Abstract
Context:
The utility of peritoneal washing cytology in patients with gastroesophageal junction cancer has not been thoroughly evaluated.
Aims:
The study aimed to determine the incidence of free peritoneal tumor cells by peritoneal washing cytology before and after neoadjuvant chemotherapy using conventional cytopathological methods and immunohistochemical staining for the analysis of peritoneal washings.
Settings and Design:
A prospective study conducted at a single tertiary referral hospital.
Materials and Methods:
Patients with gastroesophageal junction cancer and without suspicion of intra- or extraabdominal metastases before the staging laparoscopy were prospectively and consecutively enrolled. Peritoneal washing cytology was performed at staging laparoscopy (primary cytology) and after neoadjuvant chemotherapy during robot-assisted or open resection (secondary cytology). Peritoneal fluid samples were analyzed by conventional cytology and an immunohistochemical panel.
Results:
Overall, 81 patients met the primary inclusion criteria. During primary cytology, positive cytology without overt metastases (C1M0) was detected in three patients (3.8%) while five patients (6.3%) had overt intra-abdominal metastases but negative cytology (C0M1). None of the patients with C1M0 underwent surgery due to extra-abdominal (n = 1) or intra-abdominal metastases (n = 2), and the overall survival was 4, 7, and 14 months. During secondary cytology, no patients with free peritoneal tumor cells were identified, but seven patients were classified as C0M1 (10.9%).
Conclusions:
The incidence of C1M0 was 3.8% and 0% before and after neoadjuvant chemotherapy, respectively in patients with gastroesophageal junction cancer. Free peritoneal tumor cells were not identified in several patients with intra-abdominal metastases suggesting that peritoneal washing cytology with conventional cytology and immunohistochemical staining lack sensitivity.
Keywords: Cancer, esophagogastric junction, neoadjuvant chemotherapy, peritoneal lavage
INTRODUCTION
Peritoneal carcinomatosis is a negative prognostic factor for the patients with gastroesophageal junction (GEJ) cancer, and intended curative surgery is rarely indicated.[1] During diagnostic workup, the implementation of staging laparoscopy has improved the detection rate of patients with occult metastatic disease not verified by conventional CT-scans.[2,3] Yet, despite no signs of metastatic disease, peritoneal seeding often occurs before surgery and even during neoadjuvant chemotherapy. This is possibly due to the shedding of free peritoneal tumor cells (FPTCs) into the abdominal cavity when the primary tumor breach the serosal layer.[4,5,6] To detect FPTCs before overt metastases are formed, peritoneal washing cytology (PWC) has been introduced as a part of the staging program for gastric cancer. In gastric cancer, peritoneal fluid with tumor cells (positive cytology, C1) is a negative prognostic factor for the survival and recurrence even if macroscopic metastases are not present (C1M0).[7,8] According to The American Joint Committee on Cancer[9] and The Japanese Gastric Cancer Association,[10] C1 is classified as M1, that is, treatment should be nonsurgical. However, the conversion of C1M0 to negative cytology (C0) during neoadjuvant chemotherapy seems to improve survival indicating that the eradication of FPTCs is of importance, and surgery could be indicated for these patients.[11] Evaluations for the use of PWC in GEJ cancer are lacking, and only small studies have been published.[12] This exploratory study aimed to determine the incidence of C1M0 in patients with GEJ cancer at staging laparoscopy and after neoadjuvant chemotherapy during primary surgery using conventional cytopathological methods and immunohistochemical staining for the analysis of peritoneal washings.
MATERIALS AND METHODS
The study was conducted in accordance with the Helsinki Declaration of 1975 and was approved by the Danish Data Protection Agency (2012-58-0004) as registered at Clinical Trials (No. NCT02085564). Approval from the Ethics Committee was not obtained because the peritoneal samples were collected routinely according to the Danish clinical guidelines for the management of GEJ cancer.[13] Informed consent from all patients was provided verbally before initiating examinations and was documented in the patient record. The primary endpoint was the incidence of C1M0 before and after neoadjuvant chemotherapy.
Patients with a biopsy-verified tumor of the GEJ were enrolled consecutively between February 2014 and June 2015 at a single tertiary high-volume center. The diagnostic workup included a physical examination, an esophagogastro-duodenoscopy with biopsies, a thoraco-abdominal CT scan, an ultrasound examination of the neck, and/or PET-CT for tumor staging. A staging laparoscopy was only scheduled if the patient was considered operable and resectable at the multidisciplinary team conference without suspicion of intra- or extra-abdominal metastases.
Staging laparoscopy and primary PWC
Staging laparoscopy was performed during general anesthesia by insertion of a single 5 mm sub- or supraumbilical trocar. After the establishment of pneumoperitoneum, the four abdominal quadrants were inspected for any malignancy, that is, peritoneal carcinomatosis and/or liver metastases. If required, a second trocar was placed for the visualization of the stomach or GEJ. Intraoperative ultrasound was not used, thus, only superficial lesions could be assessed. For primary PWC, a pigtail catheter (Fr 10 × 25 cm, Argon Medical Devices Inc., Athens, USA) was inserted in the subhepatic space at the midclavicular line before the abdominal content was manipulated or biopsies obtained. Then, 500 mL warm isotonic saline was infused with the operation table in a neutral position. For equal distribution of fluid, the operation table was tilted in different positions for at least 30 sec at an angle of approximately 10°. First, the patient was head-up tilted on the right side and then on the left side. Second, the patients were head-down tilted on the left side and then on the right side. The saline was aspirated through the pigtail catheter for cytopathological workup, and retrieval of at least 100 mL of fluid was considered successful.
Neoadjuvant chemotherapy and surgery
Patients with no visible signs of metastatic disease on staging laparoscopy were treated with a neoadjuvant chemotherapy regimen consisting of three cycles of epirubicin, cisplatin or oxaliplatin, and capecitabine.[14] If metastases were identified during staging laparoscopy and verified histologically, palliative chemotherapy was offered.
For tumor resection, the Ivor Lewis esophagectomy procedure was used consisting of an abdominal and thoracic part.[15] The abdominal procedures were most often carried out by open resections while some were robot-assisted (da Vinci, S model, 5.0 robotic, Intuitive Surgical Inc., Sunnyvale, CA, USA) [Table 1]. The tumor was removed en bloc with a D1+ lymphadenectomy.
Table 1.
Patient characteristics
| Variable | n (%) or mean [SD] |
|---|---|
| Age, years | 66 [9.4] |
| Male sex | 64 (79.0) |
| CT-stage† | |
| T2 | 23 (28.4) |
| T3 | 50 (61.7) |
| T4 | 8 (9.9) |
| CN-stage† | |
| N0 | 31 (38.3) |
| N1 | 36 (44.4) |
| N2-3 | 14 (17.3) |
| Tumor histology | |
| Adenocarcinoma | 79 (97.5) |
| Neuroendocrine carcinoma | 1 (1.2) |
| Manec | 1 (1.2) |
| Neoadjuvant Chemotherapy | |
| None | 8 (10.8) |
| Started, not completed | 12 (16.2) |
| Completed | 54 (73.0) |
| Type of operation | |
| Open | 46 (70.0) |
| Robot-assisted | 20 (30.0) |
| Surgical results | |
| R0 | 59 (89.4) |
| Non-resectable | 7 (10.6) |
Tumors were classified according to the 7th edition of TNM-classification[9]. †T/N stage evaluated by CT-scan at the primary diagnostic workup. Manec: mixed adenoneurocrine carcinoma
Secondary PWC
The secondary PWC was performed immediately after the laparotomy before the manipulation of the tumor, and 500 mL of warm saline was equally distributed in the abdomen. The operation table was tilted as described for the primary PWC. A mechanical suction was used for aspiration in the subhepatic and the subphrenic spaces while manipulation around the GEJ area was carefully avoided. During robot-assisted resection, the same procedure as described for the primary PWC was applied. Secondary PWC was not performed if neoadjuvant chemotherapy had been abandoned or if the tumor was located distal to the GEJ as verified during surgery.
Cytopathological workup
The peritoneal fluid was centrifuged for 5 min at 1500 rpm immediately after the collection of the sample in the operation room. The specimens were smeared onto two glass slides and stained with hematoxylin-eosin (H and E) and May-Grünwald Giemsa. Furthermore, an artificial cell block was constructed and paraffin embedded. 4 μm sections were cut and mounted onto glass slides and stained with H and E. As a supplement, a panel of immunohistochemical stains consisting of CK7, EP4, calretinin, WT-1, D2-40, and CD68 was used. All slides were examined by a pathologist specialized in gastroesophageal cancer. The cytological findings were graded from I to V, where grade IV-V was considered as C1, that is, at least solid or certain evidence of malignancy.[16]
Statistics
Statistics were carried out with SPSS® version 22.0.0 (IBM Inc., IL, USA). The distribution of variables was performed with descriptive statistics and data were presented as means with SD or numbers with percentages.
RESULTS
The study population constituted 86 patients, but 5 patients were excluded because the tumors were located in the gastric fundus determined at the time of resection [Figure 1]. Thus, data from 81 patients were analyzed. Patient characteristics are presented in Table 1.
Figure 1.
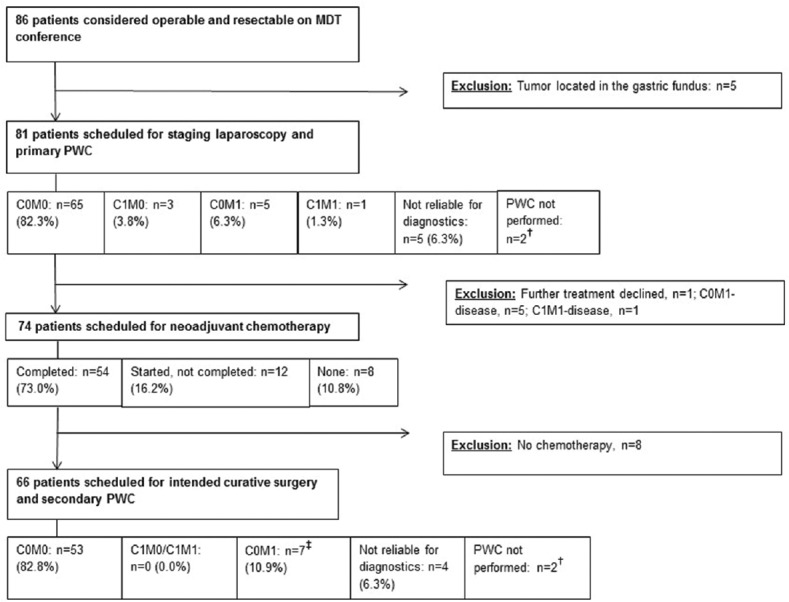
Flowchart of patient selection. †Technical assistance for peritoneal washing cytology (PWC) not available (n = 2). ‡Include two patients with C1M0 identified at staging laparoscopy and one patient with M1 with metastases in the thoracic cavity only
Primary PWC
Overall, primary PWC yielded three patients (3.8%) with C1M0 [Table 2]. Two of these patients completed neoadjuvant chemotherapy, and PWC reverted to C0 in both cases. However, definitive surgery was not attempted in any of the two cases due to peritoneal carcinomatosis and retroperitoneal tumor infiltration verified following laparotomy [Table 3]. The third patient presented with a cT2N1M0 stage with no overt intra-abdominal metastases on the initial CT-scan or staging laparoscopy. However, due to a suspicion of vertebral column metastases, a bone scintigraphy was conducted displaying multiple vertebral lesions. The patient was referred for palliative treatment, and secondary PWC was not repeated. Survival for the first, second, and third patients with C1M0 was 4, 7, and 14 months, respectively.
Table 2.
PWC results
| Primary PWC | Secondary PWC | |
|---|---|---|
| Aspirated fluid, mL | 140 [49.6] | 175.0 [47.3] |
| C0M0 | 65 (82.3) | 53 (82.8) |
| C1M0 | 3 (3.8) | 0 (0.0) |
| C0M1 | 5 (6.3) | 7 (10.9) |
| C1M1 | 1 (1.3) | 0 (0.0) |
| Not reliable for diagnostics | 5 (6.3) | 4 (6.3) |
| PWC not performed† | 2 | 2 |
Variables are numbers (%) or mean [SD]. †Technical assistance for PWC not available. PWC: peritoneal washing cytology; C1/C0: positive and negative peritoneal cytology, respectively; M1/M0: macroscopic metastases detected or not detected, respectively
Table 3.
Characteristics of patients with C1M0
| Patient | Age and sex | Tumor histology | Tumor stage† | Primary PWC results | Findings | Secondary PWC results | Survival (months)‡ |
|---|---|---|---|---|---|---|---|
| #1 | 79 ♀ | ADC | CT3N2M0 | CC: Suspicion of malignancy IHC: Positive for CK7 and EP4 |
No resection due to infiltration of retroperitoneum and the celiac trunk as local carcinomatosis | C0 | 14 |
| #2 | 61 ♀ | ADC | CT4N0M0 | CC: atypical cells IHC: Not performed due to small amount of cell material |
No resection due to retroperitoneal/colonic/mesenteric tumor infiltration | C0 | 5 |
| #3 | 64 ♀ | ADC | CT2N1M0 | CC: groups of malignant cells IHC: not performed |
No resection due to multiple cervical-, thoracic-, and sacral metastases detected by bone scintigraphy before planned surgery | Not performed§ | 7 |
†TNM stage evaluated at the primary diagnostic workup. ‡Survival after verification of disseminated disease. §Disseminated disease detected before planned surgery. CC: conventional cytology; IHC: immunohistochemical stains; ADC: adenocarcinoma
In five cases (6.3%), C1 was not detected, though intra-abdominal metastases were verified (C0M1) during staging laparoscopy. In all cases, metastases were located on the surface of the liver (left liver lobe, n = 1; right liver lobe, n = 3; both, n = 1) and these patients were offered palliative chemotherapy. A single patient was positive for FPTCs concomitantly with peritoneal and liver metastases (C1M1) and was also offered palliative treatment.
SECONDARY PWC
No patients with C1 were detected with secondary PWC, despite seven patients (11%) had overt metastases verified during the surgical procedure. In one of these patients, a tumor was found non-resectable in the thorax (infiltration of the left lung, pericardium, and thoracic aorta) with no evidence of concomitant intra-abdominal lesions. For the remaining six patients, the metastases were in the peritoneum, greater omentum, or in relation to the left gastric artery, the celiac trunk or the common hepatic artery. Hence, PWC failed to detect intra-abdominal metastases in six patients (9%) during secondary PWC. Of note, two of the six cases were patients with C1M0 verified at staging laparoscopy.
DISCUSSION
In this study, 3.8% and 0% were identified with C1M0 before and after neoadjuvant chemotherapy, respectively. For patients with overt intra-abdominal metastases, FPTCs were not detected in 6.3% and 9% of cases during primary and secondary PWC, respectively.
The risk of shedding tumor cells into the abdominal cavity is higher for advanced tumors (T3-4) and also depends on the area of serosa that is infiltrated.[4,16] Thus, due to the anatomical location, a lower prevalence of FPTCs might be expected in GEJ cancer compared to gastric cancer. In part, this could explain the low yield of FPTCs in the study and may limit the utility of PWC for detecting micrometastatic disease in GEJ cancer. We accept, however, that the utility of PWC is not examined thoroughly in GEJ cancer, and to our knowledge, this study represents the largest evaluation to date.
The use of conventional cytology is the preferred cytopathological method for the analysis of peritoneal washings[16,17] but shortcomings affecting analysis sensitivity should be considered and include, for example, poor distribution of peritoneal fluid, incorrect handling of the specimen before analysis, and/or infrequent exfoliation of cells from the tumor.[18,19] Two larger studies reported a prevalence of only 41–51% of FPTCs in patients with gastric cancer and peritoneal carcinomatosis indicating that conventional cytology lacks sensitivity.[16,17] To increase the diagnostic accuracy in this study, we added immunohistochemical stains to conventional cytology.[7] Yet, FPTCs were not detected in several patients despite that overt intra-abdominal metastases were identified. The results suggest that either FPTCs were not present or more likely not identified by the applied methods. Thus, the addition of more sensitive analysis tools could have been considered, for example, reverse transcriptase polymerase chain reaction. However, this method has been criticized for being time-consuming, expensive, and perhaps oversensitive involving the risk of false-positive results.[20]
Another methodological consideration is how peritoneal fluid is aspirated. In this study, the fluid was collected from the subhepatic and subphrenic spaces leaving the pouch of Douglas untouched which could potentially have contained FPTCs. Other studies have shown that the yield of FPTCs increases when peritoneal fluid is aspirated from multiple cavities. One study reported that pelvic washings alone would under stage about 23% of patients compared to combined washings from the subphrenic and pelvic cavities.[21] Another study demonstrated that the detection of FPTCs in three or four cavities compared to one or two resulted in a significantly lower progression-free survival as overall survival in a multivariate analysis.[22] Thus, aspiration of peritoneal fluid from multiple abdominal cavities seems to increase the sensitivity of PWC while also indicating the degree of tumor spread.
An interesting finding was the single patient with a less advanced tumor stage (cT2N1M0) and C1 suggesting that shedding of FPTCs occur even without transmural tumor invasion. One study found FPTCs in 7% and 18% with stage T1 and T2 tumors, respectively.[23] Likewise, another study reported that 27% of patients with C1M0 had stage T2 tumors.[24] Of several theories proposed, spreading of tumor cells through lymphatic channels seems likely to play a role in peritoneal dissemination.[25,26] It has been demonstrated that FPTCs can migrate through lymphatic channels into the openings on the peritoneal surface termed “milky spots,” and proliferate along with neovascularization to form metastases.[26] Therefore, PWC should be applied independently of the tumor stage.
Neoadjuvant chemotherapy may eradicate FPTCs and improve prognosis.[11] In this study, two patients with C1M0 seemed to revert to C0 during neoadjuvant chemotherapy. However, none of them underwent radical resection due to peritoneal carcinomatosis and retroperitoneal tumor infiltration verified after laparotomy, suggesting that FPTCs might have been overlooked during the analysis of the peritoneal fluid rather than actual eradication of FPTCs. Accordingly, the study results do not allow to conclude the effects of neoadjuvant chemotherapy on eradication of FPTCs in GEJ cancer.
The eradication of FPTCs seems feasible in gastric cancer. One study found that 37% of C1M0 patients reverted to C0 after neoadjuvant chemotherapy consisting of folinic acid, cisplatin, and 5-fluorouracil administrated for at least six weeks.[11] Correspondingly, median survival after resection was improved to 36 months compared with 9 months for the persistent C1-group. Likewise, another study reported a reversion rate of 56% from C1 to C0 during cisplatin-based neoadjuvant chemotherapy.[27] Of note, eight of the patients who reverted to negative cytology had initial peritoneal metastases which were eradicated along with conversion to C0. Hence, repeated washings may contribute to identifying patients suitable for surgery because reversion from C1 seems to occur in more than a third of cases. On the other hand, 24% of patients remarkably progress from C0 to C1 during neoadjuvant chemotherapy detected by conventional cytology and immunohistochemical stains using an EP4 antibody,[11] thus, suggesting unresponsiveness to treatment and/or aggressive tumor biology.
Limitations of the study should be considered. Approximately, 6% of the PWC samples were judged to be non-reliable for diagnostics during primary and secondary PWC due to few cells in the peritoneal fluid. Nevertheless, we consider it less likely that FPTCs would have been present in the samples because none of the patients had overt metastases at staging laparoscopy or during the surgical procedure. Moreover, R0 resections were achieved in all cases, and therefore we estimate the risk of selection bias small. Due to the infrequent incidence of C1, follow-up was not extended beyond 14 months since none of the patients with FPTCs had radical surgery performed. Thus, it remains to be determined whether surgery improves outcome for patients with GEJ cancer and C1M0.
In conclusion, the incidence of FPTCs was 3.8% before and 0% after neoadjuvant chemotherapy. FPTCs were not identified in several patients with visible intra-abdominal metastases suggesting that PWC with conventional cytology and immunohistochemical stains lack sensitivity in GEJ cancer.
Financial support and sponsorship
This study was supported by The Danish Cancer Research Foundation (R84-A5558), Direktør Jacob Madsen and Hustru Olga Madsens Fond, and Familien Erichsens Mindefond.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors wish to thank Linda Bardram, Steen Kofoed, Jakob Holm, Pieter De Heer, and Marianne Jendresen for technical assistance during the study.
REFERENCES
- 1.Siewert JR, Bottcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: Ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449–61. doi: 10.1097/00000658-199810000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strandby RB, Svendsen LB, Fallentin E, Egeland C, Achiam MP. The multidisciplinary team conference's decision on M-staging in patients with gastric- and gastroesophageal cancer is not accurate without staging laparoscopy. Scand J Surg. 2016;105:104–8. doi: 10.1177/1457496915598760. [DOI] [PubMed] [Google Scholar]
- 3.Coccolini F, Gheza F, Lotti M, Virzi S, Iusco D, Ghermandi C, et al. Peritoneal carcinomatosis. World J Gastroenterol. 2013;19:6979–94. doi: 10.3748/wjg.v19.i41.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribeiro U, Jr, Gama-Rodrigues JJ, Bitelman B, Ibrahim RE, Safatle-Ribeiro AV, Laudanna AA, et al. Value of peritoneal lavage cytology during laparoscopic staging of patients with gastric carcinoma. Surg Laparosc Endosc. 1998;8:132–5. [PubMed] [Google Scholar]
- 5.Ribeiro U, Jr, Safatle-Ribeiro AV, Zilberstein B, Mucerino D, Yagi OK, Bresciani CC, et al. Does the intraoperative peritoneal lavage cytology add prognostic information in patients with potentially curative gastric resection? J Gastrointest Surg. 2006;10:170–6. doi: 10.1016/j.gassur.2005.11.001. discussion 6-7. [DOI] [PubMed] [Google Scholar]
- 6.Maehara Y, Hasuda S, Koga T, Tokunaga T, Sugimachi K. Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg. 2000;87:353–7. doi: 10.1046/j.1365-2168.2000.01358.x. [DOI] [PubMed] [Google Scholar]
- 7.Nekarda H, Gess C, Stark M, Mueller JD, Schenck U, Siewert JR. Immunocytochemically detected free peritoneal tumour cells (FPTC) are a strong prognostic factor in gastric carcinoma. Br J Cancer. 1999;79:611–9. doi: 10.1038/sj.bjc.6690096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukagawa T, Katai H, Saka M, Morita S, Sasajima Y, Taniguchi H, et al. Significance of lavage cytology in advanced gastric cancer patients. World J Surg. 2010;34:563–8. doi: 10.1007/s00268-009-0355-1. [DOI] [PubMed] [Google Scholar]
- 9.Washington K. 7th edition of the AJCC cancer staging manual: Stomach. Ann Surg Oncol. 2010;17:3077–9. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 10.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver.4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenzen S, Panzram B, Rosenberg R, Nekarda H, Becker K, Schenk U, et al. Prognostic significance of free peritoneal tumor cells in the peritoneal cavity before and after neoadjuvant chemotherapy in patients with gastric carcinoma undergoing potentially curative resection. Ann Surg Oncol. 2010;17:2733–9. doi: 10.1245/s10434-010-1090-4. [DOI] [PubMed] [Google Scholar]
- 12.Nath J, Moorthy K, Taniere P, Hallissey M, Alderson D. Peritoneal lavage cytology in patients with oesophagogastric adenocarcinoma. Br J Surg. 2008;95:721–6. doi: 10.1002/bjs.6107. [DOI] [PubMed] [Google Scholar]
- 13.The Danish Esophagus, Cardiac, and Gastric Cancer Group, National Clinical Guidelines 2016. [Last accessed on 2018 Jun 01]. Available from: http://decv.gicancer.dk/Content/Files/Dokumenter/DECV_rapport2016_til%20regionerne.pdf .
- 14.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 15.Reed CE. Technique of open ivor lewis esophagectomy. Oper Tech Thorac Cardiovasc Surg. 2009;14:160–75. [Google Scholar]
- 16.Bando E, Yonemura Y, Takeshita Y, Taniguchi K, Yasui T, Yoshimitsu Y, et al. Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. Am J Surg. 1999;178:256–62. doi: 10.1016/s0002-9610(99)00162-2. [DOI] [PubMed] [Google Scholar]
- 17.Burke EC, Karpeh MS, Jr, Conlon KC, Brennan MF. Peritoneal lavage cytology in gastric cancer: An independent predictor of outcome. Ann Surg Oncol. 1998;5:411–5. doi: 10.1007/BF02303859. [DOI] [PubMed] [Google Scholar]
- 18.Lin O. Challenges in the interpretation of peritoneal cytologic specimens. Arch Pathol Lab Med. 2009;133:739–42. doi: 10.5858/133.5.739. [DOI] [PubMed] [Google Scholar]
- 19.Runyon BA. Malignancy-related ascites and ascitic fluid “humoral tests of malignancy”. J Clin Gastroenterol. 1994;18:94–8. doi: 10.1097/00004836-199403000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Leake PA, Cardoso R, Seevaratnam R, Lourenco L, Helyer L, Mahar A, et al. A systematic review of the accuracy and indications for diagnostic laparoscopy prior to curative-intent resection of gastric cancer. Gastric Cancer. 2012;15(Suppl 1):S38–47. doi: 10.1007/s10120-011-0047-z. [DOI] [PubMed] [Google Scholar]
- 21.Munasinghe A, Kazi W, Taniere P, Hallissey MT, Alderson D, Tucker O. The incremental benefit of two quadrant lavage for peritoneal cytology at staging laparoscopy for oesophagogastric adenocarcinoma. Surg Endosc. 2013;11:4049–53. doi: 10.1007/s00464-013-3058-5. [DOI] [PubMed] [Google Scholar]
- 22.Homma Y, Ushida S, Yamada M, Kobayashi H, Suzuki K. Positive peritoneal washing cytology in multiple cavities can predict poor prognosis of advanced gastric cancer patients. Ann Surg Oncol. 2010;17:455–60. doi: 10.1245/s10434-009-0764-2. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg R, Nekarda H, Bauer P, Schenck U, Hoefler H, Siewert JR. Free peritoneal tumour cells are an independent prognostic factor in curatively resected stage IB gastric carcinoma. Br J Surg. 2006;93:325–31. doi: 10.1002/bjs.5196. [DOI] [PubMed] [Google Scholar]
- 24.Oh CA, Bae JM, Oh SJ, Choi MG, Noh JH, Sohn TS, et al. Long-term results and prognostic factors of gastric cancer patients with only positive peritoneal lavage cytology. J Surg Oncol. 2012;105:393–9. doi: 10.1002/jso.22091. [DOI] [PubMed] [Google Scholar]
- 25.Yonemura Y, Canbay E, Endou Y, Ishibashi H, Mizumoto A, Miura M, et al. Peritoneal cancer treatment. Expert Opin Pharmacother. 2014;15:623–36. doi: 10.1517/14656566.2014.879571. [DOI] [PubMed] [Google Scholar]
- 26.Yonemura Y, Kawamura T, Bandou E, Tsukiyama G, Endou Y, Miura M. The natural history of free cancer cells in the peritoneal cavity. Recent Results Cancer Res. 2007;169:11–23. doi: 10.1007/978-3-540-30760-0_2. [DOI] [PubMed] [Google Scholar]
- 27.Mezhir JJ, Shah MA, Jacks LM, Brennan MF, Coit DG, Strong VE. Positive peritoneal cytology in patients with gastric cancer: Natural history and outcome of 291 patients. Indian J Surg Oncol. 2011;2:16–23. doi: 10.1007/s13193-011-0074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


