Abstract
Study Design:
Narrative review.
Objective:
To summarize relevant studies regarding the utilization of intraoperative neurophysiological monitoring (IONM) techniques in spine surgery implemented in recent years.
Methods:
A literature search of the Medline database was performed. Relevant studies from all evidence levels have been included. Titles, abstracts, and reference lists of key articles were included.
Results:
Multimodal intraoperative neurophysiological monitoring (MIONM) has the advantage of compensating for the limitations of each individual technique and seems to be effective and accurate for detecting perioperative neurological injury during spine surgery.
Conclusion:
Although there are no prospective studies validating the efficacy of IONM, there is a growing body of evidence supporting its use during spinal surgery. However, the lack of validated protocols to manage intraoperative alerts highlights a critical knowledge gap. Future investigation should focus on developing treatment methodology, validating practice protocols, and synthesizing clinical guidelines.
Keywords: MEP, SSEP, EMG, monitoring, spine surgery
Introduction
Although relatively infrequent, neurological injury is a much dreaded complication in spine surgery and has the potential to result in serious postoperative motor and sensory deficits.1 In recent years, an increase in the utilization of intraoperative neurophysiological monitoring (IONM) has been noted in an effort to avert these neurological complications. This technology allows intraoperative assessment of spinal cord function through real-time feedback from sensory tracts, motor tracts, and individual nerve roots. Currently, the most commonly employed IONM techniques for spinal procedures include (1) somatosensory sensory evoked potentials (SSEPs), (2) motor-evoked potentials (MEPs), and (3) spontaneous and triggered electromyography (EMG).2
Despite advancements in the understanding of IONM and the popularity of this technique in modern spine surgery, controversies still exist regarding its effectiveness and the necessity for its use in routine spinal procedures.3-6 Also, as modern health care shifts toward value-based systems, questions arise as to the exact cost-effectiveness of IONM.7,8 Therefore, the purpose of this review is to summarize relevant studies examining the effectiveness of IONM in spine surgery published in recent years with a comprehensive evaluation of the cost-benefit of this technology.
IONM Modalities
SSEP monitoring is among the most frequently used intraoperative spinal monitoring modality in the contemporary era.9,10 It consists of cortical responses generated by peripheral stimulating electrodes,11 which allows for monitoring of sensory pathways and detection of perioperative neurologic changes with excellent reliability.10 Although SSEPs are monitored continuously throughout the surgery, its interpretation requires temporal summation, which can delay the detection of a signal change by up to 16 minutes.12 Moreover, there have been several reports of false-positives and false-negatives with this technique12-14 that have raised safety concerns as to whether SSEPs can be used as a standalone monitoring technique.
In contrast to SSEPs, MEPs involve generating a stimulus either at the level of the spinal cord (D-wave)15,16 or at the motor cortex, often referred to as transcranial MEPs (tcMEPs).17 Subsequently, the signal is measured peripherally at multiple predetermined upper and lower extremity muscle groups by recording electrodes.18 With this protocol, MEPs allow for monitoring and tracking of the corticospinal tract activity during the operative procedure.18 Although MEPs have been shown to be reliable for detecting new postoperative deficits,12,19,20 the extreme sensitivity to inhaled volatile gases prevents the practice of routine anesthesia and necessitates the use of intravenous anesthetic agents.21,22 Additionally, the requirement of a triggered stimulus to register the MEP prevents continuous ongoing monitoring.9
EMG, which comes in the form of spontaneous (sEMG) or triggered (tEMG), is a valuable tool for neuromonitoring specific nerve roots at risk of injury during spinal instrumentation.23 Similar to SSEPs, sEMG is recorded continuously, thus giving the advantage of real-time feedback throughout the entire procedure.24 However, in order to have a proper sEMG response, neuromuscular blockade is prohibited.24 On the other hand, tEMGs are obtained by stimulating the center of the tulip of the pedicle screw. The response is then recorded from the appropriate muscle group. Lower thresholds might be suggestive of cortical breach, putting the nerve root at risk.25 Since its introduction, this technique has also been shown to be an effective tool for detecting cortical bone breaches during pedicle screw insertion.26
While each modality has its own intrinsic advantages and weaknesses,9,27 summarized in Table 1, together the strategies complement each other allowing for comprehensive monitoring of the anatomical areas of the spinal cord.28 Hence, the concept of multimodal intraoperative neurophysiological monitoring (MIONM) has gained in popularity and has become the standard practice for a variety of surgical procedures.9,10,29,30 However, despite the advancements in these techniques, reports still exist of false-positive alerts that can lead to unnecessary precautionary actions taken by the surgical team.31 Therefore, the purpose of this review is to summarize relevant studies regarding the effectiveness of IONM in spine surgery published in recent years (Tables 2 and 3).
Table 1.
Summary of Strengths and Weaknesses of IONM Modalities.
| Type of Monitoring | Strengths | Weaknesses |
|---|---|---|
| SSEP |
|
|
| tcMEP |
|
|
| Spontaneous EMG |
|
|
| Triggered EMG |
|
|
Abbreviations: IONM, intraoperative neurophysiological monitoring; SSEP, somatosensory sensory evoked potential; tcMEP, transcranial motor-evoked potential; EMG, electromyography.
Table 2.
Significant Studies Reporting Sensitivity, Specificity, Positive Predictive Value (PPV), and Negative Predictive Value (NPV) of Various IONM Techniques.
| Reference | Year | Design | Type of Monitoring | No. of Cases | Type of Cases | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|---|
| Clark et al | 2016 | Retrospective review | MEP | 144 | Cervical spine: degenerative CSM and CSM of non-degenerative causes | 71 | 94 | NA | NA |
| Hilibrand et al | 2004 | Retrospective review | SSEP, tcMEP | 427 | Cervical spine (CSM, OPLL) | tcMEP: 100; SSEP: 25 | tcMEP:100; SSEP: 100 | NA | NA |
| Kim et al | 2017 | Retrospective review | Multi-channel tcMEP, SSEP | 200 | Anterior cervical spine (ACDF) | 80 | 97 | 44.4 | 99.4 |
| Fujiwara et al | 2016 | Prospective study | tcMEP | 160 | Open door cervical laminoplasty | NA | NA | NA | NA |
| Eggspuehler et al | 2007 | Prospective study | SSEP, MEP, EMG | 246 | Cervical pathologies | 83.3 | 99.2 | NA | NA |
| Lee et al | 2016 | Retrospective review | SSEP, tcMEP, sEMG | 182 | Posterior cervical survey | 50 | 100 | 100 | 97 |
| Traynelis et al | 2012 | Retrospective case series | SSEP, MEP | 720 | Cervical spine | NA | NA | NA | NA |
| Ajiboye et al | 2017 | Retrospective review | SSEP, MEP | 15 395 | Anterior cervical spine (ACDF) | NA | NA | NA | NA |
| Nuwer et al | 1995 | Retrospective review. SRS survey | SSEP | 51 263 | Variety of pathologies | 92 | 98.9 | 42 | 99.9 |
| Quraishi et al | 2009 | Retrospective review | SSEP, MEP, EMG | 102 | Scoliosis and kyphosis | 100 | 84 | 14 | 97 |
| Pastorelli et al | 2011 | Retrospective review | SSEP, TcMEP | 172 | Spinal deformity | 100 | 98 | NA | NA |
| Hamilton et al | 2011 | Retrospective review; SRS mortality and morbidity database | SSEP, MEP, EMG | 108 419 | Variety of spinal pathologies | 43 | 98 | 21 | 99 |
| Bhagat et al | 2015 | Retrospective review | SSEP, MEP | 354 | Spinal deformity | 100 | 93.3 | NA | NA |
| Gunnarsson et al | 2004 | Retrospective review | SSEP, sEMG | 213 | Thoracolumbar procedures | sEMG: 100; SSEP: 28.6 | sEMG: 23.7; SSEP: 94.7 | NA | NA |
| Sutter et al | 2007 | Retrospective review | SSEP, tcMEP, EMG | 109 | Intradural spinal tumors of various types | 92 | 99 | NA | NA |
| Forster et al | 2012 | Retrospective review | SSEP, MEP | 203 | Intradural spinal tumors of various types | SSEP: 94.4; MEP: 95 | SSEP: 96.8; MEP: 98.9 | NA | NA |
| Korn et al | 2015 | Retrospective review | SSEP, tcMEP, EMG, D-waves | 100 | Intradural extramedullary tumors | 82 | 95 | 82 | 95 |
| Harel et al | 2017 | Retrospective review | SSEP, tcMEP, EMG | 41 | Intradural extramedullary tumors | 75 | 100 | 100 | 97 |
| Sala et al | 2006 | Retrospective review | SSEP, tcMEP, D-wave, EMG | 50 | Intramedullary spinal cord tumor | NA | NA | NA | NA |
| Jin et al | 2015 | Retrospective review | SSEP, mMEP, and fEMG | 25 | Intramedullary spinal cord tumor | 100 | 91 | 60 | 100 |
Abbreviations: MEP, motor-evoked potential; CSM, cervical spondylosis; NA, not applicable; SSEP, somatosensory sensory evoked potential; tcMEP, transcranial MEP; OPLL, ossification of the posterior longitudinal ligament; ACDF, anterior cervical discectomy and fusion; EMG, electromyography; SRS, Scoliosis Research Society; sEMG, spontaneous EMG; mMEP, muscle motor evoked potential; fEMG, free-running electromyography.
Table 3.
Summary of Significant Studies Reporting on the Cost-Effectiveness of IONM Techniques.
| Reference | Year | Design | Type of Monitoring | No. of Cases | Type of Cases | Conclusions |
|---|---|---|---|---|---|---|
| Sala et al | 2007 | Cost-benefit analysis | ND | ND | Scoliosis surgery | Considering injury rate of 0.1%, IONM would be cost-effective if the costs did not exceed $977 per surgery given the lifetime direct health care costs for a paraplegic patient. |
| Ayoub et al | 2010 | Retrospective review | SSEP | 210 | Cervical spine | Cost savings to the hospital was $64 074 to $102 192 per patient injured per year at an expense of $31 546 per year on SSEP monitoring. |
| Ney et al | 2012 | Hypothetical cost- effectiveness model | ND | ND | ND | IONM was associated with a 49% reduction in in relative risk for neurological complications. The cost of monitoring to prevent a single neurologic injury was $63 387. |
| Ney et al | 2013 | Hypothetical cost-effectiveness model | ND | ND | ND | MIONM was found to be cost-effective when neurologic complication rate from surgery exceeded 0.3%. |
| Traynelis et al | 2012 | Retrospective review | SSEP, MEP | 720 | Cervical spine | Significant savings of more than $1 million in the study cohort by not using IONM in simple cervical spine procedures. |
| Ney et al | 2018 | Retrospective review | Combination of SSEP, MEP, or EMG | 8413 | Single-level cervical spine | Initially found to have greater cost with IONM. However, it was found to be cost-effective in the year after surgery, with a net decrease in cost of $387 per patient. |
| Cole et al | 2014 | Retrospective review | Combination of SSEP, MEP, or EMG | 85 640 | Single-level spinal procedures | IONM was associated with higher spending, ranged from $2859 to $3841. |
| Ney et al | 2015 | Retrospective analysis | Combination of SSEP, MEP, or EMG | 234 067 (unweighted observations) | Simple spinal decompressions and fusions | IONM was associated with fewer neurologic complications and 9% increased hospital charges. |
Abbreviations: IONM, intraoperative neurophysiological monitoring; ND, not determined; SSEP, somatosensory sensory evoked potential; MIONM, multimodal intraoperative neurophysiological monitoring; MEP, motor-evoked potential; EMG, electromyography.
The Use of IONM During Cervical Spine Surgery
Although the likelihood of neurological complication is low, the use of IONM in degenerative cervical spine surgery has been increasingly adopted.32 Currently, SSEP is the most commonly used technique among the modalities of IONM.2 Its use in cervical spine surgery is not only limited to the monitoring of sensory tracts during the operation but also for the assessment of the spinal cord and nerve roots after surgical positioning.33-35 However, due to its reportedly low specificity,34 SSEP changes during surgery are not always associated with postoperative neurological deficits. Consequently, many investigators recommend against its use as the single monitoring modality in complex cervical procedures.9
The accuracy of MEPs in detecting potential neurological injury has been demonstrated by several studies. Clark et al36 reported a sensitivity of 71% and a specificity of 94% in predicting postoperative deficits in patients undergoing operative procedures for degenerative cervical myelopathy. In 2004, Hilibrand et al12 compared the use of both SSEP and tcMEP monitoring in 427 consecutive patients who underwent cervical spine surgery. The authors reported 12 patients experiencing significant changes on monitoring, among which 2 were found to have new neurological deficits postoperatively. Their reported sensitivity and specificity were 100% for tcMEP; however, since SSEP changes were present in only 1 of the 2 patients with a deficit, the authors concluded only 25% sensitivity but 100% specificity. Similarly, Kim et al,37 in a retrospective review of 200 consecutive patients, reported the usefulness of multi-channel tcMEPs in the detection of spinal cord injury during anterior cervical surgery. Additionally, Fujiwara et al38 showed the effectiveness of tcMEP in identifying acute-type postoperative C5 palsy following cervical laminoplasty; however, the rate of delayed palsy was unpredictable.
Given the safety concerns with isolated use of SSEP, and the limitations of MEPs as previously discussed, a combination of the two was therefore sought as a complementary method to improve the efficacy of IONM during cervical decompression surgery.9 In a prospective study of 246 patients with cervical pathologies, the majority of which being cervical stenosis, Eggspuehler et al27 reported the use of MIONM with 83.3% sensitivity and 99.2% specificity. On the other hand, Lee et al39 retrospectively reviewed the use of MIONM in 182 patients undergoing posterior cervical surgery. The overall sensitivity of MIONM in this study was 50%, the specificity was 100%, while the positive predictive value (PPV) and the negative predictive value (NPV) were 100% and 97%, respectively. Although the sensitivity was found to be lower than previously reported, the authors nonetheless supported the use of MIONM in posterior cervical procedures, acknowledging that the false-negative results caused by delayed C5 palsy may have reduced the overall detectability of true events.
Although many investigators have demonstrated the usefulness of IONM, others argue against its use in noncomplex cervical spine procedures. In a retrospective analysis of 720 patients, Traynelis et al3 concluded that surgical decompression and reconstruction for symptomatic cervical spine disease may be safely performed without utilizing IONM. In line with this study, Ajiboye et al4 also found no benefit of IONM in the prevention of new postoperative neurological complications after anterior cervical surgery.
Therefore, although the evidence appears to support increased detection of neurological injuries in cervical procedures using MIONM, controversy remains within the spine community as to whether monitoring is needed for routine noncomplex cases. At our institution, the use of MIONM for complex spinal procedures has been adopted into routine practice. Here, we present an illustrative case to demonstrate the utility of MIONM in a trauma case with unstable fracture of the upper cervical spine (see section “Clinical Case”).
The Use of IONM During Deformity Surgery
IONM in spinal deformity surgery has been well described in numerous studies. Since the introduction of SEP monitoring in the 1970s, the rate of neurological injuries in scoliosis surgery has been significantly reduced.10 Nuwer et al10 reported the results of a survey conducted by the Scoliosis Research Society (SRS), in which members were asked to submit surgical data on outcomes of operated patients, including the use of IONM. SSEP monitoring was used in 51 263 of 97 586 spinal cases (53%) with reported sensitivity of 92%, specificity of 98.9%, while PPV and NPV were 42% and 99.9%, respectively. The authors’ findings were strongly in support of SSEP monitoring during scoliosis procedures.
Nevertheless, false-negative SSEP changes have been reported in several studies,40,41 thus raising many concerns as to the use of SSEP as the singular tool for neuromonitoring. To address this issue, MIONM was introduced in complex procedures. In a retrospective analysis of 102 patients operated for adult spinal deformity, Quraishi et al29 reported an overall sensitivity of MIONM of 100%, specificity of 84%, while PPV and NPV were 14% and 97%, respectively. In a subgroup analysis of patients undergoing complex deformity surgery involving osteotomies, the combined sensitivity was 67%, specificity 98%, PPV 80%, and NPV 96%. Similarly, Pastorelli et al42 published the results of 172 patients who underwent surgical treatment for scoliosis. Their results confirmed the improved sensitivity and specificity, reported at 100% and 98%, respectively, with the combination of SSEP and MEP monitoring.
Despite the previous evidence for MIONM, Hamilton et al23 reviewed the rates of new neurological deficits in more than 100 000 spine surgery procedures registered in the SRS morbidity and mortality database. For cases using MIONM (65%), the overall sensitivity and specificity for new spinal cord deficits was 43% and 98%, respectively, with calculated PPV at 21% and NPV at 99%. The results of this study stirred controversies within the spine community, thus highlighting the need for further studies.
More recently, Bhagat et al,43 in a large retrospective review of 354 consecutive patients who underwent spinal deformity surgery, demonstrated the superiority of combined MIONM over either single modality in early detection of impending neurological injuries. The overall sensitivity and specificity of combined SSEPs and MEPs in this study was found to be 100% and 99.3%, respectively, strongly supporting its use.
The Use of IONM During Degenerative Lumbar Surgery
Although IONM is commonly used during spinal deformity in the modern era, its use in degenerative lumbar surgery, especially in uncomplicated procedures, remains controversial.44 Supporters of IONM point out its value in detecting spinal nerve root injuries with high sensitivity and specificity, especially in revision and instrumented fusions cases.23,45-47 Gunnarsson et al13 retrospectively reviewed the sensitivity and specificity of detecting new postoperative motor deficits using MIONM during thoracolumbar procedures in a cohort of 213 patients. They reported that sEMG had a sensitivity of 100% and a specificity of 23.7%, while SSEPs had a sensitivity of 28.6% and a specificity of 94.7%. Based on their findings, the authors recommended combining the 2 monitoring techniques for thoracolumbar procedures.
Despite advancements, the topic of monitor spinal nerve root function remains controversial.44,48 Additionally, although numerous studies have supported the use of IONM in lumbar fusion surgery, it is still unclear whether the improved detection of crisis events intraoperatively translates to a decreased rate of postoperative neurological deficits.49-51
The Use of IONM During Spinal Tumor Surgery
The value of IONM in detecting neurologic injuries during resection of spinal cord tumors has been well described.52 In 2007, Sutter et al53 published the results of 109 patients who were monitored with MIONM during surgical treatment for intradural spinal tumors of various types. The authors found high sensitivity and specificity of 92% and 99%, respectively, for predicting adverse neurological outcomes. Forster et al,54 in a retrospective analysis of 203 patients undergoing intradural tumor removal, reported IONM changes in 47 patients (23.2%). In this series, SSEP monitoring showed a sensitivity of 94.4% and a specificity of 96.8% in detecting postoperative deficits, whereas the sensitivity and specificity of MEPs monitoring was 95.0% and 98.9%, respectively. Both authors concluded that MIONM is an effective method for spinal cord monitoring during surgery for spinal tumors.
Furthermore, in a retrospective analysis of 100 patients who underwent intradural extramedullary tumor (IDEM) resection, Korn et al55 reported a sensitivity of 82%, specificity of 95%, PPV of 82%, and a NPV of 95% with the use of MIONM. Similar results were reported by Harel et al56 in their retrospective review of 41 patients with IDEM spinal tumors. When their study group was compared to that of a historical cohort of 70 patients, the authors demonstrated the utility of MIONM in predicting neurological injury with sensitivity, specificity, and positive and negative predicted values of 75%, 100%, 100%, and 97%, respectively. Both studies demonstrated high accuracy for MIONM in predicting neurological adverse events in resection of IDEM.
In 2006, Sala et al57 compared 50 patients operated for intramedullary tumor resection with the use of MIONM with a historical surgical cohort of 50 patients without monitoring. The authors reported significantly better neurological outcome in the MIONM group (mean McCormick grade variation +0.28 vs −0.16, P = .0016) at follow-up. This difference was noticeable even at discharge, where a trend was found toward better outcomes in favor of the MIONM group (P = .1224). Recently, Jin et al58 further strengthened the evidence for the use of MIONM as a diagnostic adjunct in intramedullar tumor resection, reporting 100% sensitivity, 91% specificity, 60% PPV, and 100% NPV in their cohort of 25 patients.
Finally, in a retrospective study, Costa et al59 reported the results of 103 patients operated for spinal cord pathologies that included intramedullary, extramedullary tumors as well as cervical myelopathy. Intraoperative monitoring was obtained with MIONM including SSEP and MEP. In this study, D-wave was obtained in 97 of the cases. The authors showed that the presence of a persistent and stable D wave was predictive of good functional outcome even in the absence of MEP signals during the surgery.
As presented, in spinal tumor resection the use of MIONM is well supported by the literature. The evidence strongly suggests that intraoperative alerts accurately predict neurological injury despite the location of the pathology. As such, the use of MIONM is strongly advised. The combined use of SSEPs and MEPs seems to provide increased accuracy for detecting injury to sensory and motor pathways reaching a high sensitivity, specificity, PPV, and NPV.53,55,60
Cost-Effectiveness of IONM in Spine Surgery
In recent years, there has been a significant increase in the use of IONM and MIONM within the global spine community.32 Although the literature has supported its use for predicting postoperative neurological deficits,61 the strength of evidence is poor regarding the effect of MIONM or response to an MIONM alert in reducing the overall rate of neurological injury.62 Unfortunately, to date, the management of MIONM alerts is still inconsistent in the spine community, which could in part explain the lack of detectable improvement in the overall rate of neurological events. Although numerous algorithms and protocols exist, none have seen widespread acceptance.63-66
In the absence of randomized trials, and given the importance of rising health care costs, cost-effectiveness analyses have been proposed as an alternative method to evaluate the possible benefit of IONM.61 There are several studies published over the past years that address the cost-effectiveness of IONM in spine surgery. Sala et al,67 based on a reported paraplegia rate of 0.1% in young adults after scoliosis surgery, and taking into account the lifetime health care costs, estimated that IONM would be cost-effective if the costs did not exceed $977 per surgery. However, in their analysis model, the authors assumed that IONM prevents every injury (100% prevention rate). Indirect costs that could be accrued by false IONM alerts were not considered.
In 2010, Ayoub et al68 performed a cost-effectiveness analysis on a cohort of 210 patients who underwent cervical spine surgery with SSEP monitoring. Total cost of the surgery, hospital stay, neuromonitoring, as well as medical expenditures associated with postoperative neurologic injury was factored into the cost analysis. Given an incidence of 0.1% for spinal cord injury, the authors assumed that without SSEP monitoring 1 out of 201 patients would have had a permanent spinal cord injury. In their estimation, the total annual cost savings for a single injured patient would range from $64 074 to $102 192 for their institution, while the yearly expenditure on SSEP amounted to only $31 546.
Recently, Ney et al69 constructed a hypothetical cost-effectiveness model with the scope to calculate the value of MIONM to avoid postoperative neurological deficits. This model was built with considerations taken for parameters such as the surgical risk, prevention rate, and cost per case estimates. The authors reported that use of IONM in spinal procedures was associated with a 49% reduction in relative risk for neurological complications. They further concluded that the cost of monitoring to prevent a single neurologic injury was $63 387. Using the same cost-benefit model, the authors demonstrated that IONM usage was cost-neutral even at a 0.3% complication rate.70
Although numerous studies have demonstrated the benefit of IONM or MIONM in their cost-effectiveness analysis, several others have found the opposite. Traynelis et al,3 in a single-center study, reported a case series of 720 consecutive patients who underwent routine cervical spine procedures without the use of IONM. The authors reported a 0.4% rate of postoperative neurological deficits. However, at the 1-year follow up, all patients were significantly improved, and their neurological deficits resolved completely. The authors, therefore, questioned the utility of IONM during routine cervical spine surgery. Additionally, further analysis was performed to explore the economic impact of IONM during cervical spine procedures. This analysis was calculated by using the Current Procedural Terminology reimbursement code. They concluded that significant savings could be achieved by not using IONM in simple cervical spine procedures. They estimated a saving of more than a million dollars for not using IONM in this group of patients.
These results were recently challenged.71 Using a commercial claims dataset, 8413 patients who were treated with single-level cervical spine surgeries with or without IONM in the years 2008 to 2013 were identified. In this retrospective analysis, IONM usage was associated with a 0.4% absolute reduction in the rate of neurological complications, a shorter length of stay, fewer readmissions, and a 1.7% reduction rate in opiate consumption in the year following surgery. After multiple regression adjustment, the authors estimated IONM usage to be associated with a $1229 increase in cost during the index admission year. However, in the year after surgery, a substantial reduction of $1615 in spending was observed in the IONM group, which resulted in a net decrease in cost of $387 per patient.
In a large retrospective propensity score matched analysis based on a national database (MarketScan),72 Cole et al looked at single-level spinal procedures, with and without the use of IONM, in order to compare the rates of neurological complications. Trauma, spinal tumor, and revision cases were excluded from the analysis. A total of 85 640 patients were included in the analysis with a minority (12.66%) receiving IONM during the surgery. The authors found no difference in neurological complication rates between those who did and did not receive IONM. They concluded that the use of IONM was associated with higher spending ranged from $2859 to $3841.
In a more recent analysis using the Nationwide Inpatient Sample (NIS), a public dataset of hospitalization in the United States, Ney et al73 also investigated the value of IONM in noncomplex spinal decompression and fusion surgeries. They found that IONM was used in 4.9% of the approximately 1.1 million patients. The IONM group had fewer neurologic complications (0.8% vs 1.4%) while the adjusted hospital charges were greater by 9%.
Cost-effectiveness analysis is a method for evaluating the outcomes and costs of interventions designed to improve health.74 In order for a new surgical intervention to be cost-effective, additional costs need to be balanced by demonstrable benefits that can either improve surgical outcomes or reduce long-term associated costs.74 Currently, the strength of evidence is poor regarding the effect of IONM or response to an IONM alert in reducing the overall rate of neurological injury.62 Moreover, IONM can hardly be cost-effective when only direct costs are taken into consideration.71 Further studies are needed in order to investigate IONM’s health and cost benefits on a longer timeline.
Conclusion
There is a growing body of evidence supporting the effectiveness of IONM in detecting adverse outcomes during spinal surgery. In the absence of prospective studies with high-level evidence to validate its efficacy, the use of IONM is guided by surgeon preference and local institutional policies and recommendations. Further studies are needed to investigate the therapeutic role of IONM in spine surgery. However, as prospective studies designed to determine the efficacy of post-IONM alert interventions are highly unlikely to occur in the future due to ethical limitations and medicolegal implications, the need for large prospective observational cohort studies is warranted. Finally, evidence-based protocols to respond to alerts in MIONM are still lacking, creating a critical knowledge gap in the management both during the event and after the fact. Therefore, future studies are needed in this regard to explore new treatments, synthetize previous literature, and develop clinical practice guidelines.
Clinical Case
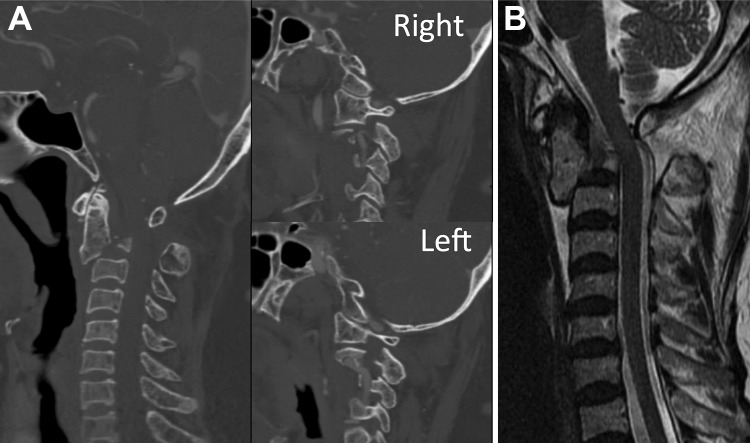
A 77-year-old female sustained a fall down a flight of stairs. She initially presented to the emergency department of her local hospital. On finding a displaced and angulated bilateral fracture of the pars interarticularis of the C2 vertebrae, also known as “Hangman’s fracture” (Figure 1A), she was urgently transferred to our center.
Figure 1.
(A) Sagittal CT scan of the cervical spine showing a “Hangman’s fracture” with displaced and angulated fracture of the bilateral C2 pars interarticularis. (B) MRI sagittal image showing no compression of the spinal cord or neural elements.
On presentation, the patient’s neck was stabilized with a rigid cervical collar, her neurological examination revealed strong motor and intact sensation in all extremities. Magnetic resonance imaging of the spine revealed no compression of the spinal cord or neural elements (Figure 1B). After comprehensive discussion of the diagnosis and treatment options, surgical consent was obtained from the patient, and an urgent reduction and operative fixation of the fracture was planned.
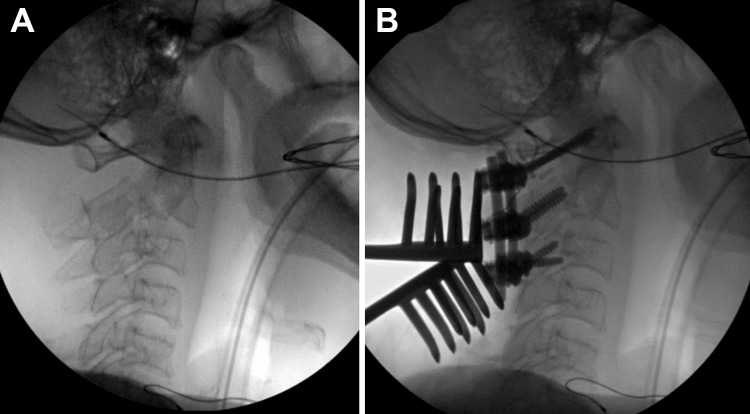
Intraoperatively, with the assistance of operative room staff, the patient was carefully transferred onto a rotating surgical bed locked in the supine position. The rigid cervical collar was kept during transfer to maintain stability and her neck was supported in a comfortable position while supine without any attempt for reduction. Next, induction and completion of awake intubation by the anesthesia team was performed. Neurophysiological monitoring leads were placed on the patient and baseline MEPs and SSEPs were obtained confirming good signals in both upper and lower extremities. The rigid collar was then removed, and the neck was carefully positioned into extension by the surgeon. Neurophysiological monitoring parameters were reassessed immediately after the maneuver and confirmed no changes to the SSEP and MEP. With satisfactory reduction confirmed by fluoroscopy (Figure 2A), a Mayfield clamp was used to lock the patient’s neck position onto the rotating surgical platform. The surgical frame was then placed over the patient and a Jackson 360° flip was performed repositioning the patient in a prone position. Neuromonitoring SSEP and MEP again reassured no changes occurred and the maintenance of the reduction was confirmed using fluoroscopy.
Figure 2.
(A) Intraoperative fluoroscopy showing closed reduction of the fracture by positioning of the head into extension. (B) Instrumentation from C1 to C3 achieved good reduction of the C2 fracture and restoration of overall cervical alignment.
The surgical procedure took place with instrumented stabilization from C1 to C3 (Figure 2B). The entire procedure was largely uncomplicated with the exception of a break out of the right C3 lateral mass screw on initial insertion using the Magerl’s technique. Subsequently, the trajectory was adjusted, and the screw was placed using the Roy-Camille method without any additional issues. MIONM was stable for the entire duration of the procedure. Postoperatively, the patient was well and remained neurologically intact throughout the entire hospital stay.
Key Points
Growing evidence supports the detection of neurological injuries with MIONM in cervical spinal procedures. However, controversies exist as to the need for monitoring in non-complex routine operations.
The use of MIONM in deformity surgery is well described in the literature. Although some controversies exist concerning the sensitivity, overall the evidence appears supportive of its use in complex deformity surgeries.
The monitoring of spinal nerve roots in lumbar spinal procedures remains controversial. Additional research is needed to further define the relationship between IONM alerts and the rate of postoperative neurological deficits.
The effectiveness of MIONM in spinal cord monitoring during spinal tumor resections is strongly supported by the literature and its use in routine practice is strongly advised.
The overall cost-effectiveness of IONM in spine surgery remains controversial within the current literature. Due to the heterogeneity of studies, reaching a consensus is difficult without further research.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MGF would like to acknowledge support from the Gerry and Tootsie Halbert Chair in Neural Repair and Regeneration as well as the DeZwirek Family Foundation. This supplement was supported by funding from AO Spine North America.
ORCID iD: Fan Jiang, MD, FRCSC  https://orcid.org/0000-0002-7673-0267
https://orcid.org/0000-0002-7673-0267
Jamie R. F. Wilson, BMBCh, FRCS  https://orcid.org/0000-0002-3488-7506
https://orcid.org/0000-0002-3488-7506
Michael G. Fehlings, MD, PhD, FRCSC, FACS  https://orcid.org/0000-0002-5722-6364
https://orcid.org/0000-0002-5722-6364
References
- 1. Ahn H, Fehlings MG. Prevention, identification, and treatment of perioperative spinal cord injury. Neurosurg Focus. 2008;25:E15. [DOI] [PubMed] [Google Scholar]
- 2. Magit DP, Hilibrand AS, Kirk J, et al. Questionnaire study of neuromonitoring availability and usage for spine surgery. J Spinal Disord Tech. 2007;20:282–289. [DOI] [PubMed] [Google Scholar]
- 3. Traynelis VC, Abode-Iyamah KO, Leick KM, Bender SM, Greenlee JD. Cervical decompression and reconstruction without intraoperative neurophysiological monitoring. J Neurosurg Spine. 2012;16:107–113. [DOI] [PubMed] [Google Scholar]
- 4. Ajiboye RM, D’Oro A, Ashana AO, et al. Routine use of intraoperative neuromonitoring during ACDFs for the treatment of spondylotic myelopathy and radiculopathy is questionable: a review of 15,395 cases. Spine (Phila Pa 1976). 2017;42:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ajiboye RM, Zoller SD, D’Oro A, et al. Utility of intraoperative neuromonitoring for lumbar pedicle screw placement is questionable: a review of 9957 cases. Spine (Phila Pa 1976). 2017;42:1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daniel JW, Botelho RV, Milano JB, et al. Intraoperative neurophysiological monitoring in spine surgery: a systematic review and meta-analysis. Spine (Phila Pa 1976). 2018;43:1154–1160. [DOI] [PubMed] [Google Scholar]
- 7. Sala F, Di Rocco C. Intraoperative neurophysiological monitoring in neurosurgery: moving the debate from evidence and cost-effectiveness to education and training. World Neurosurg. 2015;83:32–34. [DOI] [PubMed] [Google Scholar]
- 8. Howick J, Cohen BA, McCulloch P, Thompson M, Skinner SA. Foundations for evidence-based intraoperative neurophysiological monitoring. Clin Neurophysiol. 2016;127:81–90. [DOI] [PubMed] [Google Scholar]
- 9. Deletis V, Sala F. Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery: a review focus on the corticospinal tracts. Clin Neurophysiol. 2008;119:248–264. [DOI] [PubMed] [Google Scholar]
- 10. Nuwer MR, Dawson EG, Carlson LG, Kanim LE, Sherman JE. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96:6–11. [DOI] [PubMed] [Google Scholar]
- 11. Nash CL, Jr, Lorig RA, Schatzinger LA, Brown RH. Spinal cord monitoring during operative treatment of the spine. Clin Orthop Relat Res. 1977;(126):100–105. [PubMed] [Google Scholar]
- 12. Hilibrand AS, Schwartz DM, Sethuraman V, Vaccaro AR, Albert TJ. Comparison of transcranial electric motor and somatosensory evoked potential monitoring during cervical spine surgery. J Bone Joint Surg Am. 2004;86:1248–1253. [DOI] [PubMed] [Google Scholar]
- 13. Gunnarsson T, Krassioukov AV, Sarjeant R, Fehlings MG. Real-time continuous intraoperative electromyographic and somatosensory evoked potential recordings in spinal surgery: correlation of clinical and electrophysiologic findings in a prospective, consecutive series of 213 cases. Spine (Phila Pa 1976). 2004;29:677–684. [DOI] [PubMed] [Google Scholar]
- 14. Minahan RE, Sepkuty JP, Lesser RP, Sponseller PD, Kostuik JP. Anterior spinal cord injury with preserved neurogenic “motor” evoked potentials. Clin Neurophysiol 2001;112:1442–1450. [DOI] [PubMed] [Google Scholar]
- 15. Boyd SG, Rothwell JC, Cowan JM, et al. A method of monitoring function in corticospinal pathways during scoliosis surgery with a note on motor conduction velocities. J Neurol Neurosurg Psychiatry. 1986;49:251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hicks RG, Burke DJ, Stephen JP. Monitoring spinal cord function during scoliosis surgery with Cotrel-Dubousset instrumentation. Med J Aust. 1991;154:82–86. [DOI] [PubMed] [Google Scholar]
- 17. Merton PA, Morton HB. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980;285:227. [DOI] [PubMed] [Google Scholar]
- 18. Sloan TB, Janik D, Jameson L. Multimodality monitoring of the central nervous system using motor-evoked potentials. Curr Opin Anaesthesiol. 2008;21:560–564. [DOI] [PubMed] [Google Scholar]
- 19. Hsu B, Cree AK, Lagopoulos J, Cummine JL. Transcranial motor-evoked potentials combined with response recording through compound muscle action potential as the sole modality of spinal cord monitoring in spinal deformity surgery. Spine (Phila Pa 1976). 2008;33:1100–1106. [DOI] [PubMed] [Google Scholar]
- 20. Park P, Wang AC, Sangala JR, et al. Impact of multimodal intraoperative monitoring during correction of symptomatic cervical or cervicothoracic kyphosis. J Neurosurg Spine. 2011;14:99–105. [DOI] [PubMed] [Google Scholar]
- 21. Owen JH. The application of intraoperative monitoring during surgery for spinal deformity. Spine (Phila Pa 1976). 1999;24:2649–2662. [DOI] [PubMed] [Google Scholar]
- 22. Sloan TB, Heyer EJ. Anesthesia for intraoperative neurophysiologic monitoring of the spinal cord. J Clin Neurophysiol. 2002;19:430–443. [DOI] [PubMed] [Google Scholar]
- 23. Hamilton DK, Smith JS, Sansur CA, et al. Scoliosis Research Society Morbidity and Mortality Committee. Rates of new neurological deficit associated with spine surgery based on 108,419 procedures: a report of the scoliosis research society morbidity and mortality committee. Spine (Phila Pa 1976). 2011;36:1218–1228. [DOI] [PubMed] [Google Scholar]
- 24. Padberg AM, Thuet ED. Intraoperative electrophysiologic monitoring: considerations for complex spinal surgery. Neurosurg Clin N Am. 2006;17:205–226. [DOI] [PubMed] [Google Scholar]
- 25. Calancie B, Madsen P, Lebwohl N. Stimulus-evoked EMG. monitoring during transpedicular lumbosacral spine instrumentation. Initial clinical results. Spine (Phila Pa 1976). 1994;19:2780–2786. [DOI] [PubMed] [Google Scholar]
- 26. Devlin VJ, Schwartz DM. Intraoperative neurophysiologic monitoring during spinal surgery. J Am Acad Orthop Surg. 2007;15:549–560. [DOI] [PubMed] [Google Scholar]
- 27. Eggspuehler A, Sutter MA, Grob D, Jeszenszky D, Porchet F, Dvorak J. Multimodal intraoperative monitoring (MIOM) during cervical spine surgical procedures in 246 patients. Eur Spine J. 2007;16(suppl 2):S209–S215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sala F, Bricolo A, Faccioli F, Lanteri P, Gerosa M. Surgery for intramedullary spinal cord tumors: the role of intraoperative (neurophysiological) monitoring. Eur Spine J. 2007;16(suppl 2):S130–S139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quraishi NA, Lewis SJ, Kelleher MO, Sarjeant R, Rampersaud YR, Fehlings MG. Intraoperative multimodality monitoring in adult spinal deformity: analysis of a prospective series of one hundred two cases with independent evaluation. Spine (Phila Pa 1976). 2009;34:1504–1512. [DOI] [PubMed] [Google Scholar]
- 30. Bello JP, Perez-Lorensu PJ, Roldán-Delgado H, et al. Role of multimodal intraoperative neurophysiological monitoring during positioning of patient prior to cervical spine surgery. Clin Neurophysiol. 2015;126:1264–1270. [DOI] [PubMed] [Google Scholar]
- 31. Kim DH, Zaremski J, Kwon B, et al. Risk factors for false positive transcranial motor evoked potential monitoring alerts during surgical treatment of cervical myelopathy. Spine (Phila Pa 1976). 2007;32:3041–3046. [DOI] [PubMed] [Google Scholar]
- 32. James WS, Rughani AI, Dumont TM. A socioeconomic analysis of intraoperative neurophysiological monitoring during spine surgery: national use, regional variation, and patient outcomes. Neurosurg Focus. 2014;37:E10. [DOI] [PubMed] [Google Scholar]
- 33. Appel S, Korn A, Biron T, et al. Efficacy of head repositioning in restoration of electrophysiological signals during cervical spine procedures. J Clin Neurophysiol. 2017;34:174–178. [DOI] [PubMed] [Google Scholar]
- 34. May DM, Jones SJ, Crockard HA. Somatosensory evoked potential monitoring in cervical surgery: identification of pre- and intraoperative risk factors associated with neurological deterioration. J Neurosurg. 1996;85:566–573. [DOI] [PubMed] [Google Scholar]
- 35. Uribe JS, Kolla J, Omar H, et al. Brachial plexus injury following spinal surgery. J Neurosurg Spine. 2010;13:552–558. [DOI] [PubMed] [Google Scholar]
- 36. Clark AJ, Safaee M, Chou D, et al. Comparative sensitivity of intraoperative motor evoked potential monitoring in predicting postoperative neurologic deficits: nondegenerative versus degenerative myelopathy. Global Spine J. 2016;6:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim DG, Jo SR, Park YS, et al. Multi-channel motor evoked potential monitoring during anterior cervical discectomy and fusion. Clin Neurophysiol Pract. 2017;2:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fujiwara Y, Manabe H, Izumi B, Tanaka H, Kawai K, Tanaka N. The Efficacy of intraoperative neurophysiological monitoring using transcranial electrically stimulated muscle-evoked potentials (TcE-MsEPs) for predicting postoperative segmental upper extremity motor paresis after cervical laminoplasty. Clin Spine Surg. 2016;29:E188–E195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee HJ, Kim IS, Sung JH, Lee SW, Hong JT. Significance of multimodal intraoperative monitoring for the posterior cervical spine surgery. Clin Neurol Neurosurg. 2016;143:9–14. [DOI] [PubMed] [Google Scholar]
- 40. Pelosi L, Lamb J, Grevitt M, Mehdian SM, Webb JK, Blumhardt LD. Combined monitoring of motor and somatosensory evoked potentials in orthopaedic spinal surgery. Clin Neurophysiol. 2002;113:1082–1091. [DOI] [PubMed] [Google Scholar]
- 41. Schwartz DM, Auerbach JD, Dormans JP, et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am. 2007;89:2440–2449. [DOI] [PubMed] [Google Scholar]
- 42. Pastorelli F, Di Silvestre M, Plasmati R, et al. The prevention of neural complications in the surgical treatment of scoliosis: the role of the neurophysiological intraoperative monitoring. Eur Spine J. 2011;20(suppl 1):S105–S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bhagat S, Durst A, Grover H, et al. An evaluation of multimodal spinal cord monitoring in scoliosis surgery: a single centre experience of 354 operations. Eur Spine J. 2015;24:1399–1407. [DOI] [PubMed] [Google Scholar]
- 44. Alemo S, Sayadipour A. Role of intraoperative neurophysiologic monitoring in lumbosacral spine fusion and instrumentation: a retrospective study. World Neurosurg. 2010;73:72–76. [DOI] [PubMed] [Google Scholar]
- 45. Sutter MA, Eggspuehler A, Grob D, Porchet F, Jeszenszky D, Dvorak J. Multimodal intraoperative monitoring (MIOM) during 409 lumbosacral surgical procedures in 409 patients. Eur Spine J. 2007;16(suppl 2):S221–S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Resnick DK, Choudhri TF, Dailey AT, et al. American Association of Neurological Surgeons/Congress of Neurological Surgeons. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 15: electrophysiological monitoring and lumbar fusion. J Neurosurg Spine. 2005;2:725–732. [DOI] [PubMed] [Google Scholar]
- 47. Bose B, Wierzbowski LR, Sestokas AK. Neurophysiologic monitoring of spinal nerve root function during instrumented posterior lumbar spine surgery. Spine (Phila Pa 1976). 2002;27:1444–1450. [DOI] [PubMed] [Google Scholar]
- 48. Macdonald DB, Stigsby B, Al Homoud I, Abalkhail T, Mokeem A. Utility of motor evoked potentials for intraoperative nerve root monitoring. J Clin Neurophysiol. 2012;29:118–125. [DOI] [PubMed] [Google Scholar]
- 49. Weiss DS. Spinal cord and nerve root monitoring during surgical treatment of lumbar stenosis. Clin Orthop Relat Res. 2001;(384):82–100. [DOI] [PubMed] [Google Scholar]
- 50. Akay KM, Onder S. Continuous neural monitoring in lumbar spine surgery: experience with 101 patients. Minim Invasive Neurosurg. 2002;45:97–101. [DOI] [PubMed] [Google Scholar]
- 51. Sharan A, Groff MW, Dailey AT, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 15: electrophysiological monitoring and lumbar fusion. J Neurosurg Spine. 2014;21:102–105. [DOI] [PubMed] [Google Scholar]
- 52. Gonzalez AA, Jeyanandarajan D, Hansen C, Zada G, Hsieh PC. Intraoperative neurophysiological monitoring during spine surgery: a review. Neurosurg Focus. 2009;27:E6. [DOI] [PubMed] [Google Scholar]
- 53. Sutter M, Eggspuehler A, Grob D, et al. The validity of multimodal intraoperative monitoring (MIOM) in surgery of 109 spine and spinal cord tumors. Eur Spine J. 2007;16(suppl 2):S197–S208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Forster MT, Marquardt G, Seifert V, Szelényi A. Spinal cord tumor surgery—importance of continuous intraoperative neurophysiological monitoring after tumor resection. Spine (Phila Pa 1976). 2012;37:E1001–E1008. [DOI] [PubMed] [Google Scholar]
- 55. Korn A, Halevi D, Lidar Z, Biron T, Ekstein P, Constantini S. Intraoperative neurophysiological monitoring during resection of intradural extramedullary spinal cord tumors: experience with 100 cases. Acta Neurochir (Wien). 2015;157:819–830. [DOI] [PubMed] [Google Scholar]
- 56. Harel R, Schleifer D, Appel S, Attia M, Cohen ZR, Knoller N. Spinal intradural extramedullary tumors: the value of intraoperative neurophysiologic monitoring on surgical outcome. Neurosurg Rev. 2017;40:613–619. [DOI] [PubMed] [Google Scholar]
- 57. Sala F, Palandri G, Basso E, et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery. 2006;58:1129–1143. [DOI] [PubMed] [Google Scholar]
- 58. Jin SH, Chung CK, Kim CH, Choi YD, Kwak G, Kim BE. Multimodal intraoperative monitoring during intramedullary spinal cord tumor surgery. Acta Neurochir (Wien). 2015;157:2149–2155. [DOI] [PubMed] [Google Scholar]
- 59. Costa P, Peretta P, Faccani G. Relevance of intraoperative D wave in spine and spinal cord surgeries. Eur Spine J. 2013;22:840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hyun SJ, Rhim SC. Combined motor and somatosensory evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in 17 consecutive procedures. Br J Neurosurg. 2009;23:393–400. [DOI] [PubMed] [Google Scholar]
- 61. Nuwer MR, Emerson RG, Galloway G, et al. American Association of Neuromuscular and Electrodiagnostic Medicine. Evidence-based guideline update: intraoperative spinal monitoring with somatosensory and transcranial electrical motor evoked potentials. J Clin Neurophysiol. 2012;29:101–108. [DOI] [PubMed] [Google Scholar]
- 62. Fehlings MG, Brodke DS, Norvell DC, Dettori JR. The evidence for intraoperative neurophysiological monitoring in spine surgery: does it make a difference? Spine (Phila Pa 1976). 2010;35(9 suppl):S37–S46. [DOI] [PubMed] [Google Scholar]
- 63. Vitale MG, Skaggs DL, Pace GI, et al. Best practices in intraoperative neuromonitoring in spine deformity surgery: development of an intraoperative checklist to optimize response. Spine Deform. 2014;2:333–339. [DOI] [PubMed] [Google Scholar]
- 64. Jarvis JG, Strantzas S, Lipkus M, et al. Responding to neuromonitoring changes in 3-column posterior spinal osteotomies for rigid pediatric spinal deformities. Spine (Phila Pa 1976). 2013;38:E493–E503. [DOI] [PubMed] [Google Scholar]
- 65. Pahys JM, Guille JT, D’Andrea LP, Samdani AF, Beck J, Betz RR. Neurologic injury in the surgical treatment of idiopathic scoliosis: guidelines for assessment and management. J Am Acad Orthop Surg. 2009;17:426–434. [DOI] [PubMed] [Google Scholar]
- 66. Ziewacz JE, Berven SH, Mummaneni VP, et al. The design, development, and implementation of a checklist for intraoperative neuromonitoring changes. Neurosurg Focus. 2012;33:E11. [DOI] [PubMed] [Google Scholar]
- 67. Sala F, Dvorak J, Faccioli F. Cost effectiveness of multimodal intraoperative monitoring during spine surgery. Eur Spine J. 2007;16(suppl 2):S229–S231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ayoub C, Zreik T, Sawaya R, Domloj N, Sabbagh A, Skaf G. Significance and cost-effectiveness of somatosensory evoked potential monitoring in cervical spine surgery. Neurol India. 2010;58:424–428. [DOI] [PubMed] [Google Scholar]
- 69. Ney JP, van der Goes DN, Watanabe JH. Cost-effectiveness of intraoperative neurophysiological monitoring for spinal surgeries: beginning steps. Clin Neurophysiol. 2012;123:1705–1707. [DOI] [PubMed] [Google Scholar]
- 70. Ney JP, van der Goes DN, Watanabe JH. Cost-benefit analysis: intraoperative neurophysiological monitoring in spinal surgeries. J Clin Neurophysiol. 2013;30:280–286. [DOI] [PubMed] [Google Scholar]
- 71. Ney JP, Kessler DP. Neurophysiological monitoring during cervical spine surgeries: longitudinal costs and outcomes. Clin Neurophysiol. 2018;129:2245–2251. [DOI] [PubMed] [Google Scholar]
- 72. Cole T, Veeravagu A, Zhang M, Li A, Ratliff JK. Intraoperative neuromonitoring in single-level spinal procedures: a retrospective propensity score-matched analysis in a national longitudinal database. Spine (Phila Pa 1976). 2014;39:1950–1959. [DOI] [PubMed] [Google Scholar]
- 73. Ney JP, van der Goes DN, Nuwer MR. Does intraoperative neurophysiologic monitoring matter in noncomplex spine surgeries? Neurology. 2015;85:2151–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. Panel on cost-effectiveness in health and medicine. JAMA. 1996;276:1172–1177. [PubMed] [Google Scholar]