD/BMD) result in progressive weakness of skeletal and cardiac muscles due to the deficiency of functional dystrophin. Respiratory failure is a leading cause of mortality in DMD patients; however, improved management of the respiratory symptoms have increased patients’ life expectancy, thereby also increasing the clinical relevance of heart disease. In fact, the prevalence of cardiomyopathy, which significantly contributes to mortality in DMD patients, increases with age and disease progression, so that over 95% of adult patients has cardiomyopathy signs. We here review the current literature featuring the metabolic alterations observed in the dystrophic heart of the mdx mouse, i.e., the best-studied animal model of the disease, and discuss their pathophysiological role in the DMD heart. It is well assessed that dystrophin deficiency is associated with pathological alterations of lipid metabolism, intracellular calcium levels, neuronal nitric oxide (NO) synthase localization, and NO and reactive oxygen species production. These metabolic stressors contribute to impair the function of the cardiac mitochondrial bulk, which has a relevant pathophysiological role in the development of cardiomyopathy. In fact, mitochondrial dysfunction becomes more severe as the dystrophic process progresses, thereby indicating it may be both the cause and the consequence of the dystrophic process in the DMD heart.
Keywords: cardiomyopathy, Duchenne and Becker muscular dystrophy, metabolic alterations, mitochondrial dysfunction
1. Introduction
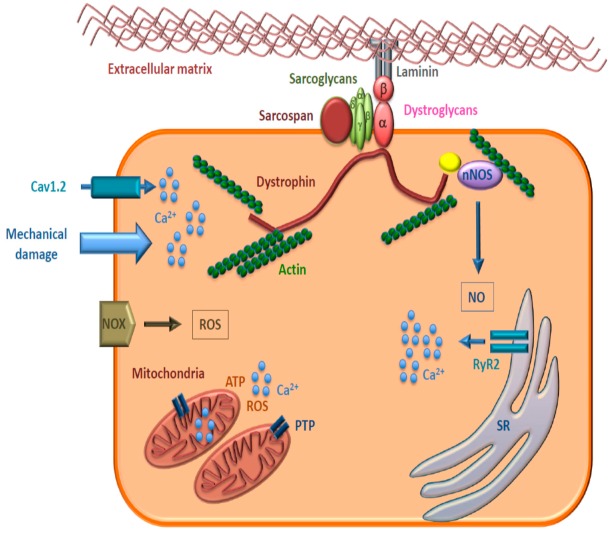
Duchenne (DMD, OMIM #301200) and Becker muscular dystrophies (BMD, OMIM #300376) are X-linked recessive degenerative disorders caused by mutations in the dystrophin gene (DMD, HGNC:2928). Dystrophin (DMD, OMIM *300377) is a part of the dystrophin-associated glycoprotein complex (DGC) that connects the cytoskeleton to the extracellular matrix [1]. Dystrophin binds to actin filaments in the cytoskeleton and to DGC proteins in the plasma membrane and is essential for the sarcolemmal structure and for protection from mechanical stress [2]. DGC also interacts with proteins implicated in signaling pathways, such as neuronal nitric oxide synthase (nNOS or NOS1), phosphoinositol triphosphate 2, calmodulin, and growth factor receptor-bound protein 2 [3]. Moreover, DGC is involved in the extracellular signal-regulated kinases (ERK)/mitogen-activated protein kinase (MAPK) signaling cascade [4] and is required for the clustering of ion channels and Ca2+ homeostasis [5]. The loss of functional dystrophin disrupts the DGC and NOS1, causing a decrease in sarcolemmal structural integrity, as well as susceptibility to myofiber injury and dystrophy development (Figure 1).
Figure 1.
Schematic representation of the dystrophin-associated glycoprotein complex in cardiomyocytes. Dystroglycans, sarcoglycans, and other key proteins involved are shown. nNOS: neuronal nitric oxide synthase, NO: nitric oxide, Cav1.2: cardiac voltage-dependent L-type calcium channel, NOX: NADPH oxidase, ROS: reactive oxygen species, PTP: permeability transition pore, RyR2: ryanodine receptor type 2, SR: sarcoplasmic reticulum.
The incidence of DMD and BMD is approximately 1 in 3500 and 1 in 20,000–30,000 live male births, respectively [6]. DMD and, often, BMD are lethal conditions. Effective treatments are limited for DMD patients, and research for genetic-based therapies is ongoing [7]. As a consequence, the analysis of the DMD gene is of utmost importance for the identification of the underlying molecular defect, because it can confirm the clinical diagnosis, reveal patients’ genotype, address patients to the most opportune therapeutic options, and allow the identification of carrier females and the application of prenatal tests [8,9,10].
Patients affected by DMD lack the dystrophin protein and show progressive degeneration of skeletal muscles at 3–5 years of age and inability to walk at the age of about 10–12 years; their average life expectancy is of about 30 years of age. BMD patients have reduced content of the dystrophin protein and show a broad spectrum of clinical symptoms, a later disease onset, and a slower progression, with difficulties in ambulation that appear at a median age of 20 years [6]. The cardiomyocytes of DMD patients exhibit susceptibility to mechanical stress that contributes to heart fibrosis [11] and to the development of the often-lethal dilated cardiomyopathy (DCM) [12,13]. The cardiac pathology and the altered respiratory function caused by diaphragm damage leading to DCM are present in almost all DMD patients over 30 years of age and are the major causes of death at about 40 years of age [14]. In patients with mild forms of BMD, symptoms are evident at the age of 30, and patients may be still ambulant at 60 years of age; however, they experience worse cardiomyopathy than DMD patients, and about 70% of them have left ventricular dysfunction [14]. DMD/BMD carrier females are usually asymptomatic, although some of them have clinical symptoms, due to X-chromosome rearrangements involving the dystrophin locus or to unbalanced X inactivation [15].
Sarcolemmal fragility due to the absence of dystrophin is associated with elevated cellular Ca2+ that causes altered cell signaling, necrosis of myofibrils, fibrosis, inflammation, and vascular dysfunction in DMD patients. Therefore, dystrophinopathies are systemic diseases involving skeletal and cardiac muscle myopathy, chronic inflammation, impaired signaling and metabolism. Mouse models of DMD presenting disease progression similar to that in humans have been developed [16,17]. Recently, the hypothesis has been advanced that DMD/BMD are primarily metabolic diseases, and the sarcolemmal damage is a downstream sequela [18].
The aim of this paper is to review the main metabolic alterations occurring in the cardiomyocytes of DMD patients.
2. Materials and Methods
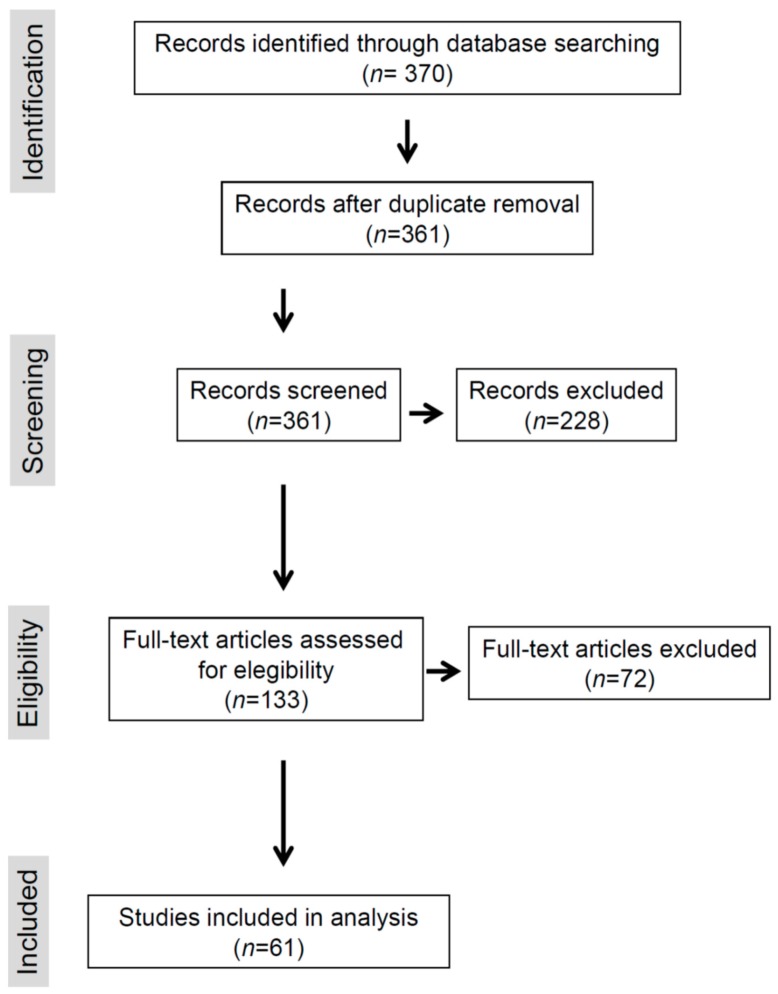
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology [19]. The search covered the PubMed database (Figure 2). The publications’ search period ranged from 2008 to 2019. The search term used was “Duchenne Becker” combined with “metabolism”, “cardiomyopathy, metabolism”, or “cardiovascular cardiomyopathy metabolism”. The selection of papers was based first on the titles and then on the abstracts. A word file containing the selected papers was developed and available to all the co-authors. To be included in the analysis, studies had to report alterations of cardiac metabolism in DMD/BMD patients or in animal models. Full texts of selected articles were then analyzed, and only papers that reported and discussed results supported by stringent experimental data carried out with appropriate methodologies were included. Any issue encountered by an author when extracting the data was discussed collectively, and a consensus was adopted to harmonize the extraction process. Manual searches were also made using reference lists from the recovered articles. In total, 51 references found by manual search were included; they concerned either articles reporting alterations of cardiac metabolism in DMD/BMD patients or in animal models published before 2008 or articles dealing with general matter related to the topic. Figure 2 describes the flowchart of this process.
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow chart.
3. Results
3.1. Lipid Metabolism
Alterations in plasma and tissue lipids have been reported in DMD patients [20], and it has been proposed that plasma lipids significantly contribute to pathology and that DMD patients could benefit from lipid-lowering and vascular targeted therapies [21] (Table 1). Interestingly, it has been reported that statins are pleiotropic drugs, and, in addition to their effect on lowering cholesterol, they are involved in the regulation of processes implicated in DMD progression, such as autophagy and NADPH oxidase 2-mediated oxidative stress (see also Section 3.3 Reactive Oxygen/Nitrogen Species (ROS/RNS)) [22], and angiogenesis [23].
Table 1.
Metabolic targets to treat cardiomyopathy in DMD/BMD patients.
| Dysfunctional Metabolism | Molecular Alteration | Therapeutic Target | Available Drugs | Potential Therapeutic Strategy |
|---|---|---|---|---|
| Lipids | Increased cholesterol-to-phospholipid ratio | Cholesterol synthesis | Statin | |
| Mitochondria | Increased O2•− production | |||
| Impaired Ca2+ handling | ||||
| Impaired oxidative phosphorylation | Respiratory complex I function | Idebenone [24,25,26] | ||
| ROS | Increased expression of NOX2 Increased O2•− production | NOX2 | Statin [22] | NOX2 inhibition |
| RNS | Lower NO levels Impaired NOS1 activity | NO delivery NO synthesis | NO donors [27] |
Accumulation of phosphatidylcholine, cholesterol, sphingomyelin, triglyceride, and increase in monounsaturated fatty acid species have been detected in the muscles of DMD patients, while no major modification of lipid metabolism was observed in BMD patients’ muscles, except for reduced carnitine concentrations.
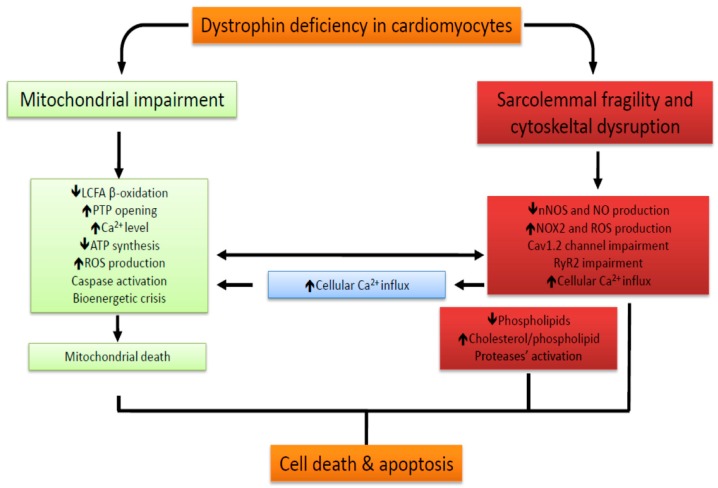
In particular, the cardiac involvement in DMD was investigated using animal models of DMD with congestive cardiomyopathy [28,29]. In these animals, the total phospholipid content is significantly reduced only in the heart and not in skeletal muscles; however, the phosphatidylcholine amount and the cholesterol-to-phospholipid ratio are increased in both cardiac and skeletal muscles [30,31] (Figure 3). Moreover, lower activity and expression of fatty acid synthase and stearoyl-CoA desaturase in the liver, as well as decreased insulin levels have been detected in DMD mice compared to control animals [32]. Insulin deficiency may in part cause impaired fatty acid metabolism. The increase in cholesterol-to-phospholipid ratio could be ascribed to the release of phospholipids during muscle degeneration, to increased activity of phospholipase A, and to increased cholesterol synthesis during muscle regeneration [33].
Figure 3.
Metabolic alterations in Duchenne muscular dystrophy/Becker muscular dystrophy (DMD/BMD) cardiomyocytes. Dystrophin deficiency leads to sarcolemmal and cytoskeletal disruption and is associated with mitochondrial dysfunction. As a consequence, metabolic alterations, which are mainly represented by impaired Ca2+ homeostasis, oxidative stress, and bioenergetic impairment, occur both in the cytosol (red box) and in the mitochondria (in green) and lastly cause cell death and apoptosis.
3.2. Mitochondrial Impairment/Dysfunction
Cardiomyocytes are cells with elevated energy requirements that therefore are highly dependent on mitochondrion activity. In these cells, mitochondria occupy approximately one-third of the cell volume, actively synthesize ATP by oxidative phosphorylation, and are the major source of reactive oxygen species (ROS) that can trigger oxidative stress and affect cell survival and death [34]. Moreover, mitochondria are site for Ca2+ storage, because they release and withdraw Ca2+ to and from the cell [35].
Dystrophin deficiency disrupts sarcolemmal stability and cytoskeletal organization, thereby triggering a variety of cellular stress factors. Among these, increased oxidative stress, impaired handling of cellular Ca2+, and strong decrease in nitric oxide (NO) signaling due to an impaired activity of nNOS (or NOS1, OMIM *163731) have been reported in DMD patients and in a dystrophin-deficient mouse model of DMD (mdx mouse) [36]. Moreover, in the initial compensatory phase that anticipates clinical heart manifestations, cardiac remodeling shifts the energy production from mitochondrial β-oxidation of long-chain fatty acids, which provides about three-quarters of the heart’s energy requirement, to extra-mitochondrial oxidation of carbohydrates [37]. All these findings indicate that mitochondrial metabolic alterations are present in DMD hearts before cardiomyopathy becomes overt.
Another prominent early factor in cardiomyopathy progression in DMD is the increased susceptibility of mitochondria to open the permeability transition pore (PTP), a cyclosporine A- sensitive high-conductance channel in the inner mitochondrial membrane (IMM) (Figure 1). As consequence of PTP opening, which plays a key role in the pathogenesis of diseases due to necrotic cell death after ischemic injuries or to muscle and brain degeneration [38], a nonspecific channel with an exclusion size of 1.5 kDa is formed within the IMM. Physiological transient PTP opening allows a rapid Ca2+ release and metabolite exchange between mitochondrial matrix and cytosol; in contrast, persistent opening leads to pathological wasting of the IMM potential, resting of ATP synthesis, bioenergetic crisis, and cell death—a main feature of mitochondrial disorders [38]. In the hearts of young mdx mice, before any clinical evidence of cardiomyopathy, mitochondria that undergo PTP opening are significantly more numerous than in normal hearts, thereby indicating that PTP opening has a key role in the pathogenesis of dystrophic cardiomyopathy (Figure 3). Accumulation of Ca2+ in the mitochondrial matrix of dystrophic muscle cells is one the main triggers of permeability transition [38]. In these cells, Ca2+ levels can increase as a consequence of the lack of dystrophin that favors sarcolemma disruption during mechanical stress and, in the absence of sarcolemma disruption, through the voltage-independent stretch-sensitive Ca2+ leak channels, the store-operated Ca2+ channels, and the ryanodine receptor [37].
Also, the increase in ROS production may effectively induce PTP opening [38]. A major source of ROS production in the muscle is the NADPH oxidase (NOX) enzyme. Expression and activity of the NOX2 isoform are increased in cardiac myocytes from mdx mice (see also Section 3.3 Reactive Oxygen/Nitrogen Species (ROS/RNS)) [39]. The activation of NOX2 produces extracellularly the superoxide anion (O2•−), which is converted to membrane-permeant H2O2 by extracellular superoxide dismutase [39,40]. High levels of ROS lead to apoptosis or necrosis [34]. Oxidative stress that is present in mitochondria from dystrophic hearts, increased PTP opening, and activation of caspase 9/3 reported in young mdx hearts before the onset of cardiac impairment are all suggestive of mitochondria-derived apoptosis [41] (Figure 3).
A very recent study demonstrates that, during altered oxidative phosphorylation, complex I-sustained emission of mitochondrial H2O2 increases in the left ventricle of dystrophin-deficient young mice, before any evidence of cardiac dysfunction [42]. Therefore, the identification of early mitochondria-specific impairments may lead to the development of mitochondria-targeted therapies able to recover respiratory chain activity and bioenergetic control, with the aim to delay the onset of cardiomyopathy and the consequent progression to heart failure in DMD.
3.3. Reactive Oxygen/Nitrogen Species (ROS/RNS)
ROS-induced oxidative stress contributes to damage in Ca2+ handling and correlates with cardiomyopathy progression and the severity of heart failure in DMD patients. Superoxide anion (O2•−) is the main free radical formed in muscles.
The membrane-bound enzyme NOX is the major source of O2•− in the cardiovascular system (Figure 1). Increased expression of the isoform NOX2 and O2•− production have been reported in the hearts of mdx mice compared to those of wild-type controls [43]. NOX2 inhibition determines the reduction of ROS levels toward levels similar to those of wild-type mice and restores the sarcoplasmic reticulum (SR) Ca2+ content and the amplitude of evoked intracellular Ca2+ concentration transients that are decreased in mdx mice [39,43]. The impairment of Ca2+ handling by oxidative stress occurs essentially at the level of the RyR2 and Cav1.2 calcium channels, which are redox-sensitive (see also Section 3.4 Calcium Handling). Consequently, increased ROS levels may in part explain the Ca2+ channel abnormalities in dystrophic cardiomyocytes [36,44] (Figure 3 and Table 1).
The RNS NO is synthesized by NOS from L-arginine and oxygen and modulates a wide range of physiological functions. NOS1 is a member of the DGC and is lost in a situation of dystrophin deficiency [45]. Therefore, NOS1 deficiency could be the proximal cause of many of the poorly understood features of the dystrophic phenotype. In particular, NO protects the cardiac muscle through vascular relaxation and prevention of pathological hypertrophy [46]. However, there is no evidence indicating that dystrophin and NOS1 co-localize at the membrane of wild-type mouse cardiomyocytes [47]; on the contrary, NOS1 is localized at the intercalated discs, and its mislocalization is associated with DMD cardiomyopathy [48]. This implies that the physical proximity of dystrophin and NOS1 is not a key factor in NOS1 regulation in the heart and suggests an indirect, though efficient, role for dystrophin in modulating NOS1 activity. Selective pharmacological inhibition of NOS1 in wild-type cardiomyocytes lowers NO production, indicating that NOS1 is the main NOS isoform in cardiomyocytes [47]. Cardiomyocytes from mdx mice produce significantly lower NO levels than cardiomyocytes from wild-type mice (Figure 3) [47]. The use of transgenic NOS1 over-expression in mdx mice prevents the development of many signs of cardiomyopathy [49]. Therapeutic strategies have been developed to address neuronal nitric oxide synthase deficiency and the loss of nitric oxide availability in DMD [27] (Table 1).
3.4. Calcium Handling
Changes in excitation–contraction coupling and intracellular Ca2+ handling have been reported in the hearts of mdx mice.
The ryanodine receptor type 2 (RyR2, OMIM *180902) calcium channel and the voltage-dependent L-type calcium channel (Cav1.2, OMIM *114205) are the two principal channels involved in excitation–contraction coupling in the cardiac muscle. The activation of Cav1.2 by plasma membrane depolarization allows Ca2+ to flow into the cell; Ca2+ binds to RyR2, a large homotetrameric Ca2+ release channel expressed in cardiomyocytes and located on the SR membrane (Figure 1), and induces it to open and to release Ca2+ from the SR, thus triggering muscle contraction. This mechanism is called calcium-induced calcium release (CICR). Several proteins, and also ATP, cations, such as Ca2+, Mg2+, and pharmacological ligands regulate RyR2 activity.
Enhanced RyR2 activity has been associated with the pathogenesis of heart dysfunction in DMD [50,51,52]. RyR2 protein levels are two- to three-fold greater in the hearts of dystrophin-deficient mdx mice compared to those of wild-type mice [53]. Moreover, the increased protein kinase A (PKA)-mediated phosphorylation of RyR2 at serine 2808 (S2808) after β-adrenergic activation contributes to SR Ca2+ leak and to the development of heart failure [50]. In fact, inhibition of RyR2-S2808 phosphorylation in mdx mice largely prevents the development of age-related cardiomyopathy. Indeed, progressive cardiac impairment in mdx mice seems to depend on the synergistic contribution of both phosphorylation and oxidation of RyR2 [54]. Consistently, inhibition of RyR2 phosphorylation suppresses SR Ca2+ leak in the mdx mouse heart in part by reducing RyR2 oxidation [54]. Therefore, increased Ca2+ leak from the SR plays a crucial role in the development of cardiomyopathy in DMD. Despite the lower NO levels produced in the cardiomyocytes of mdx mice compared to those of wild-type mice [47], it has been reported that RyR2 is S-nitrosylated in mdx mice, resulting in a functional remodeling of the RyR2 complex and in the dissociation of RyR2 from its stabilizing subunit calstabin 2. These changes destabilize the RyR2 structure and increase SR Ca2+ leakage, leading to intracellular Ca2+ increase and a diastolic SR Ca2+ leak (Figure 3) [52]. This remodeling is analogous to the one observed in the skeletal muscle RyR1 channel complex following RyR1 S-nitrosylation [55]. The inhibition of calstabin 2 dissociation from the RyR2 complex suppresses the SR Ca2+ leak in cardiomyocytes and prevents arrhythmias in vivo. Then, rescue of the RyR2-mediated diastolic SR Ca2+ leak prevents fatal sudden arrhythmias in DMD hearts [52].
The voltage-dependent L-type calcium channel Cav1.2 co-localizes with dystrophin [56] and is linked to F-actin networks by subsarcolemmal stabilizing proteins that finely regulate the channel function [57]. Disruption of actin filaments significantly alters the Ca2+-L current [58]. Cardiomyocytes from adult mdx mice show enhanced Ca2+ current densities and impaired Ca2+- and voltage-dependent inactivation of the Cav1.2 channel compared to wild-type cardiomyocytes [59]. Ca2+ channel alterations in dystrophic cardiomyocytes seem to be dependent on mice age and become more severe in the adult age; in fact, a significantly reduced Ca2+ channel inactivation has been observed in the cardiomyocytes of neonatal mdx mice [60]. Therefore, enhanced Ca2+ influx through Cav1.2 may contribute to cardiomyopathy development (Figure 3) [60]. However, the functional properties of Cav1.2 are similar in the cardiomyocytes from aged (>1 year of age) mdx and wild-type mice [61]. The loss of Cav1.2 dysregulation in the heart during aging can be ascribed to the significant decrease of dystrophin protein in the senescent murine heart. In fact, the altered L-type Ca2+ currents in dystrophic hearts have been explained by an impaired Cav1.2 regulation in the absence of dystrophin. Moreover, increased basal phosphorylation of the Cav1.2 alpha1C subunit and enhanced PKA activity after β-adrenergic activation in the hearts of mdx mice have been reported [62]. PKA-mediated phosphorylation of Cav1.2 enhances the L-type Ca2+ currents and also affects the channel inactivation properties [63]. The enhanced PKA activity in dystrophic cardiomyocytes also explains the enhanced PKA-mediated phosphorylation of RyR2 associated with dystrophic cardiomyopathy in mdx mice.
Another potential source of L-type Ca2+ currents alterations in dystrophic cardiomyocytes is the redox modification of the Cav1.2 alpha1 subunit (cysteine 543 oxidation) during oxidative stress [64], which results in an increase in the channel-mediated Ca2+ influx [63].
Although it is well accepted that Cav1.2 is inhibited by NO via NOS1 activity in the heart, some recent data do not confirm or even contradict this view [65]; therefore, the role of NO and NOS activity in regulating Cav1,2 in the heart is still debated [65].
Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pumps Ca2+ from the cytosol into the lumen of the SR, using the energy derived from ATP hydrolysis and is essential for the maintenance of a low cytosolic Ca2+ concentration. SERCA2 protein expression in mdx mice is not significantly different compared to that in wild-type hearts [53]. However, SERCA2 is reversibly inhibited by phospholamban (PLN); phosphorylation of PLN at serine 16 and threonine 17 reverses this inhibition. The phosphorylated PLN monomer content is lower in mdx compared to wild-type hearts. This suggests that SERCA2 activity is affected in mdx mice, leading to increased decay constants of Ca2+ transients [53].
Intracellular calcium overload in DMD cardiomyocytes can also arise form an altered function of other channels, such as Na+–H+ exchanger (NHE-1) and proton channels [66]. An increased Na+ influx through NHE-1 leads to an intracellular accumulation that, in turn, promotes calcium influx through the Na+–Ca+2 exchanger.
4. Discussion
Metabolic impairment is evident not only in skeletal muscle but also in many tissues and cells from DMD patients and animal models, including heart, liver, and brain. Therefore, it has been proposed that DMD is characterized by a systemic metabolic impairment (Figure 3)—which is central to the etiology of the disease and not secondary to its pathophysiology—and is primarily a mitochondrial myopathy [18]. Disruption of sarcolemmal membrane and cytoskeletal organization are associated with several cellular alterations, i.e., elevated cytosolic Ca2+, oxidative stress, and cell death, that may cause mitochondrial dysfunctions and ultimately contribute to muscle fiber degeneration. Already in 1992, Bonsett and Rudman [67] provided stringent evidence that adenylosuccinic acid (ASA) treatment can induce positive effects in DMD patients because it restores the mitochondrial metabolic impairment; indeed, ASA stimulates the Krebs and purine nucleotide cycles for ADP resynthesis, thereby increasing mitochondrial ATP synthesis. ASA treatment actually induces broad improvements in creatine retention and in the histology, energy levels, and strength of dystrophic muscles [67], which are partially lost if the ASA therapy is suspended.
Very recently, it has been demonstrated that mitochondrial impairment anticipates the onset of cardiomyopathy in a mouse model of DMD [42]. In particular, elevated mitochondrial H2O2 emission and impaired oxidative phosphorylation have been detected in the left ventricle muscle of these mice at an early age, when signs of cardiac dysfunction are absent, suggesting that mitochondrial dysfunction plays a role in the etiology of the heart disease occurring at an older age [42].
Although changes induced by dystrophin deficiency, i.e., oxidative stress and impairment of Ca2+ homeostasis, are involved in the development of cardiomyopathy in DMD patients, mitochondrial dysfunction potentially contributes to cardiac dysfunction. Indeed, treatment with idebenone—a quinone-based electron shuttle—improved cardiac and respiratory performance in DMD patients [24,25,26] (Table 1), thereby providing further proof that the mitochondria are implicated in DMD-associated heart dysfunction. Therefore, considering and treating DMD as a metabolic disease could improve DMD therapies and the quality of life of patients.
Author Contributions
G.E. consulted the PubMed database from 2008 to 2019 and studied in deep the issues related to the mitochondrial impairment/dysfunction; A.C. critically examined the issues related to the lipid metabolism and calcium handling. Both authors equally contributed to the organization, writing, supervision and critical revision of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Ervasti J.M., Campbell K.P. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J. Cell. Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrof B.J., Shrager J.B., Stedman H.H., Kelly A.M., Sweeney H. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. Natl. Acad. Sci. USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rando T.A. The dystrophin-glycoprotein complex, cellular signaling, and the regulation of cell survival in the muscular dystrophies. Muscle Nerve. 2001;24:1575–1594. doi: 10.1002/mus.1192. [DOI] [PubMed] [Google Scholar]
- 4.Spence H.J., Dhillon A.S., James M., Winder S.J. Dystroglycan, a scaffold for the ERK–MAP kinase cascade. EMBO Rep. 2004;5:484–489. doi: 10.1038/sj.embor.7400140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillis J.M. Membrane abnormalities and Ca homeostasis in muscles of the mdx mouse, an animal model of the Duchenne muscular dystrophy: A review. Acta Physiol. Scand. 1996;156:397–406. doi: 10.1046/j.1365-201X.1996.201000.x. [DOI] [PubMed] [Google Scholar]
- 6.Worton R.G., Molnar M.J., Brais B., Karpati G. The muscular dystrophies. In: Scriver C.R., Beaudet A.L., Valle D., Sly W.S., editors. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. McGraw Hill; New York, NY, USA: 2001. pp. 5493–5523. [Google Scholar]
- 7.Vita G., Vita G.L., Musumeci O., Rodolico C., Messina S. Genetic neuromuscular disorders: Living the era of a therapeutic revolution. Part 2: Diseases of motor neuron and skeletal muscle. Neurol. Sci. 2019;40:671–681. doi: 10.1007/s10072-019-03764-z. [DOI] [PubMed] [Google Scholar]
- 8.Esposito G., Ruggiero R., Savarese M., Savarese G., Tremolaterra M.R., Salvatore F., Carsana A. Prenatal molecular diagnoses of inherited neuromuscular diseases: Duchenne/Becker muscular dystrophy, myotonic dystrophy type 1 and spinal muscular atrophy. Clin. Chem. Lab. Med. 2013;51:2239–2245. doi: 10.1515/cclm-2013-0209. [DOI] [PubMed] [Google Scholar]
- 9.Esposito G., Tremolaterra M.R., Marsocci E., Tandurella I.C.M., Fioretti T., Savarese M., Carsana A. Precise mapping of 17 deletion breakpoints within the central hotspot deletion region (introns 50 and 51) of the DMD gene. J. Hum. Genet. 2017;62:1057–1063. doi: 10.1038/jhg.2017.84. [DOI] [PubMed] [Google Scholar]
- 10.Carsana A., Frisso G., Tremolaterra M.R., Ricci E., De Rasmo D., Salvatore F. A larger spectrum of intragenic STRs improves linkage analysis and localization of intragenic recombination detection in the dystrophin gene: An analysis of 93 families from Southern Italy. J. Mol. Diagn. 2007;9:64–69. doi: 10.2353/jmoldx.2007.060056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend D., Yasuda S., Metzger J. Cardiomyopathy of Duchenne muscular dystrophy: Pathogenesis and prospect of membrane sealants as a new therapeutic approach. Expert. Rev. Cardiovasc. Ther. 2007;5:99–109. doi: 10.1586/14779072.5.1.99. [DOI] [PubMed] [Google Scholar]
- 12.De Kermadec J.M., Bécane H.M., Chénard A., Tertrain F., Weiss Y. Prevalence of left ventricular systolic dysfunction in Duchenne muscular dystrophy: An echocardiographic study. Am. Heart J. 1994;127:618–623. doi: 10.1016/0002-8703(94)90672-6. [DOI] [PubMed] [Google Scholar]
- 13.Danialou G., Comtois A.S., Dudley R., Karpati G., Vincent G., Des Rosiers C., Petrof B.J. Dystrophin-deficient cardiomyocytes are abnormally vulnerable to mechanical stress-induced contractile failure and injury. Faseb J. 2001;15:1655–1657. doi: 10.1096/fj.01-0030fje. [DOI] [PubMed] [Google Scholar]
- 14.Verhaert D., Richards K., Rafael-Fortney J.A., Raman S.V. Cardiac involvement in patients with muscular dystrophies: Magnetic resonance imaging phenotype and genotypic considerations. Circ. Cardiovasc. Imaging. 2011;4:67–76. doi: 10.1161/CIRCIMAGING.110.960740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carsana A., Frisso G., Intrieri M., Tremolaterra M.R., Savarese G., Scapagnini G., Esposito G., Santoro L., Salvatore F. A 15-year molecular analysis of Duchenne/Becker muscular dystrophy: Genetic features in a large cohort. Front. Biosci. 2010;2E:2547–2558. doi: 10.2741/e113. [DOI] [PubMed] [Google Scholar]
- 16.Coley W.D., Bogdanik L., Vila M.C., Yu Q., Van Der Meulen J.H., Rayavarapu S., Novak J.S., Nearing M., Quinn J.L., Saunders A., et al. Effect of genetic background on the dystrophic phenotype in mdx mice. Hum. Mol. Genet. 2016;25:130–145. doi: 10.1093/hmg/ddv460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts N.W., Holley-Cuthrell J., Gonzalez-Vega M., Mull A.J., Heydemann A. Biochemical and Functional Comparisons of mdx and Sgcg(-/-) Muscular Dystrophy Mouse Models. BioMed Res. Int. 2015;2015:1–11. doi: 10.1155/2015/131436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timpani C.A., Hayes A., Rybalka E. Revisiting the dystrophin-ATP connection: How half a century of research still implicates mitochondrial dysfunction in Duchenne Muscular Dystrophy aetiology. Med. Hypotheses. 2015;85:1021–1033. doi: 10.1016/j.mehy.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Hutton B., Salanti G., Caldwell D.M., Chaimani A., Schmid C.H., Cameron C., Ioannidis J.P., Straus S., Thorlund K., Jansen J.P., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava N.K., Yadav R., Mukherjee S., Pal L., Sinha N. Abnormal lipid metabolism in skeletal muscle tissue of patients with muscular dystrophy: In vitro, high-resolution NMR spectroscopy based observation in early phase of the disease. Magn. Reson. Imaging. 2017;38:163–173. doi: 10.1016/j.mri.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Milad N., White Z., Tehrani A.Y., Sellers S., Rossi F.M.V., Bernatchez P. Increased plasma lipid levels exacerbate muscle pathology in the mdx mouse model of Duchenne muscular dystrophy. Skelet. Muscle. 2017;7:19. doi: 10.1186/s13395-017-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitehead N.P. Enhanced autophagy as a potential mechanism for the improved physiological function by simvastatin in muscular dystrophy. Autophagy. 2016;12:705–706. doi: 10.1080/15548627.2016.1144005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Podkalicka P., Mucha O., Dulak J., Loboda A. Targeting angiogenesis in Duchenne muscular dystrophy. Cell Mol. Life Sci. 2019;76:1507–1528. doi: 10.1007/s00018-019-03006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buyse G.M., Goemans N., van den Hauwe M., Thijs D., de Groot I.J., Schara U., Ceulemans B., Meier T., Mertens L. Idebenone as a novel, therapeutic approach for Duchenne muscular dystrophy: Results from a 12 month, double-blind, randomized placebo-controlled trial. Neuromuscul. Disord. 2011;21:396–405. doi: 10.1016/j.nmd.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Buyse G.M., Voit T., Schara U., Straathof C.S., D’Angelo M.G., Bernert G., Cuisset J.M., Finkel R.S., Goemans N., McDonald C.M., et al. Efficacy of idebenone on respiratory function in patients with Duchenne muscular dystrophy not using glucocorticoids (DELOS): A double-blind randomized placebo-controlled phase 3 trial. Lancet. 2015;385:1748–1757. doi: 10.1016/S0140-6736(15)60025-3. [DOI] [PubMed] [Google Scholar]
- 26.Buyse G.M., Voit T., Schara U., Straathof C.S., D’Angelo M.G., Bernert G., Cuisset J.M., Finkel R.S., Goemans N., Rummey C., et al. Treatment effect of idebenone on inspiratory function in patients with Duchenne muscular dystrophy. Pediatr. Pulmonol. 2017;52:508–515. doi: 10.1002/ppul.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timpani C.A., Hayes A., Rybalka E. Therapeutic strategies to address neuronal nitric oxide synthase deficiency and the loss of nitric oxide bioavailability in Duchenne Muscular Dystrophy. Orphanet J. Rare Dis. 2017;12:100. doi: 10.1186/s13023-017-0652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Homburger F., Nixon C.W., Eppenberger M., Baker J.R. Hereditary myopathy in the Syrian hamster: Studies on pathogenesis. Ann. N. Y. Acad. Sci. 1966;138:14–27. doi: 10.1111/j.1749-6632.1966.tb41151.x. [DOI] [PubMed] [Google Scholar]
- 29.Homburger F. Disease models in Syrian hamsters. Prog. Exp. Tumor Res. 1972;16:69–86. doi: 10.1159/000393365. [DOI] [PubMed] [Google Scholar]
- 30.Borowski I.F., Harrow J.A., Pritchard E.T., Dhalla N.S. Changes in electrolyte and lipid contents of the myopathic hamster (UM-X7.1) skeletal and cardiac muscles. Res. Commun. Chem. Pathol. Pharmacol. 1974;7:443–451. [PubMed] [Google Scholar]
- 31.Owens K., Weglicki W.B., Sonnenblick E.H., Gerz E.W. Phospholipid and cholesterol content of ventricular tissue from the cardiomyopathic Syrian hamster. J. Mol. Cell. Cardiol. 1972;4:229–236. doi: 10.1016/0022-2828(72)90060-0. [DOI] [PubMed] [Google Scholar]
- 32.Vecchini A., Binaglia L., Bibeau M., Minieri M., Carotenuto F., Di Nardo P. Insulin deficiency and reduced expression of lipogenic enzymes in cardiomyopathic hamster. J. Lipid Res. 2001;42:96–105. [PubMed] [Google Scholar]
- 33.Srivastava N.K., Pradhan S., Mittal B., Gowda G.A. High resolution NMR based analysis of serum lipids in Duchenne muscular dystrophy patients and its possible diagnostic significance. NMR Biomed. 2010;23:13–22. doi: 10.1002/nbm.1419. [DOI] [PubMed] [Google Scholar]
- 34.Barbieri E., Sestili P. Reactive oxygen species in skeletal muscle signaling. J. Signal. Transduct. 2012:982794. doi: 10.1155/2012/982794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou B., Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018;128:3716–3726. doi: 10.1172/JCI120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen D.G., Whitehead N.P., Froehner S.C. Absence of Dystrophin Disrupts Skeletal Muscle Signaling: Roles of Ca2+, Reactive Oxygen Species, and Nitric Oxide in the Development of Muscular Dystrophy. Physiol. Rev. 2016;96:253–305. doi: 10.1152/physrev.00007.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burelle Y., Khairallah M., Ascah A., Allen B.G., Deschepper C.F., Petrof B.J., Des Rosiers C. Alterations in mitochondrial function as a harbinger of cardiomyopathy: Lessons from the dystrophic heart. J. Mol. Cell. Cardiol. 2010;48:310–321. doi: 10.1016/j.yjmcc.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Šileikytė J., Forte M. The Mitochondrial Permeability Transition in Mitochondrial Disorders. Oxid. Med. Cell. Longev. 2019;2019:3403075. doi: 10.1155/2019/3403075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matecki S., Fauconnier J., Lacampagne A. Reactive Oxygen Species and Muscular Dystrophy. In: Laher I., editor. Systems Biology of Free Radicals and Antioxidants. Springer; Heidelberg/Berlin, Germany: 2014. pp. 3055–3079. [DOI] [Google Scholar]
- 40.Cattaneo F., Castaldo M., Parisi M., Faraonio R., Esposito G., Ammendola R. Formyl peptide receptor 1 modulates endothelial cell functions by NADPH oxidase-dependent VEGFR2 transactivation. Oxid. Med. Cell Longev. 2018;2018:2609847. doi: 10.1155/2018/2609847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ascah A., Khairallah M., Daussin F., Bourcier-Lucas C., Godin R., Allen B.G., Petrof B.J., Des Rosiers C., Burelle Y. Stress-induced opening of the permeability transition pore in the dystrophin-deficient heart is attenuated by acute treatment with sildenafil. Am. J. Physiol. Heart. Circ. Physiol. 2010;300:H144–H153. doi: 10.1152/ajpheart.00522.2010. [DOI] [PubMed] [Google Scholar]
- 42.Hughes M.C., Ramos S.V., Turnbull P.C., Edgett B.A., Huber J.S., Polidovitch N., Schlattner U., Backx P.H., Simpson J.A., Perry C.G.R. Impairments in left ventricular mitochondrial bioenergetics precede overt cardiac dysfunction and remodelling in Duchenne muscular dystrophy. J. Physiol. 2019 doi: 10.1113/JP277306. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez D.R., Treuer A.V., Lamirault G., Mayo V., Cao Y., Dulce R.A., Hare J.M. NADPH oxidase-2 inhibition restores contractility and intracellular calcium handling and reduces arrhythmogenicity in dystrophic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2014;307:H710–H721. doi: 10.1152/ajpheart.00890.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams I.A., Allen D.G. The Role of Reactive Oxygen Species in the Hearts of Dystrophin-Deficient Mdx Mice. Am. J. Physiol. Circ. Physiol. 2007;293:H1969–H1977. doi: 10.1152/ajpheart.00489.2007. [DOI] [PubMed] [Google Scholar]
- 45.Brenman J.E., Chao D.S., Xia H., Aldape K., Bredt D.S. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- 46.Danson E.J., Choate J.K., Paterson D.J. Cardiac nitric oxide: Emerging role for nNOS in regulating physiological function. Pharmacol. Ther. 2005;106:57–74. doi: 10.1016/j.pharmthera.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Ramachandran J., Schneider J.S., Crassous P.-A., Zheng R., Gonzalez J.P., Xie L.-H., Beuve A., Fraidenraich D., Peluffo R.D. Nitric Oxide Signalling Pathway in Duchenne Muscular Dystrophy Mice: Up-Regulation of L-Arginine Transporters. Biochem. J. 2013;449:133–142. doi: 10.1042/BJ20120787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonzalez J.P., Crassous P.A., Schneider J.S., Beuve A., Fraidenraich D. Neuronal nitric oxide synthase localizes to utrophin expressing intercalated discs and stabilizes their structural integrity. Neuromuscul. Disord. 2015;25:964–976. doi: 10.1016/j.nmd.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Wehling-Henricks M., Jordan M.C., Roos K.P., Deng B., Tidball J.G. Cardiomyopathy in dystrophin-deficient hearts is prevented by expression of a neuronal nitric oxide synthase transgene in the myocardium. Hum. Mol. Genet. 2005;14:1921–1933. doi: 10.1093/hmg/ddi197. [DOI] [PubMed] [Google Scholar]
- 50.Sarma S., Li N., van Oort R.J., Reynolds C., Skapura D.G., Wehrens X.H. Genetic inhibition of PKA phosphorylation of RyR2 prevents dystrophic cardiomyopathy. Proc. Natl. Acad. Sci. USA. 2010;107:13165–13170. doi: 10.1073/pnas.1004509107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ather S., Wang W., Wang Q., Li N., Anderson M.E., Wehrens X.H. Inhibition of CaMKII phosphorylation of RyR2 prevents inducible ventricular arrhythmias in mice with Duchenne muscular dystrophy. Heart Rhythm. 2013;10:592–599. doi: 10.1016/j.hrthm.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fauconnier J., Thireau J., Reiken S., Cassan C., Richard S., Matecki S., Marks A.R., Lacampagne A. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. USA. 2010;107:1559–1564. doi: 10.1073/pnas.0908540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams I.A., Allen D.G. Intracellular calcium handling in ventricular myocytes from mdx mice. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H846–H855. doi: 10.1152/ajpheart.00688.2006. [DOI] [PubMed] [Google Scholar]
- 54.Wang Q., Wang W., Wang G., Rodney G.G., Wehrens X.H. Crosstalk between RyR2 oxidation and phosphorylation contributes to cardiac dysfunction in mice with Duchenne muscular dystrophy. J. Mol. Cell Cardiol. 2015;89:177–184. doi: 10.1016/j.yjmcc.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zullo A., Frisso G., Carsana A. Influence of physical activity on structure and function of the RyR1 calcium channel: A systematic review. Gazz. Med. Ital. Arch. Sci. Med. 2019 in press. [Google Scholar]
- 56.Sadeghi A., Doyle A.D., Johnson B.D. Regulation of the cardiac L-type Ca2+ channel by the actin-binding proteins alpha-actinin and dystrophin. Am. J. Physiol. Cell Physiol. 2002;282:C1502–C1511. doi: 10.1152/ajpcell.00435.2001. [DOI] [PubMed] [Google Scholar]
- 57.Hohaus A., Person V., Behlke J., Schaper J., Morano I., Haase H. The carboxyl-terminal region of ahnak provides a link between cardiac L-type Ca2+ channels and the actin-based cytoskeleton. FASEB J. 2002;16:1205–1216. doi: 10.1096/fj.01-0855com. [DOI] [PubMed] [Google Scholar]
- 58.Rueckschloss U., Isenberg G. Cytochalasin D reduces Ca2+ currents via cofilin-activated depolymerization of F-actin in guinea-pig cardiomyocytes. J. Physiol. 2001;537:363–370. doi: 10.1111/j.1469-7793.2001.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koenig X., Rubi L., Obermair G.J., Cervenka R., Dang X.B., Lukacs P., Kummer S., Bittner R.E., Kubista H., Todt H., et al. Enhanced Currents through L-Type Calcium Channels in Cardiomyocytes Disturb the Electrophysiology of the Dystrophic Heart. Am. J. Physiol. Heart Circ. Physiol. 2014;306:H564–H573. doi: 10.1152/ajpheart.00441.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koenig X., Dysek S., Kimbacher S., Mike A.K., Cervenka R., Lukacs P., Nagl K., Dang X.B., Todt H., Bittner R.E., et al. Voltage-gated ion channel dysfunction precedes cardiomyopathy development in the dystrophic heart. PLoS ONE. 2011;6:e20300. doi: 10.1371/journal.pone.0020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rubi L., Todt H., Kubista H., Koenig X., Hilber K. Calcium Current Properties in Dystrophin-deficient Ventricular Cardiomyocytes from Aged Mdx Mice. Physiol. Rep. 2018;6:e13567. doi: 10.14814/phy2.13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y., Zhang S., Zhang X., Li J., Ai X., Zhang L., Yu D., Ge S., Peng Y., Chen X. Blunted Cardiac Beta-Adrenergic Response as an Early Indication of Cardiac Dysfunction in Duchenne Muscular Dystrophy. Cardiovasc. Res. 2014;103:60–71. doi: 10.1093/cvr/cvu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cserne Szappanos H., Muralidharan P., Ingley E., Petereit J., Millar A.H., Hool L.C. Identification of a novel cAMP dependent protein kinase A phosphorylation site on the human cardiac calcium channel. Sci. Rep. 2017;7:15118. doi: 10.1038/s41598-017-15087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muralidharan P., Cserne Szappanos H., Ingley E., Hool L.C. The Cardiac L-Type Calcium Channel AlphaSubunit Is a Target for Direct Redox Modification during Oxidative Stress-the Role of Cysteine Residues in the Alpha Interacting Domain. Clin. Exp. Pharmacol. Physiol. 2017;44:46–54. doi: 10.1111/1440-1681.12750. [DOI] [PubMed] [Google Scholar]
- 65.Koenig X., Ebner J., Hilber K. Voltage-Dependent Sarcolemmal Ion Channel Abnormalities in the Dystrophin-Deficient Heart. Int. J. Mol. Sci. 2018;19:3296. doi: 10.3390/ijms19113296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bkaily G., Jacques J. Na+-H+ exchanger and proton channel in heart failure associated with Becker and Duchenne muscular dystrophies. Can. J. Physiol. Pharmacol. 2017;95:1213–1223. doi: 10.1139/cjpp-2017-0265. [DOI] [PubMed] [Google Scholar]
- 67.Bonsett C., Rudman A. The dystrophin connection—ATP? Med. Hypotheses. 1992;38:139–154. doi: 10.1016/0306-9877(92)90087-S. [DOI] [PubMed] [Google Scholar]