Abstract
Objective:
The aim of this study is to investigate the clinical data from history and endometrial pathology by endometrial sampling in patients with postmenopausal bleeding and to identify risk factors associated with future development of endometrial cancer (EC).
Methods:
We prospectively studied 76 postmenopausal women with vaginal bleeding and endometrial thickness (ET) >5 mm undergoing endometrial biopsy or dilatation and curettage. Patient characteristics and endometrial assessment of women with or without EC and hyperplasia were compared. Univariate and multivariate logistic regression identified factors associated with risks of endometrial neoplasia.
Results:
In this study, the mean age at the time of presentation was 57.17 ± 7.11 years, mean menopausal age was 49.18 ± 3.69 years, and mean thickness of endometrial was 11.13 ± 6.37 mm. The histopathological analysis showed atrophic endometrium (30.3%), proliferative endometrium (27.6%), EC (15.8%), endometrium hyperplasia (11.8%), disordered proliferative endometrium (9.2%), and endometrial polyp (5.3%). Women of EC and hyperplasia group were more likely to be multiparous, diabetic, hypertensive, obese or overweight, has a history of recurrent bleeding episodes or thick endometrium. Using multivariate logistic regression, we found ET (adjusted odds ratio [AOR] = 17.76, confidence interval [CI] 1.91–165.02, P < 0.011, criterion ≥11 mm), recurrent episode of bleeding (AOR = 13.21, CI 1.10–158.91, P < 0.042), diabetes (AOR = 8.03, CI 1.15–55.78, P < 0.035) the best predictors of EC.
Conclusion:
As clinical characteristics are possible predictors of EC, these should also be taken into account in risk estimations and in the formulation of management plans. This not only has benefit in the process of disease detection but also may result in improved the efficiency of care.
KEYWORDS: Cancer, endometrial, hyperplasia, postmenopausal, risk factors
INTRODUCTION
Postmenopausal bleeding (PMB) is defined as abnormal uterine bleeding occurring after 1 year of permanent cessation of menstruation resulting from loss of ovarian follicular activity.[1] About 90%–95% of postmenopausal women with endometrial cancer (EC) experience a vaginal bleeding, whereas about 10% of symptomatic postmenopausal women reveal an intrauterine malignancy.[2]
Endometrial atrophy is the most common cause of vaginal bleeding among postmenopausal women, whereas endometrial hyperplasia and polyps are other common causes.[3] Several risk factors such as obesity, tamoxifen use, increasing age, early menarche, late menopause, hypertension, diabetes, hereditary nonpolyposis colorectal cancer, and unopposed use of exogenous oestrogens are strongly associated with increased risk of type-I EC.[4] Type-I cancers have an endometrioid histology and account for 70%–80% of endometrial carcinomas. Type-II cancers have a nonendometrioid histology and arise in women who are less likely to have the clinical associations as seen in Type-I cancers.[5]
Therefore, PMB should always be investigated no matter how minimal or nonpersistent. Transvaginal sonography (TVS) is an efficient and acceptable noninvasive method for the early detection of endometrial pathology. A woman with PMB has a pretest probability of 10% for EC. A negative endometrial thickness (ET) test result can reduce the posttest probability of EC to 2.4% (95% confidence interval [CI] 1.3–3.9) at ≥4 mm and 5.0% (95% CI 2.9–9.1) at ≥5 mm. With a threshold of 5 mm for ET, the sensitivity for detecting any endometrial disease was 92%, and the sensitivity for detecting EC was 96%. Currently, controversy exists as to whether transvaginal ultrasonography or endometrial biopsy should be used as the initial diagnostic step for clinical evaluation of women presenting with PMB.[6] In addition, decisions made about the most appropriate investigations that needs to be performed, are not always guided by clinical history.
Therefore, PMB requires complete assessment to ensure the absence of malignancy and to identify and treat high risk patients, this will leads to reduction in the considerable societal burden imposed by EC. Hence, this study was undertaken to investigate the clinical data from history and endometrial pathology by endometrial sampling in patients with PMB with a secondary objective to identify risk factors associated with future development of EC.
METHODS
This study was a prospective study of the patients with PMB admitted for evaluation in Obstetrics and Gynaecology department of tertiary care hospital and medical college, Puducherry.
The present study was done after approval of Institutional Ethics Board and after taking informed consent from women. For this study, we included patients who were new referrals for the symptom of PMB. We excluded all symptomatic postmenopausal women with a vaginal bleeding arising from a cervical or vaginal or vulvar disease, women with bleeding disorders, on anticoagulants on menopausal hormone therapy (MHT). All patients satisfying the above inclusion criteria were enrolled in the study and detailed history regarding age of the patient at presentation, age at menopause, time since menopause, body mass index, any unscheduled vaginal bleeding with use of MHT, presence of hypertension and diabetes, single episode or recurrent episodes of vaginal bleeding. Recurrent episodes were defined as any bleeding episode lasting 7 or more days or two or more separate bleeding events within the past 12 months. Then, the patients were subjected to routine gynecological check-up where abdominal and pelvic examination was performed, followed by TVS for uterine volume, ovarian volume, and ET. The double-wall ET was measured in an anterioposterior dimension from one basalis layer to the other. In keeping with departmental guidelines, women with ET equal to or >5 mm were admitted for dilatation and curettage (D and C) under anesthesia to yield sufficient tissue for histological diagnosis. The histopathological examinations were performed by pathologists and the reports of the curettage were reviewed for all patients.
Sample size estimation
In a study done by Kadakola B et al., it was observed that the mean ET in postmenopausal group with PMB was 8.84 mm with a standard deviation (SD) of 8.04 mm.[7] We estimated sample size required to show the significant difference in means at 3 mm with a desired level of power of 90% and level of significance 0.05, by using the formula:
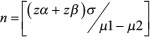
Where:
zα= 1.96 (critical value that divides the central 95% of z distribution from 5% in the tails).
zβ = 1.28 (critical value that separates the lower 10% of distribution from upper 90%), σ = SD, μ1−μ2 = difference of two means.
Accordingly, it was estimated that 76 patients are required to show the difference of 3 mm from established mean. We achieved this sample size by studying the patients from June 2015 to November 2016.
Statistical analysis
Data were summarized using standard descriptive methods, frequency and percentages for categorical variables, and mean and SD or median and range for continuous variables. Comparisons between categorical variables were tested by the use of contingency tables and by the calculation of the Chi-square test. Comparisons between normally distributed continuous variables and categorical variables were performed using Student's t-test and analysis of variance, whereas the nonparametric Mann–Whitney and Kruskal–Wallis tests were used for asymmetric continuous variables. For each factor of interest, a separate conditional logistic regression model was fit to evaluate the association between the factor and the case–control status. In addition, a multivariable, conditional logistic model was fit using stepwise and backward variable selection methods. Associations were summarized using the adjusted odds ratio (AOR) and the corresponding 95% CI. All calculated P values were 2 sided and P < 0.05 were considered statistically significant.
RESULTS
A total of 76 women with complaints of PMB and having ET ≥5 mm were referred to D and C. The basic characteristics (mean, SD, 95% CIs, range, and IQR) of the postmenopausal women are shown in [Table 1]. The mean age at the time of presentation was 57.17 ± 7.11 years. The mean menopausal age was 49.18 ± 3.69 years and the duration of menopause was 7.95 ± 6.52 years. Of these 76 women, all are multiparous. About 47.4% (36/76) of the study participants were diabetic, 38.2% (29/76) were hypertensive, 53.9% (41/76) were either obese or overweight, 61.8% (47/76) has recurrent episodes of vaginal bleeding, 1.3%(1/76) has unscheduled vaginal bleeding with the use of HRT. In this study, the mean thickness of endometrial was 11.13 ± 6.37 mm.
Table 1.
Basic characteristics of women with postmenopausal bleeding
| Basic characteristics of women (76) | Median (IQR) or n (%) or mean±SD 95% CI with range (which ever applicable for data) |
|---|---|
| Age at presentation | 57.17±7.11 |
| Age of menopause | 47.95±6.52 |
| YSM | 7.95±6.52 |
| Diabetes | 36 (47.4) |
| Hypertension | 29 (38.2) |
| Obesity/overweight | 41 (53.9) |
| Recurrent episode of bleeding | 47 (61.8) |
| Use of HRT | 1 (1.3) |
| Endometrial thickness in PMW (mm) | 11.13±6.37 |
Data are presented as mean±SD or n (%). The mean age at the time of presentation of was 57.17±7.11 years. The mean menopausal age was 49.18±3.69 years and the duration of menopause (YSM) was 7.95±6.52 years. All are multiparous with risk factors of diabetes, hypertension, obesity or overweight, recurrent episodes of vaginal bleeding, unscheduled vaginal bleeding with use of HRT. In this study the mean thickness of endometrial was 11.13±6.37 mm, SD: Standard deviation, PMW: Postmenopausal women, HRT: Hormone replacement therapy, IQR: Interquartile range, CI: Confidence interval, YSM: Year Since menopause
Histological examination revealed the presence of 23 (30.3%) women with endometrial atrophy, 21 (27.6%) with proliferative endometrium, 12 (15.8%) with EC, 9 (11.8%) with endometrial hyperplasia of which 4 (5.3%) cases of simple endometrial hyperplasia, 3 (3.9%) cases of complex hyperplasia with atypia, 2 (2.6%) cases of atypical hyperplasia, 7 (9.2%) with disordered proliferative endometrium, and 4 (5.3%) cases of endometrial polyps [Table 2].
Table 2.
Histopathology of endometrium
| Histopathology of endometrium | Cases (17) | Controls (57) | Total |
|---|---|---|---|
| Atropic (%) | 0 (0) | 23 (39) | 23 (30.3) |
| Proliferative (%) | 0 (0) | 21 (35.6) | 21 (35.6) |
| Carcinoma (%) | 12 (70.6) | 0 (0) | 12 (15.8) |
| Disordered proliferative (%) | 0 (0) | 7 (11.9) | 7 (9.2) |
| Polyp (%) | 0 (0) | 4 (6.8) | 4 (5.3) |
| Simple hyperplasia (%) | 0 (0) | 4 (6.8) | 4 (5.3) |
| Complex hyperplasia (%) | 3 (17.6) | 0 (0) | 3 (3.9) |
| Atypical hyperplasia (%) | 2 (11.8) | 0 (0) | 2 (2.6) |
Histological examination revealed the most common cause of bleeding in postmenopause women in this study is endometrial atrophy followed by other causes in descending sequence of proliferative endometrium, endometrial cancer, endometrial hyperplasia, disordered proliferative endometrium, endometrial polyps
The final sample consisted of 17 cases and 59 controls, a total of 76 participants. The 17 cases included 12 women with EC, 3 with complex hyperplasia with atypia, 2 with atypical hyperplasia. In 57 controls, 55 had benign pathologies and 4 with simple hyperplasia.
The results of the univariate analysis to assess for correlation between individual clinical characteristics and the development of EC are given in [Table 3]. Patient characteristics showed no significant differences with regard to age at presentation, age at menopause, year since menopause, past menstrual cycle, parity, and HRT use. Conversely, significant differences were present with regard to diabetes (P = 0.006), recurrent vaginal bleeding episodes (P = 0.002), presence of hypertension (P = 0.047), presence of obesity/overweight (P = 0.008), and ET (P < 0.001).
Table 3.
Basic characteristics of the population
| Variables | Cases (17) | Controls (57) | P |
|---|---|---|---|
| Age (years) | 59.12±6.57 | 56.61±7.21 | 0.202 |
| Age at menopause (years) | 49.82±4.28 | 49.00±3.52 | 0.421 |
| YSM | 9.24±6.65 | 7.58±6.49 | 0.369 |
| Diabetes (%) | |||
| Yes | 13 (76.5) | 23 (39) | 0.006** |
| No | 4 (23.5) | 36 (61) | |
| Hypertension (%) | |||
| Yes | 10 (58.8) | 19 (32.2) | 0.047* |
| No | 7 (41.2) | 40 (67.8) | |
| Obesity (%) | |||
| Yes | 14 (82.4) | 27 (45.8) | 0.008** |
| No | 3 (17.6) | 32 (54.2) | |
| PMC (%) | |||
| Regular | 12 (70.6) | 52 (88.1) | 0.126 |
| Irregular | 5 (29.4) | 7 (11.9) | |
| HRT use (%) | |||
| Yes | 0 (0) | 1 (1.7) | 1.00 |
| No | 17 (100) | 58 (98.3) | |
| Frequency of bleeding (%) | |||
| Single | 1 (5.9) | 28 (47.5) | 0.002** |
| Recurrent | 16 (94.1) | 31 (52.5) | |
| Endometrial thickness (mm) | |||
| <10 | 2 (11.8) | 38 (64.4) | <0.001** |
| 10-25 | 13 (76.5) | 19 (32.2) | |
| >25 | 2 (11.8) | 2 (3.4) | |
| Mean endometrial thickness | 18.35±7.32 | 9.05±4.25 |
Univariate comparison. **Strongly Significant,*Moderately significant. Patient characteristics showed significant differences were present with regard to diabetes, recurrent vaginal bleeding episodes, presence of hypertension, presence of obesity/overweight, and endometrial thickness. PMC: Past menstrual cycle, HRT: Hormone replacement therapy
The five variables that showed significant difference in univariate analysis were included in multivariate analysis (diabetes, presence of hypertension, obesity/overweight, recurrent vaginal bleeding, and ET). Then, multivariate logistic regression analysis showed the significant predictive variables associated with EC: ET (AOR = 17.76, CI 1.91–165.02, P < 0.011, criterion ≥11 mm), recurrent episode of bleeding (AOR = 13.21, CI 1.10–158.91, P < 0.042), diabetes (AOR = 8.03, CI 1.15–55.78, P < 0.035) [Table 4].
Table 4.
Multivariate logistic regression model showing the adjusted predictors of cancer (odds ratio)
| Variables | AOR | 95% CI | P |
|---|---|---|---|
| Diabetes | 8.03 | 1.15-55.78 | 0.035 |
| Hypertension | 0.67 | 0.12-3.84 | 0.655 |
| Obesity | 2.30 | 0.33-15.74 | 0.398 |
| Recurrent episode of bleeding | 13.21 | 1.10-158.91 | 0.042 |
| Endometrial thickness (mm) | 17.76 | 1.91-165.02 | 0.011 |
Adjusted odds ratio >1.0, positively associated. Diabetes, recurrent episodes of bleeding, endometrial thickness are significantly associated. Multivariate logistic regression analysis showed the significant predictive variables associated with endometrial cancer with endometrial thickness, recurrent episode of bleeding, diabetes. AOR: Adjusted odds ratio, CI: Confidence interval
DISCUSSION
Women with postmenopausal uterine bleeding may be assessed initially with either endometrial biopsy or transvaginal ultrasonography. Initial evaluation does not require the performance of both tests.[8] No further investigations need to be performed in women with ET <5 mm as suggested by recent evidence.[9,10] This study was undertaken to investigate the clinical significance, to identify the risk factors and to study the endometrial pathology in PMB. We tried to gain information from the clinical history for performing risk assessment for postmenopausal women with vaginal bleeding so that individualized risk prediction will allow clinicians to make more efficient use of the available diagnostic resources and simultaneously minimize false-negative results from various investigations.
In the present study, the age at presentation was 45–75 years with a mean age was 57.17 ± 7.11, the age at menopause was 42–58 years with mean age was 49.18 ± 3.69 years and the mean year since menopause was 7.95 ± 6.52 years. The results are in accordance with the study done by Lidor et al. in 226 PMB cases and revealed that the ages of patients ranged from 40 to 81 years, with a mean of 56 years.[11] Whereas a similar study done by Ubeja and Singh in 100 PMB cases, it was observed that the age of presentation was 41–70 years with a mean age of 54.51 years and the mean year since menopause was 7.20 years which was similar to our study.[12]
In our study, PMB was most commonly found in multiparous associated with risk factors of obesity/overweight (53.9%), diabetes (47.4%), and hypertension (38.2%); these results were similar to studies done by Kothapally and Bhashyakarla and Nirupama et al.[13,14] Kadakola et al. reported that most of the PMW with bleeding had ET 1–5 mm (<4 mm) with mean ± SD of 8.84 ± 8.04. The mean ET in PMW with bleeding in our study was 11.13 ± 6.37 mm, as we had excluded participants with ET of ≤5 mm for further evaluation.[7]
The most important finding of our study is that, despite identifying clinical factors significantly associated with the risk of endometrial neoplasia in univariate analysis, the results of multivariate logistic regression analysis showed the significant predictive variables associated with endometrial neoplasia were ET, recurrent episode of bleeding, diabetes with moderate ability to identify endometrial hyperplasia or cancer in women with PMB. The above-mentioned clinical and imaging criteria warrant further diagnostic testing as these women with PMB are at increased risk for endometrial hyperplasia or cancer. We also observed based on our results that diagnostic testing cannot be withheld safely from women without these characteristics.
We performed a prospective assessment of our patient which allowed us to standardize any type of examination, so as to have more reliable data and all our women had a definitive histological diagnosis with an optimal reference standard which is the strength of the study.
Some patient-related data were collected retrospectively, with clinical questions to our women about past events (e.g., recurrent vaginal bleeding). We choose symptomatic postmenopausal women with ET >5 mm because women with a lower ET have a very low incidence of cancer and usually, we do not perform further examinations in our centers as per institutional protocol so, we selected only women those can be subjected to endometrial biopsy which can be a limitation of our study.
With our given sample size, it is not possible to differentiate the whole spectrum of endometrial neoplasia as they are associated with varied biological behavior, different demographic parameters, and clinical risk factors.
Clinical characteristics play a major role in predicting in the diagnosis of EC and these should be considered in risk estimations and in the formulation of management plans. Postmenopausal women with new or recurrent bleeding symptoms should be advised to re-attend for evaluation.
CONCLUSION
We have seen that incorporation of clinical information aided with initial investigations like TVS allows us in guiding the subsequent investigations and treatment strategies. The beneficial effects are evident for disease detection and improved patient care.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Research on the menopause in the 1990s. Report of a who scientific group. World Health Organ Tech Rep Ser. 1996;866:1–107. [PubMed] [Google Scholar]
- 2.Giannella L, Mfuta K, Setti T, Cerami LB, Bergamini E, Boselli F. A risk-scoring model for the prediction of endometrial cancer among symptomatic postmenopausal women with endometrial thickness > 4 mm? Biomed Res Int 2014. 2014:130569. doi: 10.1155/2014/130569. doi:10.1155/2014/130569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iatrakis G, Diakakis I, Kourounis G, Sakellaropoulos G, Rammos G, Ladopoulos J, et al. Postmenopausal uterine bleeding. Clin Exp Obstet Gynecol. 1997;24:157. [PubMed] [Google Scholar]
- 4.Burbos N, Musonda P, Giarenis I, Shiner AM, Giamougiannis P, Morris EP, et al. Predicting the risk of endometrial cancer in postmenopausal women presenting with vaginal bleeding: The norwich DEFAB risk assessment tool. Br J Cancer. 2010;102:1201–6. doi: 10.1038/sj.bjc.6605620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–7. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein RB, Bree RL, Benson CB, Benacerraf BR, Bloss JD, Carlos R, et al. Evaluation of the woman with postmenopausal bleeding: Society of radiologists in ultrasound-sponsored consensus conference statement. J Ultrasound Med. 2001;20:1025–36. doi: 10.7863/jum.2001.20.10.1025. [DOI] [PubMed] [Google Scholar]
- 7.Kadakola B, Gurushankar G, Shivamurthy G, Rashmi MN. Ultrasonographic evaluation of abnormal uterine bleeding in postmenopausal women. Int J Reprod Contracept Obstet Gynecol. 2015;4:229–34. [Google Scholar]
- 8.American College of Obstetricians and Gynecologists. ACOG committee opinion no 440: The role of transvaginal ultrasonography in the evaluation of postmenopausal bleeding. Obstet Gynecol. 2009;114:409–11. doi: 10.1097/AOG.0b013e3181b48feb. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson B, Gransberg S, Wikland M, Ylo¨stalo P, Torvid K, Marsal K, et al. Transvaginal ultrasonography of the endometrium in women with postmenopausal bleeding – A Nordic multicenter study. Am J Obstet Gynecol. 1995;172:1488–94. doi: 10.1016/0002-9378(95)90483-2. [DOI] [PubMed] [Google Scholar]
- 10.Smith-Bindman R, Kerlikowske K, Feldstein VA, Subak L, Scheidler J, Segal M, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA. 1998;280:1510–7. doi: 10.1001/jama.280.17.1510. [DOI] [PubMed] [Google Scholar]
- 11.Lidor A, Ismajovich B, Confino E, David MP. Histopathological findings in 226 women with postmenopausal uterine bleeding. Acta Obstet Gynecol Scand. 1986;65:41–39. doi: 10.3109/00016348609158227. [DOI] [PubMed] [Google Scholar]
- 12.Ubeja A, Singh A. Clinicopathological evaluation of postmenopausal bleeding in rural hospital set up. Int J Reprod Contracept Obstet Gynecol. 2017;6:3556–9. [Google Scholar]
- 13.Kothapally K, Bhashyakarla U. Postmenopausal bleeding: Clinicopathologic study in a teaching hospital of Andhra Pradesh. Int J Reprod Contracept Obstet Gynecol. 2013;2:344–8. [Google Scholar]
- 14.Nirupama V, Suneetha Y, Prabha Devi K. Post menopausal bleeding: An analytic study of 100 cases. Int J Sci Res. 2015;4:2319. [Google Scholar]


