Abstract
Aims:
To propose a hierarchy of methodological criteria to consider when determining whether a study provides sufficient information to answer the question of whether e-cigarettes can facilitate cigarette smoking cessation or reduction.
Design:
A PubMed search through February 1, 2017 was conducted of all studies related to e-cigarettes and smoking cessation or reduction.
Settings:
Australia, Europe, Iran, Korea, New Zealand, United States.
Participants/Studies:
91 articles.
Measurements:
Coders organized studies according to six proposed methodological criteria: 1) examines outcome of interest (cigarette abstinence or reduction), 2) assesses e-cigarette use for cessation as exposure of interest, 3) employs appropriate control/comparison groups, 4) ensures that measurement of exposure precedes the outcome, 5) evaluates dose and duration of the exposure, and 6) evaluates the type and quality of the e-cigarette used.
Findings:
Twenty-four articles did not examine the outcomes of interest. Forty did not assess the specific reason for e-cigarette use as an exposure of interest. Twenty articles did not employ prospective study designs with appropriate comparison groups. The few observational studies meeting some of the criteria (duration, type, use for cessation) triangulated with findings from three randomized trials to suggest that e-cigarettes can help adult smokers quit or reduce cigarette smoking.
Conclusions:
Based on the proposed criteria, few studies claiming to address the effect of e-cigarettes on smoking cessation or reduction are of sufficient quality to inform the scientific question of interest. Studies with stronger measures and methods are needed to better inform the question of e-cigarette use for smoking cessation or reduction.
Keywords: electronic cigarettes, e-cigarettes, cigarettes, smoking cessation, tobacco, nicotine
INTRODUCTION
The 2014 Surgeon General’s Report on the Health Consequences of Smoking states that death is “overwhelmingly caused by cigarettes and other combusted tobacco products,” and that use of e-cigarettes and other innovative nicotine delivery products is likely to be beneficial to public health if current smokers who are unable to quit can eventually switch completely to these products (1, 2). Studies show that e-cigarettes could benefit the health of current smokers because they are substantially less harmful (3–5), more affordable (6, 7), and able to deliver nicotine efficiently (8–10), thus satisfying cravings and reducing nicotine withdrawal (9, 11). Although experimental use of e-cigarettes among adult smokers has increased and could encourage smoking cessation on a broader scale than nicotine replacement therapies,(12–14) it is not yet clear whether e-cigarettes can effectively facilitate smoking cessation at the population level.
A number of reviews and opinion pieces have examined the evidence on the impact of e-cigarettes on cigarette smoking cessation (15–29). Reviews have come to different conclusions, possibly because they differ in the types of studies included, the samples included in those studies, and the measures used to assess e-cigarette use. Reviews reporting positive results note that e-cigarettes may be helpful to some smokers in quitting smoking (15, 26), and that e-cigarettes with nicotine are more effective in helping smokers to quit than those without nicotine (19, 23, 24). Equivocal reviews note that no conclusion can be drawn from the existing data (16, 21, 27–29), or that e-cigarettes are no more effective for smoking cessation than existing cessation products (18, 22). One review reporting negative effects on smoking cessation noted that the odds of quitting smoking were lower in e-cigarette users than non- e-cigarette users (17). Four reviews included meta-analyses, with three reporting a positive effect of e-cigarette use on smoking cessation (19, 23, 24), and one reporting a negative effect (17). All reviews highlight the low quality of existing evidence as a limitation, largely focusing on the lack of randomized controlled trials (RCTs) addressing the safety and efficacy of e-cigarettes for smoking cessation. While some reviews have evaluated the quality of evidence based on a specified set of criteria (16, 17, 19, 23), none of the reviews have addressed all of the following key issues: (a) whether an adequate exposure to e-cigarette use occurred to test the smoking cessation hypothesis (i.e., not trial use on one or two occasions, but use for sufficient duration, intensity and frequency to affect cessation); (b) whether e-cigarettes were used with the intention of smoking cessation; and (c) whether e-cigarettes were used on the most recent quit attempt proximal to the outcome measurement time. As a result, reviews to-date have typically combined uninformative or methodologically weak studies with well-designed studies, resulting in a lack of clarity and contradictory conclusions.
Rigorous standards are needed to evaluate the evolving evidence base. These standards would differentiate informative from uninformative studies and decrease the likelihood of a false impression of the evidence based on an accumulation of uninformative studies on this question. The purpose of this paper is to propose a hierarchy of methodological criteria to consider when determining whether a study on e-cigarettes and smoking cessation provides sufficient information to answer the question of whether e-cigarettes can help current smokers quit or reduce smoking. We then categorize existing studies reporting on e-cigarette use and smoking cessation according to these criteria. Finally, we provide cautions and recommendations for designing high quality randomized and observational studies to address questions of the efficacy and effectiveness of e-cigarette use for smoking cessation.
METHODS
We conducted a systematic review of published scientific literature on e-cigarettes indexed in PubMed through February 1, 2017. The study protocol has been published elsewhere (30), as has the detailed presentation of the inclusion and cataloguing of articles in this overarching project (31). The search strategy consisted of the following keywords: “e-cigarette*” OR “electronic cigarette” OR “electronic cigarettes” OR “electronic nicotine delivery” OR “vape” OR “vaping.” Eligible studies were experimental studies, quasi-experimental studies, observational studies (including case control, cohort and cross-sectional studies), case reports, case series, qualitative studies and mixed methods studies that claimed to evaluate the impact of e-cigarette use on abstinence from traditional cigarettes or the reduction in number of traditional cigarettes consumed. Four coders (AV, SF, AG, LC) evaluated abstracts for inclusion and extracted data into a structured coding spreadsheet; one coder (AV) double-checked all data extraction. During our initial review of these studies in 2014, the study team identified the elements critical to answering the scientific question and developed a set of six questions requiring a yes or no response:
Does the study examine and adequately measure the primary outcome of interest (i.e., cigarette smoking abstinence or cigarette smoking reduction)?
Does the study assess e-cigarette use specifically for smoking cessation or reduction as the exposure of interest (i.e., e-cigarettes were explicitly used with the intention to quit smoking)?
Does the study use an appropriate design with control or comparison groups to address the potential impact of e-cigarette use on smoking cessation or reduction?
Does measurement of the exposure (i.e., e-cigarette use) precede measurement of the final outcome (smoking cessation or reduction)?
Does the study evaluate the dose and the duration of the exposure to determine degree of adherence and adequate delivery of active ingredients for a sufficient time period to be a reasonable test of a cessation hypothesis?
Related to criterion # 5, does the study evaluate the type and quality of e-cigarette product used (e.g., its efficiency and reliability at delivering nicotine and other subjective experiences thought to aid smoking cessation - such as chemosensory/sensorimotor satisfaction or appeal as an alternative to smoking)?
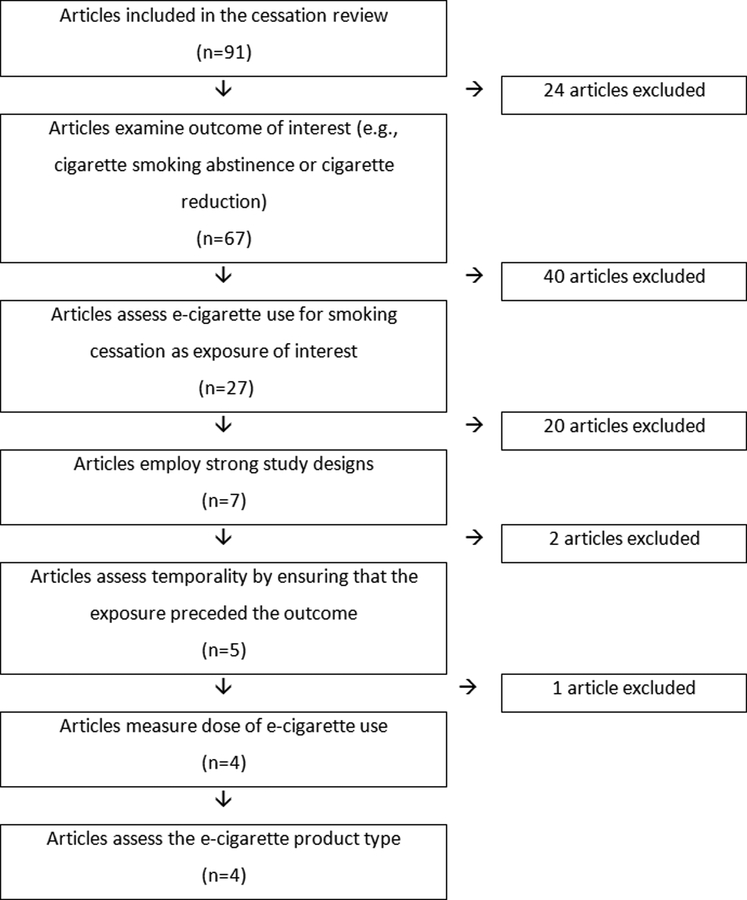
In essence, these criteria are similar to those considered when thinking about the balance between internal validity and external validity. This is often a tension in moving from individual safety and efficacy studies to community or population based studies. Such a tension is unavoidable, but must be managed, as is done when considering the strengths and weaknesses of both randomized controlled studies and observational (“real world”) studies.(32) Questions four through six relate to an assessment of whether the study precisely measured the exposure of interest as an independent variable that, when used for cessation, delivers the “active ingredients” known to facilitate cessation or reduction. These six items were used as a hierarchy to organize studies according to their methodological strengths. Studies that did not address one or more of the questions on this list fell out of the hierarchy and were considered less informative compared to those that met most of the more fundamental criteria, or ideally, met all six criteria. Figure 1 illustrates this hierarchy of methodological considerations.
Figure 1. Flowchart of included articles in the hierarchy of methodological considerations.
NOTE: Articles included towards the bottom of the flowchart have the strongest methodologies and provide the best evidence for the potential impact of e-cigarette use on cigarette smoking cessation or reduction.
RESULTS
Criterion 1: Studies must examine the outcome of interest (i.e., cigarette smoking abstinence or reduction)
To provide information regarding whether e-cigarettes can be used as an effective tool for smoking cessation, a study must appropriately define and assess the outcomes of interest (cigarette smoking reduction and cessation). We considered smoking abstinence and cigarette reduction to be the most relevant and highest-quality outcomes. However, definition of smoking abstinence and reduction varied by study. We identified 91 articles related to e-cigarette use and cigarette cessation (Table 1). Twenty-four of these articles were excluded because they addressed other outcomes, including e-cigarette nicotine concentration during quitting (33), reasons for e-cigarette use (34), use of e-cigarettes as a quit method (35–45), correlation of e-cigarette use with expectancies (46, 47), quit attempts (48–50), intention to quit (51), e-cigarette use in opioid-dependent smokers (52), cessation of e-cigarette use (53), other outcomes associated with quitting smoking (54, 55), and use of e-cigarettes during periods of forced abstinence (56).
Table 1.
List of studies included in the review and assessment of study methodologies
| Article | Study design | Outcome of interest? | Assessed Reason for Use: Cessation? | Appropriate study design? | Measurement: Exposure precedes outcome? (Timing) | Measurement: Assessed Dose of ENDS Use? (Dose) | Measurement: Assessed ENDS Product Type? |
|---|---|---|---|---|---|---|---|
| Bullen (2013) (57) | Randomized controlled trial | Yes | Yes | Yes | Yes | Yes | Yes |
| O’Brien (2015) (60) | Randomized controlled trial | Yes | Yes | Yes | Yes | Yes | Yes |
| Caponnetto (2013) (58) | Randomized controlled trial | Yes | Yes | Yes | Yes | Yes | Yes |
| Tseng (2016) (61) | Randomized controlled trial | Yes | Yes | Yes | Yes | Yes | Yes |
| Vickerman (2017) (101) | Longitudinal study with comparison group | Yes | Yes | Yes | Yes | No | Yes |
| Zawertailo (2017) (115) | Longitudinal study with comparison group | Yes | Yes | Yes | No | No | No |
| Shi (2016) (116) | Longitudinal study with comparison group | Yes | Yes | Yes | No | No | No |
| Adriens (2014) (59) | Randomized controlled trial | Yes | Yes | No | No | Yes | Yes |
| Pearson (2015) (81) | Longitudinal study with comparison group | Yes | Yes | No | No | No | No |
| Vickerman (2013) (80) | Longitudinal study with comparison group | Yes | Yes | No | No | No | No |
| Etter (2014) (63) | Longitudinal study with no comparison group | Yes | Yes | No | Yes | Yes | Yes |
| Polosa (2015) (70) | Longitudinal study with no comparison group | Yes | Yes | No | Yes | No | Yes |
| Stein (2016) (105) | Longitudinal study with no comparison group | Yes | Yes | No | Yes | Yes | Yes |
| James (2016) (106) | Longitudinal study with no comparison group | Yes | Yes | No | Yes | Yes | Yes |
| Nolan (2016) (102) | Longitudinal study with no comparison group | Yes | Yes | No | Yes | Yes | Yes |
| Goniewicz (2013) (84) | Cross-sectional survey | Yes | Yes | No | No | Yes | Yes |
| Dawkins (2013) (85) | Cross-sectional survey | Yes | Yes | No | No | Yes | Yes |
| Tackett (2015) (86) | Cross-sectional survey | Yes | Yes | No | No | Yes | Yes |
| Rutten (2015) (87) | Cross-sectional survey | Yes | Yes | No | No | Yes | No |
| Brown (2014) (91) | Cross-sectional survey | Yes | Yes | No | No | No | No |
| Zhu (2013) (90) | Cross-sectional survey | Yes | Yes | No | No | No | No |
| Allem (2015) (107) | Cross-sectional survey | Yes | Yes | No | No | No | No |
| McQueen (2016) (112) | Cross-sectional survey | Yes | Yes | No | No | No | No |
| Pechacek (2016) (113) | Cross-sectional survey | Yes | Yes | No | No | No | Yes |
| Beard (2016) (117) | Cross-sectional survey | Yes | Yes | No | No | No | No |
| Filippidis (2016) (118) | Cross-sectional survey | Yes | Yes | No | No | No | No |
| Caponnetto (2011) (100) | Case series | Yes | Yes | No | No | No | Yes |
| Hatsukami (2017) (123) | Randomized controlled trial | Yes | No | Yes | No | No | No |
| Biener (2014) (72) | Longitudinal study with comparison group | Yes | No | Yes | Yes | Yes | No |
| Brose (2015) (73) | Longitudinal study with comparison group | Yes | No | Yes | Yes | Yes | No |
| Al-Delaimy (2015) (78) | Longitudinal study with comparison group | Yes | No | Yes | Yes | No | No |
| Borderud (2014) (75) | Longitudinal study with comparison group | Yes | No | Yes | Yes | No | No |
| Choi (2014) (77) | Longitudinal study with comparison group | Yes | No | Yes | Yes | No | No |
| Grana (2014) (74) | Longitudinal study with comparison group | Yes | No | Yes | Yes | No | No |
| Prochaska (2014) (76) | Longitudinal study with comparison group | Yes | No | Yes | Yes | No | No |
| Hitchman (2015) (79) | Longitudinal study with comparison group | Yes | No | Yes | No | Yes | Yes |
| Adkison (2013) (82) | Longitudinal study with comparison group | Yes | No | Yes | No | No | No |
| Polosa (2016) (119) | Longitudinal study with comparison group | Yes | No | Yes | Yes | Yes | No |
| Manzoli (2016) (120) | Longitudinal study with comparison group | Yes | No | Yes | Yes | Yes | No |
| Polosa (2016b) (121) | Longitudinal study with comparison group | Yes | No | Yes | Yes | Yes | No |
| Zhuang (2016) (122) | Longitudinal study with comparison group | Yes | No | Yes | Yes | No | No |
| Caponnetto (2013) (64) | Longitudinal study with no comparison group | Yes | No | No | Yes | Yes | Yes |
| Nides (2014) (68) | Longitudinal study with no comparison group | Yes | No | No | Yes | Yes | Yes |
| Polosa (2014) (67) | Longitudinal study with no comparison group | Yes | No | No | Yes | Yes | Yes |
| Polosa (2011) (65) | Longitudinal study with no comparison group | Yes | No | No | Yes | Yes | Yes |
| Polosa (2014) (66) | Longitudinal study with no comparison group | Yes | No | No | Yes | Yes | Yes |
| Berg (2014) (62) | Longitudinal study with no comparison group | Yes | No | No | Yes | Yes | No |
| Polosa (2016) (103) | Longitudinal study with no comparison group | Yes | No | No | Yes | No | Yes |
| Pratt (2016) (104) | Longitudinal study with no comparison group | Yes | No | No | Yes | Yes | Yes |
| Farsalinos (2013b) (89) | Cross-sectional survey | Yes | No | No | No | Yes | Yes |
| Siegel (2011) (88) | Cross-sectional survey | Yes | No | No | No | Yes | Yes |
| Christensen (2014) (94) | Cross-sectional survey | Yes | No | No | No | No | No |
| Lee (2014) (93) | Cross-sectional survey | Yes | No | No | No | No | No |
| Popova (2013) (92) | Cross-sectional survey | Yes | No | No | No | No | No |
| Wagener (2014) (98) | Clinical laboratory study | Yes | No | No | Yes | No | Yes |
| Manzoli (2015) (83) | Longitudinal study with comparison group | Yes | No | Yes | Yes | Yes | No |
| Grace (2015) (69) | Longitudinal study with no comparison group | Yes | No | No | Yes | No | Yes |
| Pacifici (2015) (71) | Longitudinal study with no comparison group | Yes | No | No | Yes | No | Yes |
| Fraser (2015) (96) | Cross-sectional survey | Yes | No | No | No | Yes | Yes |
| Gallus (2014) (95) | Cross-sectional survey | Yes | No | No | No | Yes | Yes |
| Dutra (2014) (114) | Cross-sectional survey | Yes | No | No | No | No | No |
| Lechner (2015) (97) | Cross-sectional survey | Yes | No | No | No | Yes | No |
| Hiscock (2015) (111) | Cross-sectional survey | Yes | No | No | No | No | No |
| Etter (2016) (110) | Cross-sectional survey | Yes | No | No | No | No | No |
| Andler (2016) (108) | Cross-sectional survey | Yes | No | No | No | Yes | No |
| Busch (2016) (109) | Cross-sectional survey | Yes | No | No | No | No | No |
| McRobbie (2015) (99) | Clinical laboratory study | Yes | No | No | Yes | Yes | Yes |
| Campagna (2016) (54) | Randomized controlled trial | No | Yes | Yes | Yes | Yes | Yes |
| Cibella (2016) (55) | Randomized controlled trial | No | Yes | Yes | Yes | Yes | Yes |
| Pulvers (2014) (39) | Cross-sectional survey | No | Yes | No | No | Yes | No |
| Pepper (2014) (34) | Cross-sectional survey | No | Yes | No | No | No | No |
| Pokhrel (2013) (41) | Cross-sectional survey | No | Yes | No | No | No | No |
| Ramo (2015) (40) | Cross-sectional survey | No | No | No | No | No | No |
| Farsalinos (2013) (33) | Cross-sectional survey | No | No | No | No | Yes | No |
| Pokhrel (2014) (35) | Cross-sectional survey | No | No | No | No | No | No |
| Pokhrel (2014) (36) | Cross-sectional survey | No | No | No | No | No | No |
| Stein (2015) (52) | Cross-sectional survey | No | Yes | No | No | No | No |
| Beard (2015) (37) | Cross-sectional survey | No | Yes | No | No | No | No |
| Harrell (2015) (46) | Cross-sectional survey | No | No | No | No | Yes | Yes |
| Kinnunen (2015) (51) | Cross-sectional survey | No | No | No | No | No | No |
| Heydari (2015) (38) | Cross-sectional survey | No | No | No | No | No | No |
| Cho (2016) (49) | Cross-sectional survey | No | No | No | No | No | No |
| Lippert (2016) (48) | Cross-sectional survey | No | No | No | No | No | No |
| Camenga (2016) (42) | Cross-sectional survey | No | Yes | No | No | Yes | No |
| Huerta (2017) (50) | Cross-sectional survey | No | No | No | No | No | No |
| LeVault (2016) (44) | Cross-sectional survey | No | Yes | No | No | No | No |
| Spears (2016) (45) | Cross-sectional survey | No | Yes | No | No | No | No |
| Thrul (2017) (43) | Cross-sectional survey | No | Yes | No | No | No | No |
| Jorenby (2017) (56) | Clinical laboratory study | No | No | Yes | Yes | Yes | Yes |
| Copp (2015) (47) | Clinical laboratory study | No | No | No | No | No | Yes |
| Silver (2016) (53) | Case series | No | No | No | No | Yes | No |
Sixty-seven remaining articles reported the impact of e-cigarette use on abstinence from cigarettes or on the reduction in number of cigarettes consumed (57–123). Of these, six were from five RCTs (57–61, 123), nineteen were from longitudinal observational studies with a comparison group (72–83, 101, 115, 116, 119–122), two were from clinical laboratory studies (98, 99), fifteen were from longitudinal observational studies without a comparison group (62–71, 102–106), twenty-four were from cross-sectional surveys (84–97, 107–114, 117, 118), and one was a case series (100).
Criterion 2: Studies must assess e-cigarette use for smoking cessation or reduction as the exposure of interest
For observational studies, it is crucial to confirm that participants are using e-cigarettes for the purpose of cessation. In addition to quitting, people use e-cigarettes for many reasons, e.g., because they are cheaper than cigarettes, they are less harmful, or out of curiosity (34). If smokers are not using e-cigarettes as part of a cessation attempt, then it is unclear that e-cigarette use should be expected to result in quitting. It is possible that use of e-cigarettes may motivate desire and attempts to quit at some unspecified time in the future. It is also possible that e-cigarettes could reduce the desire to quit, for example, by allowing a person to use e-cigarettes in settings where smoking is prohibited, thereby reducing the pressure to quit. No studies have been designed to answer this question; we therefore confine our attention to studies where motivation to quit is clearly tied to use of e-cigarettes. This issue is borne out in studies on non-standard use of nicotine replacement therapy (i.e., use for purposes other than quitting smoking)(124, 125) which show no impact of NRT use on cessation (124). Pearson et al. addressed this issue by asking, “What quit methods have you used in the past 3 months?” (81). Participants who used an e-cigarette as a quit method were classified as “exposed” and those who did not were classified as “unexposed,” regardless of other e-cigarette use.
For RCTs where participants are assigned to use e-cigarettes or abstain and are followed for the cessation outcomes of interest, the primary treatment indication is for smoking cessation. Treatment assignment makes assessing reasons for use unnecessary in an RCT since they are likely to be balanced across the treatment and control groups through the randomization. Reasons for use are therefore unlikely to confound the relationship between e-cigarette exposure for cessation and cessation outcomes in an RCT.
Of the remaining 67 articles, six were from RCTs (57–61, 123); in one of the RCTs, e-cigarettes were assigned with other non-combustible products and thus, the article was excluded at this stage (123). Of the other 61 studies in the hierarchy, 39 did not assess the reason for e-cigarette use as an exposure and were excluded at this stage (62, 64–69, 71–79, 82, 83, 88, 89, 92–99, 103, 104, 108–111, 114, 119–122).
Criterion 3: Studies must use an appropriate design with control or comparison groups and measures to address the potential impact of e-cigarette use on smoking cessation or reduction with minimal confounds
Apart from RCTs, the strongest epidemiologic studies examining whether e-cigarette use leads to smoking abstinence or cigarette reduction are longitudinal study designs, where exposure precedes outcomes and with appropriate comparison groups (i.e., smokers with similar characteristics who were not using e-cigarettes to quit). Of the 27 remaining studies, 20 did not have appropriate study designs (59, 63, 70, 80, 81, 84–87, 90, 91, 100, 102, 105–107, 112, 113, 117, 118). Eleven of these studies were cross-sectional (84–87, 90, 91, 107, 112, 113, 117, 118), and one was a case series (100). Additionally, eight longitudinal studies (59, 63, 70, 80, 81, 102, 105, 106) were excluded at this point. One study randomly assigned participants to e-cigarette use or control during the initial lab phase of the study, but then provided the control group with e-cigarettes during the follow-up period (59). Since there was no longer an unexposed control group during the phase of the study in which the smoking cessation outcomes were observed, this study was excluded. Five studies were longitudinal, but lacked comparison groups (63, 70, 102, 105, 106). Two additional studies were longitudinal with appropriate comparison groups (80, 81); however, in their assessment of the association between e-cigarette use and smoking cessation outcomes, the exposure and outcome were assessed at the same follow-up time point. Thus, while the parent study design was longitudinal, analyses associating e-cigarette use and smoking cessation in these two studies were cross-sectional.
After considering whether studies assessed the outcome of interest, e-cigarette use for smoking cessation as an exposure, and study design, only seven articles – two from the same parent RCT – remained (57, 58, 60, 61, 101, 115, 116).
Criteria 4–6: Studies must precisely measure the exposure of interest (i.e., e-cigarette use)
In order to precisely measure the exposure of interest (e-cigarette use), studies should: 1) establish temporality by ensuring that the exposure (i.e., e-cigarette use) preceded the outcome (Criterion 4); 2) measure dose and duration of e-cigarette use (Criterion 5); and 3) assess the e-cigarette product type (Criterion 6). Four of the seven remaining articles met all six criteria (57, 58, 60, 61). The other three articles did not establish temporality (115, 116), or capture dose or duration of e-cigarette use in sufficient detail at baseline to reasonably test a cessation hypothesis (101).
The four articles from three RCTs meet all six criteria to be considered as addressing the scientific question of interest in an appropriately rigorous manner (57, 58, 60, 61). These three studies are consistent in their findings that e-cigarettes can help cessation in adult smokers – either through abstinence or cigarette reduction – regardless of motivation to quit smoking. Bullen et al.’s result (57) of 7-day point prevalence abstinence of 21.1% (nicotine e-cigarette) and 21.9% (placebo e-cigarette) at six months in smokers motivated to quit are in line with meta-analyses of the impact of smoking cessation medications, including nicotine replacement therapy (NRT), presented in the 2008 update to the Public Health Service (PHS) guidelines for Treating Tobacco Use and Dependence. In the PHS guidelines, the combined results of 32 RCTs of short-term (6–14 weeks) use of the nicotine patch among smokers interested in quitting, which found six-month abstinence of 23.4% (95% CI: 21.3–25.8), and six trials of use of the nicotine inhaler (24.8%) (126). Caponnetto et al.’s results (58) are also similar to findings from meta-analyses of the impact of smoking cessation medications among smokers not willing to quit (126). Specifically, the 8.7% of smokers using placebo and nicotine-containing e-cigarettes and the 11% using nicotine-containing e-cigarettes who quit at one year in this study is similar to the 8.4% abstinence rate among nicotine replacement users in five RCTs among smokers unmotivated to quit (126). Tseng et al. (61) found that young adult daily smokers interested in reducing their cigarette consumption experienced significantly greater reductions in cigarettes per day from baseline when using a nicotine-containing e-cigarette compared to a placebo e-cigarette at 3-week follow-up (p = 0.03).
DISCUSSION
Of 91 articles that claim to examine the relationship between e-cigarette use and smoking cessation or reduction, only four articles from three RCTs meet all six recommended criteria. These three studies suggest that e-cigarettes are effective in helping adult smokers to quit or to reduce their cigarette consumption, and that rates of smoking cessation with e-cigarettes are similar to rates of cessation with NRT. The criteria outlined in this article draw on “design thinking”(127) to articulate the study design elements required to answer the scientific question of interest and differentiate informative from uninformative evidence. They serve as a screening tool to identify the appropriate studies for comparison; as in a standard systematic review process, additional evaluation is needed to determine the risk of bias in these studies and potential impacts of study limitations on the outcomes reported. Our findings highlight the following methodological concerns with existing studies: first, the majority of studies that claim to address the relationship between e-cigarette use and smoking cessation or reduction do not provide evidence to answer that question; second, poor study design limits inferences about the impact of the exposure on a subsequent outcome, particularly in comparison to an unexposed group; and third, exposure measures in many studies are insufficient for assessing dose, duration (e.g., ever used an e-cigarette), product type, or reasons for use.
It is important to be cautious in drawing conclusions from the existing studies of the effect of e-cigarette use on smoking cessation or reduction. There is need for improvement in methods and measures across the board, and RCTs should not automatically be considered a gold standard if they deliver the treatment poorly or suffer from inadequate internal validity like poor treatment adherence or differential drop-out.(128) Future studies should use, for example, improved measures of exposure (e.g., frequency of use, duration, product type, product features, e-liquid used, nicotine concentration (72, 73, 79)) and 7-day point-prevalence abstinence at 6-month follow-up as the outcome measure per the PHS guidelines (129) to facilitate direct comparison to established cessation treatments. Additionally, non-randomized studies must be scrutinized with respect to the appropriateness of comparison groups used and assessment of the most plausible confounders relevant to the question at hand (i.e., beyond demographics) to rule out other likely sources of bias. Such covariates are critical to address the potential impact of e-cigarette use on smoking cessation with some confidence that the study has ruled out likely “third variable” competing explanations. Without randomization of exposure to e-cigarette use, nicotine-dependent individuals may be more likely to try multiple cessation treatments (including e-cigarettes), and may be more likely to fail at quitting smoking because of their higher nicotine dependence (130). E-cigarette users who have successfully quit smoking would also be excluded from most observational studies (131). Similar issues have been raised in observational studies about the efficacy of NRT for smoking cessation. RCTs have unequivocally established NRTs efficacy, but this effect is not substantiated in some observational studies (132, 133). This does not imply that the treatments are ineffective overall, but rather that other, extra-treatment factors need to be taken into account when smokers try to quit, such as insufficient treatment dose to address the needs of dependent smokers.
Our conclusion that the majority of existing observational studies do not constitute reliable scientific evidence is consistent with established practice for quantitative reviews. The Institute of Medicine’s Standards for Systematic Reviews notes that “the likelihood of selection bias, recall bias, and other biases are so high in certain clinical situations that no observational study could address the question with an acceptable risk of bias” (p. 113) (134). Therefore, the IOM recommends that observational studies be used to complement or supplement findings from RCTs in systematic reviews. The Cochrane Handbook for Systematic Reviews notes further that non-randomized studies which use “different study designs (or which have different design features)…should not be combined in a meta-analysis” (p. 422) (135). Two Cochrane reviews examined data from both RCTs and cohort studies, but conducted limited meta-analyses using data from only the two RCTs where the designs and populations were deemed sufficiently similar to compare (19, 24). The other two published meta-analyses included heterogeneous studies from RCTs, longitudinal, and cross-sectional designs in their pooled analyses (17, 23). There are three key issues related to combining results from non-randomized studies with respect to adjustment for confounding. First, effect estimates and standard errors from non-randomized studies do not correct for imbalance in the exposed and unexposed groups (as in randomized studies). Second, non-randomized studies on the same topics are likely to control for different confounders in their analyses; this creates further heterogeneity in the included studies. Third, adjustment for confounding in individual studies – and in pooled analyses – may yield a more precise estimate, but does not reduce bias. The Cochrane Handbook warns: “meta-analyses of studies that are at risk of bias may be seriously misleading. If bias is present in each (or some) of the individual studies, meta-analysis will simply compound the errors, and produce a ‘wrong’ result that may be interpreted as having more credibility” (p. 247) (135).
Limitations of our study include the restriction of our search to articles published in PubMed that include e-cigarette related language in the title or abstract. This may underestimate the number of studies published on e-cigarette use and cessation. More generally, the constantly evolving nature of e-cigarette products, and especially the heterogeneity and poor consumer acceptability of “first generation” products, make an accurate, evidence-based evaluation of the effectiveness of e-cigarettes as a quitting tool quite challenging.
Despite the large number of studies claiming to address the question of whether e-cigarettes are an effective smoking cessation tool, only three existing studies address the topic according to our proposed criteria. In line with guidance from the IOM and the Cochrane Collaboration, we caution against the use of meta-analysis for observational studies where the studies included are at sufficient risk of bias to produce invalid, yet precise, results. More research – especially independent, high quality RCTs with appropriate control groups and more rigorous “real world” observational studies that fit the causal question are needed to further determine whether and how e-cigarettes can be an effective cigarette cessation or harm reduction aid. This should be aided in the U.S. by the recent availability of a standardized research e-cigarette (https://www.drugabuse.gov/funding/supplemental-information-nida-e-cig). Studies to-date that do not meet at least minimal criteria for scientific rigor from both an internal and external validity perspective may be misleading and add to confusion about the state of the existing evidence rather than provide the kind of information needed to inform policy.
WHAT THIS STUDY ADDS.
Many existing studies reporting on e-cigarettes and smoking cessation are limited by methodological flaws and are uninformative with respect to the key question of the effect of e-cigarettes on smoking cessation or reduction. This study proposes a set of methodological criteria by which to identify studies that address the impact of e-cigarettes on smoking cessation or reduction. Based on the proposed criteria, few studies claiming to address the effect of e-cigarettes on smoking cessation or reduction are of sufficient quality to inform the scientific question of interest.
ACKNOWLEDGMENTS
We would like to thank Tianjing Li, MD, MHS, PhD for her guidance on methodological issues related to systematic review and meta-analysis.
FUNDING
This study was supported by Truth Initiative, the Robert Wood Johnson Foundation (Grant ID: 72208 and 72390), a Centers of Biomedical Research Excellence award from the National Institute on General Medical Sciences (Villanti; P20GM103644), a National Institutes of Health K01 Career Development Award in Tobacco Control Regulatory Research (Pearson; K01DA037950). The study sponsors did not have any role in the study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration. Authors have no other conflicts to disclose.
REFERENCES
- 1.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2.Abrams DB. Promise and peril of e-cigarettes: can disruptive technology make cigarettes obsolete? JAMA. 2014;311(2):135–6. [DOI] [PubMed] [Google Scholar]
- 3.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014;23(2):133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hecht SS, Carmella SG, Kotandeniya D, Pillsbury ME, Chen M, Ransom BW, et al. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob Res. 2015;17(6):704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nutt DJ, Phillips LD, Balfour D, Curran HV, Dockrell M, Foulds J, et al. Estimating the harms of nicotine-containing products using the MCDA approach. Eur Addict Res. 2014;20(5):218–25. [DOI] [PubMed] [Google Scholar]
- 6.Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106(11):2017–28. [DOI] [PubMed] [Google Scholar]
- 7.McQueen A, Tower S, Sumner W. Interviews with “vapers”: implications for future research with electronic cigarettes. Nicotine Tob Res. 2011;13(9):860–7. [DOI] [PubMed] [Google Scholar]
- 8.Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology (Berl). 2014;231(2):401–7. [DOI] [PubMed] [Google Scholar]
- 10.Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15(1):267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob Control. 2010;19(2):98–103. [DOI] [PubMed] [Google Scholar]
- 12.Cobb NK, Abrams DB. The FDA, e-cigarettes, and the demise of combusted tobacco. N Engl J Med. 2014;371(16):1469–71. [DOI] [PubMed] [Google Scholar]
- 13.Royal College Physicians. Nicotine without smoke: Tobacco harm reduction. London; 2016. April. [Google Scholar]
- 14.McNeill A, Brose LS, Calder R, Hitchman S, Hajek P, McRobbie H. E-cigarettes: an evidence update -- A report commissioned by Public Health England. London, England: Public Health England; 2015. [DOI] [PubMed] [Google Scholar]
- 15.Bullen C Electronic cigarettes for smoking cessation. Curr Cardiol Rep. 2014;16(11):538. [DOI] [PubMed] [Google Scholar]
- 16.Franck C, Budlovsky T, Windle SB, Filion KB, Eisenberg MJ. Electronic cigarettes in north america: history, use, and implications for smoking cessation. Circulation. 2014;129(19):1945–52. [DOI] [PubMed] [Google Scholar]
- 17.Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med. 2016;4(2):116–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee AH, Stater BJ, Close L, Rahmati R. Are e-cigarettes effective in smoking cessation? Laryngoscope. 2015;125(4):785–7. [DOI] [PubMed] [Google Scholar]
- 19.McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;12:Cd010216. [DOI] [PubMed] [Google Scholar]
- 20.Meier E, Tackett AP, Wagener TL. Effectiveness of electronic aids for smoking cessation. Curr Cardiovasc Risk Rep. 2013;7(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odum LE, O’Dell KA, Schepers JS. Electronic cigarettes: do they have a role in smoking cessation? J Pharm Pract. 2012;25(6):611–4. [DOI] [PubMed] [Google Scholar]
- 22.Orr KK, Asal NJ. Efficacy of electronic cigarettes for smoking cessation. Ann Pharmacother. 2014;48(11):1502–6. [DOI] [PubMed] [Google Scholar]
- 23.Rahman MA, Hann N, Wilson A, Mnatzaganian G, Worrall-Carter L. E-cigarettes and smoking cessation: evidence from a systematic review and meta-analysis. PLoS One. 2015;10(3):e0122544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartmann-Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2016;9:CD010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knight-West O, Bullen C. E-cigarettes for the management of nicotine addiction. Subst Abuse Rehabil. 2016;7:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malas M, van der Tempel J, Schwartz R, Minichiello A, Lightfoot C, Noormohamed A, et al. Electronic Cigarettes for Smoking Cessation: A Systematic Review. Nicotine Tob Res. 2016;18(10):1926–36. [DOI] [PubMed] [Google Scholar]
- 27.Orellana-Barrios MA, Payne D, Medrano-Juarez RM, Yang S, Nugent K. Electronic Cigarettes for Smoking Cessation. Am J Med Sci. 2016;352(4):420–6. [DOI] [PubMed] [Google Scholar]
- 28.Kaisar MA, Prasad S, Liles T, Cucullo L. A decade of e-cigarettes: Limited research & unresolved safety concerns. Toxicology. 2016;365:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zborovskaya Y E-Cigarettes and Smoking Cessation: A Primer for Oncology Clinicians. Clinical journal of oncology nursing. 2017;21(1):54–63. [DOI] [PubMed] [Google Scholar]
- 30.Glasser AM, Cobb CO, Teplitskaya L, Ganz O, Katz L, Rose SW, et al. Electronic nicotine delivery devices, and their impact on health and patterns of tobacco use: a systematic review protocol. BMJ Open. 2015;5(4):e007688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glasser AM, Collins L, Pearson JL, Abudayyeh H, Niaura RS, Abrams DB, et al. Overview of Electronic Nicotine Delivery Systems: A Systematic Review. Am J Prev Med. 2017;52(2):e33–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glasgow RE, Green LW, Klesges LM, Abrams DB, Fisher EB, Goldstein MG, et al. External validity: we need to do more. Ann Behav Med. 2006;31(2):105–8. [DOI] [PubMed] [Google Scholar]
- 33.Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluating nicotine levels selection and patterns of electronic cigarette use in a group of “vapers” who had achieved complete substitution of smoking. Subst Abuse. 2013;7:139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pepper JK, Ribisl KM, Emery SL, Brewer NT. Reasons for starting and stopping electronic cigarette use. Int J Environ Res Public Health. 2014;11(10):10345–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pokhrel P, Herzog TA. Reasons for quitting cigarette smoking and electronic cigarette use for cessation help. Psychol Addict Behav. 2015;29(1):114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pokhrel P, Little MA, Fagan P, Kawamoto CT, Herzog TA. Correlates of use of electronic cigarettes versus nicotine replacement therapy for help with smoking cessation. Addict Behav. 2014;39(12):1869–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beard E, Brown J, McNeill A, Michie S, West R. Has growth in electronic cigarette use by smokers been responsible for the decline in use of licensed nicotine products? Findings from repeated cross-sectional surveys. Thorax. 2015;70(10):974–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heydari G, Masjedi M, Ahmady AE, Leischow SJ, Harry AL, Shadmehr MB, et al. Assessment of Different Quit Smoking Methods Selected by Patients in Tobacco Cessation Centers in Iran. Int J Prev Med. 2015;6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pulvers K, Hayes RB, Scheuermann TS, Romero DR, Emami AS, Resnicow K, et al. Tobacco Use, Quitting Behavior, and Health Characteristics Among Current Electronic Cigarette Users in a National Tri-Ethnic Adult Stable Smoker Sample. Nicotine Tob Res. 2015;17(9):1085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramo DE, Young-Wolff KC, Prochaska JJ. Prevalence and correlates of electronic-cigarette use in young adults: findings from three studies over five years. Addict Behav. 2015;41:142–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pokhrel P, Fagan P, Little MA, Kawamoto CT, Herzog TA. Smokers who try e-cigarettes to quit smoking: findings from a multiethnic study in Hawaii. Am J Public Health. 2013;103(9):e57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camenga DR, Kong G, Cavallo DA, Krishnan-Sarin S. Current and Former Smokers’ Use of Electronic Cigarettes for Quitting Smoking: An Exploratory Study of Adolescents and Young Adults. Nicotine Tob Res. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thrul J, Ramo DE. Cessation Strategies Young Adult Smokers Use After Participating in a Facebook Intervention. Substance use & misuse. 2017;52(2):259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LeVault K, Mueller-Luckey G, Waters EA, Fogleman A, Crumly D, Jenkins WD. E-cigarettes: Who’s using them and why? J Fam Pract. 2016;65(6):390–7. [PubMed] [Google Scholar]
- 45.Spears CA, Jones DM, Weaver SR, Pechacek TF, Eriksen MP. Use of Electronic Nicotine Delivery Systems among Adults with Mental Health Conditions, 2015. Int J Environ Res Public Health. 2016;14(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrell PT, Simmons VN, Pineiro B, Correa JB, Menzie NS, Meltzer LR, et al. E-cigarettes and expectancies: why do some users keep smoking? Addiction. 2015;110(11):1833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Copp SR, Collins JL, Dar R, Barrett SP. The effects of nicotine stimulus and response expectancies on male and female smokers’ responses to nicotine-free electronic cigarettes. Addict Behav. 2015;40:144–7. [DOI] [PubMed] [Google Scholar]
- 48.Lippert AM. Temporal Changes in the Correlates of U.S. Adolescent Electronic Cigarette Use and Utilization in Tobacco Cessation, 2011 to 2013. Health Educ Behav. 2016. [DOI] [PubMed] [Google Scholar]
- 49.Cho JH, Paik SY. Association between Electronic Cigarette Use and Asthma among High School Students in South Korea. PLoS One. 2016;11(3):e0151022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huerta TR, Walker DM, Mullen D, Johnson TJ, Ford EW. Trends in E-Cigarette Awareness and Perceived Harmfulness in the U.S. Am J Prev Med. 2017;52(3):339–46. [DOI] [PubMed] [Google Scholar]
- 51.Kinnunen JM, Ollila H, El-Amin Sel T, Pere LA, Lindfors PL, Rimpela AH. Awareness and determinants of electronic cigarette use among Finnish adolescents in 2013: a population-based study. Tob Control. 2015;24(e4):e264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stein MD, Caviness CM, Grimone K, Audet D, Borges A, Anderson BJ. E-cigarette knowledge, attitudes, and use in opioid dependent smokers. J Subst Abuse Treat. 2015;52:73–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silver B, Ripley-Moffitt C, Greyber J, Goldstein AO. Successful use of nicotine replacement therapy to quit e-cigarettes: lack of treatment protocol highlights need for guidelines. Clinical case reports. 2016;4(4):409–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campagna D, Cibella F, Caponnetto P, Amaradio MD, Caruso M, Morjaria JB, et al. Changes in breathomics from a 1-year randomized smoking cessation trial of electronic cigarettes. European journal of clinical investigation. 2016;46(8):698–706. [DOI] [PubMed] [Google Scholar]
- 55.Cibella F, Campagna D, Caponnetto P, Amaradio MD, Caruso M, Russo C, et al. Lung function and respiratory symptoms in a randomized smoking cessation trial of electronic cigarettes. Clinical science (London, England : 1979). 2016;130(21):1929–37. [DOI] [PubMed] [Google Scholar]
- 56.Jorenby DE, Smith SS, Fiore MC, Baker TB. Nicotine levels, withdrawal symptoms, and smoking reduction success in real world use: A comparison of cigarette smokers and dual users of both cigarettes and E-cigarettes. Drug and alcohol dependence. 2017;170:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382(9905):1629–37. [DOI] [PubMed] [Google Scholar]
- 58.Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adriaens K, Van Gucht D, Declerck P, Baeyens F. Effectiveness of the electronic cigarette: An eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11(11):11220–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Brien B, Knight-West O, Walker N, Parag V, Bullen C. E-cigarettes versus NRT for smoking reduction or cessation in people with mental illness: secondary analysis of data from the ASCEND trial. Tob Induc Dis. 2015;13(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tseng TY, Ostroff JS, Campo A, Gerard M, Kirchner T, Rotrosen J, et al. A Randomized Trial Comparing the Effect of Nicotine Versus Placebo Electronic Cigarettes on Smoking Reduction Among Young Adult Smokers. Nicotine Tob Res. 2016;18(10):1937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berg CJ, Barr DB, Stratton E, Escoffery C, Kegler M. Attitudes toward E-Cigarettes, Reasons for Initiating E-Cigarette Use, and Changes in Smoking Behavior after Initiation: A Pilot Longitudinal Study of Regular Cigarette Smokers. Open J Prev Med. 2014;4(10):789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav. 2014;39(2):491–4. [DOI] [PubMed] [Google Scholar]
- 64.Caponnetto P, Auditore R, Russo C, Cappello GC, Polosa R. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health. 2013;10(2):446–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Polosa R, Caponnetto P, Morjaria JB, Papale G, Campagna D, Russo C. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Polosa R, Morjaria JB, Caponnetto P, Campagna D, Russo C, Alamo A, et al. Effectiveness and tolerability of electronic cigarette in real-life: a 24-month prospective observational study. Intern Emerg Med. 2014;9(5):537–46. [DOI] [PubMed] [Google Scholar]
- 67.Polosa R, Caponnetto P, Maglia M, Morjaria JB, Russo C. Success rates with nicotine personal vaporizers: a prospective 6-month pilot study of smokers not intending to quit. BMC Public Health. 2014;14:1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nides MA, Leischow SJ, Bhatter M, Simmons M. Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system. Am J Health Behav. 2014;38(2):265–74. [DOI] [PubMed] [Google Scholar]
- 69.Grace RC, Kivell BM, Laugesen M. Gender differences in satisfaction ratings for nicotine electronic cigarettes by first-time users. Addict Behav. 2015;50:140–3. [DOI] [PubMed] [Google Scholar]
- 70.Polosa R, Caponnetto P, Cibella F, Le-Houezec J. Quit and smoking reduction rates in vape shop consumers: a prospective 12-month survey. Int J Environ Res Public Health. 2015;12(4):3428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pacifici R, Pichini S, Graziano S, Pellegrini M, Massaro G, Beatrice F. Successful Nicotine Intake in Medical Assisted Use of E-Cigarettes: A Pilot Study. Int J Environ Res Public Health. 2015;12(7):7638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res. 2015;17(2):127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brose LS, Hitchman SC, Brown J, West R, McNeill A. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110(7):1160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grana RA, Popova L, Ling PM. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Intern Med. 2014;174(5):812–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Borderud SP, Li Y, Burkhalter JE, Sheffer CE, Ostroff JS. Electronic cigarette use among patients with cancer: characteristics of electronic cigarette users and their smoking cessation outcomes. Cancer. 2014;120(22):3527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prochaska JJ, Grana RA. E-Cigarette Use among Smokers with Serious Mental Illness. PLoS One. 2014;9(11):e113013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi K, Forster JL. Authors’ response. Am J Prev Med. 2014;46(6):e58–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Al-Delaimy WK, Myers MG, Leas EC, Strong DR, Hofstetter CR. E-cigarette use in the past and quitting behavior in the future: a population-based study. Am J Public Health. 2015;105(6):1213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hitchman SC, Brose LS, Brown J, Robson D, McNeill A. Associations Between E-Cigarette Type, Frequency of Use, and Quitting Smoking: Findings From a Longitudinal Online Panel Survey in Great Britain. Nicotine Tob Res. 2015;17(10):1187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vickerman KA, Carpenter KM, Altman T, Nash CM, Zbikowski SM. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine Tob Res. 2013;15(10):1787–91. [DOI] [PubMed] [Google Scholar]
- 81.Pearson JL, Stanton CA, Cha S, Niaura RS, Luta G, Graham AL. E-Cigarettes and Smoking Cessation: Insights and Cautions From a Secondary Analysis of Data From a Study of Online Treatment-Seeking Smokers. Nicotine Tob Res. 2015;17(10):1219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adkison SE, O’Connor RJ, Bansal-Travers M, Hyland A, Borland R, Yong HH, et al. Electronic nicotine delivery systems: international tobacco control four-country survey. Am J Prev Med. 2013;44(3):207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manzoli L, Flacco ME, Fiore M, La Vecchia C, Marzuillo C, Gualano MR, et al. Electronic Cigarettes Efficacy and Safety at 12 Months: Cohort Study. PLoS One. 2015;10(6):e0129443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goniewicz ML, Lingas EO, Hajek P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: an Internet survey. Drug Alcohol Rev. 2013;32(2):133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dawkins L, Turner J, Roberts A, Soar K. ‘Vaping’ profiles and preferences: an online survey of electronic cigarette users. Addiction. 2013;108(6):1115–25. [DOI] [PubMed] [Google Scholar]
- 86.Tackett AP, Lechner WV, Meier E, Grant DM, Driskill LM, Tahirkheli NN, et al. Biochemically verified smoking cessation and vaping beliefs among vape store customers. Addiction. 2015;110(5):868–74. [DOI] [PubMed] [Google Scholar]
- 87.Rutten LJ, Blake KD, Agunwamba AA, Grana RA, Wilson PM, Ebbert JO, et al. Use of E-Cigarettes Among Current Smokers: Associations Among Reasons for Use, Quit Intentions, and Current Tobacco Use. Nicotine Tob Res. 2015;17(10):1228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Siegel MB, Tanwar KL, Wood KS. Electronic cigarettes as a smoking-cessation: tool results from an online survey. Am J Prev Med. 2011;40(4):472–5. [DOI] [PubMed] [Google Scholar]
- 89.Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, Voudris V. Impact of flavour variability on electronic cigarette use experience: an internet survey. Int J Environ Res Public Health. 2013;10(12):7272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu SH, Gamst A, Lee M, Cummins S, Yin L, Zoref L. The Use and Perception of Electronic Cigarettes and Snus among the U.S. Population. PLoS One. 2013;8(10):e79332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brown J, Beard E, Kotz D, Michie S, West R. Real-world effectiveness of e-cigarettes when used to aid smoking cessation: a cross-sectional population study. Addiction. 2014;109(9):1531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Popova L, Ling PM. Alternative tobacco product use and smoking cessation: a national study. Am J Public Health. 2013;103(5):923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee S, Grana RA, Glantz SA. Electronic cigarette use among Korean adolescents: a cross-sectional study of market penetration, dual use, and relationship to quit attempts and former smoking. J Adolesc Health. 2014;54(6):684–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Christensen T, Welsh E, Faseru B. Profile of e-cigarette use and its relationship with cigarette quit attempts and abstinence in Kansas adults. Prev Med. 2014;69C:90–4. [DOI] [PubMed] [Google Scholar]
- 95.Gallus S, Lugo A, Pacifici R, Pichini S, Colombo P, Garattini S, et al. E-cigarette awareness, use, and harm perceptions in Italy: a national representative survey. Nicotine Tob Res. 2014;16(12):1541–8. [DOI] [PubMed] [Google Scholar]
- 96.Fraser D, Weier M, Keane H, Gartner C. Vapers’ perspectives on electronic cigarette regulation in Australia. Int J Drug Policy. 2015;26(6):589–94. [DOI] [PubMed] [Google Scholar]
- 97.Lechner WV, Tackett AP, Grant DM, Tahirkheli NN, Driskill LM, Wagener TL. Effects of duration of electronic cigarette use. Nicotine Tob Res. 2015;17(2):180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wagener TL, Meier E, Hale JJ, Oliver ER, Warner ML, Driskill LM, et al. Pilot investigation of changes in readiness and confidence to quit smoking after E-cigarette experimentation and 1 week of use. Nicotine Tob Res. 2014;16(1):108–14. [DOI] [PubMed] [Google Scholar]
- 99.McRobbie H, Phillips A, Goniewicz ML, Smith KM, Knight-West O, Przulj D, et al. Effects of Switching to Electronic Cigarettes with and without Concurrent Smoking on Exposure to Nicotine, Carbon Monoxide, and Acrolein. Cancer Prev Res (Phila). 2015;8(9):873–8. [DOI] [PubMed] [Google Scholar]
- 100.Caponnetto P, Polosa R, Russo C, Leotta C, Campagna D. Successful smoking cessation with electronic cigarettes in smokers with a documented history of recurring relapses: a case series. J Med Case Rep. 2011;5(1):585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vickerman KA, Schauer GL, Malarcher AM, Zhang L, Mowery P, Nash CM. Reasons for Electronic Nicotine Delivery System use and smoking abstinence at 6 months: a descriptive study of callers to employer and health plan-sponsored quitlines. Tob Control. 2017;26(2):126–34. [DOI] [PubMed] [Google Scholar]
- 102.Nolan M, Leischow S, Croghan I, Kadimpati S, Hanson A, Schroeder D, et al. Feasibility of Electronic Nicotine Delivery Systems in Surgical Patients. Nicotine Tob Res. 2016;18(8):1757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Polosa R, Morjaria JB, Caponnetto P, Caruso M, Campagna D, Amaradio MD, et al. Persisting long term benefits of smoking abstinence and reduction in asthmatic smokers who have switched to electronic cigarettes. Discov Med. 2016;21(114):99–108. [PubMed] [Google Scholar]
- 104.Pratt SI, Sargent J, Daniels L, Santos MM, Brunette M. Appeal of electronic cigarettes in smokers with serious mental illness. Addict Behav. 2016;59:30–4. [DOI] [PubMed] [Google Scholar]
- 105.Stein MD, Caviness C, Grimone K, Audet D, Anderson BJ, Bailey GL. An Open Trial of Electronic Cigarettes for Smoking Cessation Among Methadone-Maintained Smokers. Nicotine Tob Res. 2016;18(5):1157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.James SA, Meier EM, Wagener TL, Smith KM, Neas BR, Beebe LA. E-Cigarettes for Immediate Smoking Substitution in Women Diagnosed with Cervical Dysplasia and Associated Disorders. Int J Environ Res Public Health. 2016;13(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Allem JP, Unger JB, Garcia R, Baezconde-Garbanati L, Sussman S. Tobacco Attitudes and Behaviors of Vape Shop Retailers in Los Angeles. Am J Health Behav. 2015;39(6):794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Andler R, Guignard R, Wilquin JL, Beck F, Richard JB, Nguyen-Thanh V. Electronic cigarette use in France in 2014. Int J Public Health. 2016;61(2):159–65. [DOI] [PubMed] [Google Scholar]
- 109.Busch AM, Leavens EL, Wagener TL, Buckley ML, Tooley EM. Prevalence, Reasons for Use, and Risk Perception of Electronic Cigarettes Among Post-Acute Coronary Syndrome Smokers. J Cardiopulm Rehabil Prev. 2016;36(5):352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Etter JF. Characteristics of users and usage of different types of electronic cigarettes: findings from an online survey. Addiction. 2016;111(4):724–33. [DOI] [PubMed] [Google Scholar]
- 111.Hiscock R, Bauld L, Arnott D, Dockrell M, Ross L, McEwen A. Views from the Coalface: What Do English Stop Smoking Service Personnel Think about E-Cigarettes? Int J Environ Res Public Health. 2015;12(12):16157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McQueen N, Partington EJ, Harrington KF, Rosenthal EL, Carroll WR, Schmalbach CE. Smoking Cessation and Electronic Cigarette Use among Head and Neck Cancer Patients. Otolaryngol Head Neck Surg. 2016;154(1):73–9. [DOI] [PubMed] [Google Scholar]
- 113.Pechacek TF, Nayak P, Gregory KR, Weaver SR, Eriksen MP. The Potential That Electronic Nicotine Delivery Systems Can be a Disruptive Technology: Results From a National Survey. Nicotine Tob Res. 2016;18(10):1989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dutra LM, Glantz SA. Electronic cigarettes and conventional cigarette use among U.S. adolescents: a cross-sectional study. JAMA Pediatr. 2014;168(7):610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zawertailo L, Pavlov D, Ivanova A, Ng G, Baliunas D, Selby P. Concurrent E-Cigarette Use During Tobacco Dependence Treatment in Primary Care Settings: Association With Smoking Cessation at Three and Six Months. Nicotine Tob Res. 2017;19(2):183–9. [DOI] [PubMed] [Google Scholar]
- 116.Shi Y, Pierce JP, White M, Vijayaraghavan M, Compton W, Conway K, et al. E-cigarette use and smoking reduction or cessation in the 2010/2011 TUS-CPS longitudinal cohort. BMC Public Health. 2016;16(1):1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Beard E, West R, Michie S, Brown J. Association between electronic cigarette use and changes in quit attempts, success of quit attempts, use of smoking cessation pharmacotherapy, and use of stop smoking services in England: time series analysis of population trends. BMJ (Clinical research ed). 2016;354:i4645. [DOI] [PubMed] [Google Scholar]
- 118.Filippidis FT, Laverty AA, Vardavas CI. Experimentation with e-cigarettes as a smoking cessation aid: a cross-sectional study in 28 European Union member states. BMJ Open. 2016;6(10):e012084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Polosa R, Morjaria JB, Caponnetto P, Battaglia E, Russo C, Ciampi C, et al. Blood Pressure Control in Smokers with Arterial Hypertension Who Switched to Electronic Cigarettes. Int J Environ Res Public Health. 2016;13(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Manzoli L, Flacco ME, Ferrante M, La Vecchia C, Siliquini R, Ricciardi W, et al. Cohort study of electronic cigarette use: effectiveness and safety at 24 months. Tob Control. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Polosa R, Morjaria JB, Caponnetto P, Prosperini U, Russo C, Pennisi A, et al. Evidence for harm reduction in COPD smokers who switch to electronic cigarettes. Respiratory research. 2016;17(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhuang YL, Cummins SE, J YS, Zhu SH. Long-term e-cigarette use and smoking cessation: a longitudinal study with US population. Tob Control. 2016;25(Suppl 1):i90–i5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hatsukami DK, Luo X, Dick L, Kangkum M, Allen SS, Murphy SE, et al. Reduced nicotine content cigarettes and use of alternative nicotine products: exploratory trial. Addiction (Abingdon, England). 2017;112(1):156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Levy DE, Thorndike AN, Biener L, Rigotti NA. Use of nicotine replacement therapy to reduce or delay smoking but not to quit: prevalence and association with subsequent cessation efforts. Tob Control. 2007;16(6):384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hammond D, Reid JL, Driezen P, Cummings KM, Borland R, Fong GT, et al. Smokers’ use of nicotine replacement therapy for reasons other than stopping smoking: findings from the ITC Four Country Survey. Addiction. 2008;103(10):1696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tobacco Use and Dependence Guideline Panel. Treating Tobacco Use and Dependence: 2008 Update: Rockville (MD): US Department of Health and Human Services; 2008. [Google Scholar]
- 127.Goodman SN, Schneeweiss S, Baiocchi M. Using Design Thinking to Differentiate Useful From Misleading Evidence in Observational Research. JAMA. 2017;317(7):705–7. [DOI] [PubMed] [Google Scholar]
- 128.West R, Hall W, Marsden J. A structured approach to making causal inferences in the field of addiction. Addiction. Under review. [Google Scholar]
- 129.Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, et al. Treating Tobacco Use and Dependence Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2000. June. [Google Scholar]
- 130.Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. Am J Prev Med. 2008;34(2):102–11. [DOI] [PubMed] [Google Scholar]
- 131.Hajek P, McRobbie H, Bullen C. E-cigarettes and smoking cessation. Lancet Respir Med. 2016;4(6):e23. [DOI] [PubMed] [Google Scholar]
- 132.Alberg AJ, Patnaik JL, May JW, Hoffman SC, Gitchelle J, Comstock GW, et al. Nicotine replacement therapy use among a cohort of smokers. J Addict Dis. 2005;24(1):101–13. [DOI] [PubMed] [Google Scholar]
- 133.Pierce JP, Gilpin EA. Impact of over-the-counter sales on effectiveness of pharmaceutical aids for smoking cessation. JAMA. 2002;288(10):1260–4. [DOI] [PubMed] [Google Scholar]
- 134.Institute of Medicine . Finding What Works in Health Care: Standards for Systematic Reviews. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 135.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. West Sussex, England: Wiley-Blackwell; 2008. Cochrane Book Series. [Google Scholar]