Abstract
Objective
To explore the effectiveness, biosafety, photobleaching and mechanism of antimicrobial photodynamic therapy (aPDT) using methylene blue (MB) plus potassium iodide (KI), for root canal infections.
Methods
Different combinations and concentrations of MB, KI and 660 nm LED light were used against E. faecalis in planktonic and in biofilm states by colony-forming unit (CFU), confocal laser scanning microscopy (CLSM), scanning electron microscopy (SEM). Human gingival fibroblasts (HGF) were used for safety testing by Cell Counting Kit-8 (CCK8) and fluorescence microscopy (FLM). The photobleaching effect and mechanisms were analyzed.
Results
KI could not only enhance MB aPDT on E. faecalis in both planktonic and biofilm states even in a hypoxic environment, but also produced a long-lasting bactericidal effect after end of the illumination. KI could accelerate photobleaching to reduce tooth staining by MB, and the mixture was harmless for HGFs. Mechanistic studies showed the generation of hydrogen peroxide and free iodine, and iodine radicals may be formed in hypoxia.
Conclusion
aPDT with MB plus KI could be used for root canal disinfection and clinical studies are worth pursuing.
Keywords: antimicrobial photodynamic inactivation, potassium iodide, methylene blue, Enterococcus faecalis, root canal disinfection
1. Introduction
Dental pulp infections caused by bacteria are an important cause of the failure of root canal treatments [1]. Enterococcus faecalis is one of the most important bacteria that cause persistent dental pulp infections. It can colonize the surface of gutta-percha tips and survive in anaerobic or nutrient-poor environments[2]. These bacteria are resistant to a variety of antibiotics and disinfectants [3–5] and can penetrate into dentin tubules[6], which makes it difficult to be completely removed or killed by mechanical instruments or antiseptics.
Sodium hypochlorite (NaClO) is a powerful disinfectant used in root canal irrigation[7, 8]. On one hand, commonly used concentrations of NaClO solution can kill (> 3 log10) E. faecalis in vitro [9] and some studies have shown that the bactericidal ability of NaClO is more powerful than that of recently developed disinfectants[10, 11]. On the other hand, sodium hypochlorite may be too toxic for normal cells [12–15]. Root tip leakage or dripping of NaClO onto the gingival tissues can cause pain and burns[16–19].
Recently, antibiotic photodynamic therapy (aPDT) has been advocated for root canal treatment [20, 21] although it has not yet received regulatory approval. However there have been several clinical trials of aPDT in endodontics using MB-PDT [22, 23] and also using other PS [24]. aPDT is a method of killing microorganisms using a photosensitizer (PS) activated by a specific wavelength of light. Photoexcited PS produce reactive oxygen species (ROS) in the presence of oxygen [25]. Unlike antibiotics, which act against a single target in bacteria, ROS can damage multiple biomolecules, such as lipids, proteins and nucleic acids. Therefore, aPDT is not susceptible to the development of drug resistance [26, 27]. Studies have shown that aPDT can penetrate as deep as 600 μm into dentin tubules compared to NaClO (only 100 μm) [28].
Different photosensitizers have varied affinities to different types of microbial cells. Methylene blue (MB) is used in clinical dental treatment, with an absorption peak at 668 nm [29]. MB is a positively charged hydrophilic PS with a low molecular weight[21]. It can bind to a variety of Gram-positive and Gram-negative oral bacteria[30, 31], and has low toxicity to human gingival fibroblasts and osteoblasts[32]. Researchers have found that many photosensitizers, like Laserphyrin, Photofrin erythrosine B, crystal violet, rose Bengal, new methylene blue, have much higher phototoxicity towards neutrophils than MB. MB can retain the killing effect of neutrophils against pathogens [33]. Therefore, MB has become one of the promising PS used in the field of stomatology. It has received a Chinese drug license (Chinese Drug Approval Number:H20083164).
However, MB has some drawbacks for root canal treatment including a tooth staining effect. At present, MB concentrations have been employed as high as 0.01% (31.3 μM)[34–36] or higher than 100 μM[37]. On one hand, 0.01% MB can stain the teeth [38, 39], and on the other hand, too high MB concentration can reduce the aPDT effect due to self-quenching of the light[40].
The second, E. faecalis can colonize the deeper dentin tubules, where lack of enough oxygen can inhibit the PDT bacterial killing. Therefore, the traditional PDT effect in the hypoxic conditions inside root canals may be significantly decreased[41].
Thus, searching for more effective and safer PDT treatments in root canal infection treatment, is a continuing theme. In 2012, Huang et al [42] accidentally discovered that MB combined with sodium azide (NaN3), which was expected to quench the bactericidal singlet oxygen, unexpectedly enhanced the PDT killing of Staphylococcus aureus and Escherichia coli without the involvement of oxygen. In order to avoid the toxic effects of NaN3 on human cells, the team found that potassium iodide (KI) could also enhance the bactericidal effect in PDT. KI has no antimicrobial effect alone when used from 0 mM to 100 mM with or without 5 J/cm2 660 nm light [43, 44]. Also, oral acute toxicity values (LD50 in mg/kg) of KI are more than 3000 mg/kg in rats [43]. Oral KI is used to treat threshold thyroid radioactive exposures at a dose of 130 mg for adults and is the treatment of choice for cutaneous fungal infections such as sporotrichosis [45]. The only contraindication is hypersensitivity or allergic reactions.
There have been no studies using aPDT mediated by MB combined with KI in dentistry. The present study looks at the sterilization efficiency of photoactivated MB combined with KI on E. faecalis in planktonic and biofilm states. MB photobleaching, biosafety for host cells, activity under hypoxic conditions and mechanisms were studied.
2. Material and methods
2.1. Bacterial cultivation
Bacterial strain of Enterococcus faecalis ATCC 29212 (E. faecalis) was cultivated on brain heart infusion (BHI, Becton, Dickinson and Company, New York, USA) agar plates in an incubator at 37°C. One single colony was chosen to add to 20 mL liquid BHI medium for overnight shaken culture (120 rpm, 37°C). After that, a 1:100 dilution was enriched for 3–5 hours to reach mid-log phase. The concentration of the E. faecalis was 10(8) CFU/ml (OD630=0.1) and was centrifuged (9000 g, 2 minutes) and resuspended in PBS for subsequent experiments.
2.2. Light source and photosensitizers
A 660 nm deep red LED light source (Fig. 1A M660L4, Thorlabs, New York, USA) was used to deliver light to cover 2×2 wells (spot size 4 cm in diameter) in 48-well plate. A power meter (Thorlabs, New York, USA) was used to measure the light output power which was set at 50 mW/cm2. Methylene blue (MB), potassium iodide (KI) were purchased from Sigma-Aldrich (St. Louis MO, USA).
Figure 1. Spectrum of 660 nm deep red led light source.
(Data taken from Thorlabs.com).
2.3. Tooth preparation
The study protocol was approved by the Ethics committee of Peking University School of Stomatology (Ethics review sheet number: PKUSSIRB-201631102) with patient informed consent. Biofilm were established on teeth without other debris, or organic matter [46]. Briefly, 40 freshly extracted wisdom teeth were selected and kept in 5.25% sodium hypochlorite (NaClO) overnight, soft tissue, debris, and calculus were removed. The teeth were cut both transversely, others vertically by a diamond Wire Saw (STX-202A, Kejing, China) to form dentin blocks under cutting fluids [47]. 5.25% NaClO, 17% Ethylene Diamine Tetraacetic Acid (EDTA) were used for more than 10 minutes to clean the smear layer, then blocks were washed three times by distilled water and sterilized by autoclaving (121°C, 20 minutes).
2.4. aPDT parameter exploration in planktonic cells
The planktonic experiment was chosen for studying the dose-effect relationship of each parameter. In order to optimize the experimental parameters, two of the three factors (KI, MB, and light dosage) were initially fixed based on existing data, to test the dose-response relationship of the other parameter.
Resuspended planktonic E. faecalis cells (10(8)/ml, 200 μL) in PBS (Solarbio, Beijing, China), were incubated with different concentrations of MB (100 μL) and KI (100 μL) in a 48 well plate in the dark at room temperature. Blocks of 4 wells (2×2) were covered uniformly by the light spot at a power density of 50 mW/cm2[48]. The dark group in a separate plate was covered with aluminum foil. The incubation time in the literature has varied from 2–30 minutes [40, 49], but we choose 15 minutes as pre-irradiation time to allow the PS to penetrated into the bacterial cells and into the deeper dentinal tubules. After completion of the illumination each sample was placed in a new 96-well plate for serial 10-fold dilution from 101 to 106 times in PBS. 10 μL of diluted bacteria were streaked horizontally on square BHI agar plates in triplicate. Each group was repeated at least 3 times. The plates were placed in CO2 incubator for around 18 hours at 37°C. Colony-forming units were used to calculate the survival fraction of bacteria.
Varying MB concentration
Considering the staining ability of MB, we wanted to use lower MB concentration but more KI to reduce the staining effect, so KI (100 mM) was used to find a more suitable MB dosage compared to that used by other researchers[44]. For light, we also needed a shorter illumination time with 50mw/cm2 light, so we chose 6 J/cm2 [43] (2minutes) to test the sterilization concentration of MB needed, because many researchers have used 5–30 J/cm(2)[50–52] as the light dosage in PDT treatment. Thus, to verify whether the addition of KI could potentiate MB against E. faecalis in dark and light, a series of MB concentrations were tested (0, 0.1, 0.2, 0.3, 0.4, 1, 2 μM) with or without KI (100 mM) [48]and with or without 6 J/cm2 (50 mw/cm2, 2 minutes) 660 nm LED light[50–52]. (Table 1)
Table 1.
Parameters for varying MB concentration
| Group A | MB (μM) | KI (mM) | 660 nm (J/cm2) |
|---|---|---|---|
| 1 | 0 | 0 | |
| 2 | 0, 0.1, 0.2, 0.3, 0.4, 1, 2 | 6 | |
| 3 | 100 | 0 | |
| 4 | 6 |
Varying KI concentration
In order to find the dose-effect relationship of KI to potentiate E. faecalis sterilization, we tested different combinations of MB (0.1 and 0.4 μM, which was ineffective on its own but was effective with 100 mM KI and 6J/cm2 light respectively, according to the results above), combined with KI (0, 10, 25, 50, 100 mM) and red light (6 J/cm2) as shown in Table 2. In previous studies, the addition of KI (10 mM) to MB had been shown to give a good antibacterial effect [44]. In this study we tested a wider range of KI concentrations. (Table 2)
Table 2.
Parameters for varying KI concentration
| Group B | MB (μM) | KI (mM) | 660 nm (J/cm2) |
|---|---|---|---|
| 1 | 0 | ||
| 2 | 0.1 | 0, 10, 20, 50, 100 | 6 |
| 3 | 0.4 |
Varying light dosage
To study the dose-effect relationship of light, we tested different combinations of LED energy density. These light dosage parameters were fixed by illumination times (2, 4, 6, 8, 10 minutes) that corresponded to light dosages of 6, 12, 18, 24, 30 J/cm2 (with 50 mW/cm2) [43]. In this part, 660 nm LED light (0, 6, 12, 18, 24 J/cm2) were used to excite MB (0.1~0.4μM) with KI (100mM) as well as MB (0.4 μM) alone. (Table 3)
Table 3.
Parameters for varying light dosage
| Group C | MB (μM) | KI (mM) | 660 nm (J/cm2) |
|---|---|---|---|
| 1 | 0.4 | 0 | |
| 2 | 0.1 | ||
| 3 | 0.2 | 100 | 0, 6, 12, 18, 24 |
| 4 | 0.3 | ||
| 5 | 0.4 |
2.5. Long-lasting effects and effects of hypoxia
Long-lasting killing effect of aPDT
In order to test whether aPDT plus KI could still have a killing effect after stopping illumination, aPDT groups with or without KI were sampled at different times after the end of light delivery. Samples were incubated with with or without KI (100 mM) for 15 minutes, then exposed to 6 J/cm2 light [44, 53]. Samples took place before light started, and at 0, 5, and 10 minutes after the end of the light (Table 4).
Table 4.
Parameters for long lasting killing effect
| Group D | MB (μM) | KI (mM) | 660 nm (J/cm2) | Sampling time (minutes) |
|---|---|---|---|---|
| 1 | 0.2 | 0 | 6 | 0, 5, 10 |
| 2 | 100 |
aPDT effect in hypoxia
The deeper dentinal tubules always lack oxygen[54], causing lower efficiency of aPDT. To test whether KI could still enhance the antimicrobial effect of photoexcited MB in the absence of oxygen, MB (0.4 μM), MB (0.4 μM) with KI (100 mM) were incubated with E. faecalis for 15 minutes, then exposed to 660 nm LED (6 J/cm2) either inside or outside an anaerobic incubator (Bacbasic, Shellab Bactron, USA) (Table 5).
Table 5.
Parameters for aPDT effect in hypoxia
| Group E | MB (μM) | KI (mM) | 660 nm (J/cm2) | O2 |
|---|---|---|---|---|
| 1 |
0 |
0 |
+ |
|
| 2 | 0 | − | ||
| 3 | 0.4 | 6 | + | |
| 4 | 100 | − | ||
| 5 | + |
2.6. Biofilm sterilization study
Bacterial in the biofilm stage can resist the sterilization effect more than planktonic bacteria [55]. With the 50 mW/cm2 light, we used a total 30 J/cm2[43] fluence to sterilize the E. faecalis biofilm. Also, we used a higher MB (10 μM)[44] concentration group to enhance the killing effect for biofilms.
To find suitable concentrations of MB + KI + light to kill E. faecalis biofilm, 1 mL E. faecalis (10(8) CFU/ml) was added into a 6-well-plate and refreshed every 2 days to form biofilms. After 7 days, biofilm was established in each well and was treated with MB in PBS (0, 0.4, 10 μM), or MB (0.4, 10 μM) plus KI (100 mM) followed by 30 J/cm2 red light. After light delivery, scrapers were used to collect the biofilm into PBS with ultrasound vibration to disperse the cells before measuring CFU/mL (Table 6)
Table 6.
Parameters for biofilm experiment
| Group F | MB (μM) | KI (mM) | 660 nm (J/cm2) |
|---|---|---|---|
| 1 | 0 | 0 | |
| 2 | 0.4 | 0 | |
| 3 | 100 | 30 | |
| 4 | 10 | 0 | |
| 5 | 100 |
Dentin block infection and treatment
Dentin blocks were placed into 5 mL EP tubes with E. faecalis (10(8) CFU/ml) in 2 mL BHI medium. All samples were incubated in a CO2 incubator for 21 days and the BHI was refreshed every 2 days.
Before the PDT treatments, dentin blocks were washed lightly with PBS. Each sample was incubated with different concentrations of MB (100 μL) and KI (100 μL) in a 12 well plate for 15 minutes in the dark at room temperature. Then the samples were exposed to 660 nm LED as the group demanded. The experimental group details are shown in Table 7.
Table 7.
Parameters for E. faecalis biofilm sterilization in tooth blocks
| Group G | MB (μM) | KI (mM) | 660 nm (J/cm2) |
|---|---|---|---|
| 1 | 0 | 100 | |
| 2 | 10 | 0 | 30 |
| 3 | 100 |
Confocal laser scanning microscopy (CLSM) observation
CLSM was used to check the live and dead bacteria immediately after PDT using special stains. Bacteria with unbroken cell membranes are stained fluorescent green by SYTO 9, whereas bacteria with damaged membranes are stained fluorescent red by PI. The dentin blocks were grouped as shown in Table 7. Double distilled water was used to clean the dentin blocks. Each group was immersed in Live/Dead BacLight kit L7012 (Invitrogen, Carlsbad, CA) (PI: 535/617, SYTO 9: 485/498) and incubated for 15 minutes in darkness. Fluorescent images were obtained by confocal scanning fluorescence microscope (Zeiss LSM710, Germany).
Scanning Electron Microscopy Analysis (SEM)
The dentin blocks in the same groups as in the CLSM experiment, were slowly washed by 2.5% glutaraldehyde for fixation at 4°C overnight. After that, each simple was immersed in 30%, 50%, 70%, 80%, 90% alcohol for dehydration over 10 minutes. 100% alcohol dehydration 2 times again. After that, in vacuo metal spraying on each sample as shown in Table 7.
2.7. Photobleaching
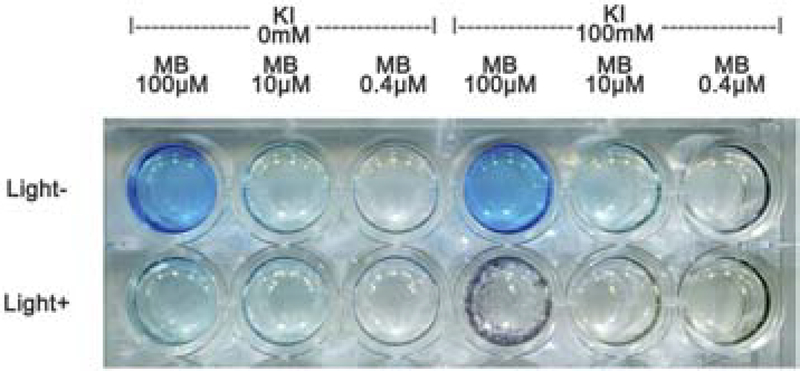
In our study, we found that MB (100, 10, 0.4 μM) combined with or without KI (100 mM) displayed pronounced color changes before and after 6 J/cm2 (Table 8).
Table 8.
Parameters for photobleaching of MB + KI
| Group H | MB (μM) | KI (100 mM) | 660 nm (6 J/cm2) |
|---|---|---|---|
| 1 | 100 | ||
| 2 | 10 | ± | ± |
| 3 | 0.4 |
2.8. Biosafety evaluation
Cell culture
Human gingival fibroblasts (HGF) were a generous gift from Central Laboratory (Peking University School and Hospital of Stomatology, Beijing, China). HGF were maintained in Dulbecco’s Modified Eagle Medium (DMEM, Gibico, Thermofisher, USA) with 10% Fetal Bovine Serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Grand Island, NY, USA), streptomycin (100 μg/ml), penicillin (100 U/ml) at 37°C in 5% CO2 incubator. Then the cells (5 × 104/well,) passage 3–5 were used for the experiment in 96 well plate (Costar; Corning Inc., NY, USA).
Cytotoxicity test
When the cell confluence was about 80%, MB (2 μM), MB (0.4 μM) plus KI (100 mM) were prepared in DMEM for 3*3 wells in a 96 well plate respectively. The doses for these groups were chosen to correspond to the doses that produced bacterial eradication in the planktonic studies in 2.4. 2*3 wells were prepared for untreated group and 3 wells for blank groups (no cells). Cells were irradiated at 660 nm wavelength with 6 J/cm2 dose[44, 53]. After treatment, the cells were washed with PBS and replaced with fresh culture medium. Cell viability in each group was measured by Cell Counting Kit-8 (CCK8, Dojindo, JAPAN) before light. Each well was incubated with 10 μL CCK8, 37 °C for 90 minutes. 90 μL of each well were transferred to a new 96 well plate for Absorbance Microplate Reader (ELX808 Biotek, Vermont, USA) at 450 nm. 3 wells data were used to calculate cell survival rate and expressed as percentage compared with untreated cells (100%). Each experiment was repeated three times. At the same time, 5.25% NaClO was added to test its toxicity to HGF cells.
After CCK-8 test, each well was washed by PBS for three times, and then added LIVE/DEAD Viability /Cytotoxicity Assay Kit (KGAF001, keygen, China), which contains Calcein AM (green florescence, Ex/Em: 495 nm/520 nm) for showing living cells and PI (red florescence, Ex/Em: 530 nm/620 nm) for showing dead cells. Cells were observed under fluorescence microscopy (ECLIPSE Tis, Nikon, Tokyo, Japan). The groups were as Table 9:
Table 9.
Parameters for safety evaluation on HGF
| Group I | MB (μM) | KI (mM) | 660 nm (J/cm2) |
|---|---|---|---|
| 1 | 0 | 0 | |
| 2 | 2 | 0 | 6 |
| 3 | 0.4 | 100 | |
| 4 | Only 5.25% NaClO | ||
2.9. Mechanistic experiments
Starch test for iodine
MB (0.4 μM) and KI (100 mM) were irradiated with 660 nm LED for 0–18 J/cm2[43]. 50 μL of each sample after illumination was added to 50 μL starch indicator (DI0636–100mL, Leagene, China) and immediately measured by microplate reader (ELx800, Biotek, USA), at 630 nm. Control groups were MB (0.4 μM), KI (100 mM), and PBS alone. Each group was retested at least three times.
Amplex-red assay for H2O2 production
Amplex Red (A22188, Invitrogen, USA) was used to detect H2O2 produced. H2O2 can react with the peroxidase in the kit to oxidize Amplex Red to form resorufin. Detection was performed according to the manufacturer’s instructions. The reaction misture contained MB (0.4 μM) combined with KI (100 mM) and irradiated with an increasing fluence of 660 nm LED as above. 50 μM Amplex red reagent in Krebs-Ringer phosphate and 0.1 U/ml horseradish peroxidase (HRP) were added. The incremental fluorescence after the LED light exposure was measured using Enspire Multimode Plate Reader (Perkin Elmer, Waltham, USA) with excitation of 570 nm and emission of 585 nm. Controls were 3 groups with MB (0.4 μM), KI (100 mM), or PBS. Each experiment was performed at least three times.
Nitroblue tetrazolium (NBT) assay for superoxide
20 mM NBT, MB (0.4 μM) and KI (100 mM) were dissolved in PBS; the absorbance of the blue product (distinguishable from MB peak at 660 nm) after increasing LED light exposure as above, was measured using an absorbance microplate reader at 570 nm absorbance. Controls were MB (0.4 μM), KI (100 mM), and PBS alone. Each experiment was performed at least three times. A positive control using C60-fullerene with β-NADH, excited by 375 nm LED light was used to demonstrate the production of superoxide.
2.10. Statistical analysis
Graphs were shown as the mean ± standard error. When necessary, one-way analysis of variance (ANOVA) and student-t tests[56–58] was carried out by SPSS 24.0. (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
3. Results
3.1. MB plus KI aPDT parameter exploration
In order to find a suitable parameter of MB-PDT + KI, we fixed two of the three factors (KI, MB, and light dosage), according to existing data, in order to test the dose-response relationship of the other parameter.
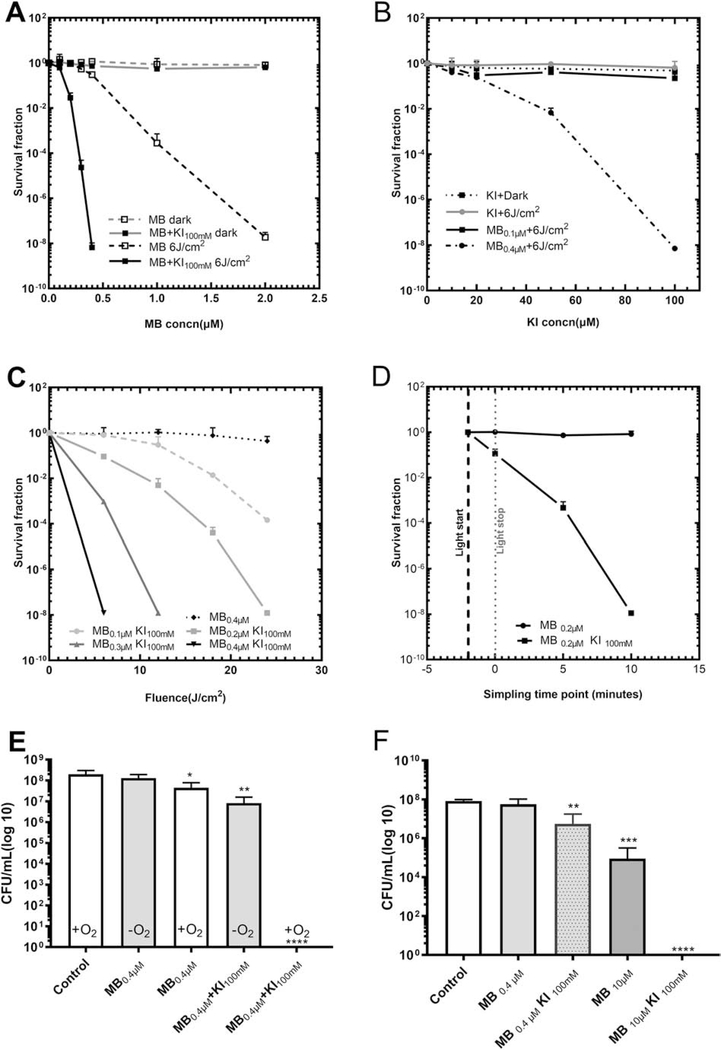
Figure 2 (A–C) shows that KI could dramatically reduce the concentration of both MB and the light dose necessary for sterilization of E. faecalis.
Figure 2. MB plus KI enhanced aPDT of E. faecalis.
(A) KI (100mM) reduced MB concentration needed for E. faecalis aPDT using 6 J/cm2. (B) KI concentration effect on potentiation of MB (0.1 or 0.4μM) aPDT using 6 J/cm2. (C) KI (100mM) reduces light dose needed for E. faecalis MB (0.1–0.4μM)-aPDT. (D) MB plus KI aPDT for E. faecalis sampled at different time after the end of illumination. (E) Hypoxic antimicrobial effect of MB (0.4μM) plus KI (100mM) aPDT for E. faecalis using 6J/cm2. (F) Photoactivation of MB with or without KI to kill E. faecalis biofilm at 30 J/cm2 of light, the biofilms were formed in 6 well plates. (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Firstly, when KI (100 mM) [48] was added, the MB concentration could be reduced by 5 times (from 2 μM to 0.4 μM) to produce the same bactericidal effect against planktonic bacteria. The concentrations of MB from 0.1–0.4 μM combined with light could hardly kill any bacteria (<101 CFU), while with added KI (100 mM) the survival fraction of E. faecalis could be decreased to 0.00000001 (10(8) logs of killing). Moreover, MB (0.4 μM) combined with KI (100 mM) and 6 J/cm2 could fully sterilize E. faecalis as efficiently as MB (2 μM) alone. The dark groups showed no obvious killing effect. (Fig. 2A)
The efficiency of the MB+KI aPDT combination was KI concentration dependent (Fig. 2B). When MB (0.4 μM) was excited with 6 J/cm2 red light, a significant potentiation of 2 log10 was observed upon the addition of KI (50 mM), and complete sterilization (7 log10 potentiation) was seen with KI (100 mM).
As many researchers have used 5–30 J/cm(2)[50–52] as the energy density in aPDT, the addition of KI (100mM) to MB(0.1–0.4μM) aPDT could also dramatically reduce the light dose required for equivalent bacterial killing at different light dosage( 6–24 J/cm2). (Fig. 2C)
3.2. Long lasting killing effect, hypoxia effect and biofilm studies
KI plus MB could produce a long-lasting killing effect beyond the end of the illumination period. If the mixture was incubated for an extra 5 minutes after the end of the light, an extra 3 Log10 of killing was observed. If it was incubated for an extra 10 minutes after the light, complete sterilization (8 log10) was obtained. (Fig. 2D)
The addition of KI could preserve some of the antimicrobial killing effect of MB aPDT in an hypoxic environment as shown in Fig. 2E. In the case of MB aPDT alone, the removal of oxygen almost entirely quenched the killing. However, in the presence of added KI, there was still 1–2 log10 of killing remaining in the anaerobic chamber.
The effects of added KI on the killing of E. faecalis biofilm, which are known to be considerably more resistant to aPDT than planktonic cells. MB (10 μM) plus KI (100 mM) and red light could sterilize the biofilm, giving almost complete killing effect compared to MB aPDT alone (Fig. 2F)
3.3. E. faecalis biofilm observed by CLSM
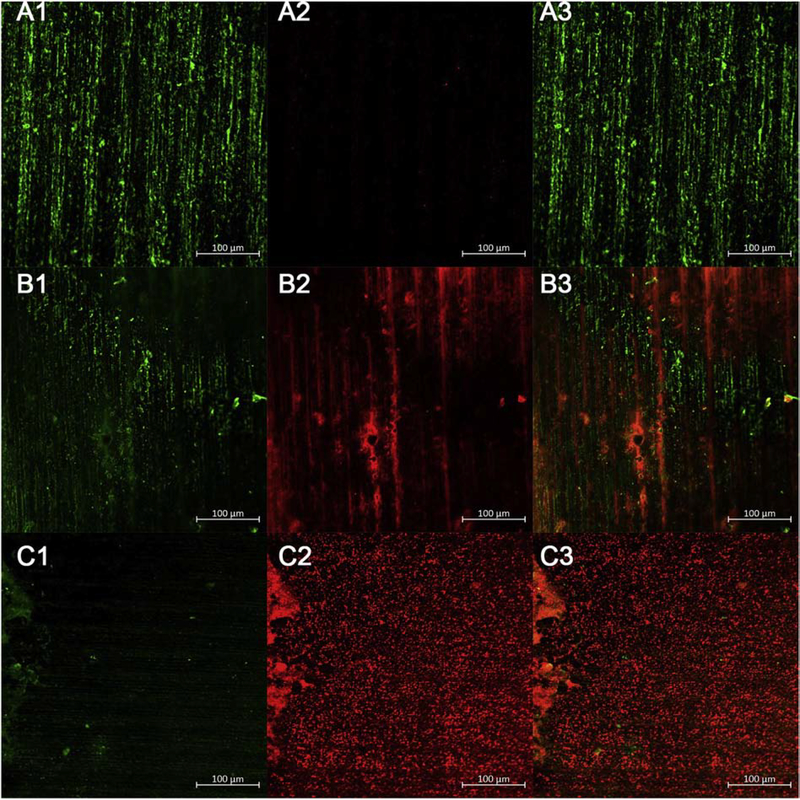
The fluorescence images of the live-dead cells in the biofilm are shown in Figure 3. (A1-C1) represent live cells, while (A2-C2) represent dead cells. (A3-C3) shows live and dead cells merged. Image J v. 1.80 (available as freeware from http://rsbweb.nih.gov/ij/) was used to measure the percentage of live or dead cells in each image.
Figure 3. CLSM images for E. faecalis biofilm growing on dentin blocks.
Biofilm were treated after 21 days using the following groups. (A1-A3) Control group; (B1-B3) MB (10 μM) plus 30 J/cm2; (C1-C3) MB (10 μM) plus KI (100 mM) plus 30 J/cm2. Images are representative examples of three separate images
Fig. 3 A1–A3 shows the untreated control group, with 20.4% area of live cells (99.5%) and 0.1% area for dead cells (0.5%).
Fig. 3 B1–B3 is aPDT using MB (10 μM) alone showing 12.4% area for live cells (38.9%) and 19.5% area for dead cells (61.1%).
Fig. 3 C1–C3 is aPDT with MB (1 μM) plus KI 100 mM showing only a 1.1% area for living cells (3.2%) but a 33.3% area for dead cells (96.8%).
3.4. Bacterial morphology by SEM
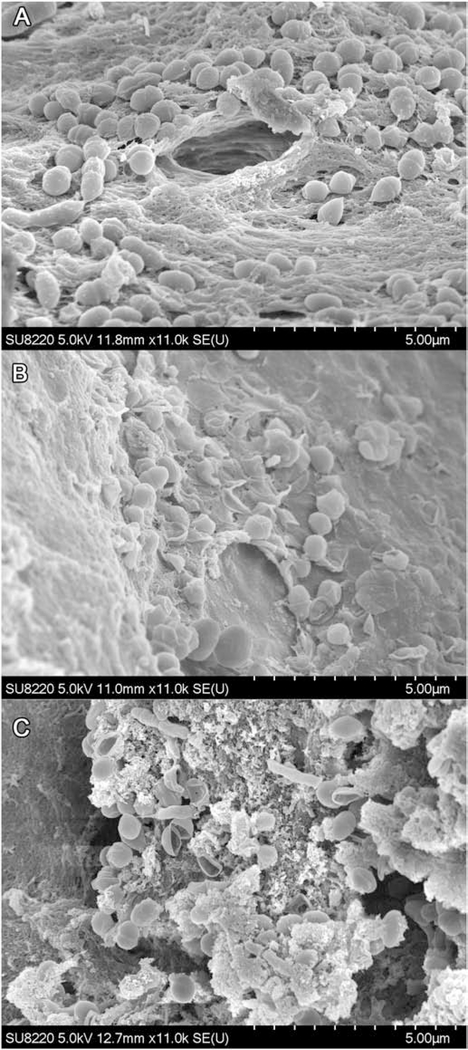
The SEM images are shown in Figure 4. Untreated E. faecalis cells (Fig. 4A) have intact membranes, while MB (10 μM) with light (Fig. 4B) has caused E. faecalis cell membranes were being damaged and atrophied. However, in aPDT with MB (10 μM) + KI (100 mM) (Fig. 4C), bacterial membranes were totally ruptured.
Figure 4. SEM images of E. faecalis biofilm.
(A) Control group; (B) MB (10 μM) plus 30 J/cm2; (C) MB (10 μM) plus KI (100 mM) plus 30 J/cm2. Images are representative examples of three separate images
3.5. Photobleaching
As can be seen from Figure 5, a light blue color is visible if the MB concentration was 10 μM. A deep blue color could be seen when the concentration was 100 μM. After application of light, the reduction of the blue color in the case of 10 μM MB was minor without KI, but more pronounced with the addition of KI (100 mM). In the case of 100 μM MB alone plus light showed a reduction of the blue color, but when KI was added there was overt precipitation to give an insoluble dark solid.
Figure 5.
Light-induced color changes of MB ± KI with or without 6 J/cm2.
3.6. HGF cell safety testing
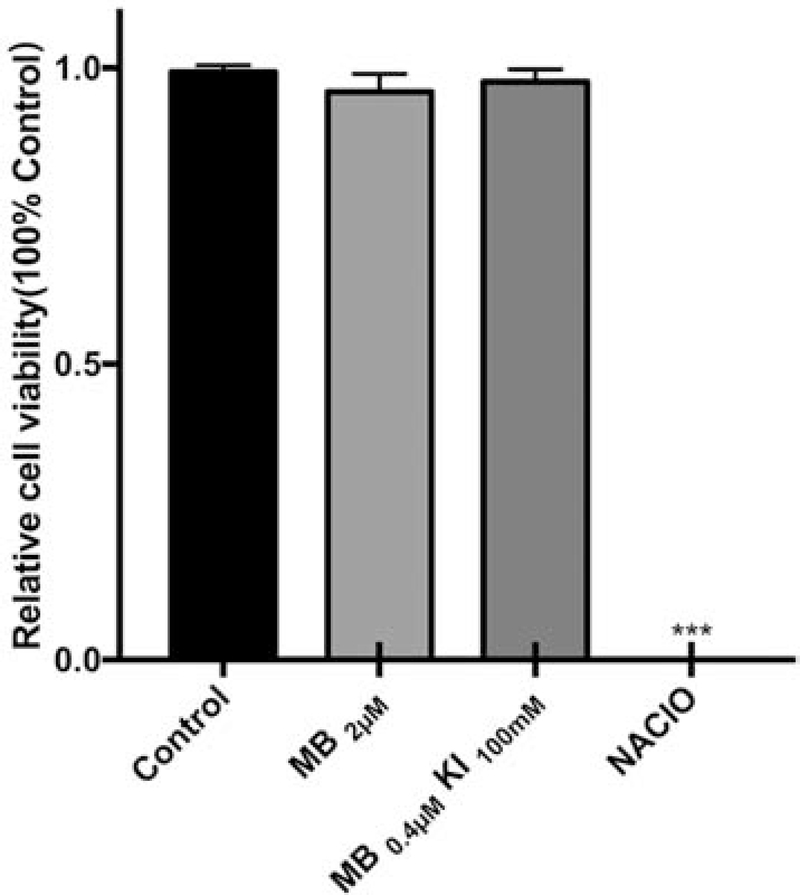
Unlike NaClO, which killed all the cells, the ideal PDT treatment should provide effective sterilization of E. faecalis without significant damage to normal HGF cells. Figure 6 has shown that aPDT with MB (2 μM) alone or MB (0.4 μM) plus KI (100 mM), which both had similar killing ability for E. faecalis, do not cause any significant loss of viability of HGF cells. In contrast a bactericidal concentration of NaClO produced 100% loss of viability of HGF cells.
Figure 6. Survival fractions of HGFs.
Untreated control group; MB (2 μM) plus 6 J/cm2 light: MB (0.4 μM) plus KI (100 mM) plus 6 J/cm2 light; NaClO (5.25%)
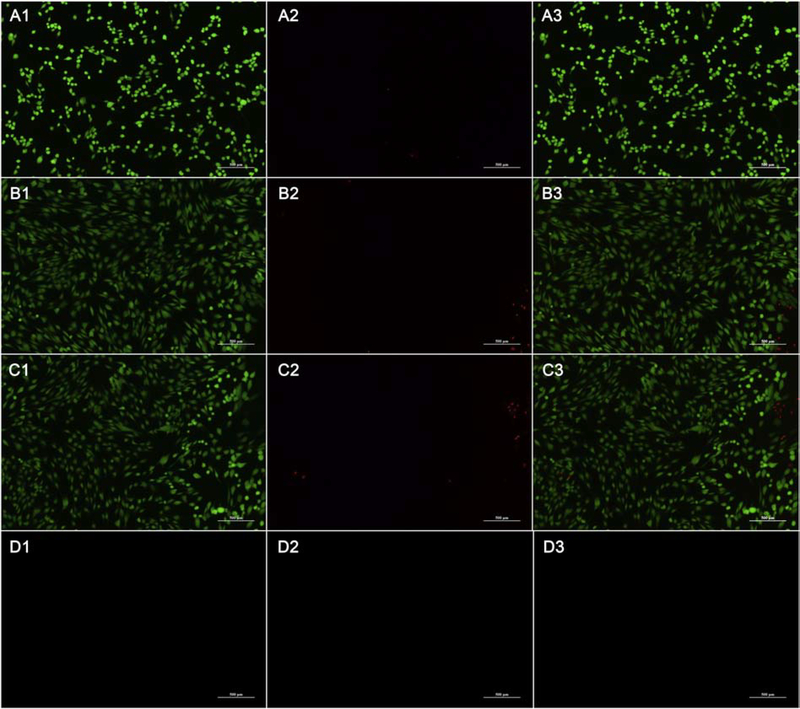
The fluorescence microscopy images of the HGF cells are shown in Figure 7. The aPDT MB alone group, MB+KI aPDT group showed no obvious differences. Green fluorescence means live HGF cells and red fluorescence means dead HGF cells. The MB group and MB+KI group showed no obvious cytotoxicity compare to the untreated group. But NaClO destroyed all the cells so that no red or green fluorescence was visible.
Figure 7. Fluorescence microscopic images for HGF cells in groups.
(A1-A3) untreated group; (B1-B3) MB (2 μM) + 6 J/cm2; (C1-C3) MB (0.4 μM) + KI (100 mM) + 6 J/cm2; (D1-D3) 5.25% NaClO. A1-D1, green cells are living cells; A2-D2, red cells are dead cells; A3-D3 are merged image.
3.7. Mechanistic experiments
A previous study showed that MB plus light primarily works via a Type 1 photochemical mechanism. In other words, the production of singlet oxygen is less important compared to hydroxyl radicals and hydrogen peroxide when it comes to killing bacteria. The mechanism by which photoexcited MB interacts with inorganic ions such as iodide was originally proposed to be a Type I electron transfer process[44], but findings with other photosensitizers (Photofrin and Rose Bengal) showed it could also be a type II process involving singlet oxygen [50, 59]. Both Type I and Type photochemical processes can oxidize iodide to produce free molecular iodine. In Figure 8 we shown the results of the mechanistic studies.
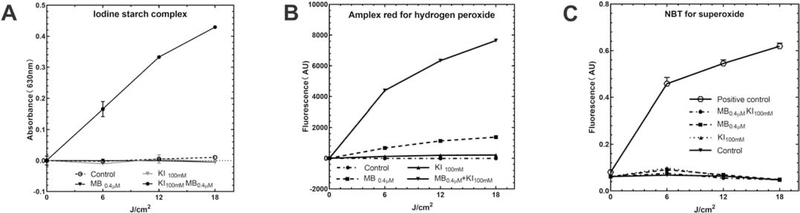
Figure 8. Mechanistic experiments.
A) Production of free iodine measured by starch indicator assay. B) Production of hydrogen peroxide by Amplex red assay. C) Production of superoxide anion by the NBT test.
Fig. 8A shows that increasing amounts of light produced more free iodine from KI (100 mM) in the presence of MB assessed by the absorption of the iodine starch complex at 630nm. Higher MB concentration produced more free iodine with increasing light dose
Fig. 8B shows the Amplex red assay demonstrating that hydrogen peroxide (H2O2) was generated in much higher quantities when KI was added. A previous study has shown that Photofrin or Rose Bengal with added KI could also generate more H2O2 than without KI.
There are two possible ways to form H2O2. One possibility is that iodide anion underwent a one-electron transfer to singlet oxygen to produce an iodine radical and superoxide radical anion. Superoxide could then undergo dismutation to give H2O2.
Fig. 8C shows superoxide production using nitro blue tetrazolium (NBT). If no superoxide was found, the second reaction pathway, singlet oxygen addition to iodine anion to produce peroxyiodide, which decomposes to produce H2O2 and iodine, is more likely. The results showed that compared to the positive control group (photoactivated fullerene plus NADH), MB plus KI did not form superoxide therefore the second pathway was more likely.
4. Discussion
aPDT is a treatment that could be employed in root canal therapy[20, 21]. Recently, researchers have found that KI could enhance the aPDT effect of photoactivated MB to kill bacteria and fungal infection. However, whether KI can still function in a hypoxic environment or could produce a long-lasting effect after light, and whether it could affect photobleaching of the MB color as well as biosafety were still unknown.
First, this study has carried out a lot of experiments and explored the suitable parameters of this complex system. The planktonic bacteria experiments showed that KI significantly enhanced the effect of aPDT with MB on E. faecalis. The killing effect was much better with increased concentrations of MB, KI, and increasing illumination time. MB with KI still has a continuing bactericidal effect after stopping the illumination, while carrying out the aPDT with the same concentration of MB had no obvious long-lasting bactericidal effect. In a hypoxic environment, the bactericidal effect of aPDT MB was completely abolished, but MB with added KI was still able to exert a bactericidal effect, although the killing was reduced compared to that found in the presence of oxygen. That observation suggests that aPDT with MB plus Ki could damage E. faecalis in an hypoxic environment, as in the deeper dentinal tubules[54]. The explanation is probably that photoactivated MB could carry out electron transfer reactions not involving oxygen, thus producing iodine radicals from iodide anions.
We observed significant damage to the E. faecalis biofilm using confocal laser scanning microscopy and scanning electron microscope. This observation shows that photoactivation of 10μM MB plus KI may be a suitable method to destroy biofilm.
MB is a colored photosensitizer that can change the color of teeth by staining them blue [38]. We found that adding KI to the MB could increase the photobleaching effect thus reducing the blue color of MB.
For the biosafety study, we compared MB, MB with KI and NaClO added to HGF cells, and found that aPDT with MB plus KI showed no obvious toxicity to HGF cells, while NaClO could destroy almost all the cells.
The synergistic mechanism by which KI and MB-PDT may involve the production of short-lived reactive iodine species, such as iodine radicals, in addition to the bactericidal long-lived free molecular iodine. Previous studies have found that MB+KI does not produce more singlet oxygen, but the formation of hydroxyl radicals is reduced. Therefore, it is not believed that ROS are increased by the addition of KI [44]. In the present study, it was confirmed that the addition of KI to aPDT with MB could increase the production of free iodine and increase the production of hydrogen peroxide. Hydrogen peroxide has a bactericidal effect, and its production is believed to be caused by the addition of singlet oxygen to the iodide anion to produce peroxyiodide (Equation 1). Peroxyiodide then gains a proton to form iodine hydroperoxide (Equation 2). Iodine hydroperoxide then reacts with an additional iodide anion to form HOOI2− (Equation 3). Dalmazio et al. [60] from Brazil used mass spectrometry and ab initio free energy calculations to study the decomposition of hydrogen peroxide in the presence of iodide anions. They detected a species with m/z = 287 that was proposed to be HOOI2−. They carried out free energy calculations revealing that the most thermodynamically favored decomposition pathway of HOOI2− was via Equation 4, to give two radicals, I2•− and HOO•. Both these short-lived radicals would be expected to cause significant damage to the bacteria. The net result of these steps is the production of free iodine (actually, tri-iodide anion I3− in the presence of excess iodide) plus H2O2 as shown in Equation 5. These two bactericidal species are likely to be responsible for the long-lasting bactericidal effect that continues after cessation of illumination.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
In the absence of oxygen (in the anaerobic incubator) we propose that the excited state of MB can undergo a 1-electron transfer to form the MB radical cation and the iodine radical anion as shown in Equation 6. These two highly reactive radicals could damage the bacterial cells.
| (6) |
In conclusion, we found that addition of KI (100 mM) to MB PDT (0.4μM for planktonic, and 10μM for biofilm) dramatically increased the sterilization effect against E. faecalis, while still preserving the safety of the treatment towards human cells. The combination was able to sterilize bacterial biofilm growing on human teeth, was able to preserve a bactericidal effect after switching off the light and could still produce bacterial killing in the absence of oxygen. If lighting time can be increased, the aPDT effect can be more. These data suggest the combination approach could be developed for root canal disinfection in root canal therapy. These data suggest the combination approach could be developed for root canal disinfection in root canal therapy. This would likely be carried out after conventional chemo‐mechanical debridement had removed the bulk of the infected material [61]. Moreover considering the lack of toxicity towards gingival fibroblasts, the addition of KI to MB aPDT alone[62, 63] could be tested in other dental indications such as peri-implantitis or periodontitis.
Highlights.
KI potentiates MB aPDT of E. faecalis in planktonic cells and dentine biofilm.
MB + KI aPDT produces long-lasting effects and could work in hypoxic root canals
The combination is safe for human cells compared to 5.25% NaClO.
KI accelerates MB photobleaching avoiding tooth staining.
Mechanism involves production of free iodine and hydrogen peroxide
Acknowledgments
FUNDING INFORMATION
YW was supported by New Technology and New Therapy Project in Peking University School of Stomatology. (PKUSSNCT-16A0) National Natural Science Foundation of China. (51972003). Peking University Health Science Center project Beijing high precision discipline. (BMU2019GJJXK024. Sub-project of national key research and development plan. (2018YFE0192500. This work was funded by The Clinical Characteristics Application of Research Projects in Capital (Z181100001718186). Smart Medical Discipline Construction Project in Peking University Health Science Center (BMU2018ZHYL013). New Technology and New Therapy Project in Peking University School of Stomatology. (PKUSSNCT-16A06)
MRH was supported by US NIH Grants R01AI050875 and R21AI121700.
Footnotes
Conflicts of interest
MRH declares the following potential conflicts of interest. Scientific Advisory Boards: Transdermal Cap Inc, Cleveland, OH; BeWell Global Inc, Wan Chai, Hong Kong; Hologenix Inc. Santa Monica, CA; LumiThera Inc, Poulsbo, WA; Vielight, Toronto, Canada; Bright Photomedicine, Sao Paulo, Brazil; Quantum Dynamics LLC, Cambridge, MA; Global Photon Inc, Bee Cave, TX; Medical Coherence, Boston MA; NeuroThera, Newark DE; JOOVV Inc, Minneapolis-St. Paul MN; AIRx Medical, Pleasanton CA; FIR Industries, Inc. Ramsey, NJ; UVLRx Therapeutics, Oldsmar, FL; Ultralux UV Inc, Lansing MI; Illumiheal & Petthera, Shoreline, WA; MB Lasertherapy, Houston, TX; ARRC LED, San Clemente, CA; Varuna Biomedical Corp. Incline Village, NV; Niraxx Light Therapeutics, Inc, Boston, MA. Consulting; Lexington Int, Boca Raton, FL; USHIO Corp, Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland B.V. Eindhoven, Netherlands; Johnson & Johnson Inc, Philadelphia, PA; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany. Stockholdings: Global Photon Inc, Bee Cave, TX; Mitonix, Newark, DE.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gutmann JL, Manjarres V, Historical and Contemporary Perspectives on the Microbiological Aspects of Endodontics, Dentistry journal, 6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Figdor D, Davies JK, Sundqvist G, Starvation survival, growth and recovery of Enterococcus faecalis in human serum, Oral Microbiol Immunol, 18 (2003) 234–239. [DOI] [PubMed] [Google Scholar]
- [3].Lins RX, de Oliveira Andrade A, Hirata Junior R, Wilson MJ, Lewis MA, Williams DW, Fidel RA, Antimicrobial resistance and virulence traits of Enterococcus faecalis from primary endodontic infections, J Dent, 41 (2013) 779–786. [DOI] [PubMed] [Google Scholar]
- [4].Asadollahi P, Razavi S, Asadollahi K, Pourshafie MR, Talebi M, Rise of antibiotic resistance in clinical enterococcal isolates during 2001–2016 in Iran: a review, New microbes and new infections, 26 (2018) 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Moghimbeigi A, Moghimbeygi M, Dousti M, Kiani F, Sayehmiri F, Sadeghifard N, Nazari A, Prevalence of vancomycin resistance among isolates of enterococci in Iran: a systematic review and meta-analysis, Adolescent health, medicine and therapeutics, 9 (2018) 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sun X, Wang S, Yang Y, Luo C, Hou B, Study of invasion E faecalis in microtubes by a novel device, Biomed Microdevices, 18 (2016) 82. [DOI] [PubMed] [Google Scholar]
- [7].Estrela C, Silva JA, de Alencar AHG, Leles CR, Decurcio DA, Efficacy of sodium hypochlorite and chlorhexidine against Enterococcus faecalis--a systematic review., in: J Appl Oral Sci, 2008, pp. 364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tejada S, Baca P, Ferrer-Luque CM, Ruiz-Linares M, Valderrama MJ, Arias-Moliz MT, Influence of dentine debris and organic tissue on the properties of sodium hypochlorite solutions, Int Endod J, 52 (2019) 114–122. [DOI] [PubMed] [Google Scholar]
- [9].Estrela C, Estrela CR, Barbin EL, Spano JC, Marchesan MA, Pecora JD, Mechanism of action of sodium hypochlorite, Braz Dent J, 13 (2002) 113–117. [DOI] [PubMed] [Google Scholar]
- [10].Wang Z, Shen Y, Ma J, Haapasalo M, The effect of detergents on the antibacterial activity of disinfecting solutions in dentin, J Endod, 38 (2012) 948–953. [DOI] [PubMed] [Google Scholar]
- [11].Rodrigues CT, de Andrade FB, de Vasconcelos L, Midena RZ, Pereira TC, Kuga MC, Duarte MAH, Bernardineli N, Antibacterial properties of silver nanoparticles as a root canal irrigant against Enterococcus faecalis biofilm and infected dentinal tubules, Int Endod J, 51 (2018) 901–911. [DOI] [PubMed] [Google Scholar]
- [12].Alkahtani A, Alkahtany SM, Anil S, An in vitro evaluation of the cytotoxicity of varying concentrations of sodium hypochlorite on human mesenchymal stem cells, J Contemp Dent Pract, 15 (2014) 473–481. [DOI] [PubMed] [Google Scholar]
- [13].Hidalgo E, Bartolome R, Dominguez C, Cytotoxicity mechanisms of sodium hypochlorite in cultured human dermal fibroblasts and its bactericidal effectiveness, Chem Biol Interact, 139 (2002) 265–282. [DOI] [PubMed] [Google Scholar]
- [14].Hidalgo E, Dominguez C, Growth-altering effects of sodium hypochlorite in cultured human dermal fibroblasts, Life Sci, 67 (2000) 1331–1344. [DOI] [PubMed] [Google Scholar]
- [15].Navarro-Escobar E, Gonzalez-Rodriguez MP, Ferrer-Luque CM, Cytotoxic effects of two acid solutions and 2.5% sodium hypochlorite used in endodontic therapy, Med Oral Patol Oral Cir Bucal, 15 (2010) e90–94. [DOI] [PubMed] [Google Scholar]
- [16].Bashetty K, Hegde J, Comparison of 2% chlorhexidine and 5.25% sodium hypochlorite irrigating solutions on postoperative pain: a randomized clinical trial, Indian J Dent Res, 21 (2010) 523–527. [DOI] [PubMed] [Google Scholar]
- [17].Matthews J, Merrill RL, Sodium hypochlorite-related injury with chronic pain sequelae, J Am Dent Assoc, 145 (2014) 553–555. [DOI] [PubMed] [Google Scholar]
- [18].Mehdipour O, Kleier DJ, Averbach RE, Anatomy of sodium hypochlorite accidents, Compend Contin Educ Dent, 28 (2007) 544–546, 548, 550. [PubMed] [Google Scholar]
- [19].Gursoy UK, Bostanci V, Kosger HH, Palatal mucosa necrosis because of accidental sodium hypochlorite injection instead of anaesthetic solution, Int Endod J, 39 (2006) 157–161. [DOI] [PubMed] [Google Scholar]
- [20].Plotino G, Grande NM, Mercade M, Photodynamic therapy in endodontics, Int Endod J, 52 (2019) 760–774. [DOI] [PubMed] [Google Scholar]
- [21].Trindade AC, De Figueiredo JA, Steier L, Weber JB, Photodynamic therapy in endodontics: a literature review, Photomed Laser Surg, 33 (2015) 175–182. [DOI] [PubMed] [Google Scholar]
- [22].Coelho MS, Vilas-Boas L, Tawil PZ, The effects of photodynamic therapy on postoperative pain in teeth with necrotic pulps, Photodiagnosis Photodyn Ther, 27 (2019) 396–401. [DOI] [PubMed] [Google Scholar]
- [23].Rabello DGD, Corazza BJM, Ferreira LL, Santamaria MP, Gomes APM, Martinho FC, Does supplemental photodynamic therapy optimize the disinfection of bacteria and endotoxins in one-visit and two-visit root canal therapy? A randomized clinical trial, Photodiagnosis Photodyn Ther, 19 (2017) 205–211. [DOI] [PubMed] [Google Scholar]
- [24].Garcez AS, Nunez SC, Hamblin MR, Ribeiro MS, Antimicrobial effects of photodynamic therapy on patients with necrotic pulps and periapical lesion, Journal of endodontics, 34 (2008) 138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gursoy H, Ozcakir-Tomruk C, Tanalp J, Yilmaz S, Photodynamic therapy in dentistry: a literature review, Clinical oral investigations, 17 (2013) 1113–1125. [DOI] [PubMed] [Google Scholar]
- [26].Alves E, Faustino MA, Neves MG, Cunha A, Tome J, Almeida A, An insight on bacterial cellular targets of photodynamic inactivation, Future medicinal chemistry, 6 (2014) 141–164. [DOI] [PubMed] [Google Scholar]
- [27].Tegos GP, Hamblin MR, Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps, Antimicrob Agents Chemother, 50 (2006) 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bumb SS, Bhaskar DJ, Agali CR, Punia H, Gupta V, Singh V, Kadtane S, Chandra S, Assessment of Photodynamic Therapy (PDT) in Disinfection of Deeper Dentinal Tubules in a Root Canal System: An In Vitro Study, Journal of clinical and diagnostic research : JCDR, 8 (2014) ZC67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Whang TJ, Huang HY, Hsieh MT, Chen JJ, Laser-induced silver nanoparticles on titanium oxide for photocatalytic degradation of methylene blue, Int J Mol Sci, 10 (2009) 4707–4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wainwright M, Phoenix DA, Marland J, Wareing DR, Bolton FJ, A study of photobactericidal activity in the phenothiazinium series, FEMS immunology and medical microbiology, 19 (1997) 75–80. [DOI] [PubMed] [Google Scholar]
- [31].Garcez AS, Nunez SC, Azambuja N Jr., Fregnani ER, Rodriguez HM, Hamblin MR, Suzuki H, Ribeiro MS, Effects of photodynamic therapy on Gram-positive and Gram-negative bacterial biofilms by bioluminescence imaging and scanning electron microscopic analysis, Photomed Laser Surg, 31 (2013) 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xu Y, Young MJ, Battaglino RA, Morse LR, Fontana CR, Pagonis TC, Kent R, Soukos NS, Endodontic antimicrobial photodynamic therapy: safety assessment in mammalian cell cultures, J Endod, 35 (2009) 1567–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tanaka M, Kinoshita M, Yoshihara Y, Shinomiya N, Seki S, Nemoto K, Hirayama T, Dai T, Huang L, Hamblin MR, Morimoto Y, Optimal photosensitizers for photodynamic therapy of infections should kill bacteria but spare neutrophils, Photochem Photobiol, 88 (2012) 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nunes MR, Mello I, Franco GCN, de Medeiros JMF, dos Santos SSF, Habitante SM, Lage-Marques JL, Raldi DP, Effectiveness of Photodynamic Therapy Against Enterococcus faecalis, With and Without the Use of an Intracanal Optical Fiber: An In Vitro Study, Photomedicine and Laser Surgery, 29 (2011) 803–808. [DOI] [PubMed] [Google Scholar]
- [35].Lim Z, Cheng JL, Lim TW, Teo EG, Wong J, George S, Kishen A, Light activated disinfection: an alternative endodontic disinfection strategy, Aust Dent J, 54 (2009) 108–114. [DOI] [PubMed] [Google Scholar]
- [36].Komine C, Tsujimoto Y, A small amount of singlet oxygen generated via excited methylene blue by photodynamic therapy induces the sterilization of Enterococcus faecalis, J Endod, 39 (2013) 411–414. [DOI] [PubMed] [Google Scholar]
- [37].Ng R, Singh F, Papamanou DA, Song XQ, Patel C, Holewa C, Patel N, Klepac-Ceraj V, Fontana CR, Kent R, Pagonis TC, Stashenko PP, Soukos NS, Endodontic Photodynamic Therapy Ex Vivo, J Endodont, 37 (2011) 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Costa LM, Matos Fde S, Correia AM, Carvalho NC, Faria ESAL, Paranhos LR, Ribeiro MA, Tooth color change caused by photosensitizers after photodynamic therapy: An in vitro study, J Photochem Photobiol B, 160 (2016) 225–228. [DOI] [PubMed] [Google Scholar]
- [39].Figueiredo RA, Anami LC, Mello I, Carvalho Edos S, Habitante SM, Raldi DP, Tooth discoloration induced by endodontic phenothiazine dyes in photodynamic therapy, Photomed Laser Surg, 32 (2014) 458–462. [DOI] [PubMed] [Google Scholar]
- [40].Garcez AS, Hamblin MR, Methylene Blue and Hydrogen Peroxide for Photodynamic Inactivation in Root Canal - A New Protocol for Use in Endodontics, Eur Endod J, 2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Uekubo A, Hiratsuka K, Aoki A, Takeuchi Y, Abiko Y, Izumi Y, Effect of antimicrobial photodynamic therapy using rose bengal and blue light-emitting diode on Porphyromonas gingivalis in vitro: Influence of oxygen during treatment, Laser therapy, 25 (2016) 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Huang L, St Denis TG, Xuan Y, Huang YY, Tanaka M, Zadlo A, Sarna T, Hamblin MR, Paradoxical potentiation of methylene blue-mediated antimicrobial photodynamic inactivation by sodium azide: role of ambient oxygen and azide radicals, Free radical biology & medicine, 53 (2012) 2062–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hamblin MR, Potentiation of antimicrobial photodynamic inactivation by inorganic salts, Expert review of anti-infective therapy, 15 (2017) 1059–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Vecchio D, Gupta A, Huang L, Landi G, Avci P, Rodas A, Hamblin MR, Bacterial Photodynamic Inactivation Mediated by Methylene Blue and Red Light Is Enhanced by Synergistic Effect of Potassium Iodide, in: Antimicrob Agents Chemother, 2015, pp. 5203–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sandhu K, Gupta S, Potassium iodide remains the most effective therapy for cutaneous sporotrichosis, J Dermatolog Treat, 14 (2003) 200–202. [DOI] [PubMed] [Google Scholar]
- [46].Kishen A, Shrestha A, Del Carpio-Perochena A, Validation of Biofilm Assays to Assess Antibiofilm Efficacy in Instrumented Root Canals after Syringe Irrigation and Sonic Agitation, J Endod, 44 (2018) 292–298. [DOI] [PubMed] [Google Scholar]
- [47].Sadek FT, Monticelli F, Muench A, Ferrari M, Cardoso PE, A novel method to obtain microtensile specimens minimizing cut flaws, J Biomed Mater Res B Appl Biomater, 78 (2006) 7–14. [DOI] [PubMed] [Google Scholar]
- [48].Huang YY, Wintner A, Seed PC, Brauns T, Gelfand JA, Hamblin MR, Antimicrobial photodynamic therapy mediated by methylene blue and potassium iodide to treat urinary tract infection in a female rat model, Sci Rep, 8 (2018) 7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Xuan W, He Y, Huang L, Huang YY, Bhayana B, Xi L, Gelfand JA, Hamblin MR, Antimicrobial Photodynamic Inactivation Mediated by Tetracyclines in Vitro and in Vivo: Photochemical Mechanisms and Potentiation by Potassium Iodide, Sci Rep, 8 (2018) 17130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wen X, Zhang X, Szewczyk G, El-Hussein A, Huang YY, Sarna T, Hamblin MR, Potassium Iodide Potentiates Antimicrobial Photodynamic Inactivation Mediated by Rose Bengal in In Vitro and In Vivo Studies, Antimicrob Agents Chemother, 61 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wu X, Huang YY, Kushida Y, Bhayana B, Hamblin MR, Broad-spectrum antimicrobial photocatalysis mediated by titanium dioxide and UVA is potentiated by addition of bromide ion via formation of hypobromite, Free radical biology & medicine, 95 (2016) 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hamblin MR, Abrahamse H, Tetracyclines: light-activated antibiotics?, Future medicinal chemistry, 11 (2019) 2427–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Xuan W, Huang L, Wang Y, Hu X, Szewczyk G, Huang YY, El-Hussein A, Bommer JC, Nelson ML, Sarna T, Hamblin MR, Amphiphilic tetracationic porphyrins are exceptionally active antimicrobial photosensitizers: In vitro and in vivo studies with the free-base and Pd-chelate, J Biophotonics, 12 (2019) e201800318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sakko M, Tjaderhane L, Rautemaa-Richardson R, Microbiology of Root Canal Infections, Prim Dent J, 5 (2016) 84–89. [DOI] [PubMed] [Google Scholar]
- [55].She P, Zhou L, Li S, Liu Y, Xu L, Chen L, Luo Z, Wu Y, Synergistic Microbicidal Effect of Auranofin and Antibiotics Against Planktonic and Biofilm-Encased S. aureus and E. faecalis, Front Microbiol, 10 (2019) 2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Shany-Kdoshim S, Polak D, Houri-Haddad Y, Feuerstein O, Killing mechanism of bacteria within multi-species biofilm by blue light, J Oral Microbiol, 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Luo W, Liu RS, Zhu JG, Li YC, Liu HC, Subcellular location and photodynamic therapeutic effect of chlorin e6 in the human tongue squamous cell cancer Tca8113 cell line, Oncol Lett, 9 (2015) 551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lee K, Roberts JS, Choi CH, Atanasova KR, Yilmaz O, Porphyromonas gingivalis traffics into endoplasmic reticulum-rich-autophagosomes for successful survival in human gingival epithelial cells, Virulence, 9 (2018) 845–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Huang L, Szewczyk G, Sarna T, Hamblin MR, Potassium Iodide Potentiates Broad-Spectrum Antimicrobial Photodynamic Inactivation Using Photofrin, ACS infectious diseases, 3 (2017) 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Dalmázio I, MouraI FC, AraújoI MH, AlvesII TM, LagoI RM, de LimaI GF, DuarteI HA;, AugustiI R, The iodide-catalyzed decomposition of hydrogen peroxide: mechanistic details of an old reaction as revealed by electrospray ionization mass spectrometry monitoring, J. Braz. Chem. Soc, 19 (2006) 1105–1110. [Google Scholar]
- [61].da Silva CC, Chaves Junior SP, Pereira GLD, Fontes K, Antunes LAA, Povoa HCC, Antunes LS, Iorio N, Antimicrobial Photodynamic Therapy Associated with Conventional Endodontic Treatment: A Clinical and Molecular Microbiological Study, Photochemistry and photobiology, 94 (2018) 351–356. [DOI] [PubMed] [Google Scholar]
- [62].Deppe H, Mucke T, Wagenpfeil S, Kesting M, Sculean A, Nonsurgical antimicrobial photodynamic therapy in moderate vs severe peri-implant defects: a clinical pilot study, Quintessence Int, 44 (2013) 609–618. [DOI] [PubMed] [Google Scholar]
- [63].Alvarenga LH, Gomes AC, Carribeiro P, Godoy-Miranda B, Noschese G, Simoes Ribeiro M, Kato IT, Bussadori SK, Pavani C, Geraldo YGE, Silva D, Horliana A, Wainwright M, Prates RA, Parameters for antimicrobial photodynamic therapy on periodontal pocket-Randomized clinical trial, Photodiagnosis Photodyn Ther, 27 (2019) 132–136. [DOI] [PubMed] [Google Scholar]